Nitrogen Source Preference and Growth Carbon Costs of Leucaena leucocephala (Lam.) de Wit Saplings in South African Grassland Soils
Abstract
:1. Introduction
2. Results
2.1. Soil Characteristics
2.2. Soil Bacteria and Plant Endophytic Bacterial Isolates Identification
2.3. Biomass and Mineral Contents
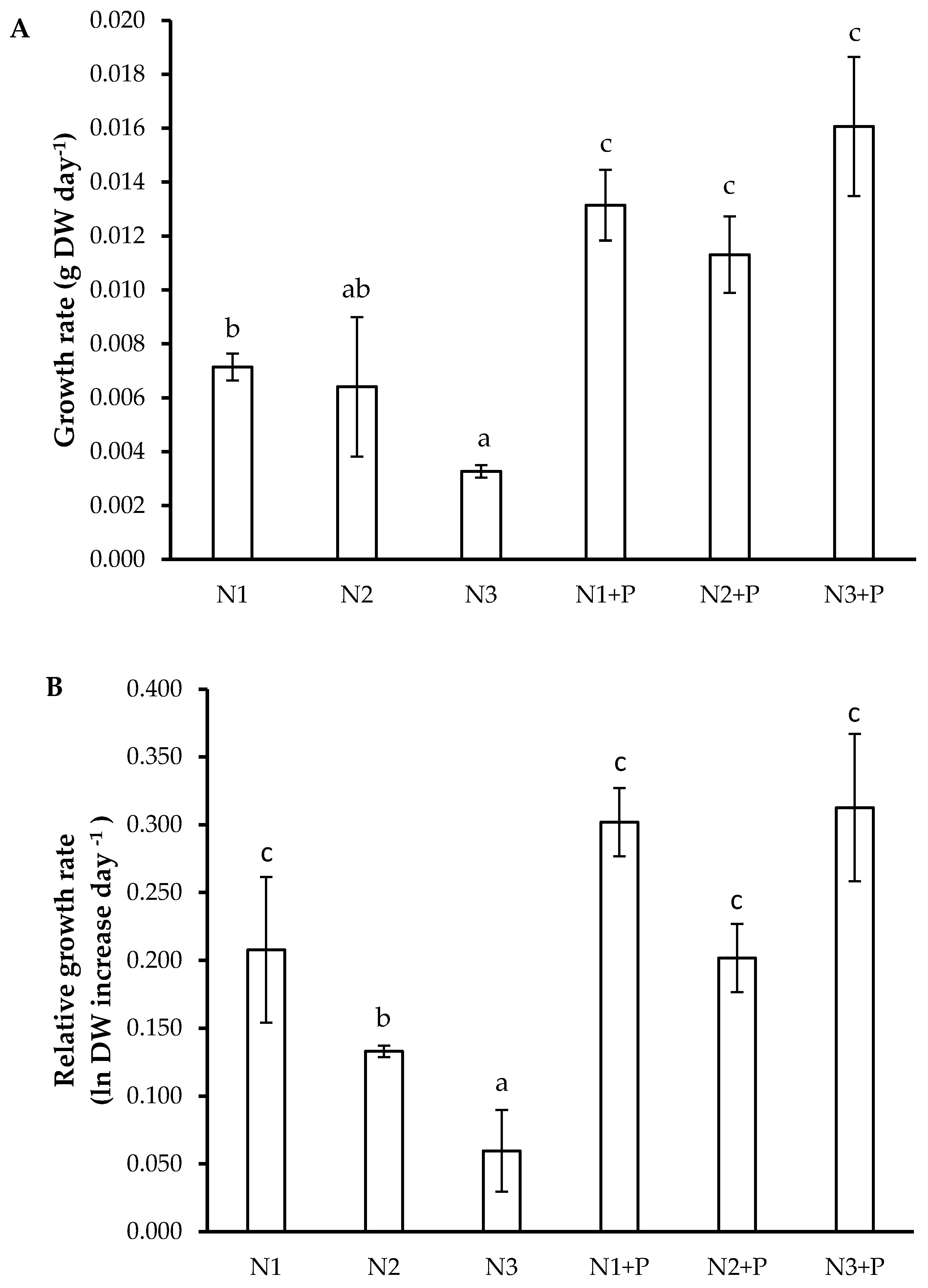
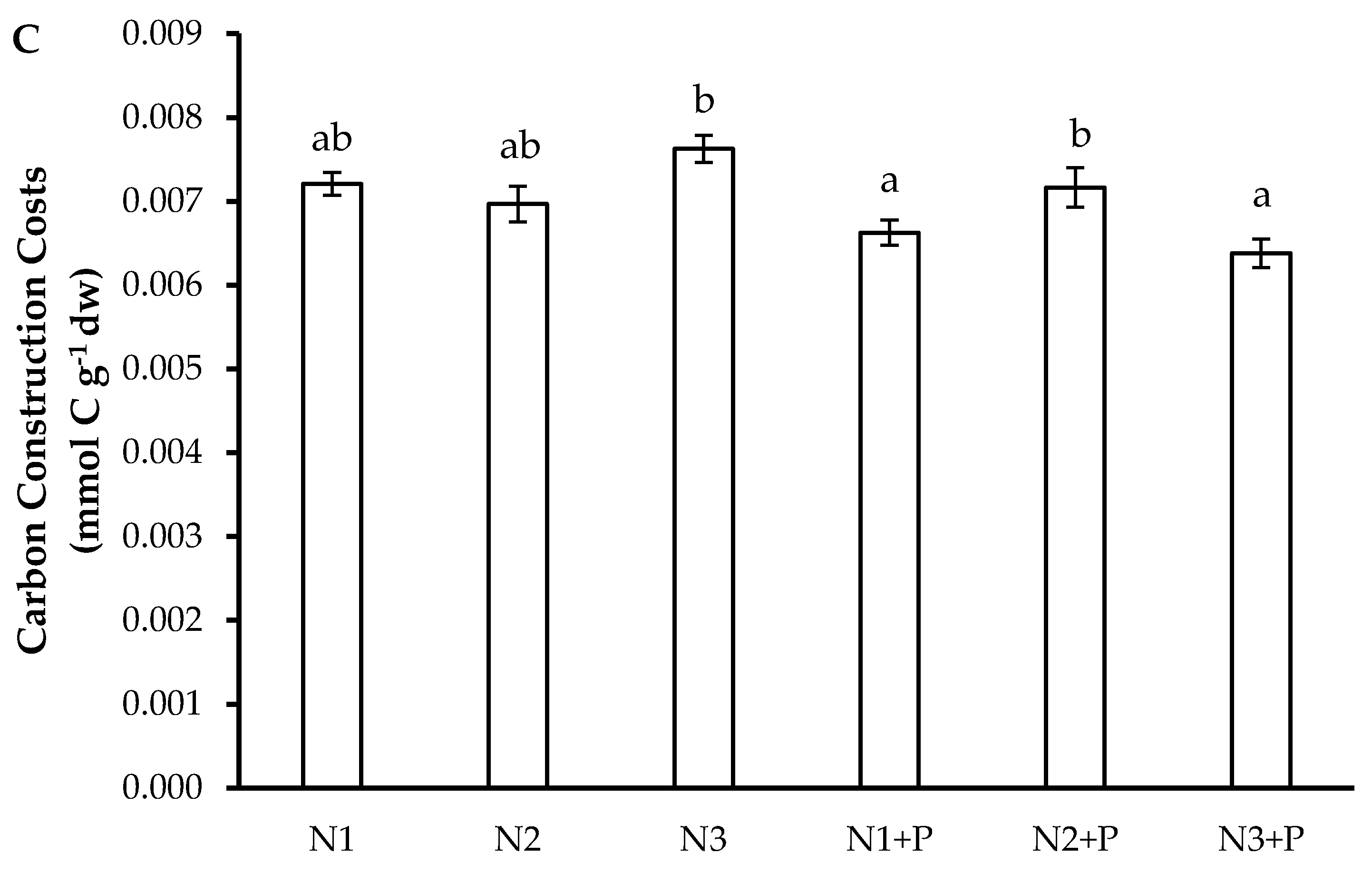
2.4. Growth Rates and Carbon Construction Costs
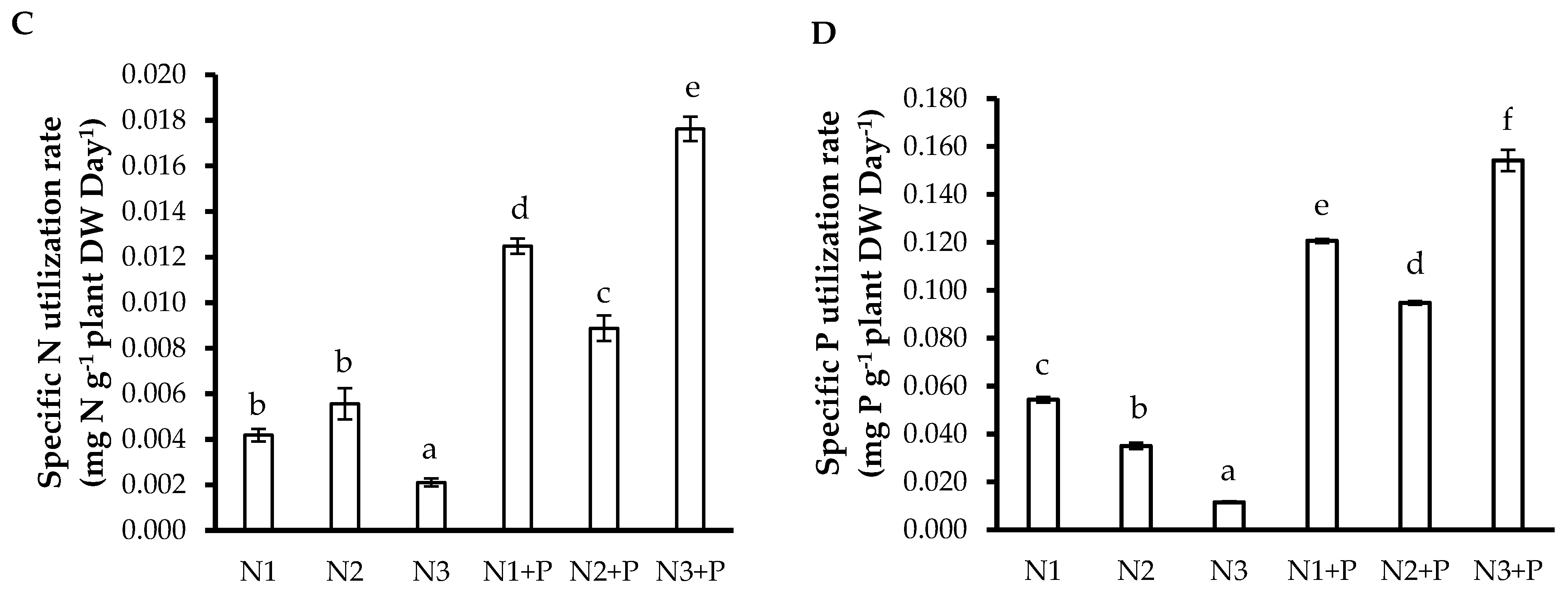
2.5. N and P Use Efficiencies
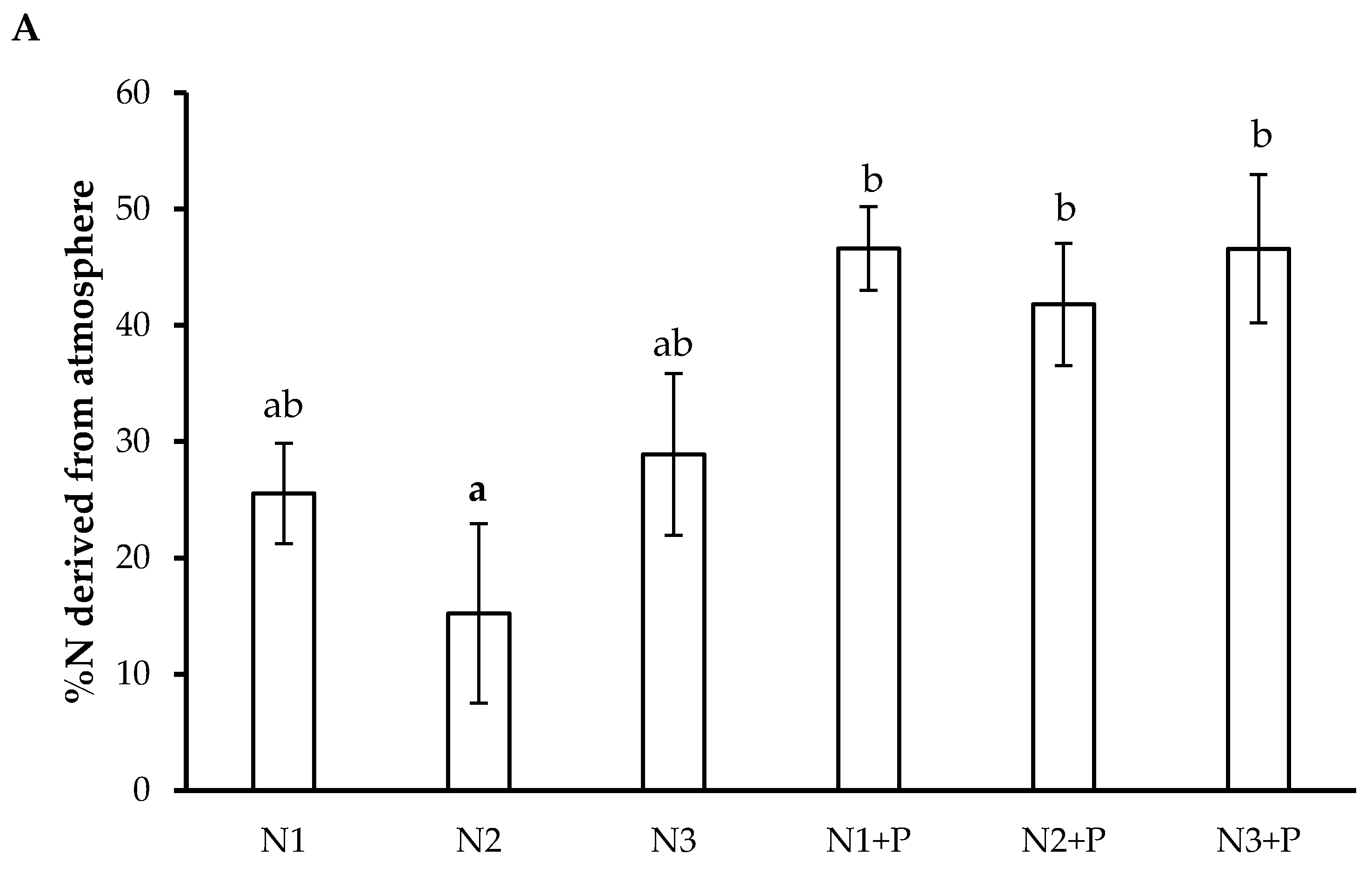
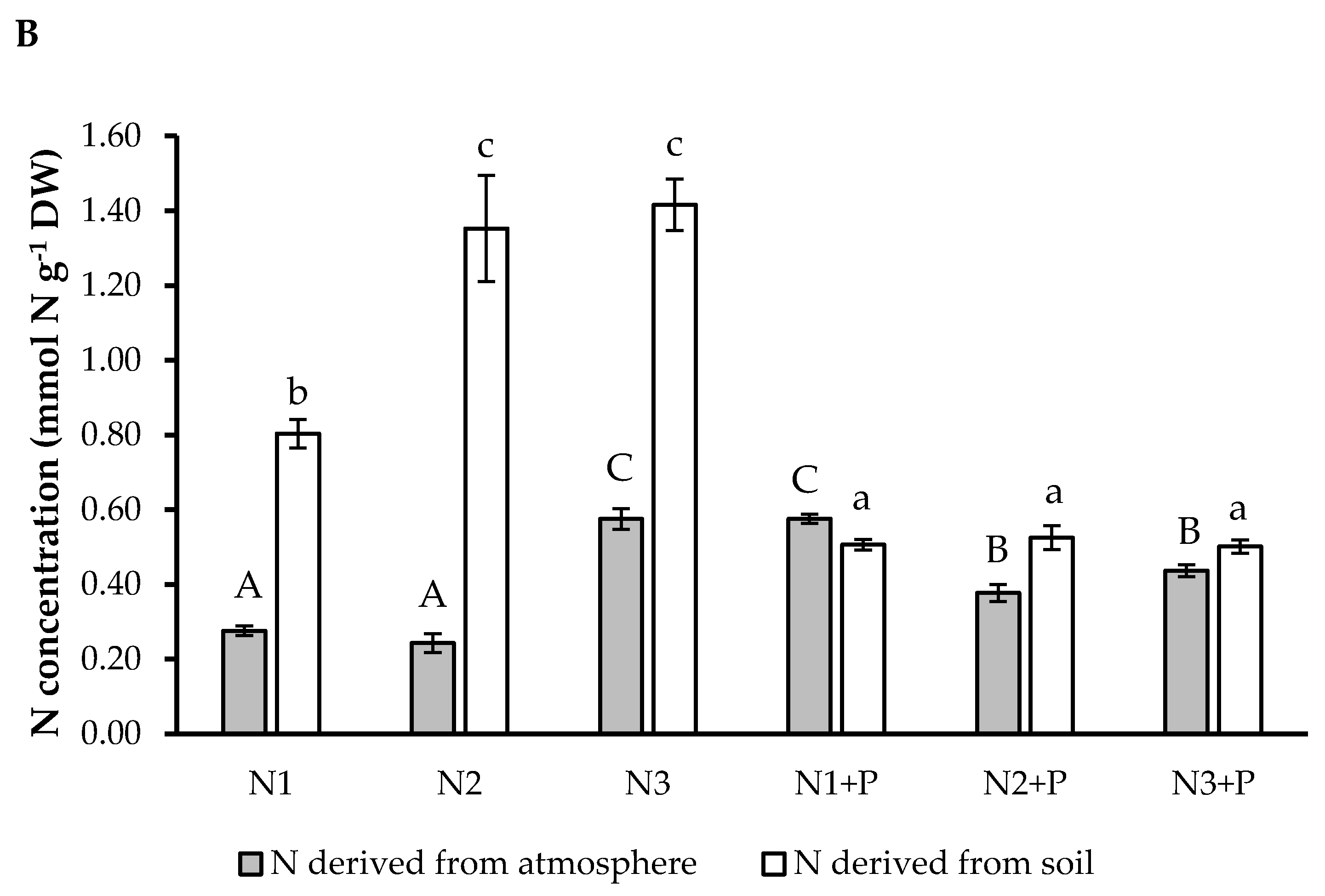
2.6. N Preference
3. Discussion
4. Materials and Methods
4.1. Study Site
4.2. Experimental Design
4.3. Soil Characteristics Analysis and Bacterial Identification
4.4. Seed Collection, Germination and Growth Conditions
4.5. Plant Harvesting and Nutrient Analysis
4.6. Bacterial Extraction and Identification
4.7. Growth Calculations
4.7.1. Calculation of the Specific N/P Absorption and Utilization Rates
4.7.2. Carbon Construction Costs
4.7.3. Determination and Calculation of N Derived from the Atmosphere
5. Statistical Analysis
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hughes, C.; Department of Plant Sciences, University of Oxford, Oxford, UK. Compiler: IUCN/SSC Invasive Species Specialist Group (ISSG), Global Invasive Species Database: 2010. Available online: http://www.iucngisd.org/gisd/speciesname/Leucaena+leucocephala (accessed on 2 April 2014).
- Olckers, T. Biological control of Leucaena leucocephala (Lam.) de Wit (Fabaceae) in South Africa: A tale of opportunism, seed feeders and unanswered questions. Afr. Entomol. 2011, 19, 356–365. [Google Scholar] [CrossRef]
- Henderson, L. Invasive, naturalized and casual alien plants in southern Africa: A summary based on the Southern African Plant Invaders Atlas (SAPIA). Bothalia 2007, 37, 215–248. [Google Scholar] [CrossRef]
- Bond, W.J. Ancient grasslands at risk. Science 2016, 351, 120–122. [Google Scholar] [CrossRef] [PubMed]
- Duke, J. Handbook of Legumes of World Economic Importance; Springer Science & Business Media: New York, NY, USA, 2012. [Google Scholar]
- Leakey, R.R.; Tchoundjeu, Z.; Schreckenberg, K.; Shackleton, S.E.; Shackleton, C.M. Agroforestry tree products (AFTPs): Targeting poverty reduction and enhanced livelihoods. Int. J. Agric. Sustain. 2005, 3, 1–23. [Google Scholar] [CrossRef]
- Craine, J.M.; Morrow, C.; Stock, W.D. Nutrient concentration ratios and co-limitation in South African grasslands. New Phytol. 2008, 179, 829–836. [Google Scholar] [CrossRef]
- Sankaran, M.; Ratnam, J.; Hanan, N. Woody cover in African savannas: The role of resources, fire and herbivory. Glob. Ecol. Biogeogr. 2008, 17, 236–245. [Google Scholar] [CrossRef]
- Mafongoya, P.; Bationo, A.; Kihara, J.; Waswa, B.S. Appropriate technologies to replenish soil fertility in southern Africa. Nutr. Cycl. Agroecosyst. 2006, 76, 137–151. [Google Scholar] [CrossRef]
- Bargaz, A.; Drevon, J.-J.; Oufdou, K.; Mandri, B.; Faghire, M.; Ghoulam, C. Nodule phosphorus requirement and O2 uptake in common bean genotypes under phosphorus deficiency. Acta Agric. Scand. Sect. B-Soil Plant Sci. 2011, 61, 602–611. [Google Scholar] [CrossRef]
- Ndlovu, J.; Richardson, D.M.; Wilson, J.R.; Le Roux, J.J. Co-invasion of South African ecosystems by an Australian legume and its rhizobial symbionts. J. Biogeogr. 2013, 40, 1240–1251. [Google Scholar] [CrossRef]
- Reinhart, K.O.; Callaway, R.M. Soil biota and invasive plants. New Phytol. 2006, 170, 445–457. [Google Scholar] [CrossRef]
- Rodríguez-Echeverría, S.; Crisóstomo, J.A.; Nabais, C.; Freitas, H. Belowground mutualists and the invasive ability of Acacia longifolia in coastal dunes of Portugal. Biol. Invasions 2009, 11, 651–661. [Google Scholar] [CrossRef] [Green Version]
- Kaschuk, G.; Kuyper, T.W.; Leffelaar, P.A.; Hungria, M.; Giller, K.E. Are the rates of photosynthesis stimulated by the carbon sink strength of rhizobial and arbuscular mycorrhizal symbioses? Soil Biol. Biochem. 2009, 41, 1233–1244. [Google Scholar] [CrossRef]
- Magadlela, A.; Pérez-Fernández, M.A.; Kleinert, A.; Dreyer, L.L.; Valentine, A.J. Source of inorganic N affects the cost of growth in a legume tree species (Virgilia divaricata) from the Mediterrean-type Fynbos ecosystem. J. Plant Ecol. 2016, 9, 752–761. [Google Scholar] [CrossRef] [Green Version]
- Mortimer, P.E.; Pérez-Fernández, M.A.; Valentine, A.J. Arbuscular mycorrhiza maintains nodule function during external NH 4+ supply in Phaseolus vulgaris (L.). Mycorrhiza 2012, 22, 237–245. [Google Scholar] [CrossRef]
- Minchin, F.R.; Witty, J.F. Respiratory/carbon costs of symbiotic nitrogen fixation in legumes. In Plant Respiration; Springer: Dordrecht, The Netherlands, 2005; pp. 195–205. [Google Scholar] [CrossRef]
- Magadlela, A. Variation in Phosphorus Supply Alters Nitrogen Metabolism in the Nodules and Roots of Virgilia Divaricata, a Cape Fynbos Indigenous Legume from the Cape Floristic Region; Stellenbosch University: Stellenbosch, South Africa, 2016. [Google Scholar]
- Abbadie, L.; Mariotti, A.; Menaut, J.-C. Independence of savanna grasses from soil organic matter for their nitrogen supply. Ecology 1992, 73, 608–613. [Google Scholar] [CrossRef]
- Marchante, E.; Kjøller, A.; Struwe, S.; Freitas, H. Soil recovery after removal of the N 2-fixing invasive Acacia longifolia: Consequences for ecosystem restoration. Biol. Invasions 2009, 11, 813–823. [Google Scholar] [CrossRef] [Green Version]
- Stock, W.; Wienand, K.; Baker, A. Impacts of invading N 2-fixing Acacia species on patterns of nutrient cycling in two Cape ecosystems: Evidence from soil incubation studies and 15 N natural abundance values. Oecologia 1995, 101, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Mortimer, P.E.; Le Roux, M.R.; Pérez-Fernández, M.A.; Benedito, V.A.; Kleinert, A.; Xu, J.; Valentine, A.J. The dual symbiosis between arbuscular mycorrhiza and nitrogen fixing bacteria benefits the growth and nutrition of the woody invasive legume Acacia cyclops under nutrient limiting conditions. Plant Soil 2013, 366, 229–241. [Google Scholar] [CrossRef]
- Blackburn, T.M.; Pyšek, P.; Bacher, S.; Carlton, J.T.; Duncan, R.P.; Jarošík, V.; Wilson, J.R.; Richardson, D.M. A proposed unified framework for biological invasions. Trends Ecol. Evol. 2011, 26, 333–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macdonald, I.; Reaser, J.; Bright, C.; Neville, L.; Howard, G.; Murphy, S.; Preston, G. Invasive alien species in Southern Africa: National reports and directory of resources. Invasive Alien Species South. Afr. Natl. Rep. Dir. Resour. 2003, 125. [Google Scholar]
- Morris, T.L.; Esler, K.J.; Barger, N.N.; Jacobs, S.M.; Cramer, M.D. Ecophysiological traits associated with the competitive ability of invasive Australian acacias. Divers. Distrib. 2011, 17, 898–910. [Google Scholar] [CrossRef]
- Lawrie, A. Nitrogen fixation by native Australian legumes. Aust. J. Bot. 1981, 29, 143–157. [Google Scholar] [CrossRef]
- Ferguson, B.; Lin, M.-H.; Gresshoff, P.M. Regulation of legume nodulation by acidic growth conditions. Plant Signal. Behav. 2013, 8, e23426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lira Junior, M.A. Legume-rhizobia signal exchange: Promiscuity and environmental effects. Front. Microbiol. 2015, 6, 945. [Google Scholar] [CrossRef]
- Britto, D.T.; Kronzucker, H.J. NH4+ toxicity in higher plants: A critical review. J. Plant Physiol. 2002, 159, 567–584. [Google Scholar] [CrossRef] [Green Version]
- Jebara, M.; Aouani, M.E.; Payre, H.; Drevon, J.-J. Nodule conductance varied among common bean (Phaseolus vulgaris) genotypes under phosphorus deficiency. J. Plant Physiol. 2005, 162, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Hellsten, A.; Huss-Danell, K. Interaction effects of nitrogen and phosphorus on nodulation in red clover (Trifolium pratense L.). Acta Agric. Scand. Sect. B-Plant Soil Sci. 2000, 50, 135–142. [Google Scholar] [CrossRef]
- Vance, C.P.; Uhde-Stone, C.; Allan, D.L. Phosphorus acquisition and use: Critical adaptations by plants for securing a nonrenewable resource. New Phytol. 2003, 157, 423–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, C.; Peng, R.; Luo, Y.; Zhou, X.; Wu, X.; Fang, C.; Chen, J.; Li, B. Altered ecosystem carbon and nitrogen cycles by plant invasion: A meta-analysis. New Phytol. 2008, 177, 706–714. [Google Scholar] [CrossRef]
- Van Der Heijden, M.G.; Bardgett, R.D.; Van Straalen, N.M. The unseen majority: Soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol. Lett. 2008, 11, 296–310. [Google Scholar] [CrossRef]
- Yadav, A.N.; Verma, P.; Singh, B.; Chauhan, V.S.; Suman, A.; Saxena, A.K. Plant growth promoting bacteria: Biodiversity and multifunctional attributes for sustainable agriculture. Adv. Biotechnol. Microbiol. 2017, 5, 1–16. [Google Scholar] [CrossRef]
- Elser, J.J.; Bracken, M.E.; Cleland, E.E.; Gruner, D.S.; Harpole, W.S.; Hillebrand, H.; Ngai, J.T.; Seabloom, E.W.; Shurin, J.B.; Smith, J.E. Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol. Lett. 2007, 10, 1135–1142. [Google Scholar] [CrossRef] [Green Version]
- Keerthisinghe, G.; Hocking, P.; Ryan, P.; Delhaize, E. Effect of phosphorus supply on the formation and function of proteoid roots of white lupin (Lupinus albus L.). Plant Cell Environ. 1998, 21, 467–478. [Google Scholar] [CrossRef]
- Ward, D.; Kirkman, K.P.; Tsvuura, Z.; Morris, C.; Fynn, R.W. Are there common assembly rules for different grasslands? Comparisons of long-term data from a subtropical grassland with temperate grasslands. J. Veg. Sci. 2020, 31, 780–791. [Google Scholar] [CrossRef]
- Mucina, L.; Rutherford, M.C. The Vegetation of South Africa, Lesotho and Swaziland; South African National Biodiversity Institute: Pretoria, South Africa, 2006; p. 807.
- Bilskie, J.; Scientific, C. Soil Water Status: Content and Potential. Campbell Scientific, Inc. App. Note: 2S-1. 2001. Available online: http://s.campbellsci.com/documents/ca/technical-papers/soilh20c.pdf (accessed on 2 April 2014).
- Aagot, N.; Nybroe, O.; Nielsen, P.; Johnsen, K. An altered pseudomonas diversity is recovered from soil by using nutrient-poorpseudomonas-selective soil extract media. Appl. Environ. Microbiol. 2001, 67, 5233–5239. [Google Scholar] [CrossRef] [Green Version]
- Zungu, N.S.; Egbewale, S.O.; Olaniran, A.O.; Pérez-Fernández, M.; Magadlela, A. Soil nutrition, microbial composition and associated soil enzyme activities in KwaZulu-Natal grasslands and savannah ecosystems soils. Appl. Soil Ecol. 2020, 155, 103663. [Google Scholar] [CrossRef]
- Nielsen, K.L.; Eshel, A.; Lynch, J.P. The effect of phosphorus availability on the carbon economy of contrasting common bean (Phaseolus vulgaris L.) genotypes. J. Exp. Bot. 2001, 52, 329–339. [Google Scholar] [CrossRef]
- ÅGREN, G.I.; Franklin, O. Root: Shoot ratios, optimization and nitrogen productivity. Ann. Bot. 2003, 92, 795–800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mortimer, P.; Archer, E.; Valentine, A. Mycorrhizal C costs and nutritional benefits in developing grapevines. Mycorrhiza 2005, 15, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Eissenstat, D.M.; Graham, J.H.; Williams, K.; Hodge, N.C. Growth depression in mycorrhizal citrus at high-phosphorus supply (analysis of carbon costs). Plant Physiol. 1993, 101, 1063–1071. [Google Scholar] [CrossRef]
- Williams, K.; Percival, F.; Merino, J.; Mooney, H. Estimation of tissue construction cost from heat of combustion and organic nitrogen content. Plant Cell Environ. 1987, 10, 725–734. [Google Scholar] [CrossRef]

| Parameter | Treatment Trials | |||||
|---|---|---|---|---|---|---|
| N1 | N2 | N3 | N1 + P | N2 + P | N3 + P | |
| Biomass (g DW) | ||||||
| Total plant | 1.285 ± 0.090 b | 1.154 ± 0.465 ab | 0.589 ± 0.042 a | 2.366 ± 0.236 c | 2.036 ± 0.236 bc | 2.891 ± 0.503 c |
| Leaves | 0.403 ± 0.034 b | 0.373 ± 0.126 a | 0.176 ± 0.008 a | 0.737 ± 0.106 b | 0.516 ± 0.087 b | 0.643 ± 0.098 b |
| Shoot | 0.305 ± 0.026 b | 0.272 ± 0.086 ab | 0.179 ± 0.026 a | 0.579 ± 0.056 c | 0.555 ± 0.075 c | 0.761 ± 0.099 c |
| Roots | 0.576 ± 0.040 bc | 0.509 ± 0.257 ab | 0.233 ± 0.020 a | 1.049 ± 0.122 d | 0.964 ± 0.098 cd | 1.487 ± 0.345 d |
| Root–shoot ratio | 0.819 ± 0.039 ab | 0.667 ± 0.100 a | 0.659 ± 0.039 a | 0.804 ± 0.063 ab | 0.924 ± 0.054 ab | 1.049 ± 0.138 b |
| Mineral contents | ||||||
| Total plant N (mmol N g−1 DW) | 1.080 ± 0.051 a | 1.596 ± 0.168 b | 1.992 ± 0.096 c | 0.948 ± 0.027 a | 0.902 ± 0.055 a | 0.938 ± 0.034 a |
| Standard corrected 15N/14N | 2.550 ± 0.151 ab | 3.262 ± 0.177 b | 2.319 ± 0.230 ab | 1.099 ± 0.169 a | 1.430 ± 0.200 a | 1.101 ± 0.322 a |
| Total plant P (µmol P g−1 DW) | 60.92 ± 5.615 b | 21.37 ± 2.739 a | 15.63 ± 0.912 a | 76.60 ± 2.896 b | 59.22 ± 2.372 b | 82.99 ± 12.99 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sithole, N.; Tsvuura, Z.; Kirkman, K.; Magadlela, A. Nitrogen Source Preference and Growth Carbon Costs of Leucaena leucocephala (Lam.) de Wit Saplings in South African Grassland Soils. Plants 2021, 10, 2242. https://doi.org/10.3390/plants10112242
Sithole N, Tsvuura Z, Kirkman K, Magadlela A. Nitrogen Source Preference and Growth Carbon Costs of Leucaena leucocephala (Lam.) de Wit Saplings in South African Grassland Soils. Plants. 2021; 10(11):2242. https://doi.org/10.3390/plants10112242
Chicago/Turabian StyleSithole, Nonkululeko, Zivanai Tsvuura, Kevin Kirkman, and Anathi Magadlela. 2021. "Nitrogen Source Preference and Growth Carbon Costs of Leucaena leucocephala (Lam.) de Wit Saplings in South African Grassland Soils" Plants 10, no. 11: 2242. https://doi.org/10.3390/plants10112242







