Targeting the B1 Gene and Analysis of Its Polymorphism Associated with Awned/Awnless Trait in Russian Germplasm Collections of Common Wheat
Abstract
:1. Introduction
2. Results
2.1. Analysis of C2H2Zf, Putative Candidate for the B1- Gene
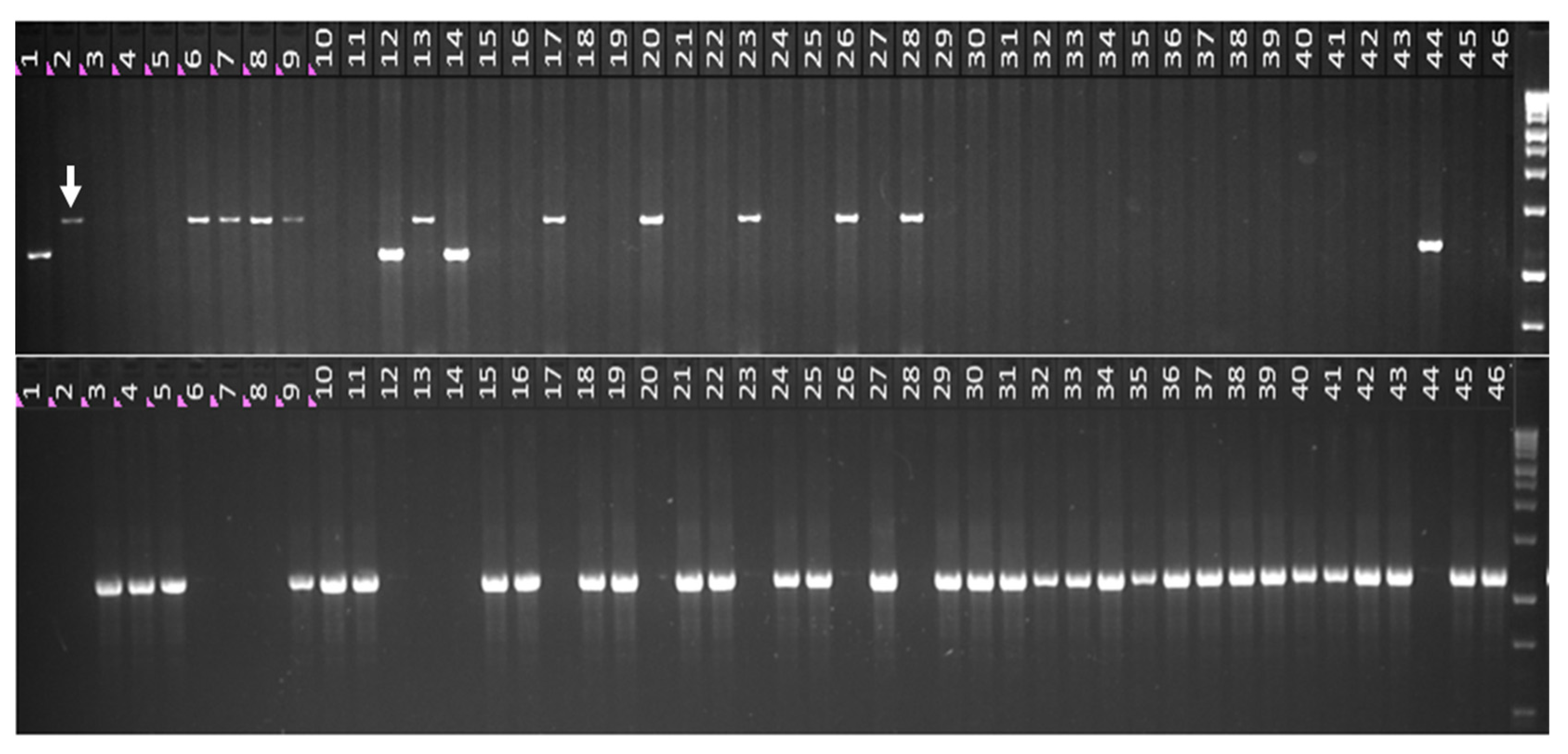
2.2. Design and Confirmation of Molecular B1- Markers
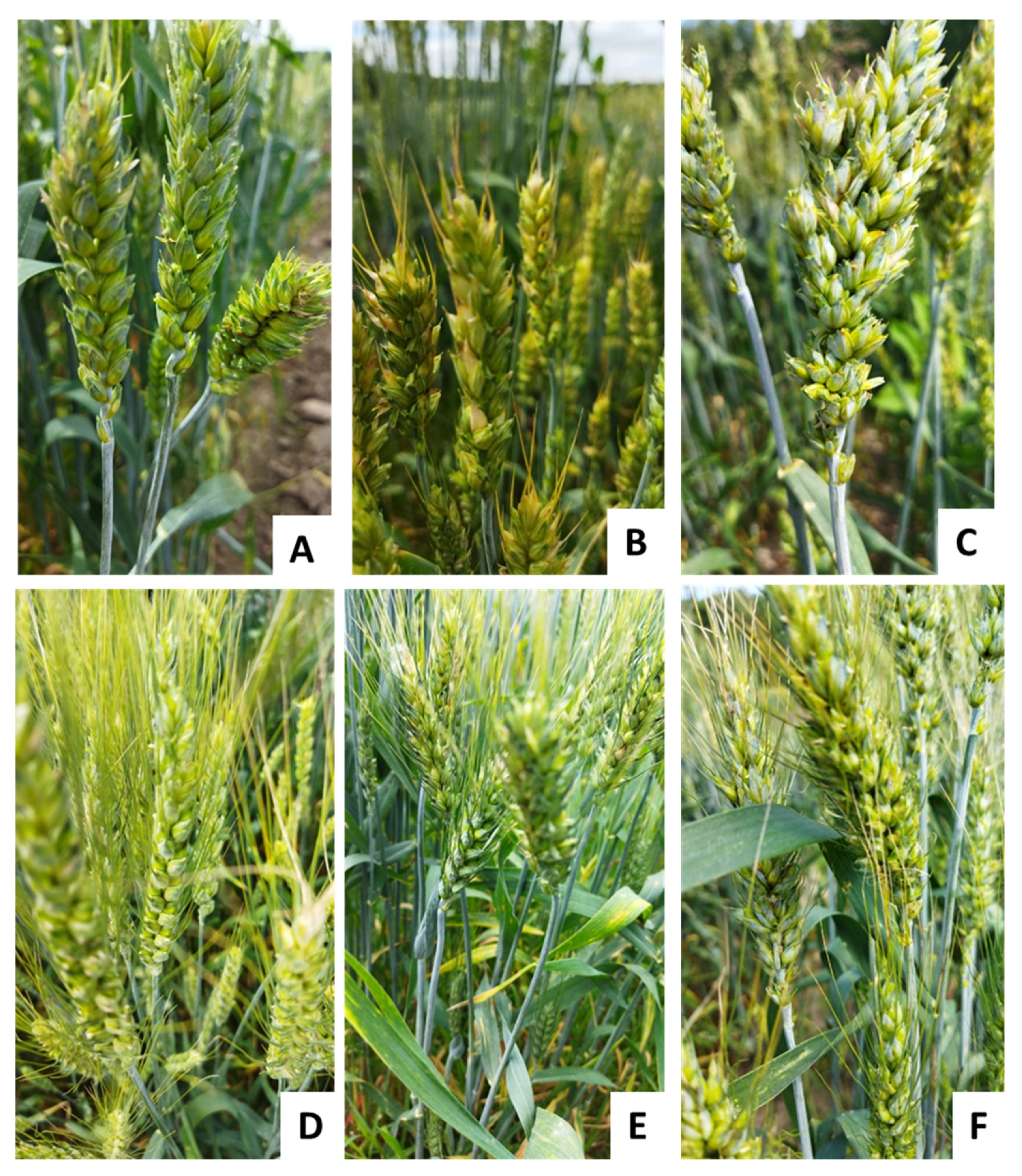
2.3. Genotyping and Phenotyping of Russian Wheat Germplasm Collection of VIR
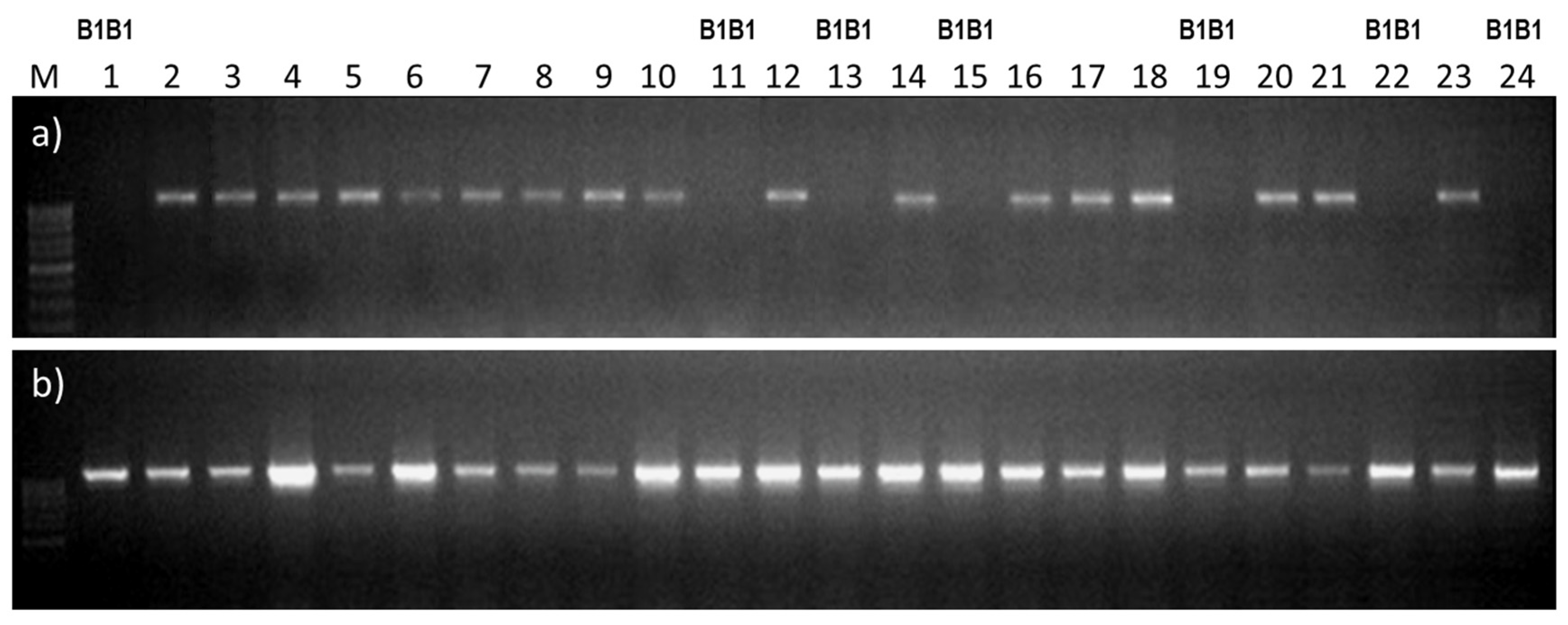
2.4. Using the B1/b1 Markers for Identification of Heterozygotes and Homozygotes in the Breeding Population
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. PCR
4.3. Sequencing of PCR Products
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jiang, Q.; Roche, D.; Durham, S.; Hole, D. Awn contribution to gas exchanges of barley ears. Photosynthetica 2006, 44, 536–541. [Google Scholar] [CrossRef]
- Li, X.; Bin, D.; Hong-gang, W. Awn anatomy of common wheat (Triticum aestivum L.) and its relatives. Caryologia 2010, 63, 391–397. [Google Scholar] [CrossRef] [Green Version]
- Simkin, A.J.; Faralli, M.; Ramamoorthy, S.; Lawson, T. Photosynthesis in non-foliar tissues: Implications for yield. Plant J. 2020, 101, 1001–1015. [Google Scholar] [CrossRef] [PubMed]
- Olugbemi, R.B.; Bingham, J. Effects of awns on the photosynthesis and yield of wheat. Ann. Appl. Biol. 1976, 84, 241–250. [Google Scholar] [CrossRef]
- Tambussi, E.A.; Bort, J.; Guiamet, J.J.; Nogués, S.; Araus, J.L. The Photosynthetic Role of Ears in C3 Cereals: Metabolism, Water Use Efficiency and Contribution to Grain Yield. Crit. Rev. Plant Sci. 2007, 26, 1–16. [Google Scholar] [CrossRef]
- Motzo, R.; Giunta, F. Awnedness affects grain yield and kernel weight in near-isogenic lines of durum wheat. Crop. Pasture Sci. 2002, 53, 1285–1293. [Google Scholar] [CrossRef]
- Martin, J.N.; Carver, B.F.; Hunger, R.M.; Cox, T.S. Contributions of leaf rust resistance and awns to agronomic and grain quality performance in winter wheat. Crop Sci. 1993, 43, 1712–1717. [Google Scholar] [CrossRef] [Green Version]
- Khaliq, I.; Irshad, A.; Ahsan, M. Awns and Flag Leaf Contribution towards Grain Yield in Spring Wheat (Triticum aestivum L.). Cereal Res. Commun. 2008, 36, 65–76. [Google Scholar] [CrossRef]
- Chhabra, A.K.; Sethi, S.K. Contribution and association of awns and flag-leaf with yield and its components in durum wheat. Cereal Res. Commun. 1989, 17, 265–271. [Google Scholar]
- Ibrahim, H.A.; Abo Elenein, R.A. The relative contribution of different wheat leaves and awns to the grain yield and its protein content. J. Agron. Crop Sci. 1977, 144, 1–7. [Google Scholar]
- Sharma, S.K.; Dhaliwal, H.S.; Randhawa, A.S.; Singh, R.P.; Saxena, A.K. Effect of leafblades and awns on the protein content and Pelshenke value in wheat. J. Crop Improv. 1983, 10, 53–55. [Google Scholar]
- Tambussi, E.A.; Nogués, S.; Araus, J.L. Ear of durum wheat under water stress: Water relations and photosynthetic metabolism. Planta 2005, 221, 446–458. [Google Scholar] [CrossRef]
- Sorensen, A.E. Seed dispersal by adhesion. Annu. Rev. Ecol. Evol. Syst. 1986, 17, 443–463. [Google Scholar] [CrossRef]
- Watkins, A.E.; Ellerton, S. Variation and genetics of the awn in Triticum. J. Genet. 1940, 40, 243–270. [Google Scholar] [CrossRef]
- McIntosh, R.A.; Hart, G.E.; Devos, K.M.; Gale, M.D.; Rogers, W.J. Catalogue of gene symbols for wheat. In Proceedings of the 9th International Wheat Genetics Symposium, Saskatoon, SK, 2–7 August 1998; Volume 5, p. 235. [Google Scholar]
- Sears, E.R. The Aneuploids of Common Wheat; University of Missouri, College of Agriculture, Agricultural Experiment Station: Columbia, MO, USA, 1954; 572p. [Google Scholar]
- Yoshioka, M.; Iehisa, J.C.M.; Ohno, R.; Kimura, T.; Enoki, H.; Nishimura, S.; Nasuda, S.; Takumi, S. Three dominant awnless genes in common wheat: Fine mapping, interaction and contribution to diversity in awn shape and length. PLoS ONE 2017, 12, e0176148. [Google Scholar] [CrossRef] [Green Version]
- Iehisa, J.C.M.; Ohno, R.; Kimura, T.; Enoki, H.; Nishimura, S.; Okamoto, Y.; Nasuda, S.; Takumi, S. A high-density genetic map with array-based markers facilitates structural and quantitative trait locus analyses of the common wheat genome. DNA Res. 2014, 21, 555–567. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Yu, K.; Jin, D.; Sun, L.; Chu, J.; Wu, W.; Xin, P.; Li, X.; Sun, J.; Yang, W.; et al. ALI-1, candidate gene of B1 locus, is associated with awn length and grain weight. BioRxiv 2019. [Google Scholar] [CrossRef] [Green Version]
- Huang, D.; Zheng, Q.; Melchkart, T.; Bekkaoui, Y.; Konkin, D.J.F.; Kagale, S.; Martucci, M.; You, F.M.; Clarke, M.; Adamski, N.M.; et al. Dominant inhibition of awn development by a putative zinc-finger transcriptional repressor expressed at the B1 locus in wheat. New Phytol. 2020, 225, 340–355. [Google Scholar] [CrossRef] [Green Version]
- DeWitt, N.; Guedira, M.; Lauer, E.; Sarinelli, M.; Tyagi, P.; Fu, D.; Hao, Q.; Murphy, J.P.; Marshall, D.; Akhunova, A.; et al. Sequence-based mapping identifies a candidate transcription repressor underlying awn suppression at the B1 locus in wheat. New Phytol. 2020, 225, 326–339. [Google Scholar] [CrossRef] [Green Version]
- Kato, K.; Miura, H.; Akiyama, M.; Kuroshima, M.; Sawada, S. RFLP mapping of the three major genes, Vrn1, Q and B1, on the long arm of chromosome 5A of wheat. Euphytica 1998, 101, 91–95. [Google Scholar] [CrossRef]
- Sourdille, P.; Cadalen, T.; Gay, G.; Gill, B.; Bernard, M. Molecular and physical mapping of genes affecting awning in wheat. Plant Breed. 2002, 121, 320–324. [Google Scholar] [CrossRef]
- Mackay, I.J.; Bansept-Basler, P.; Barber, T.; Bentley, A.R.; Cockram, J.; Gosman, N.; Greenland, A.J.; Horsnell, R.; Howells, R.; O’Sullivan, D.M.; et al. An Eight-Parent Multiparent Advanced Generation Inter-Cross Population for Winter-Sown Wheat: Creation, Properties, and Validation. G3 Genes Genom. Genet. 2014, 4, 1603–1610. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, P.; Arora, R.; Ray, S.; Singh, A.K.; Singh, V.P.; Takatsuji, H.; Kapoor, S.; Tyagi, A.K. Genome-wide identification of C2H2 zinc-finger gene family in rice and their phylogeny and expression analysis. Plant Mol. Biol. 2007, 65, 467–485. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Tang, J.; Li, Y.; Wang, W.; Li, X.; Jin, L.; Xie, R.; Luo, H.; Zhao, X.; Meng, Z.; et al. STAMENLESS 1, encoding a single C2H2 zinc finger protein, regulates floral organ identity in rice. Plant J. 2009, 59, 789–801. [Google Scholar] [CrossRef] [PubMed]
- Yaakov, B.; Ben-David, S.; Kashkush, K. Genome-wide analysis of Stowaway-like MITEs in wheat reveals high sequence conservation, gene association, and genomic diversification. Plant Physiol. 2013, 161, 486–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Packa, D.; Załuski, D.; Graban, L.; Lajszner, W. An Evaluation of Spelt Crosses for Breeding New Varieties of Spring Spelt. Agronomy 2019, 9, 167. [Google Scholar] [CrossRef] [Green Version]
- Gulyás, G.; Rakszegi, M.; Bognár, Z.; Láng, L.; Bedő, Z. Evaluation of genetic diversity of spelt breeding materials based on AFLP and quality analyses. Cereal Res. Commun. 2012, 40, 185–193. [Google Scholar] [CrossRef]
- Goncharov, N.P. Comparative Genetics of Wheats and Their Related Species; Siberian University Press: Novosibirsk, Russia, 2002; p. 251. [Google Scholar]
- Sun, L.; Zhang, A.; Zhou, Z.; Zhao, Y.; Yan, A.; Bao, S.; Yu, H.; Gan, Y. GLABROUS INFLORESCENCE STEMS3 (GIS3) regulates trichome initiation and development in Arabidopsis. New Phytol. 2015, 206, 220–230. [Google Scholar] [CrossRef]
- Toriba, T.; Hirano, H.Y. The DROOPING LEAF and OsETTIN2 genes promote awn development in rice. Plant J. 2014, 77, 616–626. [Google Scholar] [CrossRef]
- Wang, T.; Zou, T.; He, Z.; Yuan, G.; Luo, T.; Zhu, J.; Liang, Y.; Deng, Q.; Wang, S.; Zheng, A.; et al. GRAIN LENGTH and AWN 1 negatively regulates grain size in rice. J. Integr. Plant Biol. 2019, 61, 1036–1042. [Google Scholar] [CrossRef]
- Kiseleva, A.A.; Shcherban, A.B.; Leonova, I.N.; Frenkel, Z.; Salina, E.A. Identification of new heading date determinants in wheat 5B chromosome. BMC Plant Biol. 2016, 16 (Suppl 1), 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shcherban, A.B.; Kuvaeva, D.D.; Mitrofanova, O.P.; Khverenets, S.E.; Pryanishnikov, A.I.; Salina, E.A. Targeting the B1 Gene and Analysis of Its Polymorphism Associated with Awned/Awnless Trait in Russian Germplasm Collections of Common Wheat. Plants 2021, 10, 2285. https://doi.org/10.3390/plants10112285
Shcherban AB, Kuvaeva DD, Mitrofanova OP, Khverenets SE, Pryanishnikov AI, Salina EA. Targeting the B1 Gene and Analysis of Its Polymorphism Associated with Awned/Awnless Trait in Russian Germplasm Collections of Common Wheat. Plants. 2021; 10(11):2285. https://doi.org/10.3390/plants10112285
Chicago/Turabian StyleShcherban, Andrey B., Diana D. Kuvaeva, Olga P. Mitrofanova, Svetlana E. Khverenets, Alexander I. Pryanishnikov, and Elena A. Salina. 2021. "Targeting the B1 Gene and Analysis of Its Polymorphism Associated with Awned/Awnless Trait in Russian Germplasm Collections of Common Wheat" Plants 10, no. 11: 2285. https://doi.org/10.3390/plants10112285
APA StyleShcherban, A. B., Kuvaeva, D. D., Mitrofanova, O. P., Khverenets, S. E., Pryanishnikov, A. I., & Salina, E. A. (2021). Targeting the B1 Gene and Analysis of Its Polymorphism Associated with Awned/Awnless Trait in Russian Germplasm Collections of Common Wheat. Plants, 10(11), 2285. https://doi.org/10.3390/plants10112285






