Reproductive Cycle of the Seagrass Zostera noltei in the Ria de Aveiro Lagoon
Abstract
:1. Introduction
2. Materials and Methods
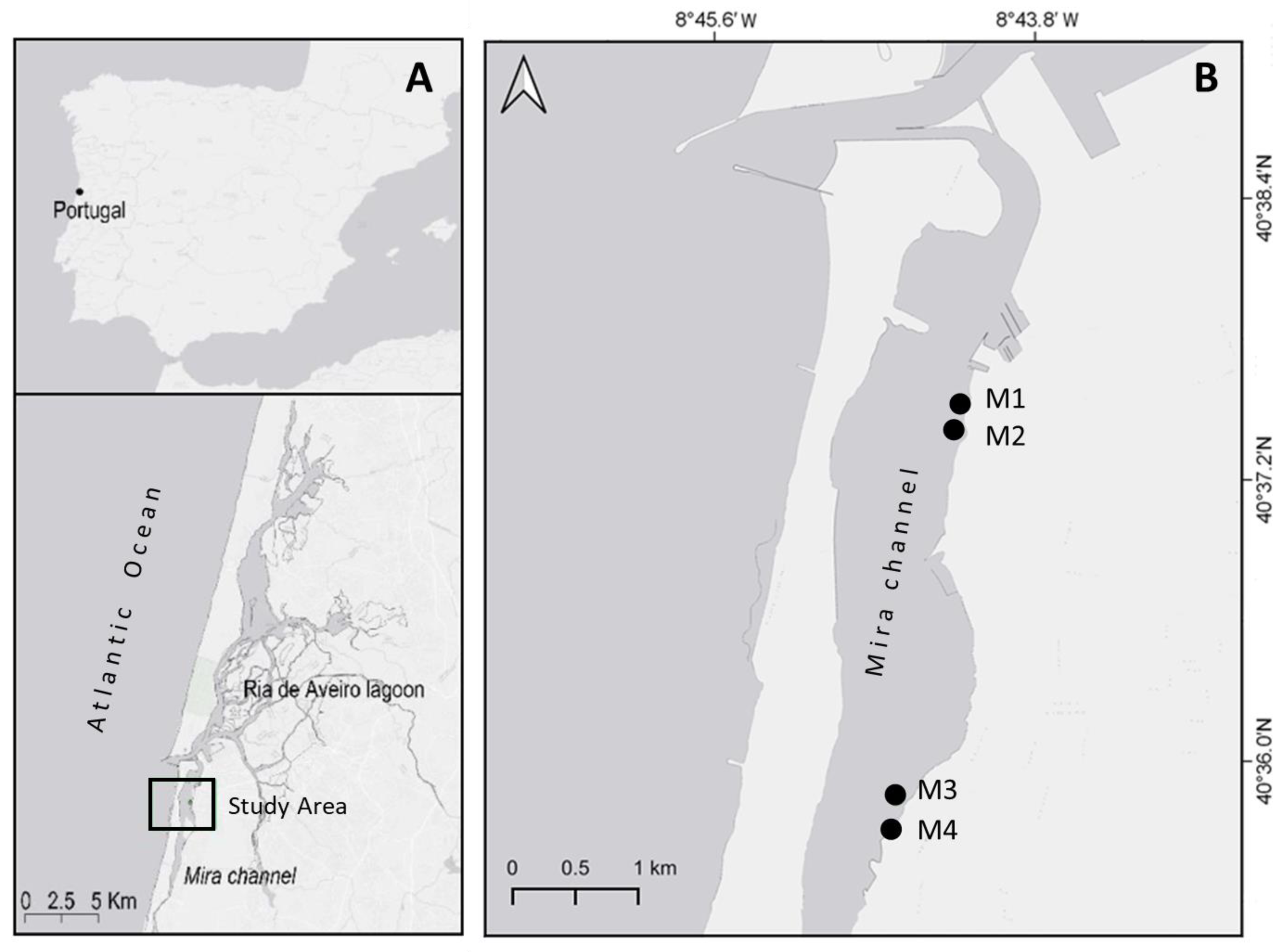
2.1. Study Area
2.2. Flowering Effort and Reproductive Phenology
2.3. Germination Ability
2.4. Data Analyses
3. Results
3.1. Spatio-Temporal Variability in Environmental Conditions
3.2. General Reproductive Phenology of Z. noltei in the Ria de Aveiro Lagoon
3.3. Spatio-Temporal Variability in the Flowering Effort and Reproductive Phenology
3.4. Germination Ability
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Short, F.T.; Short, C.A.; Novak, A.B. Seagrasses. In The Wetland Book; Springer: Dordrecht, The Netherlands, 2016; pp. 1–19. [Google Scholar]
- Hemminga, M.A.; Duarte, C.M. Seagrass Ecology; Cambridge University Press: Cambridge, UK, 2000; ISBN 9780521661843. [Google Scholar]
- Waycott, M.; Duarte, C.M.; Carruthers, T.J.B.; Orth, R.J.; Dennison, W.C.; Olyarnik, S.; Calladine, A.; Fourqurean, J.W.; Heck, K.L.; Hughes, A.R.; et al. Accelerating loss of seagrasses across the globe threatens coastal ecosystems. Proc. Natl. Acad. Sci. USA 2009, 106, 12377–12381. [Google Scholar] [CrossRef] [Green Version]
- Orth, R.J.W.; Carruthers, J.B.; Dennison, W.C.; Duarte, C.M.; Fourqurean, J.W.; Heck, K.L.; Hughes, A.R.; Kendrick, G.A.; Kenworthy, W.J.; Olyarnik, S.; et al. A Global Crisis for Seagrass Ecosystems. BioScience 2006, 56, 987. [Google Scholar] [CrossRef] [Green Version]
- Oprandi, A.; Mucerino, L.; De Leo, F.; Bianchi, C.N.; Morri, C.; Azzola, A.; Benelli, F.; Besio, G.; Ferrari, M.; Montefalcone, M. Effects of a severe storm on seagrass meadows. Sci. Total Environ. 2020, 748, 141373. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.Z.; Kim, S.H.; Song, H.J.; Kim, H.G.; Suonan, Z.; Kwon, O.; Kim, Y.K.; Park, S.R.; Park, J.I.; Lee, K.S. Long-term variability in the flowering phenology and intensity of the temperate seagrass Zostera marina in response to regional sea warming. Ecol. Indic. 2020, 119, 106821. [Google Scholar] [CrossRef]
- Guerrero-Meseguer, L.; Marín, A.; Sanz-Lázaro, C. Heat wave intensity can vary the cumulative effects of multiple environmental stressors on Posidonia oceanica seedlings. Mar. Environ. Res. 2020, 159, 105001. [Google Scholar] [CrossRef] [PubMed]
- Hughes, A.R.; Stachowicz, J.J. Genetic diversity enhances the resistance of a seagrass ecosystem to disturbance. Proc. Natl. Acad. Sci. USA 2004, 101, 8998–9002. [Google Scholar] [CrossRef] [Green Version]
- Reusch, T.B.H.; Ehlers, A.; Ha, A.; Hammerli, A.; Worm, B. Ecosystem recovery after climatic extremes enhanced by genotypic diversity. Proc. Natl. Acad. Sci. USA 2005, 102, 2826–2831. [Google Scholar] [CrossRef] [Green Version]
- Inglis, G.J. Variation in the recruitment behaviour of seagrsses seeds: Implications for population dynamics and resource managment. Pac. Conserv. Biol. 2000, 5, 251–259. [Google Scholar] [CrossRef]
- Orth, R.J.; Harwell, M.C.; Inglis, G.J. Ecology of seagrass seeds and dispersal strategies. Seagrasses Biol. Ecol. Conserv. 2006, 111–133. [Google Scholar] [CrossRef]
- Hootsmans, M.J.M.; Vermaat, J.E.; Van Vierssen, W. Seed-bank development, germination and early seedling survival of two seagrass species from The Netherlands: Zostera marina L. and Zostera noltii hornem. Aquat. Bot. 1987, 28, 275–285. [Google Scholar] [CrossRef]
- Zipperle, A.M.; Coyer, J.A.; Reise, K.; Stam, W.T.; Olsen, J.L. Evidence for persistent seed banks in dwarf eelgrass Zostera noltii in the German Wadden Sea. Mar. Ecol. Prog. Ser. 2009, 380, 73–80. [Google Scholar] [CrossRef] [Green Version]
- Zipperle, A.M.; Coyer, J.A.; Reise, K.; Gitz, E.; Stam, W.T.; Olsen, J.L. Clonal architecture in an intertidal bed of the dwarf eelgrass Zostera noltii in the Northern Wadden Sea: Persistence through extreme physical perturbation and the importance of a seed bank. Mar. Biol. 2009, 156, 2139–2148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Short, F.T. The response of interstitial ammonium in eelgrass (Zostera marina L.) beds to environmental perturbations. J. Exp. Mar. Biol. Ecol. 1983, 68, 195–208. [Google Scholar] [CrossRef]
- Green, E.P.; Short, F.T. World Atlas of Seagrasses; Copub: Unep-Wcmc; University of California Press: Berkeley, CA, USA, 2003; ISBN 9780520240476. [Google Scholar]
- Cabaço, S.; Machás, R.; Santos, R. Individual and population plasticity of the seagrass Zostera noltii along a vertical intertidal gradient. Estuar. Coast. Shelf Sci. 2009, 82, 301–308. [Google Scholar] [CrossRef]
- Peralta, G.; Brun, F.; Pérez-Lloréns, J.; Bouma, T. Direct effects of current velocity on the growth, morphometry and architecture of seagrasses: A case study on Zostera noltii. Mar. Ecol. Prog. Ser. 2006, 327, 135–142. [Google Scholar] [CrossRef] [Green Version]
- Alexandre, A.; Santos, R.; Serrão, E. Effects of clam harvesting on sexual reproduction of the seagrass Zostera noltii. Mar. Ecol. Prog. Ser. 2005, 298, 115–122. [Google Scholar] [CrossRef] [Green Version]
- Cabaço, S.; Santos, R.; Sprung, M. Population dynamics and production of the seagrass Zostera noltii in colonizing versus established meadows. Mar. Ecol. 2012, 33, 280–289. [Google Scholar] [CrossRef]
- Guerrero-Meseguer, L.; Veiga, P.; Sampaio, L.; Rubal, M. Sediment characteristics determine the flowering effort of Zostera noltei meadows inhabiting a human-dominated lagoon. Plants 2021, 10, 1387. [Google Scholar] [CrossRef]
- Cunha, A.H.; Assis, J.F.; Serrão, E.A. Seagrasses in Portugal: A most endangered marine habitat. Aquat. Bot. 2013, 104, 193–203. [Google Scholar] [CrossRef]
- Sousa, A.I.; da Silva, J.F.; Azevedo, A.; Lillebø, A.I. Blue Carbon stock in Zostera noltei meadows at Ria de Aveiro coastal lagoon (Portugal) over a decade. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azevedo, A.; Dias, J.M.; Lillebø, A.I. Thriving of Zostera noltei under intertidal conditions: Implications for the modelling of seagrass populations. Mar. Biol. 2016, 163, 1–8. [Google Scholar] [CrossRef]
- Sousa, A.I.; Calado, R.; Cleary, D.F.R.; Nunes, C.; Coimbra, M.A.; Serôdio, J.; Lillebø, A.I. Effect of spatio-temporal shifts in salinity combined with other environmental variables on the ecological processes provided by Zostera noltei meadows. Sci. Rep. 2017, 7, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Dias, J.M.; Lopes, J.F.; Dekeyser, I. Hydrological characterisation of Ria de Aveiro, Portugal, in early summer. Oceanol. Acta 1999, 22, 473–485. [Google Scholar] [CrossRef] [Green Version]
- Moreira, M.H.; Queiroga, H.; Machado, M.M.; Cunha, M.R. Environmental gradients in a southern Europe estuarine system: Ria de Aveiro, Portugal implications for soft bottom macrofauna colonization. Neth. J. Aquat. Ecol. 1993, 27, 465–482. [Google Scholar] [CrossRef]
- Wentworth, C.K. A scale of grade and class terms for clastic sediments. J. Geol. 1922, 30, 377–392. [Google Scholar] [CrossRef]
- Loques, F.; Caye, G.; Meinesz, A. Germination in the marine phanerogam Zostera noltii Hornemann at Golfe Juan, French Mediterranean. Aquat. Bot. 1990, 38, 249–260. [Google Scholar] [CrossRef]
- Underwood, A.J. Experiments in Ecology: Their Logical Design and Interpretation Using Analysis of Variance; Cambridge University Press: Cambridge, UK, 1997; ISBN 9780521556965. [Google Scholar]
- R Core Team. R: A language and Environment for Statistical Computing; Version 4.0.0; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Cabaço, S.; Santos, R. Effects of burial and erosion on the seagrass Zostera noltii. J. Exp. Mar. Biol. Ecol. 2007, 340, 204–212. [Google Scholar] [CrossRef]
- Loques, F.; Caye, G.; Meinesz, A. Flowering and fruiting of Zostera noltii in Golfe Juan (French Mediterranean). Aquat. Bot. 1988, 32, 341–352. [Google Scholar] [CrossRef]
- Buia, M.C.; Mazzella, L. Reproductive phenology of the Mediterranean seagrasses Posidonia oceanica (L.) Delile, Cymodocea nodosa (Ucria) Aschers., and Zostera noltii Hornem. Aquat. Bot. 1991, 40, 343–362. [Google Scholar] [CrossRef]
- Auby, I.; Labourg, P.J. Seasonal dynamics of Zostera noltii Hornem. In the Bay of Arcachon (France). J. Sea Res. 1996, 35, 269–277. [Google Scholar] [CrossRef]
- Van Tussenbroek, B.I.; Soissons, L.M.; Bouma, T.J.; Asmus, R.; Auby, I.; Brun, F.G.; Cardoso, P.G.; Desroy, N.; Fournier, J.; Ganthy, F.; et al. Pollen limitation may be a common Allee effect in marine hydrophilous plants: Implications for decline and recovery in seagrasses. Oecologia 2016, 182, 595–609. [Google Scholar] [CrossRef]
- Cabaço, S.; Santos, R. Seagrass reproductive effort as an ecological indicator of disturbance. Ecol. Indic. 2012, 23, 116–122. [Google Scholar] [CrossRef]
- Lee, K.; Park, J.; Kim, Y.; Park, S.; Kim, J. Recolonization of Zostera marina following destruction caused by a red tide algal bloom: The role of new shoot recruitment from seed banks. Mar. Ecol. Prog. Ser. 2007, 342, 105–115. [Google Scholar] [CrossRef]
- Silva, J.F.; Duck, R.W. Changing use and hydromorphological adjustment in a coastal lagoon-estuarine system, the Ria de Aveiro, Portugal. In Sediment Dynamics and the Hydromorphology of Fluvial Systems; Rowan, J.S., Duck, R.W., Alan, W., Eds.; IAHS Publication 306: Dundee, UK, 2006; pp. 197–204. [Google Scholar]
- Middelburg, J.J.; Soetaert, K.; Herman, P.M.J. Empirical relationships for use in global diagenetic models. Deep Sea Res. Part I Oceanogr. Res. Pap. 1997, 44, 327–344. [Google Scholar] [CrossRef]
- Dias, J.M.; Lopes, J.F.; Dekeyser, I. A numerical system to study the transport properties in the Ria de Aveiro lagoon. Ocean Dyn. 2003, 53, 220–231. [Google Scholar] [CrossRef]
- Reynolds, L.K.; Waycott, M.; McGlathery, K.J.; Orth, R.J. Ecosystem services returned through seagrass restoration. Restor. Ecol. 2016, 24, 583–588. [Google Scholar] [CrossRef]




| Period | Stage | Description |
|---|---|---|
| Flowering | I | Yellow-green spathe, sheath closed; pistils and stamina are visible, aligned onto the stem |
| II | Pistils (IIa) and/or stamina erected (IIb); styles and stigma and/or anthers are outside the sheath | |
| III | Stigma brown, start to depart from spathe; often with stamina already detached from the spathe | |
| Seed formation | IV | Green spathe with immature seeds; sheath closed |
| V | Green/brown spathe, dark brown seeds are visible, sheath open |
| Variable | Meadow (df = 3) | Time (df = 2) | Meadow × Time (df = 6) |
|---|---|---|---|
| Salinity | 16.59 (<0.001) | 1.632 (0.236) | 0.474 (0.815) |
| OM | 18.87 (<0.001) | 4.820 (0.029) | 10.33 (<0.001) |
| Fine gravel | 17.42 (<0.001) | 0.211 (0.812) | 13.38 (<0.001) |
| Very coarse sand | 12.50 (<0.001) | 0.368 (0.699) | 11.79 (<0.001) |
| Coarse sand | 25.76 (<0.001) | 0.516 (0.609) | 0.460 (0.830) |
| Medium sand * | 42.99 (<0.001) | 0.677 (0.527) | 7.480 (0.002) |
| Fine sand | 196.5 (<0.001) | 0.808 (0.467) | 3.506 (0.031) |
| Very fine sand * | 446.1 (<0.001) | 3.187 (0.078) | 4.176 (0.017) |
| Slit and clay | 52.27 (<0.001) | 0.488 (0.626) | 5.631 (0.005) |
| Variable | Meadow (df = 3) | Time (df = 3) | Meadow × Time (df = 9) |
|---|---|---|---|
| Flowering effort | 2.853 (0.047) | 2.495 (0.071) | 0.708 (0.699) |
| Stage I | 1.274 (0.294) | 2.290 (0.090) | 1.782 (0.097) |
| Stage II | 0.408 (0.748) | 10.37 (>0.001) | 1.010 (0.446) |
| Stage III | 0.287 (0.834) | 1.470 (0.235) | 2.282 (0.032) |
| Stage IV | 1.150 (0.339) | 10.92 (>0.001) | 0.807 (0.612) |
| Stage V | 1.266 (0.297) | 1.595 (0.203) | 1.047 (0.418) |
| Aborted spathes | 0.774 (0.514) | 3.375 (0.026) | 0.534 (0.842) |
| Meadow | Germinated (%) | No Germinated (%) |
|---|---|---|
| M1 | 24 | 76 |
| M2 | 43.9 | 56.1 |
| M3 | 33.3 | 66.7 |
| Fisher’s exact test of independence | 2.69 | p = 0.253 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ankel, M.; Rubal, M.; Veiga, P.; Sampaio, L.; Guerrero-Meseguer, L. Reproductive Cycle of the Seagrass Zostera noltei in the Ria de Aveiro Lagoon. Plants 2021, 10, 2286. https://doi.org/10.3390/plants10112286
Ankel M, Rubal M, Veiga P, Sampaio L, Guerrero-Meseguer L. Reproductive Cycle of the Seagrass Zostera noltei in the Ria de Aveiro Lagoon. Plants. 2021; 10(11):2286. https://doi.org/10.3390/plants10112286
Chicago/Turabian StyleAnkel, Manuel, Marcos Rubal, Puri Veiga, Leandro Sampaio, and Laura Guerrero-Meseguer. 2021. "Reproductive Cycle of the Seagrass Zostera noltei in the Ria de Aveiro Lagoon" Plants 10, no. 11: 2286. https://doi.org/10.3390/plants10112286
APA StyleAnkel, M., Rubal, M., Veiga, P., Sampaio, L., & Guerrero-Meseguer, L. (2021). Reproductive Cycle of the Seagrass Zostera noltei in the Ria de Aveiro Lagoon. Plants, 10(11), 2286. https://doi.org/10.3390/plants10112286









