A Study of Phytochemistry, Genoprotective Activity, and Antitumor Effects of Extracts of the Selected Lamiaceae Species
Abstract
:1. Introduction
2. Results and Discussion
2.1. Phytochemical Characterization
2.2. Antioxidant Activity In Vitro
2.3. Genoprotective Activity in Acellular System
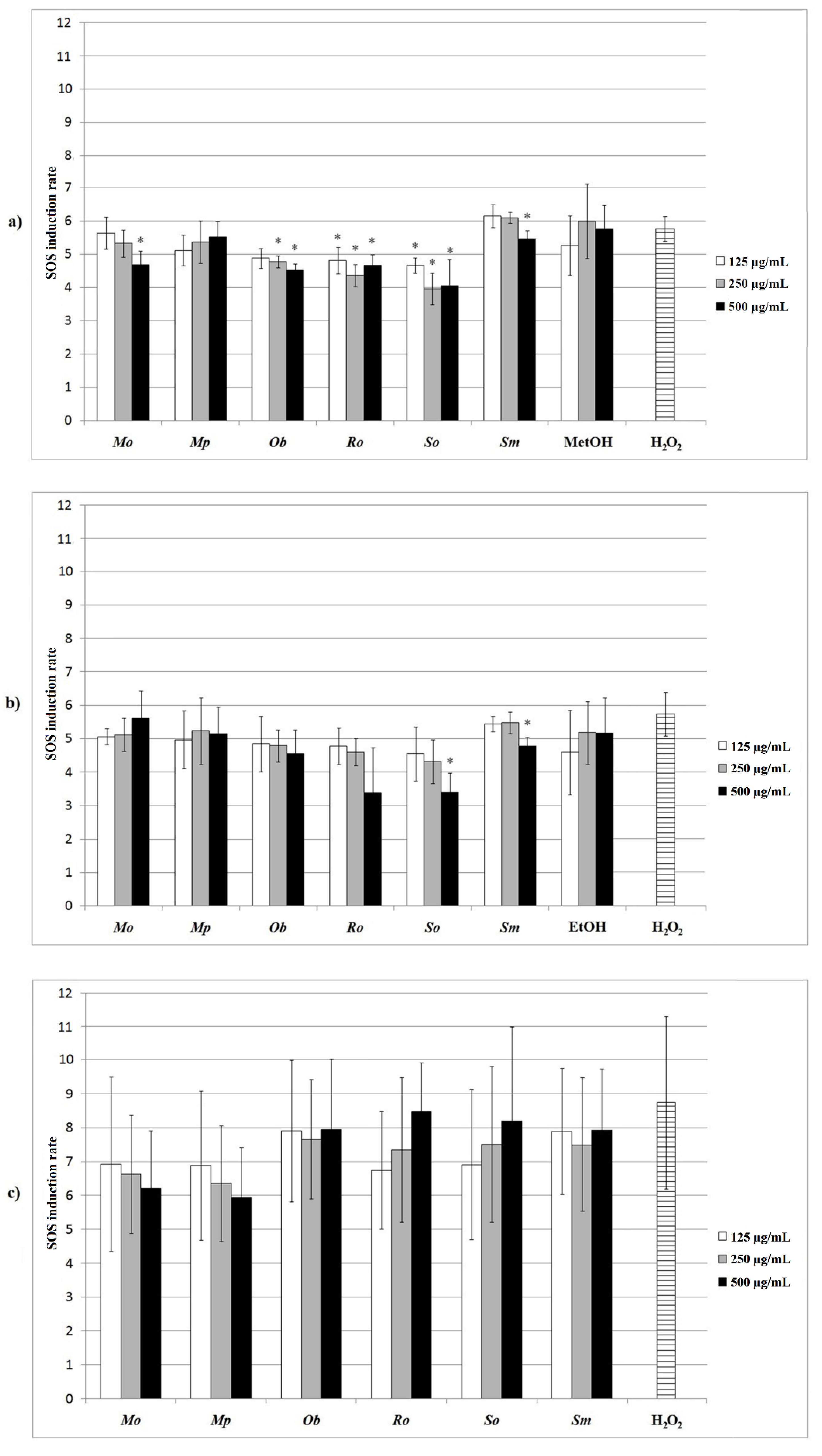
2.4. Protective Effect of the Extracts against Hydrogen-Peroxide-Induced DNA Damage in the Prokaryotic Model
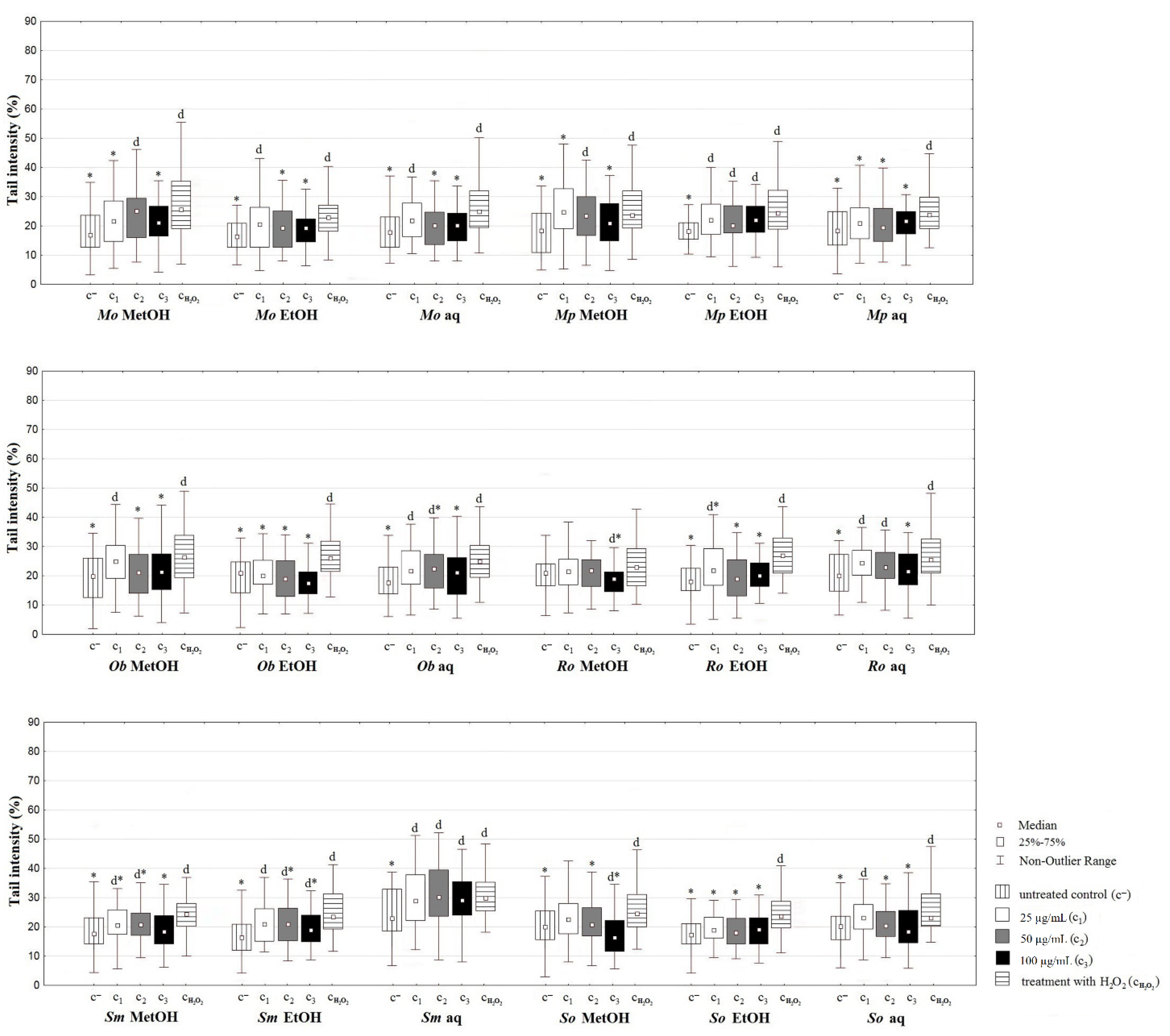
2.5. Protective Effect of the Extracts against Hydrogen-Peroxide-Induced DNA Damage in the Eukaryotic Model
2.5.1. Determination of Cell Proliferation/Metabolic Viability
2.5.2. Comet Assay
2.6. Antitumor Potential of the Extracts
2.6.1. Determination of Antiproliferative/Cytotoxic Effect on Tumor Cells
2.6.2. Determination of ROS-Modulating Activity in Tumor Cells
2.6.3. Determination of NO-Modulating Activity in Tumor Cells
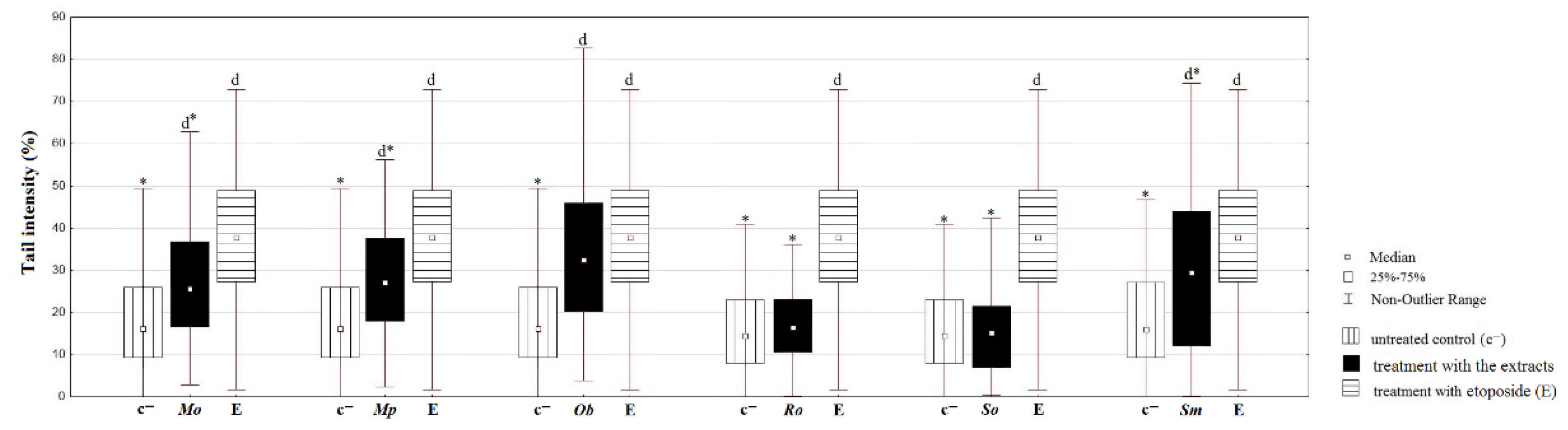
2.6.4. Comet Assay
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Plant Material
3.3. Determination of Polyphenolic Components
3.4. HPLC-DAD Quantification of Polyphenols
3.5. Evaluation of DPPH-Scavenging Activity
3.6. Genoprotective Activity in Acellular System
3.7. Protective Effect of the Extracts against Hydrogen Peroxide-Induced DNA Damage in the Prokaryotic Model
3.8. The Effects of Extracts on Normal and Tumor Cell Lines
3.8.1. Cell Culture
3.8.2. Determination of Cell Proliferation/Metabolic Viability (MTT Assay)
3.8.3. Comet Assay
3.8.4. Determination of Superoxide Anion Radical (NBT Assay)
3.8.5. Determination of the Levels of Nitrites in Supernatants (Griess Assay)
3.9. Statistical Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Perillo, B.; Di Donato, M.; Pezone, A.; Di Zazzo, E.; Giovannelli, P.; Galasso, G.; Castoria, G.; Migliaccio, A. ROS in cancer therapy: The bright side of the moon. Exp. Mol. Med. 2020, 52, 192–203. [Google Scholar] [CrossRef]
- Deutschmann, B.; Kolarević, S.; Brack, W.; Kaisarević, S.; Kostić, J.; Kračun-Kolarević, M.; Liska, I.; Paunović, M.; Seiler, T.B.; Shao, Y.; et al. Longitudinal profile of the genotoxic potential of the River Danube on erythrocytes of wild common bleak (Alburnus alburnus) assessed using the comet and micronucleus assay. Sci. Total Environ. 2016, 573, 1441–1449. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef] [Green Version]
- Sitarek, P.; Merecz-Sadowska, A.; Śliwiński, T.; Zajdel, R.; Kowalczyk, T. An in vitro evaluation of the molecular mechanisms of action of medical plants from the Lamiaceae family as effective sources of active compounds against human cancer cell lines. Cancers 2020, 12, 2957. [Google Scholar] [CrossRef] [PubMed]
- Patrignani, F.; Prasad, S.; Novaković, M.; Marin, P.D.; Bukvički, D. Lamiaceae in the treatment of cardiovascular diseases. Front. Biosci. 2021, 26, 612–643. [Google Scholar] [CrossRef]
- Luca, T.; Napoli, E.; Privitera, G.; Musso, N.; Ruberto, G.; Castorina, S. Antiproliferative effect and cell cycle alterations induced by Salvia officinalis essential oil and its three main components in human colon cancer cell lines. Chem. Biodivers 2020, 17, e2000309. [Google Scholar] [CrossRef]
- Salami, A.; Seydi, E.; Pourahmad, J. Use of nutraceuticals for prevention and treatment of cancer. Iran. J. Pharm. Res. 2013, 12, 219–220. [Google Scholar] [PubMed]
- Yeung, A.W.K.; Tzvetkov, N.T.; El-Tawil, O.S.; Bungǎu, S.G.; Abdel-Daim, M.M.; Atanasov, A.G. Antioxidants: Scientific literature landscape analysis. Oxidative Med. Cell. Longev. 2019, 2019, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Pérez-González, C.; Pérez-Ramos, J.; Méndez-Cuesta, C.A.; Serrano-Vega, R.; Martell-Mendoza, M.; Pérez-Gutiérrez, S. Cytotoxic activity of essential oils of some species from Lamiaceae family. In Cytotoxicity: Definition, Identification, and Cytotoxic Compounds; Istifli, E.S., İla, H.B., Eds.; IntechOpen: London, UK, 2019; pp. 29–43. [Google Scholar]
- Vladimir-Knežević, S.; Blažeković, B.; Kindl, M.; Vladić, J.; Lower-Nedza, A.D.; Brantner, A.H. Acetylcholinesterase inhibitory, antioxidant and phytochemical properties of selected medicinal plants of the Lamiaceae family. Molecules 2014, 19, 767–782. [Google Scholar] [CrossRef] [Green Version]
- Alimpić, A.; Oalđe, M.; Matevski, V.; Marin, P.D.; Duletić-Laušević, S. Antioxidant activity and total phenolic flavonoid contents of Salvia amplexicaulis Lam. extracts. Arch. Biol. Sci. 2014, 66, 307–316. [Google Scholar] [CrossRef]
- Alimpić Aradski, A.Z.; Oalđe, M.M.; Duletić-Laušević, S.N. Salvia officinalis: Traditional medicinal plant with novel therapeutical perspectives. In Salvia officinalis: Production, Cultivation and Uses; Ashton, C., Ed.; Nova Science Publishers, Inc.: New York, NY, USA, 2021; pp. 1–72. [Google Scholar]
- Oalđe, M.M.; Kolarević, S.M.; Živković, J.C.; Vuković-Gačić, B.S.; Jovanović Marić, J.M.; Kračun Kolarević, M.J.; Đorđević, J.Z.; Alimpić Aradski, A.Z.; Marin, P.D.; Šavikin, K.P.; et al. The impact of different extracts of six Lamiaceae species on deleterious effects of oxidative stress assessed in acellular, prokaryotic and eukaryotic models in vitro. Saudi Pharm. J. 2020, 28, 1592–1604. [Google Scholar] [CrossRef]
- Oalđe, M.; Kolarević, S.; Živković, J.; Alimpić Aradski, A.; Jovanović Marić, J.; Kračun Kolarević, M.; Đorđević, J.; Marin, P.D.; Šavikin, K.; Vuković-Gačić, B.; et al. A comprehensive assessment of the chemical composition, antioxidant, genoprotective and antigenotoxic activities of Lamiaceae species using different experimental models in vitro. Food Funct. 2021, 12, 3233–3324. [Google Scholar] [CrossRef] [PubMed]
- Wink, M. Evolution of secondary metabolites from an ecological and molecular phylogenetic perspective. Phytochemistry 2003, 64, 3–19. [Google Scholar] [CrossRef]
- Shakeri, A.; Sahebkar, A.; Javadi, B. Melissa officinalis L.–A review of its traditional uses, phytochemistry and pharmacology. J. Ethnopharmacol. 2016, 188, 204–228. [Google Scholar] [CrossRef] [PubMed]
- Sipos, S.; Moacă, E.A.; Pavel, I.Z.; Avram, Ş.; Crețu, O.M.; Coricovac, D.; Racoviceanu, R.M.; Ghiulai, R.; Pană, R.D.; Şoica, C.M.; et al. Melissa officinalis L. aqueous extract exerts antioxidant and antiangiogenic effects and improves physiological skin parameters. Molecules 2021, 26, 2369. [Google Scholar] [CrossRef] [PubMed]
- Alexa, E.; Danciu, C.; Radulov, I.; Obistioiu, D.; Sumalan, R.M.; Morar, A.; Dehelean, C.A. Phytochemical screening and biological activity of Mentha × piperita L. and Lavandula angustifolia Mill. extracts. Anal. Cell. Pathol. 2018, 2018, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miraj, S.; Kiani, S. Study of pharmacological effect of Ocimum basilicum: A review. Pharm. Lett. 2016, 8, 276–280. [Google Scholar]
- Seyed, M.A.; Ayesha, S.; Azmi, N.; Al-Rabae, F.M.; Al-Alawy, A.I.; Al-Zahrani, O.R.; Hawsawi, Y. The neuroprotective attribution of Ocimum basilicum: A review on the prevention and management of neurodegenerative disorders. Future J. Pharm. Sci. 2021, 7, 1–14. [Google Scholar]
- Andrade, J.M.; Faustino, C.; Garcia, C.; Ladeiras, D.; Reis, C.P.; Rijo, P. Rosmarinus officinalis L.: An update review of its phytochemistry and biological activity. Future Sci. OA 2018, 4, FSO283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghorbani, A.; Esmaeilizadeh, M. Pharmacological properties of Salvia officinalis and its components. J. Tradit. Complement. Med. 2017, 7, 433–440. [Google Scholar] [CrossRef]
- Mabrouki, H.; Duarte, C.M.M.; Akretche, D.E. Estimation of total phenolic contents and in vitro antioxidant and antimicrobial activities of various solvent extracts of Melissa officinalis L. Arab. J. Sci. Eng. 2018, 43, 3349–3357. [Google Scholar] [CrossRef]
- Stagos, D.; Portesis, N.; Spanou, C.; Mossialos, D.; Aligiannis, N.; Chaita, E.; Panagoulis, C.; Reri, E.; Skaltsounis, L.; Tsatsakis, A.M.; et al. Correlation of total polyphenolic content with antioxidant and antibacterial activity of 24 extracts from Greek domestic Lamiaceae species. Food Chem. Toxicol. 2012, 50, 4115–4124. [Google Scholar] [CrossRef] [PubMed]
- Sytar, O.; Hemmerich, I.; Zivcak, M.; Rauh, C.; Brestic, M. Comparative analysis of bioactive phenolic compounds composition from 26 medicinal plants. Saudi J. Biol. Sci. 2016, 25, 631–641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skendi, A.; Irakli, M.; Chatzopoulou, P. Analysis of phenolic compounds in Greek plants of Lamiaceae family by HPLC. J. Appl. Res. Med. Aromat. Plants 2017, 6, 62–69. [Google Scholar] [CrossRef]
- Lee, C.J.; Chen, L.G.; Chang, T.L.; Ke, W.M.; Lo, Y.F.; Wang, C.C. The correlation between skin-care effects and phytochemical contents in Lamiaceae plants. Food Chem. 2011, 124, 833–841. [Google Scholar] [CrossRef]
- Khoddami, A.; Wilkes, M.A.; Roberts, T.H. Techniques for analysis of plant phenolic compounds. Molecules 2013, 18, 2328–2375. [Google Scholar] [CrossRef] [PubMed]
- Milevskaya, V.V.; Prasad, S.; Temerdashev, Z.A. Extraction and chromatographic determination of phenolic compounds from medicinal herbs in the Lamiaceae and Hypericaceae families: A review. Microchem. J. 2019, 145, 1036–1049. [Google Scholar] [CrossRef]
- Sharma, Y.; Velamuri, R.; Fagan, J.; Schaefer, J.; Streicher, C.; Stimson, J. Identification and characterization of polyphenols and volatile terpenoid compounds in different extracts of garden sage (Salvia officinalis L.). Pharmacogn. Res. 2020, 12, 149–157. [Google Scholar] [CrossRef]
- Vieira, S.F.; Ferreira, H.; Neves, N.M. Antioxidant and anti-inflammatory activities of cytocompatible Salvia officinalis extracts: A comparison between traditional and Soxhlet extraction. Antioxidants 2020, 9, 1157. [Google Scholar] [CrossRef]
- Silva, B.N.; Cadavez, V.; Ferreira-Santos, P.; Alves, M.J.; Ferreira, I.C.; Barros, L.; Teixeira, J.A.; Gonzales-Barron, U. Chemical profile and bioactivities of extracts from edible plants readily available in Portugal. Foods 2021, 10, 673. [Google Scholar] [CrossRef]
- Elansary, H.O.; Szopa, A.; Kubica, P.; Ekiert, H.; El-Ansary, D.O.; Al-Mana, F.A.; Mahmoud, E.A. Saudi Rosmarinus officinalis and Ocimum basilicum L. polyphenols and biological activities. Processes 2020, 8, 446. [Google Scholar] [CrossRef] [Green Version]
- Taylor, R. Interpretation of the correlation coefficient: A basic review. J. Diagn. Med. Sonogr. 1990, 6, 35–39. [Google Scholar] [CrossRef]
- Nadeem, M.; Imran, M.; Aslam Gondal, T.; Imran, A.; Shahbaz, M.; Muhammad Amir, R.; Wasim Sajid, M.; Batool Qaisrani, T.; Atif, M.; Hussain, G.; et al. Therapeutic potential of rosmarinic acid: A comprehensive review. Appl. Sci. 2019, 9, 3139. [Google Scholar] [CrossRef] [Green Version]
- Marchev, A.S.; Vasileva, L.V.; Amirova, K.M.; Savova, M.S.; Koycheva, I.K.; Balcheva-Sivenova, Z.P.; Vasileva, S.M.; Georgiev, M.I. Rosmarinic acid-From bench to valuable applications in food industry. Trends Food Sci. Technol. 2021, in press. [Google Scholar] [CrossRef]
- Russo, A.; Cardile, V.; Lombardo, L.; Vanella, L.; Acquaviva, R. Genistin inhibits UV light-induced plasmid DNA damage and cell growth in human melanoma cells. J. Nutr. Biochem. 2006, 17, 103–108. [Google Scholar] [CrossRef]
- Asadi, N.S.; Heidari, M.M.; Khatami, M. Protective effect of Berberis vulgaris on Fenton reaction-induced DNA cleavage. Avicenna J. Phytomed. 2019, 9, 213–220. [Google Scholar]
- Kolarević, S.; Milovanović, D.; Kračun-Kolarević, M.; Kostić, J.; Sunjog, K.; Martinović, R.; Đorđević, J.; Novaković, I.; Sladić, D.; Vuković-Gačić, B. Evaluation of genotoxic potential of avarol, avarone, and its methoxy and methylamino derivatives in prokaryotic and eukaryotic test models. Drug Chem. Toxicol. 2018, 2018, 1–10. [Google Scholar] [CrossRef]
- Simić, D.; Vuković-Gačić, B.; Knežević-Vukčević, J. Detection of natural bioantimutagens and their mechanisms of action with bacterial assay-system. Mutat. Res. 1998, 402, 51–57. [Google Scholar] [CrossRef]
- Stanojković, T.; Kolundžija, B.; Ćirić, A.; Soković, M.; Nikolić, D.; Kundaković, T. Cytotoxicity and antimicrobial activity of Satureja kitaibelii Wierzb. ex Heuff (Lamiaceae). Dig. J. Nanomater. Biostruct. 2013, 8, 845–854. [Google Scholar]
- Garcia, C.S.; Menti, C.; Lambert, A.P.F.; Barcellos, T.; Moura, S.; Calloni, C.; Branco, C.S.; Salvador, M.; Roesch-Ely, M.; Henriques, J.A. Pharmacological perspectives from Brazilian Salvia officinalis (Lamiaceae): Antioxidant, and antitumor in mammalian cells. An. Acad. Bras. Ciênc. 2016, 88, 281–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carvalho, N.C.D.; Corrêa-Angeloni, M.J.F.; Leffa, D.D.; Moreira, J.; Nicolau, V.; Amaral, P.D.A.; Rossatto, Â.E.; Andrade, V.M.D. Evaluation of the genotoxic and antigenotoxic potential of Melissa officinalis in mice. Genet. Mol. Biol. 2011, 34, 290–297. [Google Scholar] [CrossRef]
- Güez, C.M.; Souza, R.O.D.; Fischer, P.; Leão, M.F.D.M.; Duarte, J.A.; Boligon, A.A.; Athayde, M.L.; Zuravski, L.; Oliveira, L.F.S.D.; Machado, M.M. Evaluation of basil extract (Ocimum basilicum L.) on oxidative, anti-genotoxic and anti-inflammatory effects in human leukocytes cell cultures exposed to challenging agents. Braz. J. Pharm. Sci. 2017, 53, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Razavi-Azarkhiavi, K.; Behravan, J.; Mosaffa, F.; Sehatbakhsh, S.; Shirani, K.; Karimi, G. Protective effects of aqueous and ethanol extracts of rosemary on H2O2-induced oxidative DNA damage in human lymphocytes by comet assay. J. Complement. Integr. Med. 2014, 11, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Gateva, S.; Jovtchev, G.; Stankov, A.; Gregan, F. Salvia extract can decrease DNA damage induced by zeocin. Int. J. Pharma Med. Biol. Sci. 2015, 4, 1–10. [Google Scholar]
- Mosaffa, F.; Behravan, J.; Karimi, G.; Iranshahi, M. Antigenotoxic effects of Satureja hortensis L. on rat lymphocytes exposed to oxidative stress. Arch. Pharmacal. Res. 2006, 29, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Kapiszewska, M.; Soltys, E.; Visioli, F.; Cierniak, A.; Zajac, G. The protective ability of the Mediterranean plant extracts against the oxidative DNA damage. The role of the radical oxygen species and the polyphenol content. J. Physiol. Pharmacol. 2005, 56, 183–197. [Google Scholar]
- Sabahi, Z.; Soltani, F.; Moein, M. Insight into DNA protection ability of medicinal herbs and potential mechanisms in hydrogen peroxide damages model. Asian Pac. J. Trop. Biomed. 2018, 8, 120–129. [Google Scholar]
- Gęgotek, A.; Jarocka-Karpowicz, I.; Skrzydlewska, E. Cytoprotective effect of ascorbic acid and rutin against oxidative changes in the proteome of skin fibroblasts cultured in a three-dimensional system. Nutrients 2020, 12, 1074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Encalada, M.A.; Hoyos, K.M.; Rehecho, S.; Berasategi, I.; de Ciriano, M.G.Í.; Ansorena, D.; Astiasarán, I.; Navarro-Blasco, Í.; Cavero, R.Y.; Calvo, M.I. Anti-proliferative effect of Melissa officinalis on human colon cancer cell line. Plant Foods Hum. Nutr. 2011, 66, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Elansary, H.O.; Szopa, A.; Kubica, P.; Ekiert, H.; Klimek-Szczykutowicz, M.; El-Ansary, D.O.; Mahmoud, E.A. Polyphenol profile and antimicrobial and cytotoxic activities of natural Mentha × piperita and Mentha longifolia populations in Northern Saudi Arabia. Processes 2020, 8, 479. [Google Scholar] [CrossRef] [Green Version]
- Kundaković, T.; Stanojković, T.; Kolundžija, B.; Marković, S.; Šukilović, B.; Milenković, M.; Lakušić, B. Cytotoxicity and antimicrobial activity of the essential oil from Satureja montana subsp. pisidica (Lamiceae). Nat. Prod. Commun. 2014, 9, 569–572. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.H.; Kee, J.Y.; Hong, S.H. Rosmarinic acid activates AMPK to inhibit metastasis of colorectal cancer. Front. Pharmacol. 2018, 9, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Trivellini, A.; Lucchesini, M.; Maggini, R.; Mosadegh, H.; Villamarin, T.S.S.; Vernieri, P.; Mensuali-Sodi, A.; Pardossi, A. Lamiaceae phenols as multifaceted compounds: Bioactivity, industrial prospects and role of “positive-stress”. Ind. Crops Prod. 2016, 83, 241–254. [Google Scholar] [CrossRef]
- Wang, J.; Yi, J. Cancer cell killing via ROS: To increase or decrease, that is the question. Cancer Biol. Ther. 2008, 7, 1875–1884. [Google Scholar] [CrossRef] [PubMed]
- Naik, E.; Dixit, V.M. Mitochondrial reactive oxygen species drive proinflammatory cytokine production. J. Exp. Med. 2011, 208, 417–420. [Google Scholar] [CrossRef] [PubMed]
- Weidner, C.; Rousseau, M.; Plauth, A.; Wowro, S.J.; Fischer, C.; Abdel-Aziz, H.; Sauer, S. Melissa officinalis extract induces apoptosis and inhibits proliferation in colon cancer cells through formation of reactive oxygen species. Phytomedicine 2015, 22, 262–270. [Google Scholar] [CrossRef]
- Pérez-Sánchez, A.; Barrajón-Catalán, E.; Ruiz-Torres, V.; Agulló-Chazarra, L.; Herranz-López, M.; Valdés, A.; Cifuentes, A.; Micol, V. Rosemary (Rosmarinus officinalis) extract causes ROS-induced necrotic cell death and inhibits tumor growth in vivo. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef]
- Costa, T.J.; Barros, P.R.; Arce, C.; Santos, J.D.; da Silva-Neto, J.; Egea, G.; Dantas, A.P.; Tostes, R.C.; Jimenez-Altayó, F. The homeostatic role of hydrogen peroxide, superoxide anion and nitric oxide in the vasculature. Free Radic. Biol. Med. 2021, 162, 615–635. [Google Scholar] [CrossRef]
- Rao, C.V. Nitric oxide signaling in colon cancer chemoprevention. Mutat. Res. Fundam. Mol. Mech. Mutagn. 2004, 555, 107–119. [Google Scholar] [CrossRef]
- Sinha, B.K. Role of oxygen and nitrogen radicals in the mechanism of anticancer drug cytotoxicity. J. Cancer Sci. Ther. 2020, 12, 10–18. [Google Scholar]
- Grujičić, D.; Marković, A.; Vukajlović, J.T.; Stanković, M.; Jakovljević, M.R.; Ćirić, A.; Đorđević, K.; Planojević, N.; Milutinović, M.; Milošević-Đorđević, O. Genotoxic and cytotoxic properties of two medical plants (Teucrium arduini L. and Teucrium flavum L.) in relation to their polyphenolic contents. Mutat. Res. Gen. Toxicol. Environ. Mutagen. 2020, 852, 503168. [Google Scholar] [CrossRef] [PubMed]
- Mićović, T.; Topalović, D.; Živković, L.; Spremo-Potparević, B.; Jakovljević, V.; Matić, S.; Popović, S.; Baskić, D.; Stešević, D.; Samardžić, S.; et al. Antioxidant, antigenotoxic and cytotoxic activity of essential oils and methanol extracts of Hyssopus officinalis L. subsp. aristatus (Godr.) Nyman (Lamiaceae). Plants 2021, 10, 711. [Google Scholar] [CrossRef]
- Blagosklonny, M.V. Target for cancer therapy: Proliferating cells or stem cells. Leukemia 2006, 20, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Atha, D.H.; Coskun, E.; Erdem, O.; Tona, A.; Reipa, V.; Nelson, B.C. Genotoxic effects of etoposide, bleomycin, and ethyl methanesulfonate on cultured CHO cells: Analysis by GC-MS/MS and comet assay. J. Nucl. Acids 2020, 2020, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Mihailović, V.; Kreft, S.; Tavčar Benković, E.; Ivanović, N.; Stanković, M.S. Chemical profile, antioxidant activity and stability in stimulated gastrointestinal tract model system of three Verbascum species. Ind. Crops Prod. 2016, 89, 141–151. [Google Scholar] [CrossRef]
- Park, Y.K.; Koo, M.H.; Ikegaki, M.; Contado, J.L. Comparison of the flavonoid aglycone contents of Apis mellifera propolis from various regions of Brazil. Arq. Biol. Tecnol. 1997, 40, 97–106. [Google Scholar]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Birnboim, H.C. A rapid alkaline extraction method for the isolation of plasmid DNA. Methods Enzymol. 1983, 100, 243–255. [Google Scholar] [PubMed]
- Yang, C.F.; Shen, H.M.; Ong, C.N. Protective effect of ebselen against hydrogen peroxide-induced cytotoxicity and DNA damage in HepG2 cells. Biochem. Pharmacol. 1999, 57, 273–279. [Google Scholar] [CrossRef]
- Azqueta, A.; Gutzkow, K.B.; Priestley, C.C.; Meier, S.; Walker, J.S.; Brunborg, G.; Collins, A.R. A comparative performance test of standard, medium-and high-throughput comet assays. Toxicol. In Vitro 2013, 27, 768–773. [Google Scholar] [CrossRef] [PubMed]
- Auclair, C.; Voisin, E. Nitroblue tetrazolium reduction. In Handbook of Methods for Oxygen Radical Research; Bokaraton CRC Press: Boca Raton, FL, USA, 1985; pp. 123–132. [Google Scholar]
- Griess, P. Bemerkungen zu der Abhandlung der HH. Weselsky und Benedikt „Ueber einige Azoverbindungen”. Ber. Dtsch. Chem. Ges. 1879, 12, 426–428. [Google Scholar] [CrossRef] [Green Version]





| The Content of Polyphenolic Compounds | |||||
|---|---|---|---|---|---|
| Species | Extract | TPC (mg GAE/g) | PAC (mg CAE/g) | TFC (mg QE/g) | FC (mg QE/g) |
| Mo | Methanolic | 79.40 ± 3.78 b | 58.26 ± 8.34 c | 11.25 ± 1.13 c | 0.90 ± 0.29 |
| Ethanolic | 70.30 ± 1.52 a | 67.89 ± 1.92 c | 10.13 ± 0.43 c | 0.96 ± 0.23 | |
| Aqueous | 74.22 ± 1.39 | 40.48 ± 2.31 ab | 5.97 ± 0.22 ab | 1.30 ± 0.55 | |
| Mp | Methanolic | 77.12 ± 4.11 | 51.59 ± 2.80 c | 32.00 ± 0.44 c | 11.09 ± 0.20 c |
| Ethanolic | 70.79 ± 2.10 | 54.56 ± 2.94 c | 30.85 ± 0.35 c | 11.34 ± 0.26 c | |
| Aqueous | 76.27 ± 2.64 | 16.41 ± 1.70 ab | 21.31 ± 0.34 ab | 5.33 ± 0.21 ab | |
| Ob | Methanolic | 19.71 ± 0.40 b | 12.70 ± 7.40 c | 8.58 ± 0.35 b | 0.96 ± 0.47 b |
| Ethanolic | 29.14 ± 0.37 a | 19.00 ± 3.33 c | 10.28 ± 0.33 a | 2.67 ± 0.21 ac | |
| Aqueous | 24.28 ± 0.17 | nd ab | 8.76 ± 0.57 | 0.55 ± 0.54 b | |
| Ro | Methanolic | 57.32 ± 0.54 c | 68.63 ± 8.34 bc | 18.52 ± 1.15 bc | 3.75 ± 0.13 bc |
| Ethanolic | 54.09 ± 2.33 c | 33.07 ± 1.70 a | 12.09 ± 0.28 ac | 2.50 ± 0.06 ac | |
| Aqueous | 76.37 ± 0.74 ab | 33.44 ± 4.44 a | 14.82 ± 0.38 ab | 5.77 ± 0.43 ab | |
| So | Methanolic | 70.79 ± 2.16 c | 46.78 ± 6.19 | 26.93 ± 0.67 ac | 8.84 ± 0.39 b |
| Ethanolic | 69.26 ± 5.77 c | 50.11 ± 10.94 c | 19.95 ± 0.05 a | 6.91 ± 0.20 ac | |
| Aqueous | 44.63 ± 0.64 ab | 30.85 ± 2.31 b | 20.50 ± 0.14 a | 8.01 ± 0.30 b | |
| Sm | Methanolic | 55.17 ± 1.87 bc | 64.56 ± 3.33 bc | 10.47 ± 0.53 bc | 4.39 ± 0.27 c |
| Ethanolic | 66.52 ± 1.21 a | 44.93 ± 3.57 a | 12.30 ± 0.22 a | 4.06 ± 0.31 c | |
| Aqueous | 63.49 ± 1.57 a | 35.30 ± 7.23 a | 12.46 ± 0.24 a | 6.62 ± 0.29 ab | |
| Spices | Extract | Rosmarinic Acid | Caffeic Acid | Chlorogenic Acid | Quercetin | Rutin | Naringin | Luteolin-7-O-glucoside |
|---|---|---|---|---|---|---|---|---|
| Mo | Methanolic | 86.76 ± 4.69 bc | 1.33 ± 0.08 | 0.23 ± 0.01 b | 12.43 ± 0.48 c | 1.64 ± 0.05 bc | 3.36 ± 0.13 bc | 2.55 ± 0.15 |
| Ethanolic | 61.77 ± 3.58 ac | 2.54 ± 0.10 | 1.11 ± 0.04 ac | 11.56 ± 0.59 c | 3.38 ± 0.15 a | 2.94 ± 0.11 ac | 1.34 ± 0.10 | |
| Aqueous | 52.32 ± 3.18 ab | 2.87 ± 0.14 | 0.60 ± 0.01 b | 7.75 ± 0.35 ab | 3.40 ± 0.18 a | 1.44 ± 0.07 ba | 1.64 ± 0.08 | |
| Mp | Methanolic | 63.30 ± 3.21 bc | tr | 0.35 ± 0.01 c | 2.94 ± 0.17 c | tr bc | 2.42 ± 0.09 b | 5.07 ± 0.28 |
| Ethanolic | 3.81 ± 1.15 ac | tr | 0.22 ± 0.01 c | 3.36 ± 0.15 c | 0.56 ± 0.29 ac | 1.20 ± 0.05 ac | 5.09 ± 0.29 | |
| Aqueous | 84.45 ± 4.15 ab | 0.69 ± 0.02 | 1.09 ± 0.05 ab | 11.05 ± 0.42 ab | 6.40 ± 0.31 ab | 2.41 ± 0.10 b | 4.41 ± 0.18 | |
| Ob | Methanolic | 13.85 ± 0.42 c | 1.33 ± 0.03 | 0.83 ± 0.01 c | 3.03 ± 0.05 c | 2.70 ± 0.08 c | 3.26 ± 0.09 c | 1.25 ± 0.02 |
| Ethanolic | 12.02 ± 0.28 c | tr | 0.62 ± 0.01 c | 2.62 ± 0.09 | 3.09 ± 0.10 c | 3.15 ± 0.18 c | 0.99 ± 0.01 | |
| Aqueous | 4.36 ± 0.11 ab | 0.78 ± 0.02 | tr ab | 2.19 ± 0.07 a | 1.97 ± 0.05 ab | 1.83 ± 0.13 ab | 1.11 ± 0.02 | |
| Ro | Methanolic | 63.48 ± 3.87 bc | 2.14 ± 0.09 b | 4.75 ± 0.27 bc | trb | 10.04 ± 0.58 b | 1.08 ± 0.05 | 8.04 ± 0.46 |
| Ethanolic | 49.44 ± 2.90 a | 0.08 ± 0.00 ac | 0.06 ± 0.00 ac | 1.95 ± 0.05 ac | 8.09 ± 0.32 ac | 0.92 ± 0.03 | 8.46 ± 0.42 c | |
| Aqueous | 45.87 ± 2.64 a | 2.40 ± 0.11 b | 2.61 ± 0.13 ab | trb | 10.84 ± 0.48 b | 1.10 ± 0.03 | 7.02 ± 0.31 b | |
| So | Methanolic | 55.62 ± 2.52 bc | 1.97 ± 0.06 c | 0.17 ± 0.00 bc | 3.28 ± 0.23 b | 8.82 ± 0.31 bc | 12.83 ± 0.56 bc | 22.82 ± 1.05 bc |
| Ethanolic | 42.35 ± 2.66 ac | 2.38 ± 0.09 c | 0.60 ± 0.02 ac | 2.35 ± 0.10 a | tr a | 9.88 ± 0.38 a | 18.65 ± 0.53 ac | |
| Aqueous | 29.01 ± 1.13 ab | 3.47 ± 0.13 ab | 1.39 ± 0.06 ab | 2.58 ± 0.11 | tr a | 9.84 ± 0.41 a | 16.97 ± 0.69 ab | |
| Sm | Methanolic | 51.96 ± 2.41 bc | 1.61 ± 0.05 bc | tr bc | 3.37 ± 0.14 bc | 1.65 ± 0.07 bc | 1.62 ± 0.09 | 5.18 ± 0.19 bc |
| Ethanolic | 39.60 ± 1.78 ac | 2.79 ± 0.09 ac | 0.79 ± 0.03 a | 2.03 ± 0.10 a | tra | 1.48 ± 0.06 | 6.97 ± 0.32 a | |
| Aqueous | 13.44 ± 0.62 ab | 4.02 ± 0.19 ab | 0.66 ± 0.02 a | 2.22 ± 0.08 a | tra | 1.54 ± 0.40 | 7.00 ± 0.38 a |
| TPC | PAC | TFC | FC | RA | CA | ChA | Q | RUT | NAR | L-G | DPPH | PRA | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TPC | 1 | 0.90 c | 0.78 c | 0.69 c | 0.68 c | 0.22 a | −0.06 a | 0.47 b | 0.28 a | 0.00 a | 0.21 a | 0.89 c | −0.58 b |
| PAC | 1 | 0.65 b | 0.56 b | 0.60 b | 0.01 a | −0.04 a | 0.48 b | 0.06 a | −0.16 a | 0.04 a | 0.79 c | −0.33 a | |
| TFC | 1 | 0.93 c | 0.15 a | 0.11 a | 0.02 a | −0.22 a | 0.33 a | 0.40 b | 0.59 b | 0.75 c | −0.38 b | ||
| FC | 1 | −0.08 a | 0.46 b | −0.09 a | −0.33 a | 0.15 a | 0.26 a | 0.47 b | 0.65 b | −0.38 b | |||
| RA | 1 | −0.26 a | 0.16 a | 0.70 c | 0.37 b | 0.05 a | 0.08 a | 0.46 b | −0.34 a | ||||
| CA | 1 | 0.15 a | −0.15 a | 0.10 a | 0.21 a | 0.26 a | 0.58 b | −0.37 b | |||||
| ChA | 1 | 0.20 a | 0.59 b | −0.21 a | −0.01 a | −0.02 a | −0.19 a | ||||||
| Q | 1 | −0.07 a | −0.14 a | −0.36 a | 0.25 a | −0.15 a | |||||||
| RUT | 1 | 0.19 a | 0.61 b | 0.12 a | −0.39 b | ||||||||
| NAR | 1 | 0.83 c | 0.09 a | −0.02 a | |||||||||
| L-G | 1 | 0.22 a | −0.29 a | ||||||||||
| DPPH | 1 | −0.52 b | |||||||||||
| PRA | 1 |
| DPPH Assay (Percentage of Inhibition) | ||||
|---|---|---|---|---|
| Species | Extract | 100 µg/mL | 250 µg/mL | 500 µg/mL |
| Mo | Methanolic | 41.81 ± 1.70 c, xyz | 90.50 ± 1.04 xy | 93.08 ± 0.29 xy |
| Ethanolic | 38.47 ± 0.52 c, yz | 93.29 ± 0.13 xy | 93.58 ± 0.29 xy | |
| Aqueous | 48.19 ± 0.72 ab, xyz | 92.42 ± 0.68 xy | 92.36 ± 0.67 xy | |
| Mp | Methanolic | 55.86 ± 1.08 bc, xyz | 93.40 ± 0.77 c, xy | 93.33 ± 0.34 xy |
| Ethanolic | 40.09 ± 0.67 ac, yz | 92.80 ± 0.64 c, xy | 94.46 ± 0.28 c, xy | |
| Aqueous | 23.88 ± 0.66 ab, xyz | 61.27 ± 2.84 ab, xyz | 91.99 ± 0.38 b, xy | |
| Ob | Methanolic | 15.84 ± 0.94 xyz | 42.50 ± 1.70 bc, xyz | 69.95 ± 0.56 bc, xz |
| Ethanolic | 14.05 ± 1.35 xyz | 34.43 ± 0.36 ac, xyz | 54.02 ± 0.90 ac, xyz | |
| Aqueous | 11.89 ± 1.12 xyz | 27.68 ± 0.07 ab, xyz | 51.61 ± 0.24 ab, xyz | |
| Ro | Methanolic | 37.25 ± 0.51 byz | 64.14 ± 0.41 bc, xyz | 94.98 ± 0.14 b, xy |
| Ethanolic | 23.61 ± 2.16 ac, xyz | 30.35 ± 0.56 ac, xyz | 88.99 ± 1.48 ac, xy | |
| Aqueous | 35.56 ± 1.43 b, z | 56.60 ± 1.18 ab, yz | 93.74 ± 0.09 b, xy | |
| So | Methanolic | 39.41 ± 0.63 c, yz | 81.24 ± 1.02 bc, xyz | 94.90 ± 0.09 c, xy |
| Ethanolic | 37.05 ± 0.81 c, z | 77.00 ± 1.31 ac, xyz | 95.21 ± 0.05 c, xy | |
| Aqueous | 31.64 ± 0.81 ab, xz | 53.09 ± 1.32 ab, z | 92.43 ± 0.14 ab, xy | |
| Sm | Methanolic | 32.89 ± 0.61 c, z | 61.16 ± 0.86 xyz | 94.98 ± 0.05 xy |
| Ethanolic | 30.63 ± 0.59 c, xz | 63.76 ± 0.62 xyz | 95.09 ± 0.11 xy | |
| Aqueous | 37.59 ± 3.51 ab, yz | 62.52 ± 1.54 xyz | 93.74 ± 0.25 xy | |
| BHA | 36.20 ± 0.66 z | 55.43 ± 1.38 z | 75.43 ± 1.16 yz | |
| BHT | 32.73 ± 0.60 z | 52.01 ± 0.53 z | 71.90 ± 1.86 xz | |
| Ascorbic acid | 89.42 ± 0.17 xy | too high xy | too high xy | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oalđe Pavlović, M.; Kolarević, S.; Đorđević, J.; Jovanović Marić, J.; Lunić, T.; Mandić, M.; Kračun Kolarević, M.; Živković, J.; Alimpić Aradski, A.; Marin, P.D.; et al. A Study of Phytochemistry, Genoprotective Activity, and Antitumor Effects of Extracts of the Selected Lamiaceae Species. Plants 2021, 10, 2306. https://doi.org/10.3390/plants10112306
Oalđe Pavlović M, Kolarević S, Đorđević J, Jovanović Marić J, Lunić T, Mandić M, Kračun Kolarević M, Živković J, Alimpić Aradski A, Marin PD, et al. A Study of Phytochemistry, Genoprotective Activity, and Antitumor Effects of Extracts of the Selected Lamiaceae Species. Plants. 2021; 10(11):2306. https://doi.org/10.3390/plants10112306
Chicago/Turabian StyleOalđe Pavlović, Mariana, Stoimir Kolarević, Jelena Đorđević, Jovana Jovanović Marić, Tanja Lunić, Marija Mandić, Margareta Kračun Kolarević, Jelena Živković, Ana Alimpić Aradski, Petar D. Marin, and et al. 2021. "A Study of Phytochemistry, Genoprotective Activity, and Antitumor Effects of Extracts of the Selected Lamiaceae Species" Plants 10, no. 11: 2306. https://doi.org/10.3390/plants10112306
APA StyleOalđe Pavlović, M., Kolarević, S., Đorđević, J., Jovanović Marić, J., Lunić, T., Mandić, M., Kračun Kolarević, M., Živković, J., Alimpić Aradski, A., Marin, P. D., Šavikin, K., Vuković-Gačić, B., Božić Nedeljković, B., & Duletić-Laušević, S. (2021). A Study of Phytochemistry, Genoprotective Activity, and Antitumor Effects of Extracts of the Selected Lamiaceae Species. Plants, 10(11), 2306. https://doi.org/10.3390/plants10112306












