Alleviation of Malathion Toxicity Effect by Coffea arabica L. Oil and Olea europaea L. Oil on Lipid Profile: Physiological and In Silico Study
Abstract
:1. Introduction
2. Results
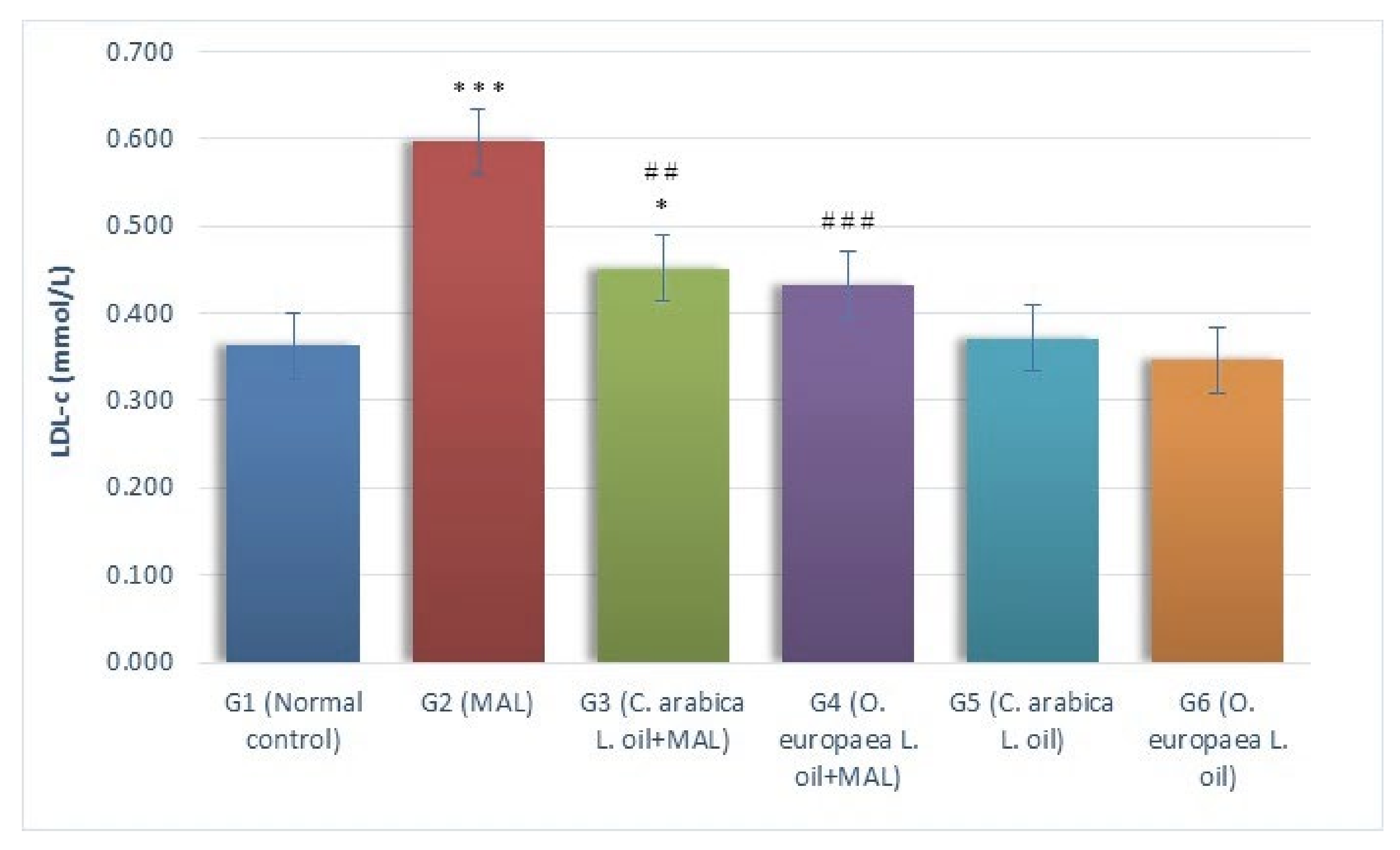
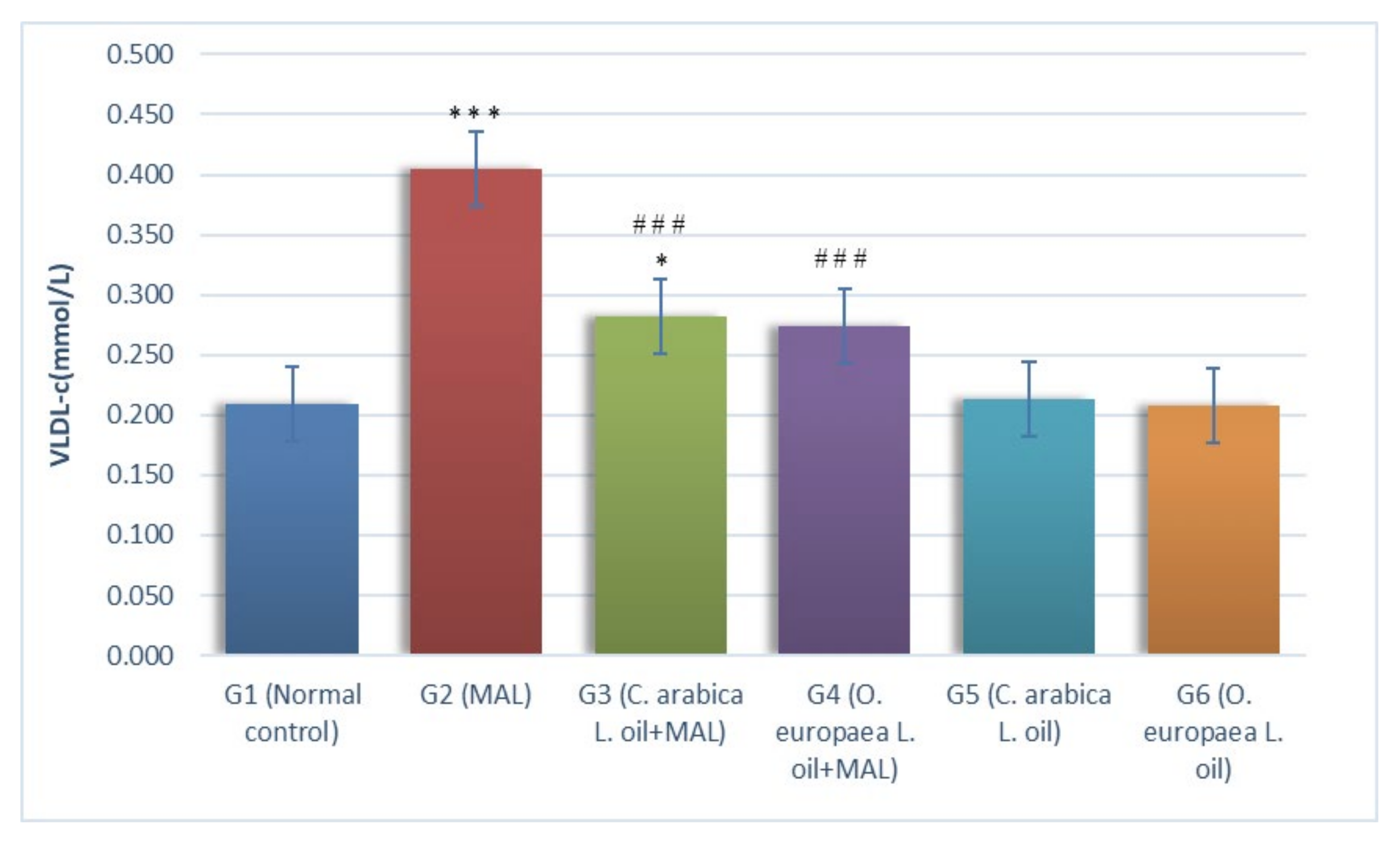
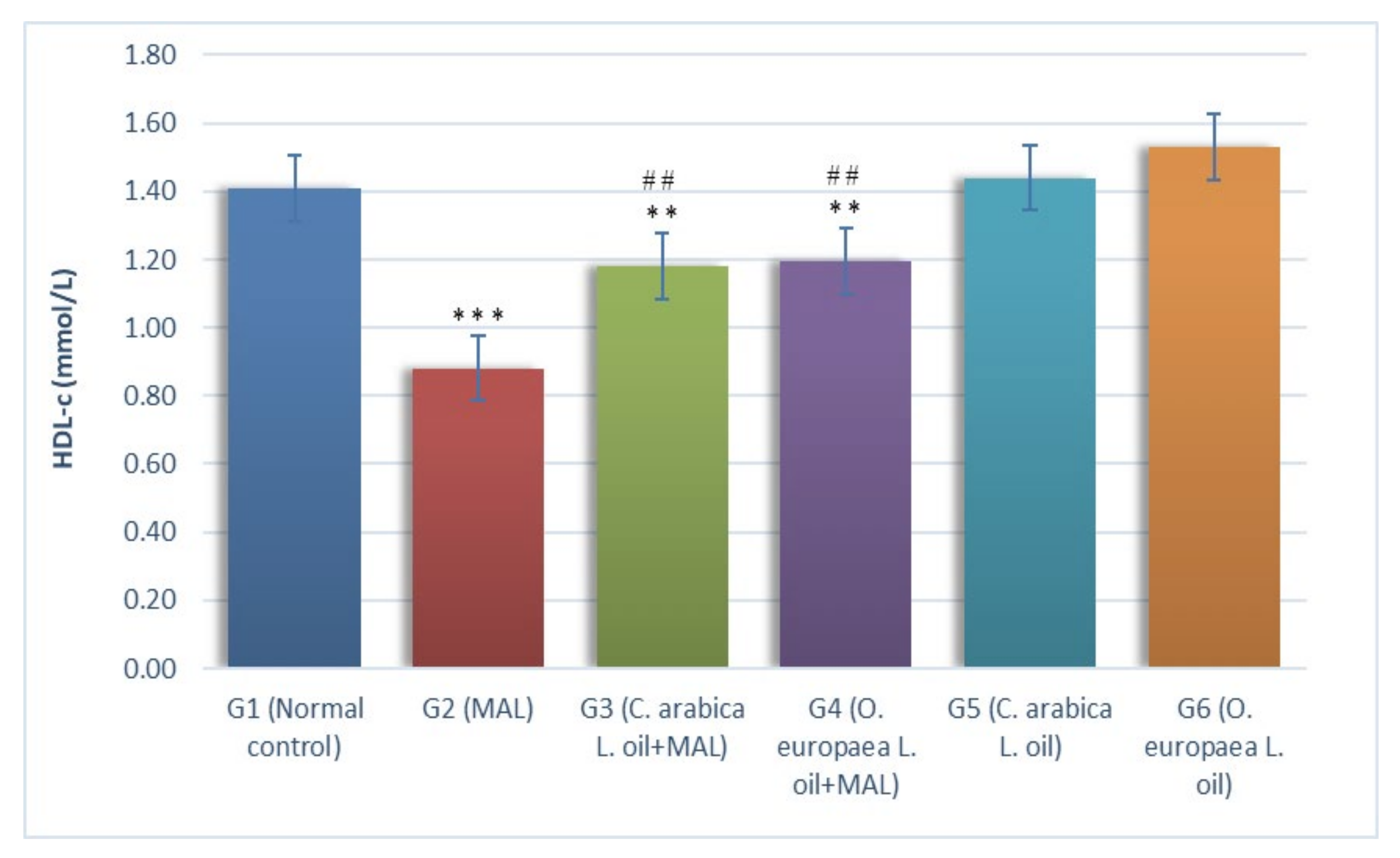
2.1. Assessment of Biochemical Markers
2.2. Molecular Docking Study
3. Discussion
4. Materials and Methods
4.1. Plant Oils
4.2. Animals Model
4.3. Experimental Protocol
- No treatment was given to the rats in the first group (group 1). They were designated as uninterfered normal control.
- Oral administration of MAL (100 mg per kg BW) for 49 days (7 weeks) was provided to the rats of the second group (group 2).
- Oral supplementation of Coffea arabica L. oil (400 mg per kg BW) and exposure to MAL (100 mg per kg BW) after three hours of Coffea arabica L. oil ingestion was daily provided to the animals of the third group (group 3) for 49 days (7 weeks).
- Oral supplementation of Olea europaea L. oil (400 mg per kg BW) and exposure to MAL (100 mg per kg BW) after three hours of Olea europaea L. oil ingestion was daily provided to the animals of the fourth group (group 4) for 49 days (7 weeks).
- Oral supplementation of Coffea arabica L. oil (400 mg per kg BW) was provided daily to the animals of the fifth group (group 5) for 49 days (7 weeks).
- Oral supplementation of Olea europaea L. oil (400 mg per kg BW) was provided daily to the animals of the sixth group (group 6) for 49 days (7 weeks).
4.4. Analysis of Blood Serum
4.5. BIOINFORMATICS Analysis
4.5.1. Selection of Enzymes for Docking Study
4.5.2. Molecular Docking Study
4.6. Analysis of the Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dawson, A.H.; Eddleston, M.; Senarathna, L.; Mohamed, F.; Gawarammana, I.; Bowe, S.J.; Manuweera, G.; Buckley, N.A. Acute human lethal toxicity of agricultural pesticides: A prospective cohort study. PLoS Med. 2010, 7, e1000357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.-J.; Mehler, L.; Beckman, J.; Diebolt-Brown, B.; Prado, J.; Lackovic, M.; Waltz, J.; Mulay, P.; Schwartz, A.; Mitchell, Y. Acute pesticide illnesses associated with off-target pesticide drift from agricultural applications: 11 States, 1998–2006. Environ. Health Perspect. 2011, 119, 1162–1169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mostafalou, S.; Abdollahi, M. Concerns of environmental persistence of pesticides and human chronic diseases. Clin. Exp. Pharmacol. Physiol. 2012, 2, 3. [Google Scholar] [CrossRef] [Green Version]
- Makris, K.C.; Konstantinou, C.; Andrianou, X.D.; Charisiadis, P.; Kyriacou, A.; Gribble, M.O.; Christophi, C.A. A cluster-randomized crossover trial of organic diet impact on biomarkers of exposure to pesticides and biomarkers of oxidative stress/inflammation in primary school children. PLoS ONE 2019, 14, e0219420. [Google Scholar] [CrossRef] [Green Version]
- Poomagal, S.; Sujatha, R.; Kumar, P.S.; Vo, D.-V.N. A fuzzy cognitive map approach to predict the hazardous effects of malathion to environment (air, water and soil). Chemosphere 2021, 263, 127926. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Zhang, R.; Liu, J.; Guo, Y.; Ma, E. Effects of malathion and chlorpyrifos on acetylcholinesterase and antioxidant defense system in Oxya chinensis (Thunberg)(Orthoptera: Acrididae). Chemosphere 2011, 83, 599–604. [Google Scholar] [CrossRef]
- Kwong, T.C. Organophosphate pesticides: Biochemistry and clinical toxicology. Ther. Drug Monit. 2002, 24, 144–149. [Google Scholar] [CrossRef]
- Brocardo, P.S.; Pandolfo, P.; Takahashi, R.N.; Rodrigues, A.L.S.; Dafre, A.L. Antioxidant defenses and lipid peroxidation in the cerebral cortex and hippocampus following acute exposure to malathion and/or zinc chloride. Toxicology 2005, 207, 283–291. [Google Scholar] [CrossRef]
- Delgado, E.H.; Streck, E.L.; Quevedo, J.L.; Dal-Pizzol, F. Mitochondrial respiratory dysfunction and oxidative stress after chronic malathion exposure. Neurochem. Res. 2006, 31, 1021–1025. [Google Scholar] [CrossRef] [PubMed]
- Akhgari, M.; Abdollahi, M.; Kebryaeezadeh, A.; Hosseini, R.; Sabzevari, O. Biochemical evidence for free radicalinduced lipid peroxidation as a mechanism for subchronic toxicity of malathion in blood and liver of rats. Hum. Exp. Toxicol. 2003, 22, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Hazarika, A.; Sarkar, S.; Hajare, S.; Kataria, M.; Malik, J. Influence of malathion pretreatment on the toxicity of anilofos in male rats: A biochemical interaction study. Toxicology 2003, 185, 1–8. [Google Scholar] [CrossRef]
- Indolfi, C.; De Rosa, S.; Colombo, A. Bioresorbable vascular scaffolds—Basic concepts and clinical outcome. Nat. Rev. Cardiol. 2016, 13, 719–729. [Google Scholar] [CrossRef]
- EL, S.G. Effect of garlic consumption on blood lipid and oxidant/antioxidant parameters in rat males exposed to chlorpyrifos. Slovak J. Anim. Sci. 2009, 42, 111–117. [Google Scholar]
- Uchendu, C.; Ambali, S.; Ayo, J.; Lasisi, I.; Umosen, A. Subacute chlorpyrifos-induced alterations in serum lipids and some oxidative stress biomarkers in male Wistar rats: Beneficial effect of acetyl-L-carnitine. Toxicol. Environ. Chem. 2013, 95, 483–494. [Google Scholar] [CrossRef]
- Uzun, F.G.; Kalender, Y. Chlorpyrifos induced hepatotoxic and hematologic changes in rats: The role of quercetin and catechin. Food Chem. Toxicol. 2013, 55, 549–556. [Google Scholar] [CrossRef]
- Chen, Z.-H.; Niki, E. Two faces of lipid peroxidation products: The-Yin and Yang-principles of oxidative stress. J. Exp. Integr. Med. 2011, 1, 215–219. [Google Scholar] [CrossRef] [Green Version]
- Aly, N.; Kawther, E.-G.; Mahmoud, F.; El-Sebae, A.K. Protective effect of vitamin C against chlorpyrifos oxidative stress in male mice. Pestic. Biochem. Physiol. 2010, 97, 7–12. [Google Scholar] [CrossRef]
- Zheng, J.; Zhou, Y.; Li, S.; Zhang, P.; Zhou, T.; Xu, D.-P.; Li, H.-B. Effects and mechanisms of fruit and vegetable juices on cardiovascular diseases. Int. J. Mol. Sci. 2017, 18, 555. [Google Scholar] [CrossRef] [Green Version]
- Shang, A.; Cao, S.-Y.; Xu, X.-Y.; Gan, R.-Y.; Tang, G.-Y.; Corke, H.; Mavumengwana, V.; Li, H.-B. Bioactive compounds and biological functions of garlic (Allium sativum L.). Foods 2019, 8, 246. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.-Y.; Zhao, C.-N.; Cao, S.-Y.; Tang, G.-Y.; Gan, R.-Y.; Li, H.-B. Effects and mechanisms of tea for the prevention and management of cancers: An updated review. Crit. Rev. Food Sci. Nutr. 2020, 60, 1693–1705. [Google Scholar] [CrossRef]
- Pimentel, G.; Micheletti, T.; Nehlig, A. Coffee intake and obesity. In Nutrition in the Prevention and Treatment of Abdominal Obesity; Watson, R.R., Ed.; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Baeza, G.; Amigo-Benavent, M.; Sarriá, B.; Goya, L.; Mateos, R.; Bravo, L. Green coffee hydroxycinnamic acids but not caffeine protect human HepG2 cells against oxidative stress. Food Res. Int. 2014, 62, 1038–1046. [Google Scholar] [CrossRef] [Green Version]
- La Lastra, C.; Barranco, M.; Motilva, V.; Herrerias, J. Mediterrranean diet and health biological importance of olive oil. Curr. Pharm. Des. 2001, 7, 933–950. [Google Scholar] [CrossRef] [Green Version]
- Bellosta, S.; Bernini, F.; Paoletti, R.; Corsini, A. Non-lipid-related effects of statins. Ann. Med. 2000, 32, 164–176. [Google Scholar] [CrossRef]
- Martín, M.J.; Pablos, F.; González, A.G.; Valdenebro, M.S.; León-Camacho, M. Fatty acid profiles as discriminant parameters for coffee varieties differentiation. Talanta 2001, 54, 291–297. [Google Scholar] [CrossRef]
- Manna, C.; D’Angelo, S.; Migliardi, V.; Loffredi, E.; Mazzoni, O.; Morrica, P.; Galletti, P.; Zappia, V. Protective effect of the phenolic fraction from virgin olive oils against oxidative stress in human cells. J. Agric. Food Chem. 2002, 50, 6521–6526. [Google Scholar] [CrossRef] [PubMed]
- Calligaris, S.; Munari, M.; Arrighetti, G.; Barba, L. Insights into the physicochemical properties of coffee oil. Eur. J. Lipid Sci. Technol. 2009, 111, 1270–1277. [Google Scholar] [CrossRef]
- Wagemaker, T.A.L.; Carvalho, C.R.L.; Maia, N.B.; Baggio, S.R.; Guerreiro Filho, O. Sun protection factor, content and composition of lipid fraction of green coffee beans. Ind. Crop. Prod. 2011, 33, 469–473. [Google Scholar] [CrossRef] [Green Version]
- Barbaro, B.; Toietta, G.; Maggio, R.; Arciello, M.; Tarocchi, M.; Galli, A.; Balsano, C. Effects of the olive-derived polyphenol oleuropein on human health. Int. J. Mol. Sci. 2014, 15, 18508–18524. [Google Scholar] [CrossRef]
- De Oliveira, P.M.A.; de Almeida, R.H.; de Oliveira, N.A.; Bostyn, S.; Goncalves, C.B.; de Oliveira, A.L. Enrichment of diterpenes in green coffee oil using supercritical fluid extraction—Characterization and comparison with green coffee oil from pressing. J. Supercrit. Fluids 2014, 95, 137–145. [Google Scholar] [CrossRef]
- Covas, M.-I.; de la Torre, R.; Fitó, M. Virgin olive oil: A key food for cardiovascular risk protection. Br. J. Nutr. 2015, 113, 19–28. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Morató, J.; Boronat, A.; Kotronoulas, A.; Pujadas, M.; Pastor, A.; Olesti, E.; Pérez-Mañá, C.; Khymenets, O.; Fitó, M.; Farré, M. Metabolic disposition and biological significance of simple phenols of dietary origin: Hydroxytyrosol and tyrosol. Drug Metab. Rev. 2016, 48, 218–236. [Google Scholar] [CrossRef] [Green Version]
- Deiana, M.; Serra, G.; Corona, G. Modulation of intestinal epithelium homeostasis by extra virgin olive oil phenolic compounds. Food Funct. 2018, 9, 4085–4099. [Google Scholar] [CrossRef]
- Marcelino, G.; Hiane, P.A.; de Cássia Freitas, K.; Santana, L.F.; Pott, A.; Donadon, J.R.; de Cássia Avellaneda Guimarães, R. Effects of olive oil and its minor components on cardiovascular diseases, inflammation, and gut microbiota. Nutrients 2019, 11, 1826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devrim, E.; Ergüder, İ.B.; Özbek, H.; Durak, İ. High-cholesterol diet increases xanthine oxidase and decreases nitric oxide synthase activities in erythrocytes from rats. Nutr. Res. 2008, 28, 212–215. [Google Scholar] [CrossRef] [PubMed]
- Sabán-Ruiz, J.; Alonso-Pacho, A.; Fabregate-Fuente, M.; de la Puerta Gonzalez-Quevedo, C. Xanthine oxidase inhibitor febuxostat as a novel agent postulated to act against vascular inflammation. Anti-Inflamm. Anti-Allergy Agents Med. Chem. 2013, 12, 94–99. [Google Scholar] [CrossRef]
- Marahatha, R.; Basnet, S.; Bhattarai, B.R.; Budhathoki, P.; Aryal, B.; Adhikari, B.; Lamichhane, G.; Poudel, D.K.; Parajuli, N. Potential natural inhibitors of xanthine oxidase and HMG-CoA reductase in cholesterol regulation: In silico analysis. BMC Complement. Med. Ther. 2021, 21, 1. [Google Scholar] [CrossRef]
- Babu, N.S.; Malik, J.; Rao, G.; Aggarwal, M.; Ranganathan, V. Effects of subchronic malathion exposure on the pharmacokinetic disposition of pefloxacin. Environ. Toxicol. Pharmacol. 2006, 22, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Paşaoğlu, Ö.; Şen, B.; Ekremoğlu, M.; Severcan, Ç.; Paşaoğlu, H. Effects of Acute Malathion Exposure on Renal Oxidant and Antioxidant Balance in Rats. Gazi Med. J. 2021, 32, 189–193. [Google Scholar]
- Kumar, D.; Sharma, B.; Rizvi, S. Protective role of black tea and vitamin C during sub-acute toxicity of carbofuran in rats. Prog. Health Sci. 2016, 6, 148–154. [Google Scholar] [CrossRef] [Green Version]
- Ince, S.; Arslan-Acaroz, D.; Demirel, H.H.; Varol, N.; Ozyurek, H.A.; Zemheri, F.; Kucukkurt, I. Taurine alleviates malathion induced lipid peroxidation, oxidative stress, and proinflammatory cytokine gene expressions in rats. Biomed. Pharmacother. 2017, 96, 263–268. [Google Scholar] [CrossRef]
- Jaiswal, S.; Gupta, V.; Siddiqi, N.; Sharma, B. Curcumin mediated attenuation of carbofuran induced toxicity in the heart of Wistar rats. Cell. Mol. Biol. 2017, 63, 12–17. [Google Scholar] [CrossRef]
- Jaiswal, S.K.; Gupta, V.K.; Ansari, M.D.; Siddiqi, N.J.; Sharma, B. Vitamin C acts as a hepatoprotectant in carbofuran treated rat liver slices in vitro. Toxicol. Rep. 2017, 4, 265–273. [Google Scholar] [CrossRef]
- Begaa, S.; Messaoudi, M. Toxicological aspect of some selected medicinal plant samples collected from Djelfa, Algeria Region. Biol. Trace Elem. Res. 2019, 187, 301–306. [Google Scholar] [CrossRef]
- ALAsmari, K.M.; Zeid, I.M.A.; Al-Attar, A.M. Medicinal Properties of Arabica coffee (Coffea arabica) Oil: An Overview. Adv. Life Sci. 2020, 8, 20–29. [Google Scholar]
- Antoniou, C.; Hull, J. The Anti-cancer Effect of Olea europaea L. Products: A Review. Curr. Nutr. Rep. 2021, 10, 99–124. [Google Scholar] [CrossRef]
- Yubero-Serrano, E.M.; Lopez-Moreno, J.; Gomez-Delgado, F.; Lopez-Miranda, J. Extra virgin olive oil: More than a healthy fat. Eur. J. Clin. Nutr. 2019, 72, 8–17. [Google Scholar] [CrossRef] [Green Version]
- Lasram, M.M.; Annabi, A.B.; El-Elj, N.; Selmi, S.; Kamoun, A.; El-Fazaa, S.; Gharbi, N. Metabolic disorders of acute exposure to malathion in adult Wistar rats. J. Hazard. Mater. 2009, 163, 1052–1055. [Google Scholar] [CrossRef] [PubMed]
- Al-Attar, A.M.; Abu Zeid, I.M. Effect of tea (Camellia sinensis) and olive (Olea europaea L.) leaves extracts on male mice exposed to diazinon. BioMed Res. Int. 2013, 2013, 461415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Attar, A.M. Effect of grapeseed oil on diazinon-induced physiological and histopathological alterations in rats. Saudi J. Biol. Sci. 2015, 22, 284–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selmi, S.; El-Fazaa, S.; Gharbi, N. Oxidative stress and alteration of biochemical markers in liver and kidney by malathion in rat pups. Toxicol. Ind. Health 2015, 31, 783–788. [Google Scholar] [CrossRef] [PubMed]
- El-Beih, N.M.; Ramadan, G.; Khorshed, M.A.; Ahmed, R.S. Biochemical alterations in insecticides-treated male albino rats: Potential modulatory effects of a standardized aged garlic extract. Indian J. Tradit. Knowl. 2017, 16, 181–188. [Google Scholar]
- Selmi, S.; Rtibi, K.; Grami, D.; Sebai, H.; Marzouki, L. Malathion, an organophosphate insecticide, provokes metabolic, histopathologic and molecular disorders in liver and kidney in prepubertal male mice. Toxicol. Rep. 2018, 5, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, F.K.; Alkhalaf, M.I. Effects of black seed and thyme leaves dietary supplements against malathion insecticide-induced toxicity in experimental rat model. J. King Saud Univ.-Sci. 2020, 32, 914–919. [Google Scholar] [CrossRef]
- Abdel-Daim, M.M.; Abushouk, A.I.; Bungău, S.G.; Bin-Jumah, M.; El-Kott, A.F.; Shati, A.A.; Aleya, L.; Alkahtani, S. Protective effects of thymoquinone and diallyl sulphide against malathion-induced toxicity in rats. Environ. Sci. Pollut. Res. 2020, 27, 10228–10235. [Google Scholar] [CrossRef] [PubMed]
- Hassan, H.A.; Yousef, M.I. Ameliorating effect of chicory (Cichorium intybus L.)-supplemented diet against nitrosamine precursors-induced liver injury and oxidative stress in male rats. Food Chem. Toxicol. 2010, 48, 2163–2169. [Google Scholar] [CrossRef]
- Kalender, S.; Uzun, F.G.; Durak, D.; Demir, F.; Kalender, Y. Malathion-induced hepatotoxicity in rats: The effects of vitamins C and E. Food Chem. Toxicol. 2010, 48, 633–638. [Google Scholar] [CrossRef]
- Reaven, G.M. Importance of identifying the overweight patient who will benefit the most by losing weight. Ann. Intern. Med. 2003, 138, 420–423. [Google Scholar] [CrossRef]
- Celik, I.; Suzek, H. The hematological effects of methyl parathion in rats. J. Hazard. Mater. 2008, 153, 1117–1121. [Google Scholar] [CrossRef]
- Medithi, S.; Jonnalagadda, P.R.; Jee, B. Predominant role of antioxidants in ameliorating the oxidative stress induced by pesticides. Arch. Environ. Occup. Health 2021, 76, 61–74. [Google Scholar] [CrossRef]
- Tajik, N.; Tajik, M.; Mack, I.; Enck, P. The potential effects of chlorogenic acid, the main phenolic components in coffee, on health: A comprehensive review of the literature. Eur. J. Nutr. 2017, 56, 2215–2244. [Google Scholar] [CrossRef]
- Al-Megrin, W.A.; El-Khadragy, M.F.; Hussein, M.H.; Mahgoub, S.; Abdel-Mohsen, D.M.; Taha, H.; Bakkar, A.A.; Abdel Moneim, A.E.; Amin, H.K. Green Coffea arabica extract ameliorates testicular injury in high-fat diet/streptozotocin-induced diabetes in rats. J. Diabetes Res. 2020, 2020, 6762709. [Google Scholar] [CrossRef]
- El Rabey, H.A.; Rezk, S.M.; Sakran, M.I.; Mohammed, G.M.; Bahattab, O.; Balgoon, M.J.; Elbakry, M.A.; Bakry, N. Green coffee methanolic extract and silymarin protect against CCl4-induced hepatotoxicity in albino male rats. BMC Complement. Med. Ther. 2021, 21, 1–11. [Google Scholar] [CrossRef]
- Alhamhany, N.N.; Alassady, E.H. Does green coffee has a positive effect on body mass index and lipid profile in a sample of obese people. J. Pharm. Sci. Res. 2018, 10, 627–630. [Google Scholar]
- Ayad, A.; Merzouk, H.; Hamed, Y.B.; Merzouk, S.A.; Gresti, J.; Narce, M. Beneficial effects of dietary olive and linseed oils on serum and tissue lipids and redox status in the aging obese rat. J. Nat. Prod. Plant Resour. 2013, 3, 61–71. [Google Scholar]
- Ghobadi, S.; Hassanzadeh-Rostami, Z.; Mohammadian, F.; Nikfetrat, A.; Ghasemifard, N.; Raeisi Dehkordi, H.; Faghih, S. Comparison of blood lipid-lowering effects of olive oil and other plant oils: A systematic review and meta-analysis of 27 randomized placebo-controlled clinical trials. Crit. Rev. Food Sci. Nutr. 2019, 59, 2110–2124. [Google Scholar] [CrossRef]
- Rezq, A.A.; Labib, F.A.; Attia, A. Effect of some dietary oils and fats on serum lipid profile, calcium absorption and bone mineralization in mice. Pak. J. Nutr. 2010, 9, 643–650. [Google Scholar] [CrossRef] [Green Version]
- Kalita, B.; Kusre, D.; Bhuyan, K. Effects of sesame oil and olive oil on the plasma total cholesterol, low density lipoprotein and high density lipoprotein cholesterol of guineapig. Int. J. Eng. Sci. Innov. Technol. 2014, 5, 217–221. [Google Scholar]
- Owen, R.W.; Giacosa, A.; Hull, W.E.; Haubner, R.; Würtele, G.; Spiegelhalder, B.; Bartsch, H. Olive-oil consumption and health: The possible role of antioxidants. Lancet Oncol. 2000, 1, 107–112. [Google Scholar] [CrossRef]
- Covas, M.-I.; Ruiz-Gutiérrez, V.; De La Torre, R.; Kafatos, A.; Lamuela-Raventós, R.M.; Osada, J.; Owen, R.W.; Visioli, F. Minor components of olive oil: Evidence to date of health benefits in humans. Nutr. Rev. 2006, 64, 20–30. [Google Scholar] [CrossRef]
- AL-Asmari, K.M.; Al-Attar, A.M.; Mohamed, I.; Zeid, A. Potential health benefits and components of olive oil: An overview. Biosci. Res. 2020, 17, 2673–2687. [Google Scholar]
- Gorinstein, S.; Leontowicz, H.; Lojek, A.; Leontowicz, M.; Ciz, M.; Krzeminski, R.; Gralak, M.; Czerwinski, J.; Jastrzebski, Z.; Trakhtenberg, S. Olive oils improve lipid metabolism and increase antioxidant potential in rats fed diets containing cholesterol. J. Agric. Food Chem. 2002, 50, 6102–6108. [Google Scholar] [CrossRef]
- Pérez-Jiménez, F.; de Cienfuegos, G.Á.; Badimón, L.; Barja, G.; Battino, M.; Blanco, Á.; Bonanome, A.; Colomer, R.; Corella-Piquer, D.; Covas, I.; et al. International conference on the healthy effect of virgin olive oil. Eur. J. Clin. Investig. 2005, 35, 421–424. [Google Scholar] [CrossRef]
- Cicerale, S.; Lucas, L.; Keast, R. Biological activities of phenolic compounds present in virgin olive oil. Int. J. Mol. Sci. 2010, 11, 458–479. [Google Scholar] [CrossRef] [Green Version]
- Farràs, M.; Canyelles, M.; Fitó, M. Effects of virgin olive oil and phenol-enriched virgin olive oils on lipoprotein atherogenicity. Nutrients 2020, 12, 601. [Google Scholar] [CrossRef] [Green Version]
- De Sotillo, D.V.R.; Hadley, M. Chlorogenic acid modifies plasma and liver concentrations of: Cholesterol, triacylglycerol, and minerals in (fa/fa) Zucker rats. J. Nutr. Biochem. 2002, 13, 717–726. [Google Scholar] [CrossRef]
- Karthikesan, K.; Pari, L.; Menon, V. Antihyperlipidemic effect of chlorogenic acid and tetrahydrocurcumin in rats subjected to diabetogenic agents. Chem.-Biol. Interact. 2010, 188, 643–650. [Google Scholar] [CrossRef]
- Kim, M.-S.; Koppula, S.; Sung, S.-J.; Lee, S.-R.; Park, Y.-D.; Lee, K.-A. Olea europaea linn (oleaceae) fruit pulp exhibits hypocholesterolemic and hepatoprotective effects via regulation of peroxisome proliferation-activated receptor alpha in high-fat diet-fed rats. Trop. J. Pharm. Res. 2014, 13, 31–39. [Google Scholar] [CrossRef] [Green Version]
- Fossati, P.; Prencipe, L. Serum triglycerides determined colorimetrically with an enzyme that produces hydrogen peroxide. Clin. Chem. 1982, 28, 2077–2080. [Google Scholar] [CrossRef]
- Richmond, W. Preparation and properties of a cholesterol oxidase from Nocardia sp. and its application to the enzymatic assay of total cholesterol in serum. Clin. Chem. 1973, 19, 1350–1356. [Google Scholar] [CrossRef]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef]
- Warnick, G.; Benderson, V.; Albers, N. Selected methods. Clin. Chem. 1983, 10, 91–99. [Google Scholar]




| Compounds | PubChem ID | XO (1N5X) | HMGR (1HWK) |
|---|---|---|---|
| Coffea arabica L. oil compounds | |||
| Chlorogenic acid | 1794427 | −8.2 | −10.6 |
| Oleic acid | 445639 | −7 | −7 |
| Linoleic acid | 5280450 | −6.7 | −6.7 |
| Palmitic acid | 985 | −6.2 | −6.2 |
| Kahweol | 114778 | −5.5 | −3.8 |
| Cafestol | 108052 | −5.2 | −3.8 |
| Caffeine | 2519 | −3.4 | −3.1 |
| Olea europea L. oil compound | |||
| Oleuropein | 5281544 | −11.36 | −8 |
| Linoleic acid | 5280450 | −8.3 | −6.7 |
| Oleic acid | 445639 | 7.3 | −7 |
| Palmitic acid | 985 | −6.2 | −6.2 |
| Stearic acid | 5281 | −6.7 | −6 |
| Hydroxytyrosol | 82755 | −5.3 | −5.4 |
| Tyrosol | 10393 | −5.12 | −4.5 |
| Squalene | 638072 | −5 | −1.9 |
| Controls | |||
| Atorvastatin | 60823 | −12.3 | |
| TEI-6720 | 134018 | −10.8 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Asmari, K.M.; Zeid, I.M.A.; Altayb, H.N.; Al-Attar, A.M.; Alomar, M.Y. Alleviation of Malathion Toxicity Effect by Coffea arabica L. Oil and Olea europaea L. Oil on Lipid Profile: Physiological and In Silico Study. Plants 2021, 10, 2314. https://doi.org/10.3390/plants10112314
Al-Asmari KM, Zeid IMA, Altayb HN, Al-Attar AM, Alomar MY. Alleviation of Malathion Toxicity Effect by Coffea arabica L. Oil and Olea europaea L. Oil on Lipid Profile: Physiological and In Silico Study. Plants. 2021; 10(11):2314. https://doi.org/10.3390/plants10112314
Chicago/Turabian StyleAl-Asmari, Khalid M., Isam M. Abu Zeid, Hisham N. Altayb, Atef M. Al-Attar, and Mohammed Y. Alomar. 2021. "Alleviation of Malathion Toxicity Effect by Coffea arabica L. Oil and Olea europaea L. Oil on Lipid Profile: Physiological and In Silico Study" Plants 10, no. 11: 2314. https://doi.org/10.3390/plants10112314
APA StyleAl-Asmari, K. M., Zeid, I. M. A., Altayb, H. N., Al-Attar, A. M., & Alomar, M. Y. (2021). Alleviation of Malathion Toxicity Effect by Coffea arabica L. Oil and Olea europaea L. Oil on Lipid Profile: Physiological and In Silico Study. Plants, 10(11), 2314. https://doi.org/10.3390/plants10112314






