Metabolites of Siberian Raspberries: LC-MS Profile, Seasonal Variation, Antioxidant Activity and, Thermal Stability of Rubus matsumuranus Phenolome
Abstract
1. Introduction
2. Results and Discussion
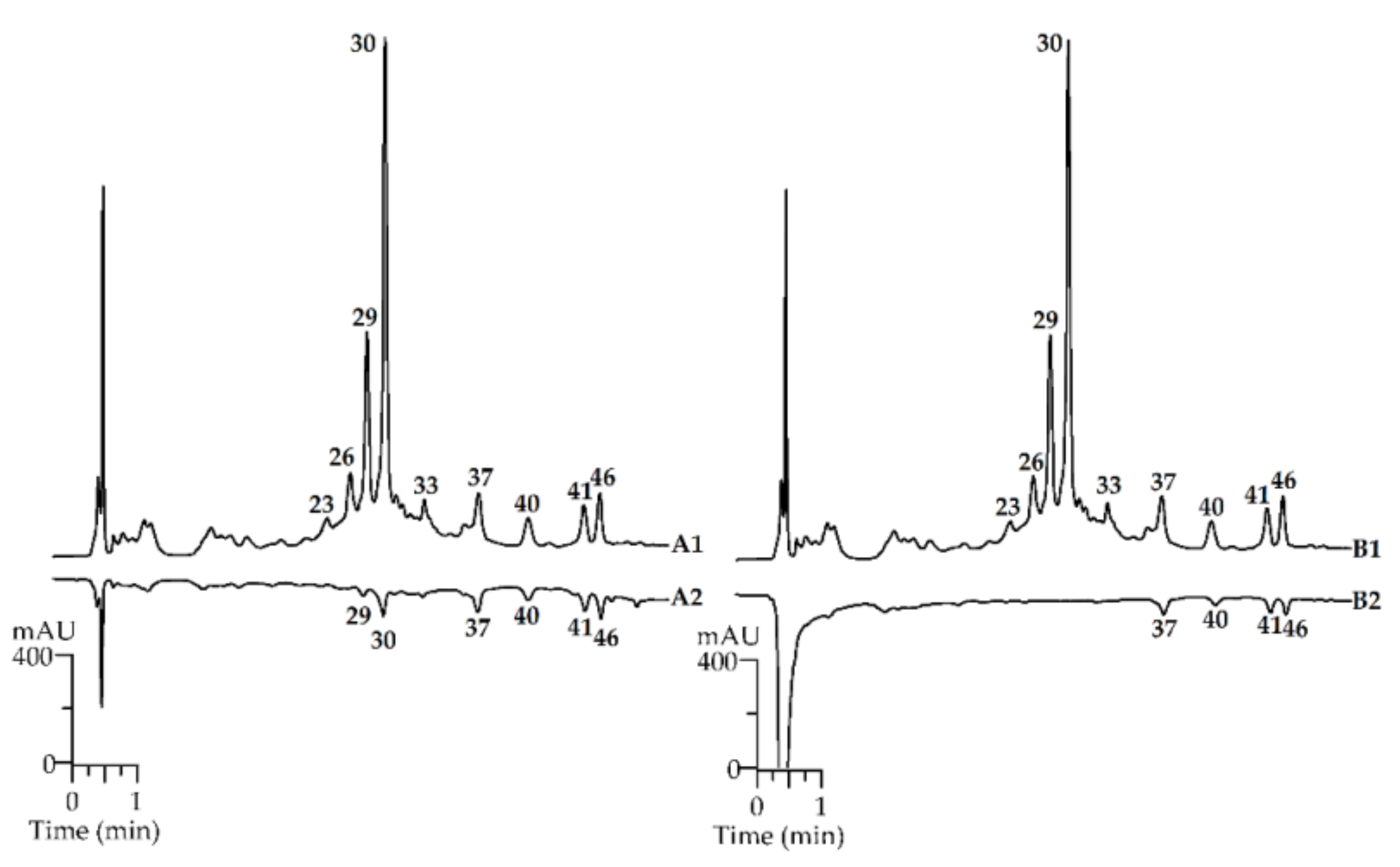
2.1. Metabolites of Rubus matsumuranus Leaves: LC-MS Profile
2.1.1. Gallic Acid Derivatives
2.1.2. Hydroxycinnamates
2.1.3. Catechins and Procyanidins
2.1.4. Flavonols
2.1.5. Ellagic Acid Derivatives and Ellagitannins
2.2. Quantitative Content and Seasonal Phenolic Profile of R. matsumuranus Leaves
2.3. Bioactivity of R. matsumuranus Leaf Extract: Antioxidant Potential
2.4. Stability of R. matsumuranus Phenolic Compounds in Water Media: Comparison of Infusion and Decoction Composition
3. Materials and Methods
3.1. Chemicals
3.2. Plant Material
3.3. High-Performance Liquid Chromatography with Photodiode Array Detection and Electrospray Ionization Triple Quadrupole Mass Spectrometric Detection (HPLC-PDA-ESI-tQ-MS): Metabolite Profiling and Quantification
3.4. Methanol Extract Preparation from R. matsumuranus Leaves
3.5. HPLC-PDA-Based Antioxidant Potential: DPPH Scavenging and Fe2+-Chelating Activity
3.6. Preparation of R. matsumuranus Leaf Infusions and Decoctions
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Indrayanto, G. Recent development of quality control methods for herbal derived drug preparations. Nat. Prod. Commun. 2018, 13, 1599–1606. [Google Scholar] [CrossRef]
- Rivera, G.; Bocanegra-García, V.; Monge, A. Traditional plants as source of functional foods: A review. Plantas tradicionales como fuente de alimentos funcionales: Una revision. CYTA J. Food 2010, 8, 159–167. [Google Scholar] [CrossRef]
- Häkkinen, S.; Heinonen, M.; Kärenlampi, S.; Mykkänen, H.; Ruuskanen, J.; Törrönen, R. Screening of selected flavonoids and phenolic acids in 19 berries. Food Res. Int. 1999, 32, 345–353. [Google Scholar] [CrossRef]
- Oszmianski, J.; Wojdylo, A.; Gorzelany, J.; Kapusta, I. Identification and characterization of low molecular weight polyphenols in berry leaf extracts by HPLC-DAD and LC-ESI/MS. J. Agric. Food Chem. 2011, 59, 12830–12835. [Google Scholar] [CrossRef]
- Verma, R.; Gangrade, T.; Punasiya, R.; Ghulaxe, C. Rubus fruticosus (blackberry) use as an herbal medicine. Pharmacogn. Rev. 2014, 8, 101–104. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Y.; Lin, H.S. Antioxidant activity in fruits and leaves of blackberry, raspberry, and strawberry varies with cultivar and developmental stage. J. Agric. Food Chem. 2000, 48, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Committee on Herbal Medicinal Products (HMPC) European Medicines Agency. Assessment Report on Rubus idaeus L., Folium; Committee on Herbal Medicinal Products (HMPC) European Medicines Agency: London, UK, 2012. [Google Scholar]
- Dugarzhapov, T.A.; Basaev, S.E. Myths and Legends of Buryats; Novaya Buryatia: Ulan-Ude, Russia, 2017; pp. 18–26. [Google Scholar]
- Rusakova, L.M. Traditions and Innovations in Life and Culture of the Siberian Peoples; SD RAS: Novosibirsk, Russia, 1983; p. 97. [Google Scholar]
- Komarov, V.L. The Flora of the U.S.S.R.; JASTOR: Moscow, Russia, 1941; Volume X, pp. 11–57. [Google Scholar]
- Ivanov, B.I. (Ed.) Atlas of Medicinal Plants of Yakutia; YaNTc SO RAN: Yakutsk, Russia, 2005; pp. 144–145. [Google Scholar]
- Batorova, S.M.; Yakovlev, G.P.; Aseeva, T.A. Reference-book of Traditional Tibetan Medicine Herbs; Nauka: Novosibirsk, Russia, 2013; p. 144. [Google Scholar]
- Ren, S.; Bao, B. Study on the flavonoids and biological activity of Rubus sachalinensis. Zhong Yao Cai 2016, 39, 2019–2023. [Google Scholar]
- Liu, B.-N.; Zhang, P.; Wu, Y.-X.; Su, R.-N.; Zhao, H.-M.; Bao, B.-Q. Separation, purification and immunomodulatory activity of polysaccharides from Rubus sachalinensis. Chin. Trad. Herb Drugs 2019, 50, 5941–5949. [Google Scholar] [CrossRef]
- Caidan, R.; Cairang, L.; Pengcuo, J.; Tong, L. Comparison of compounds of three Rubus species and their antioxidant activity. Drug Discov. Ther. 2015, 9, 391–396. [Google Scholar] [CrossRef]
- Tito, A.; Bimonte, M.; Carola, A.; De Lucia, A.; Barbulova, A.; Tortora, A.; Colucci, G.; Apone, F. An oil-soluble extract of Rubus idaeus cells enhances hydration and water homeostasis in skin cells. Int. J. Cosmet. Sci. 2015, 37, 588–594. [Google Scholar] [CrossRef]
- Rao, A.V.; Snyder, D.M. Raspberries and human health: A review. J. Agric. Food Chem. 2010, 58, 3871–3883. [Google Scholar] [CrossRef] [PubMed]
- Niero, R.; Filho, V.C. Therapeutic potential and chemical composition of plants from the genus Rubus: A mini review of the last 10 years. Nat. Prod. Comm. 2008, 3, 437–444. [Google Scholar] [CrossRef]
- Määttä-Riihinen, K.R.; Kamal-Eldin, A.; Törrönen, A.R. Identification and quantification of phenolic compounds in berries of Fragaria and Rubus species (family Rosaceae). J. Agric. Food Chem. 2004, 52, 6178–6187. [Google Scholar] [CrossRef] [PubMed]
- Määttä-Riihinen, K.R.; Kamal-Eldin, A.; Mattila, P.H.; Gonzalez-Paramas, A.M.; Törrönen, A.R. Distribution and contents of phenolic compounds in eighteen Scandinavian berry species. J. Agric. Food Chem. 2004, 52, 4477–4486. [Google Scholar] [CrossRef] [PubMed]
- Vrhovsek, U.; Guella, G.; Gasperotti, M.; Pojer, E.; Zancato, M.; Mattivi, F. Clarifying the identity of the main ellagitannin in the fruit of the strawberry, Fragaria vesca and Fragaria ananassa Duch. J. Agric. Food Chem. 2012, 60, 2507–2516. [Google Scholar] [CrossRef]
- Beekwilder, J.; Jonker, H.; Meesters, P.; Hall, R.D.; van der Meer, I.M.; de Vos, C.H.R. Antioxidant in raspberry: On-line analysis links antioxidant activity to a diversity of individual metabolites. J. Agric. Food Chem. 2005, 53, 3313–3320. [Google Scholar] [CrossRef]
- Sangiovanni, E.; Vrhovsek, U.; Rossoni, G.; Colombo, E.; Brunelli, C.; Brembati, L.; Trivulzio, S.; Gasperotti, M.; Mattivi, F.; Bosisio, E.; et al. Ellagitannins from Rubus berries for the control of gastric inflammation: In vitro and in vivo studies. PLoS ONE 2013, 8, e71762. [Google Scholar] [CrossRef]
- Ross, H.A.; McDougall, G.J.; Stewart, D. Antiproliferative activity is predominantly associated with ellagitannins in raspberry extracts. Phytochemistry 2007, 68, 218–228. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Chirikova, N.K.; Vasilieva, A.G.; Fedorov, I.A. LC-MS profile, gastrointestinal and gut microbiota stability and antioxidant activity of Rhodiola rosea herb metabolites: A comparative study with subterranean organs. Antioxidants 2020, 9, 526. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Nikolaev, V.M.; Chirikova, N.K. Sagan Dalya tea, a new “old” probable adaptogenic drug: Metabolic characterization and bioactivity potentials of Rhododendron adamsii leaves. Antioxidants 2021, 10, 863l. [Google Scholar] [CrossRef]
- Kashchenko, N.I.; Olennikov, D.N. Phenolome of Asian agrimony tea (Agrimonia asiatica Juz., Rosaceae): LC-MS profile, α-glucosidase inhibitory potential and stability. Foods 2020, 9, 1348. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Tankhaeva, L.M.; Agafonova, S.V. Antioxidant components of Laetiporus sulphureus (Bull.: Fr.) Murr. fruit bodies. Appl. Biochem. Microbiol. 2011, 47, 419–425. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Vasilieva, A.G.; Chirikova, N.K. Fragaria viridis fruit metabolites: Variation of LC-MS profile and antioxidant potential during ripening and storage. Pharmaceuticals 2020, 13, 262. [Google Scholar] [CrossRef]
- Ghareeb, M.A.; Sobeh, M.; El-Maadawy, W.H.; Mohammed, H.S.; Khalil, H.; Botros, S.; Wink, M. Chemical profiling of polyphenolics in Eucalyptus globulus and evaluation of its hepato-renal protective potential against cyclophosphamide induced toxicity in mice. Antioxidants 2019, 8, 415. [Google Scholar] [CrossRef]
- Poay, T.H.; Kiong, L.S.; Hock, C.C. Characterisation of galloylated cyanogenic glucosides and hydrolysable tannins from leaves of Phyllagathis rotundifolia by LC-ESI-MS/MS. Phytochem. Anal. 2011, 22, 516–525. [Google Scholar] [CrossRef] [PubMed]
- Ferlemi, A.-V.; Lamari, F.N. Berry leaves: An alternative source of bioactive natural products of nutritional and medicinal value. Antioxidants 2016, 5, 17. [Google Scholar] [CrossRef]
- Ponder, A.; Hallmann, E. Phenolics and carotenoid contents in the leaves of different organic and conventional raspberry (Rubus idaeus L.) cultivars and their in vitro activity. Antioxidants 2019, 8, 458. [Google Scholar] [CrossRef]
- Dall’Acqua, S.; Cervellati, R.; Loi, M.C.; Innocenti, G. Evaluation of in vitro antioxidant properties of some traditional Sardinian medicinal plants: Investigation of the high antioxidant capacity of Rubus ulmifolius. Food Chem. 2008, 106, 745–749. [Google Scholar] [CrossRef]
- Oszmiański, J.; Wojdyło, A.; Nowicka, P.; Teleszko, M.; Cebulak, T.; Wolanin, M. Determination of phenolic compounds and antioxidant activity in leaves from wild Rubus L. species. Molecules 2015, 20, 4951–4966. [Google Scholar] [CrossRef]
- Alanís, A.D.; Calzada, F.; Cedillo-Rivera, R.; Meckes, M. Antiprotozoal activity of the constituents of Rubus coriifolius. Phytother. Res. 2003, 17, 681–682. [Google Scholar] [CrossRef]
- Buřičová, L.; Andjelkovic, M.; Čermáková, A.; Réblová, Z.; Jurček, O.; Kolehmainen, E.; Verhé, R.; Kvasnička, F. Antioxidant capacity and antioxidants of strawberry, blackberry, and raspberry leaves. Czech J. Food Sci. 2011, 29, 181–189. [Google Scholar] [CrossRef]
- Krauze-Baranowska, M.; Głód, D.; Kula, M.; Majdan, M.; Hałasa, R.; Matkowski, A.; Kozłowska, W.; Kawiak, A. Chemical composition and biological activity of Rubus idaeus shoots—A traditional herbal remedy of Eastern Europe. BMC Complement. Altern. Med. 2014, 14. [Google Scholar] [CrossRef]
- Ziemlewska, A.; Zagórska-Dziok, M.; Nizioł-Łukaszewska, Z. Assessment of cytotoxicity and antioxidant properties of berry leaves as by-products with potential application in cosmetic and pharmaceutical products. Sci. Rep. 2021, 11, 3240. [Google Scholar] [CrossRef] [PubMed]
- Tzouwara-Karayanni, S.M.; Philianos, S.M. Isolation of quercetin and kaempferol from Rubus ulmifolius and their fluorometric assay. Microchem. J. 1982, 27, 155–161. [Google Scholar] [CrossRef]
- Gudej, J.; Tomczyk, M. Determination of flavonoids, tannins and ellagic acid in leaves from Rubus L. species. Arch. Pharm. Res. 2004, 27, 1114–1119. [Google Scholar] [CrossRef] [PubMed]
- Tallini, L.R.; Pedrazza, G.P.R.; de Bordignon, S.A.L.; Costa, A.C.O.; Steppe, M.; Fuentefria, A.; Zuanazzi, J.A.S. Analysis of flavonoids in Rubus erythrocladus and Morus nigra leaves extracts by liquid chromatography and capillary electrophoresis. Rev. Bras. Farmacogn. 2015, 25, 219–227. [Google Scholar] [CrossRef]
- Gudej, J. Kaempferol and quercetin glycosides from Rubus idaeus leaves. Acta Polon. Pharm. 2003, 60, 313–315. [Google Scholar]
- Regueiro, J.; Sanchez-Gonzalez, C.; Vallverdu-Queralt, A.; Simal-Gandara, J.; Lamuela-Raventos, R.; Izquierdo-Pulido, M. Comprehensive identification of walnut polyphenols by liquid chromatography coupled to linear ion trap-Orbitrap mass spectrometry. Food Chem. 2014, 152, 340–348. [Google Scholar] [CrossRef]
- Mena, P.; Calani, L.; Dall’Asta, C.; Galaverna, G.; Garcia-Viguera, C.; Bruni, R.; Crozier, A.; del Rio, D. Rapid and comprehensive evaluation of (poly)phenolic compounds in pomegranate (Punica granatum L.) juice by UHPLC-MSn. Molecules 2012, 17, 14821–14840. [Google Scholar] [CrossRef]
- Grochowski, D.M.; Strawa, J.W.; Granica, S.; Tomczyk, M. Secondary metabolites of Rubus caesius (Rosaceae). Biochem. Syst. Ecol. 2020, 92, 104111. [Google Scholar] [CrossRef]
- Okuda, T.; Yoshida, T.; Hatano, T.; Iwasaki, M.; Kubo, M.; Orime, T.; Yoshizaki, M.; Naruhashi, N. Hydrolysable tannins as chemotaxonomic markers in the Rosaceae. Phytochemistry 1992, 31, 3091–3096. [Google Scholar] [CrossRef]
- Hukkanen, A.; Kostamo, K.; Kärenlampi, S.; Kokko, H. Impact of agrochemicals on Peronospora sparsa and phenolic profiles in three Rubus arcticus cultivars. J. Agric. Food Chem. 2008, 56, 1008–1016. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Tachibana, H.; Nonaka, G.; Nishioka, I.; Hsu, F.L.; Kohda, H.; Tanaka, O. Tannins and related compounds. CXXII. New dimeric, trimeric and tetrameric ellagitannins, lambertianins A-D, from Rubus lambertianus Seringe. Chem. Pharm. Bull. 1993, 41, 1214–1220. [Google Scholar] [CrossRef] [PubMed]
- Niehaus, J.U.; Gross, G.G. A gallotannin degrading esterase from leaves of pedunculate oak. Phytochemistry 1997, 45, 1555–1560. [Google Scholar] [CrossRef]
- Schulenburg, K.; Feller, A.; Hoffmann, T.; Schecker, J.H.; Martens, S.; Schwab, W. Formation of β-glucogallin, the precursor of ellagic acid in strawberry and raspberry. J. Exp. Bot. 2016, 67, 2299–2308. [Google Scholar] [CrossRef]
- Niemetz, R.; Gross, G.G. Gallotannin biosynthesis: β-glucogallin: Hexagalloyl 3-O-galloyltransferase from Rhus typhina leaves. Phytochemistry 2001, 58, 657–661. [Google Scholar] [CrossRef]
- Niemetz, R.; Gross, G.G. Enzymology of gallotannin and ellagitannins biosynthesis. Phytochemistry 2005, 66, 2001–2011. [Google Scholar] [CrossRef]
- Grochowski, D.M.; Skalicka-Woźniak, K.; Orhan, I.E.; Xiao, J.; Locatelli, M.; Piwowarski, J.P.; Granica, S.; Tomczyk, M. A comprehensive review of agrimoniin. Ann. N. Y. Acad. Sci. 2017, 1401, 166–180. [Google Scholar] [CrossRef]
- Tuominen, A.; Salminen, J.-P. Hydrolyzable tannins, flavonol glycosides, and phenolic acids show seasonal and ontogenic variation in Geranium sylvaticum. J. Agric. Food Chem. 2017, 65, 6387–6403. [Google Scholar] [CrossRef]
- Salminen, J.-P.; Roslin, T.; Karonen, M.; Sinkkonen, J.; Pihlaja, K.; Pulkkinen, P. Seasonal variation in the content of hydrolyzable tannins, flavonoid glycosides, and proanthocyanidins in oak leaves. J. Chem. Ecol. 2004, 30, 1675–1693. [Google Scholar] [CrossRef]
- Solar, A.; Colarič, M.; Usenik, V.; Stampar, F. Seasonal variations of selected flavonoids, phenolic acids and quinones in annual shoots of common walnut (Juglans regia L.). Plant Sci. 2006, 170, 453–461. [Google Scholar] [CrossRef]
- Vagiri, M.; Conner, S.; Stewart, D.; Andersson, S.C.; Verral, S.; Johansson, E.; Rumpunen, K. Phenolic compounds in black currant (Ribes nigrum L.) leaves relative to leaf position and harvest date. Food Chem. 2015, 172, 135–142. [Google Scholar] [CrossRef]
- Rutkowska, M.; Balcerczak, E.; Świechowski, R.; Dubicka, M.; Olszewska, M.A. Seasonal variation in phenylpropanoid biosynthesis and in vitro antioxidant activity of Sorbus domestica leaves: Harvesting time optimisation for medicinal application. Ind. Crop Prod. 2020, 156, 112858. [Google Scholar] [CrossRef]
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouységu, L. Plant polyphenols: Chemical properties, biological activities, and synthesis. Angew. Chem. 2011, 50, 586–621. [Google Scholar] [CrossRef] [PubMed]
- Michel, P.; Owczarek, A.; Kosno, M.; Gontarek, D.; Matczak, M.; Olszewska, M.A. Variation in polyphenolic profile and in vitro antioxidant activity of eastern teaberry (Gaultheria procumbens L.) leaves following foliar development. Phytochem. Lett. 2017, 20, 356–364. [Google Scholar] [CrossRef]
- Melkadze, R.G.; Chichkovani, N.S.; Kakhniashvili, E.Z. Characteristics of the composition of Caucasian blackberry (Rubus caucasicus L.) leaves as a raw material for tea production. Prikl. Biokhim. Mikrobiol. 2008, 44, 714–719. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-D.; Lee, Y.; Kim, H.; Kim, H.; Park, C.-G.; Lee, S. HPLC/UV quantification of (+)-catechin in Filipendula glaberrima from different regions and flowering stages. Korean J. Pharmacogn. 2020, 51, 291–296. [Google Scholar] [CrossRef]
- Zidorn, C. Seasonal variation of natural products in European trees. Phytochem. Rev. 2018, 17, 923–935. [Google Scholar] [CrossRef]
- Mellway, R.D.; Constabel, C.P. Metabolic engineering and potential functions of proanthocyanidins in poplar. Plant Signal. Behav. 2009, 4, 790–792. [Google Scholar] [CrossRef]
- Wang, S.Y.; Zheng, W. Effect of plant growth temperature on antioxidant capacity in strawberry. J. Agric. Food Chem. 2001, 49, 4977–4982. [Google Scholar] [CrossRef]
- Mierziak, J.; Kostyn, K.; Kulma, A. Flavonoids as important molecules of plant interactions with the environment. Molecules 2014, 19, 16240–16265. [Google Scholar] [CrossRef]
- Agati, G.; Tattini, M. Multiple functional roles of flavonoids in photoprotection. New Phytol. 2010, 186, 786–793. [Google Scholar] [CrossRef] [PubMed]
- Tattini, M.; Galardi, C.; Pinelli, P.; Massai, R.; Remorini, D.; Agati, G. Differential accumulation of flavonoids and hydroxycinnamates in leaves of Ligustrum vulgare under excess light and drought stress. New Phytol. 2004, 163, 547–561. [Google Scholar] [CrossRef]
- Zhang, X.R.; Chen, Y.H.; Guo, Q.S.; Wang, W.M.; Liu, L.; Fan, J.; Cao, L.P.; Li, C. Short-term UV-B radiation effects on morphology, physiological traits and accumulation of bioactive compounds in Prunella vulgaris L. J. Plant Interact. 2017, 12, 348–354. [Google Scholar] [CrossRef]
- Remberg, S.F.; Sønsteby, A.; Aaby, K.; Heide, O.M. Influence of postflowering temperature on fruit size and chemical composition of Glen Ample raspberry (Rubus idaeus L.). J. Agric. Food. Chem. 2008, 56, 9080–9086. [Google Scholar] [CrossRef]
- Hatano, T.; Kira, R.; Yoshizaki, M.; Okuda, T. Seasonal changes in the tannins of Liquidambar formosana reflecting their biogenesis. Phytochemistry 1986, 25, 2787–2789. [Google Scholar] [CrossRef]
- Okuda, T.; Yoshida, T.; Hatano, T.; Yazaki, K.; Ashida, M. Ellagitannins of the Casuarinaceae, Stachyuraceae and Myrtaceae. Phytochemistry 1980, 21, 2871–2874. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, S.Y. Antioxidant activity and phenolic compounds in selected herbs. J. Agric. Food Chem. 2001, 49, 5165–5170. [Google Scholar] [CrossRef]
- Chirikova, N.K.; Olennikov, D.N.; Tankhaeva, L.M. Quantitative determination of flavonoid content in the aerial parts of Baikal skullcap (Scutellaria baicalensis Georgi). Russ. J. Bioorg. Chem. 2010, 36, 915–922. [Google Scholar] [CrossRef]
- Mullen, W.; McGinn, J.; Lean, M.E.J.; MacLean, M.R.; Gardner, P.; Duthie, G.G.; Yokota, T.; Crozier, A. Ellagitannins, flavonoids, and other phenolics in red raspberries and their contribution to antioxidant capacity and vasorelaxation properties. J. Agric. Food Chem. 2002, 50, 5191–5196. [Google Scholar] [CrossRef] [PubMed]
- Kähkönen, M.; Kylli, P.; Ollilainen, V.; Salminen, J.-P.; Heinonen, M. Antioxidant activity of isolated ellagitannins from red raspberries and cloudberries. J. Agric. Food Chem. 2012, 60, 1167–1174. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Kashchenko, N.I.; Chirikova, N.K. A novel HPLC-assisted method for investigation of the Fe2+-chelating activity of flavonoids and plant extracts. Molecules 2014, 19, 18296–18316. [Google Scholar] [CrossRef] [PubMed]
- Olennikov, D.N.; Kashchenko, N.I.; Chirikova, N.K.; Koryakina, L.P.; Vladimirov, L.N. Bitter gentian teas: Nutritional and phytochemical profiles, polysaccharide characterization and bioactivity. Molecules 2015, 20, 20014–20030. [Google Scholar] [CrossRef]
- Hatano, T.; Edamatsu, R.; Hiramatsu, M.; Mori, A.; Fujita, Y.; Yasuhara, T.; Yoshida, T.; Okuda, T. Effects of the interaction of tannins with co-existing substances. VI. Effects of tannins and related polyphenols on superoxide anion radical, and on 1,1-diphenyl-2-picrylhydrazyl radical. Chem. Pharm. Bull. 1989, 37, 2016–2021. [Google Scholar] [CrossRef]
- Özacar, M.; Soykan, C.; Şengil, I. Studies on synthesis, characterization, and metal adsorption of mimosa and valonia tannin resins. J. Appl. Polym. Sci. 2006, 102, 786–797. [Google Scholar] [CrossRef]
- Guimarães, R.; Barros, L.; Carvalho, A.M.; Ferreira, I.C.F.R. Infusions and decoctions of mixed herbs used in folk medicine: Synergism in antioxidant potential. Phytother. Res. 2011, 25, 1209–1214. [Google Scholar] [CrossRef] [PubMed]
- Sõukand, R.; Kalle, R. Where does the border lie: Locally grown plants used for making tea for recreation and/or healing, 1970s-1990s Estonia. J. Ethnopharmacol. 2013, 150, 162–174. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Kirillina, C.S.; Chirikova, N.K. Water-soluble melanoidin pigment as a new antioxidant component of fermented willowherb leaves (Epilobium angustifolium). Antioxidants 2021, 10, 1300. [Google Scholar] [CrossRef] [PubMed]
- Petkova, N.; Ognyanov, M.; Kirchev, M.; Stancheva, M. Bioactive compounds in water extracts prepared from rosehip-containing herbal blends. J. Food Process. Preserv. 2021, 48, e14645. [Google Scholar] [CrossRef]
- Sójka, M.; Janowski, M.; Grzelak-Błaszczyk, K. Stability and transformations of raspberry (Rubus idaeus L.) ellagitannins in aqueous solutions. Eur. Food Res. Technol. 2019, 245, 1113–1122. [Google Scholar] [CrossRef]
- Jordão, A.M.; Ricardo-da-Silva, J.; Laureano, O. Extraction of some ellagic tannins and ellagic acid from oak wood chips (Quercus pyrenaica L.) in model wine solutions: Effect of time, pH, temperature and alcoholic content. South Afr. J. Enol. Viticult. 2005, 26, 83–89. [Google Scholar] [CrossRef][Green Version]
- Zhu, Q.Y.; Zhang, A.; Tsang, D.; Huang, Y.; Chen, Z.-Y. Stability of green tea catechins. J. Agric. Food Chem. 1997, 45, 4624–4628. [Google Scholar] [CrossRef]
- De Maria, C.A.B.; Trugo, L.C.; De Mariz e Miranda, L.S.; Salvador, E. Stability of 5-caffeoylquinic acid under different conditions of heating. Food Res. Intern. 1998, 31, 475–477. [Google Scholar] [CrossRef]
- Li, Y.J.; Zhang, C.F.; Ding, G.; Huang, W.-Z.; Wang, Z.-Z.; Bi, Y.-A.; Xiao, W. Investigating the thermal stability of six caffeoylquinic acids employing rapid-resolution liquid chromatography with quadrupole time-of-flight tandem mass spectrometry. Eur. Food Res. Technol. 2015, 240, 1225–1234. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Tankhaeva, L.M.; Samuelsen, A.B. Quantitative analysis of polysaccharides from Plantago major leaves using the Dreywood method. Chem. Nat. Comp. 2006, 42, 265–268. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Kruglova, M.Y. A new quercetin glucoside and other phenolic compounds from the genus Filipendula. Chem. Nat. Comp. 2013, 49, 610–616. [Google Scholar] [CrossRef]
- Olennikov, D.N. Ellagitannins and other phenolic compounds from Comarum palustre. Chem. Nat. Comp. 2016, 52, 721–723. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Zilfikarov, I.N.; Penzina, T.A. Use of microcolumn HPLC for analysis of aloenin in Aloe arborescens raw material and related drugs. Pharm. Chem. J. 2013, 47, 494–497. [Google Scholar] [CrossRef]


| No | tR, min | Compound * [Ref.] | [M-H]−, m/z | MS/MS, m/z | Seasonal Content, mg/g DW ** ± SD | ||
|---|---|---|---|---|---|---|---|
| May (n = 32) | July (n = 54) | September (n = 44) | |||||
| 1 | 3.22 | O-Galloyl-dihexose L [25] | 493 | 331, 169 | <0.01 b | 0.25 ± 0.00 a | <0.01 b |
| 2 | 6.82 | 1-O-Caffeoylquinic acid S [26] | 353 | 191, 179, 173, 135 | 0.93 ± 0.02 a | 0.80 ± 0.02 b | 0.56 ± 0.01 c |
| 3 | 7.18 | 2-Pyrone-4,6-dicarboxyllic acid S [27] | 183 | 0.79 ± 0.02 c | 2.11 ± 0.04 a | 1.83 ± 0.04 b | |
| 4 | 8.49 | Gallic acid S [28] | 169 | 2.03 ± 0.04 a | 1.09 ± 0.02 b | 0.24 ± 0.00 c | |
| 5 | 8.79 | 1-O-Galloyl-glucose (glucogallin) S [25] | 331 | 169 | <0.01 a | <0.01 a | <0.01 a |
| 6 | 9.05 | Pedunculagin S [29] | 783, 391 *** | 633, 481, 301 | <0.01 c | 0.67 ± 0.02 b | 0.92 ± 0.02 a |
| 7 | 9.73 | O-Galloyl-hexose L [25] | 331 | 169 | <0.01 a | <0.01 a | <0.01 a |
| 8 | 10.02 | Gallocatechin S [25] | 305 | 168, 125 | 2.18 ± 0.04 c | 3.57 ± 0.07 a | 3.06 ± 0.07 b |
| 9 | 10.51 | Procyanidin B1 S [27] | 577 | 407, 289, 125 | 1.73 ± 0.04 c | 2.03 ± 0.04 b | 2.53 ± 0.05 a |
| 10 | 10.97 | Catechin S [25] | 289 | 247, 191, 123 | 0.79 ± 0.02 c | 1.52 ± 0.03 a | 1.01 ± 0.02 b |
| 11 | 11.28 | Procyanidin B2 S [27] | 577 | 407, 289, 125 | 0.18 ± 0.00 c | 0.59 ± 0.02 b | 0.79 ± 0.02 a |
| 12 | 11.53 | Epicatechin S [25] | 289 | 247, 191, 123 | 3.53 ± 0.07 c | 5.39 ± 0.11 a | 4.22 ± 0.08 b |
| 13 | 11.99 | 3-O-Caffeoylquinic acid S [26] | 353 | 191, 179, 135 | 1.79 ± 0.03 a | 1.22 ± 0.02 b | 0.93 ± 0.02 c |
| 14 | 12.53 | O-Caffeoyl-hexose L [26] | 341 | 179 | 4.38 ± 0.09 a | 3.27 ± 0.06 b | 1.40 ± 0.02 c |
| 15 | 13.02 | Tellimagrandin I1 S [27] | 785, 392 *** | 633, 483, 301 | 0.93 ± 0.02 b | 1.93 ± 0.04 a | 0.38 ± 0.00 c |
| 16 | 13.09 | 1,6-Di-O-galloyl-glucose S [25] | 483 | 331, 169 | 1.18 ± 0.02 a | 0.33 ± 0.02 c | 0.52 ± 0.01 b |
| 17 | 14.35 | 5-O-Caffeoylquinic acid S [26] | 353 | 191, 179, 165 | 3.53 ± 0.07 a | 2.81 ± 0.05 b | 2.03 ± 0.04 c |
| 18 | 14.48 | 4-O-Caffeoylquinic acid S [26] | 353 | 191, 179, 173, 135 | 2.36 ± 0.04 a | 1.73 ± 0.03 b | 1.48 ± 0.03 c |
| 19 | 14.73 | 5-O-Feruloylquinic acid S [26] | 367 | 205, 193 | 3.86 ± 0.07 a | 3.07 ± 0.05 b | 1.98 ± 0.04 c |
| 20 | 15.09 | Tellimagrandin I2 S [27] | 785, 392 *** | 633, 483, 301 | 0.26 ± 0.00 c | 0.93 ± 0.02 a | 0.31 ± 0.00 b |
| 21 | 15.46 | Tellimagrandin II1 S [27] | 937 | 785, 767,599,465, 301 | 0.12 ± 0.00 b | 0.38 ± 0.00 a | <0.01 c |
| 22 | 15.92 | Potentillin S [27] | 935, 467 *** | 633, 463, 301 | 0.45 ± 0.01 c | 1.63 ± 0.03 a | 1.28 ± 0.02 b |
| 23 | 16.03 | 1,3,6,-Tri-O-galloyl-glucose S [25] | 635 | 483, 331, 169, 125 | 2.90 ± 0.06 a | 1.67 ± 0.03 c | 1.93 ± 0.04 b |
| 24 | 16.51 | Tri-O-galloyl-hexose L [25] | 635 | 483, 331, 169, 125 | 2.27 ± 0.04 a | 1.26 ± 0.03 c | 1.48 ± 0.03 b |
| 25 | 16.77 | Sanguiin H10 L [29] | 1567,783 *** | 933, 633, 301 | 0.93 ± 0.02 b | 1.91 ± 0.04 a | 0.67 ± 0.02 c |
| 26 | 17.41 | Lambertianin A S [29] | 1869,934 *** | 1265,935,783,633,481,301 | 1.83 ± 0.03 c | 5.21 ± 0.11 a | 4.63 ± 0.09 b |
| 27 | 18.21 | Pedunculagin isomer L [29] | 783 | 481, 301 | <0.01 c | 0.14 ± 0.00 b | 0.44 ± 0.01 a |
| 28 | 18.48 | Tellimagrandin II2 S [27] | 937 | 785, 767,599,465, 301 | 0.07 ± 0.00 b | 0.36 ± 0.00 a | <0.01 c |
| 29 | 18.63 | Sanguiin H6 S [29] | 1567,783 *** | 933, 633, 301 | 6.14 ± 0.14 c | 19.62 ± 0.39 a | 15.32 ± 0.31 b |
| 30 | 19.14 | Lambertianin C S [29] | 1401 | 783, 633, 301 | 25.18 ± 0.50 c | 57.11 ± 1.14 a | 48.10 ± 0.96 b |
| 31 | 20.45 | Catechin O-gallate S [25] | 441 | 289, 125, 109 | 0.36 ± 0.00 c | 1.63 ± 0.03 b | 1.58 ± 0.03 a |
| 32 | 20.81 | Ellagic acid O-pentoside-O-hexoside L [27] | 595 | 433, 301 | 0.27 ± 0.00 c | 1.35 ± 0.02 a | 0.93 ± 0.02 b |
| 33 | 21.46 | Sanguiin H11 S [27] | 951 | 799, 481, 301 | 0.14 ± 0.00 c | 2.03 ± 0.04 a | 1.27 ± 0.03 b |
| 34 | 21.78 | Ellagic acid O-hexoside L [27] | 463 | 301 | <0.01 b | 0.52 ± 0.02 a | <0.01 b |
| 35 | 22.02 | Ellagic acid O-hexoside L [27] | 463 | 301 | <0.01 b | 0.40 ± 0.01 a | <0.01 b |
| 36 | 22.71 | Ellagic acid O-pentoside L [27] | 433 | 301 | <0.01 b | 0.49 ± 0.01 a | <0.01 b |
| 37 | 23.00 | Ellagic acid S [27] | 301 | 1.67 ± 0.03 c | 6.24 ± 0.12 b | 11.20 ± 0.23 a | |
| 38 | 23.43 | Quercetin-3-O-rutinoside (rutin) S [26,27,29] | 609 | 463, 301 | 0.09 ± 0.00 c | 0.96 ± 0.02 a | 0.11 ± 0.00 b |
| 39 | 24.69 | Quercetin-3-O-glucoside (isoquercitrin) S [26,27,29] | 463 | 301 | <0.01 b | 0.52 ± 0.01 a | <0.01 b |
| 40 | 24.42 | Quercetin-3-O-glucuronide (miquelianin) S [26,27,29] | 477 | 301 | 14.22 ± 0.29 c | 39.63 ± 0.78 a | 31.15 ± 0.63 b |
| 41 | 25.11 | Kaempferol-3-O-glucuronide S [26,27,29] | 461 | 285 | 9.23 ± 0.18 c | 31.18 ± 0.60 a | 25.67 ± 0.51 b |
| 42 | 25.44 | Quercetin O-(O-malonyl)-hexuronide L [26,27,29] | 563 | 477, 301 | 0.63 ± 0.02 b | 2.61 ± 0.05 a | 0.57 ± 0.01 c |
| 43 | 25.69 | Kaempferol O-(O-malonyl)-hexuronide L [26,27,29] | 533 | 447, 285 | <0.01 a | <0.01 a | <0.01 a |
| 44 | 26.39 | Quercetin O-(O-acetyl)-hexuronide L [26,27,29] | 519 | 477, 301 | 0.18 ± 0.00 c | 2.20 ± 0.04 a | 0.20 ± 0.00 b |
| 45 | 26.81 | Kaempferol O-(O-acetyl)-hexuronide L [26,27,29] | 503 | 461, 285 | <0.01 a | <0.01 a | <0.01 a |
| 46 | 27.31 | Quercetin O-(O-acetyl-O-malonyl)-hexuronide L | 605 | 519, 477, 301 | 18.69 ± 0.36 c | 36.82 ± 0.73 a | 21.03 ± 0.42 b |
| 47 | 27.93 | Quercetin O-(O-acetyl-O-malonyl)-hexuronide L | 605 | 519, 477, 301 | <0.01 a | <0.01 a | <0.01 a |
| 48 | 28.63 | Kaempferol O-(O-acetyl-O-malonyl)-hexuronide L | 589 | 503, 461, 285 | 0.04 ± 0.00 c | 1.83 ± 0.04 a | 0.30 ± 0.00 b |
| 49 | 29.47 | Kaempferol O-(O-acetyl-O-malonyl)-hexuronide L | 589 | 503, 461, 285 | <0.01 b | 0.31 ± 0.00 a | <0.01 b |
| 50 | 29.83 | Quercetin O-(O-acetyl-di-O-malonyl)-hexuronide L | 691 | 605, 519, 477, 301 | <0.01 a | <0.01 a | <0.01 a |
| 51 | 30.33 | Kaempferol O-(O-acetyl-di-O-malonyl)-hexuronide L | 675 | 589, 503, 461, 285 | <0.01 b | 0.26 ± 0.00 a | <0.01 b |
| 52 | 31.06 | Quercetin O-(di-O-acetyl-O-malonyl)-hexuronide L | 647 | 561, 519, 477, 301 | <0.01 a | <0.01 a | <0.01 a |
| 53 | 31.27 | Kaempferol O-(di-O-acetyl-O-malonyl)-hexuronide L | 631 | 545, 503, 461, 285 | <0.01 a | <0.01 a | <0.01 a |
| 54 | 31.90 | Ellagic acid O-methyl ester O-pentoside L [26,29] | 447 | 315, 301 | <0.01 a | <0.01 a | <0.01 a |
| 55 | 32.41 | Ellagic acid O-methyl ester O-pentoside L [26,29] | 447 | 315, 301 | <0.01 a | <0.01 a | <0.01 a |
| 56 | 32.58 | Quercetin O-(tri-O-acetyl)-hexuronide L [26,29] | 603 | 561, 519, 477, 301 | <0.01 c | 0.82 ± 0.02 a | 0.22 ± 0.00 b |
| 57 | 32.79 | Quercetin O-(tri-O-acetyl)-hexuronide L [26,29] | 603 | 561, 519, 477, 301 | <0.01 b | 0.08 ± 0.00 a | <0.01 b |
| 58 | 33.60 | Quercetin O-(tri-O-acetyl-O-malonyl)-hexuronide L | 689 | 603, 561, 519, 477, 301 | <0.01 b | <0.01 b | 0.10 ± 0.00 a |
| 59 | 34.01 | Kaempferol O-(tri-O-acetyl)-hexuronide L [26,29] | 587 | 545, 503, 461, 285 | <0.01 a | <0.01 a | <0.01 a |
| 60 | 34.99 | Kaempferol O-(tri-O-acetyl-O-malonyl)-hexuronide L | 673 | 587, 545, 503, 461, 285 | <0.01 a | <0.01 a | <0.01 a |
| 61 | 35.72 | Ellagic acid O-di-methyl ester L [26,29] | 329 | 315, 301 | <0.01 a | <0.01 a | <0.01 a |
| 62 | 36.53 | Ellagic acid O-di-methyl ester L [26,29] | 329 | 315, 301 | <0.01 a | <0.01 a | <0.01 a |
| 63 | 38.11 | Ellagic acid O-tri-methyl ester L [26,29] | 343 | 329, 315, 301 | <0.01 a | <0.01 a | <0.01 a |
| Compound | Cold Infusion (20 °C) | Warm Infusion (50 °C) | Hot Infusion (80 °C) | Boiling Infusion (100 °C) | Decoction 15 min | Decoction 30 min |
|---|---|---|---|---|---|---|
| Ellagic acid and Ellagitannins | ||||||
| Ellagic acid | 1.27 ± 0.03 f | 1.54 ± 0.03 e | 1.96 ± 0.05 d | 3.39 ± 0.07 c | 15.89 ± 0.37 b | 18.25 ± 0.37 a |
| Lambertianin A | 1.11 ± 0.02 c | 1.68 ± 0.03 a | 1.35 ± 0.03 b | 0.21 ± 0.01 d | <0.01 e | <0.01 e |
| Sanguiin H6 | 5.25 ± 0.10 c | 6.13 ± 0.12 a | 5.40 ± 0.11 b | 4.01 ± 0.08 d | 1.11 ± 0.07 e | <0.01 f |
| Lambertianin C | 13.05 ± 0.27 c | 16.32 ± 0.30 a | 14.96 ± 0.30 b | 9.03 ± 0.19 d | 2.12 ± 0.11 e | <0.01 f |
| Sanguiin H11 | 0.19 ± 0.00 c | 0.51 ± 0.01 a | 0.36 ± 0.01 b | <0.01 d | <0.01 d | <0.01 d |
| Subtotal ellagic acid and ellagitannins | 20.87 | 26.18 | 24.03 | 16.64 | 19.12 | 18.25 |
| Catechins | ||||||
| Gallocatechin | 0.19 ± 0.00 f | 0.35 ± 0.01 e | 0.60 ± 0.01 d | 0.77 ± 0.02 c | 0.92 ± 0.02 a | 0.85 ± 0.02 b |
| Epicatechin | 0.26 ± 0.01 f | 0.40 ± 0.01 e | 0.72 ± 0.02 d | 0.93 ± 0.02 c | 1.02 ± 0.02 b | 1.17 ± 0.02 a |
| Subtotal catechins | 0.45 | 0.75 | 1.32 | 1.70 | 1.94 | 2.02 |
| Hydroxycinnamates | ||||||
| O-Caffeoyl-hexose | 0.33 ± 0.01 f | 0.52 ± 0.01 e | 0.86 ± 0.02 d | 1.06 ± 0.03 a | 1.01 ± 0.03 b | 0.93 ± 0.02 c |
| 4-O-Caffeoyquinic acid | <0.01 f | 0.22 ± 0.00 e | 0.58 ± 0.01 c | 0.83 ± 0.02 a | 0.70 ± 0.01 b | 0.55 ± 0.01 d |
| 5-O-Caffeoylquinic acid | 0.35 ± 0.01 f | 0.60 ± 0.01 e | 0.88 ± 0.02 d | 1.14 ± 0.03 a | 1.07 ± 0.03 b | 0.96 ± 0.02 c |
| 5-O-Feruloylquinic acid | 0.29 ± 0.01 f | 0.52 ± 0.01 e | 0.71 ± 0.02 d | 0.94 ± 0.02 a | 0.88 ± 0.02 b | 0.76 ± 0.02 c |
| Subtotal hydroxycinnamates | 0.97 | 1.86 | 3.03 | 3.97 | 3.66 | 3.20 |
| Flavonols | ||||||
| Quercetin-3-O-glucuronide | 5.12 ± 0.10 e | 6.29 ± 0.14 d | 7.02 ± 0.15 c | 7.43 ± 0.16 b | 8.07 ± 0.16 a | 8.21 ± 0.17 a |
| Quercetin-O-(O-acetyl-O-malonyl)-hexuronide | 7.32 ± 0.15 d | 8.61 ± 0.17 b | 9.14 ± 0.21 a | 7.69 ± 0.16 c | 5.07 ± 0.10 e | 4.21 ± 0.08 f |
| Kaempferol-3-O-glucuronide | 3.17 ± 0.06 e | 5.08 ± 0.11 d | 7.39 ± 0.13 c | 8.23 ± 0.16 b | 8.51 ± 0.17 a,b | 8.66 ± 0.18 a |
| Subtotal flavonols | 15.61 | 19.98 | 23.55 | 23.35 | 21.65 | 21.08 |
| Total phenolics | 37.90 | 48.77 | 51.93 | 45.66 | 46.37 | 44.55 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kashchenko, N.I.; Olennikov, D.N.; Chirikova, N.K. Metabolites of Siberian Raspberries: LC-MS Profile, Seasonal Variation, Antioxidant Activity and, Thermal Stability of Rubus matsumuranus Phenolome. Plants 2021, 10, 2317. https://doi.org/10.3390/plants10112317
Kashchenko NI, Olennikov DN, Chirikova NK. Metabolites of Siberian Raspberries: LC-MS Profile, Seasonal Variation, Antioxidant Activity and, Thermal Stability of Rubus matsumuranus Phenolome. Plants. 2021; 10(11):2317. https://doi.org/10.3390/plants10112317
Chicago/Turabian StyleKashchenko, Nina I., Daniil N. Olennikov, and Nadezhda K. Chirikova. 2021. "Metabolites of Siberian Raspberries: LC-MS Profile, Seasonal Variation, Antioxidant Activity and, Thermal Stability of Rubus matsumuranus Phenolome" Plants 10, no. 11: 2317. https://doi.org/10.3390/plants10112317
APA StyleKashchenko, N. I., Olennikov, D. N., & Chirikova, N. K. (2021). Metabolites of Siberian Raspberries: LC-MS Profile, Seasonal Variation, Antioxidant Activity and, Thermal Stability of Rubus matsumuranus Phenolome. Plants, 10(11), 2317. https://doi.org/10.3390/plants10112317








