Photosynthesis-Related Responses of Colombian Elite Hevea brasiliensis Genotypes under Different Environmental Variations: Implications for New Germplasm Selection in the Amazon
Abstract
1. Introduction
2. Results
2.1. Variations in the Microclimatic Parameters
2.2. Changes in the Micro-Environmental Parameters at the Leaf and Soil Levels
2.3. Photosynthetic Responses to Light
2.4. Gas Exchange and Chlorophyll a Fluorescence
2.5. Leaf Water Potential and Soil Water Status
2.6. Multidimensional Analysis of the Photosynthesis-Related Traits
2.7. Girth Growth
3. Discussion
4. Materials and Methods
4.1. Study Area
4.2. Plant Material
4.3. Experiment Design and Field Trial Maintenance
4.4. Photosynthesis-Related Traits
4.4.1. Photosynthetic and Micro-Environmental Traits at the Leaf Level
4.4.2. Leaf Water Potential and Soil Water Status-Related Traits
4.4.3. Assessment of Girth Growth
4.5. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Compagnon, P. El Caucho Natural, Biología-Cultivo-Producción. Consejo Mexicano del Hule-CIRAD. Mexico, D.F. 1998. Available online: https://www.cirad.fr/en (accessed on 5 October 2021).
- Wu, C.; Lan, L.; Li, Y.; Nie, Z.; Zeng, R. The relationship between latex metabolism gene expression with rubber yield and related traits in Hevea brasiliensis. BMC Genom. 2018, 1–18. [Google Scholar] [CrossRef] [PubMed]
- MADR. Cadena de Caucho Natural Indicadores e Instrumentos; Ministerio de Agricultura y Desarrollo Rural: Bogotá, Colombia, 2018. [Google Scholar]
- Ramírez, U.; Charry, A.; Jäger, M.; Hurtado, J.; Rosas, G.; Sterling, A.; Romero, M.; Sierra, L.; Quintero, M. Estrategia Sectorial de la Cadena de Productos no Maderables del Bosque en Caquetá, con Enfoque Agroambiental y Cero Deforestación; Publicación CIAT No. 451; Centro Internacional de Agricultura Tropical (CIAT): Cali, Colombia, 2018. [Google Scholar]
- Sterling, A.; Rodríguez, C.H. Estrategias de Manejo Para las Principales Enfermedades y Plagas del Cultivo del Caucho con Énfasis en la Amazonia Colombiana; Instituto Amazónico de Investigaciones Científicas-SINCHI: Florencia, Colombia, 2018. [Google Scholar]
- Sterling, A.; Rodríguez, C.H.; Melgarejo, L.M. Evaluación Inicial del Asocio Caucho–Copoazú en el Caquetá: Una Alternativa de Enriquecimiento Agroforestal con Potencial Para la Amazonia Colombiana; Instituto Amazónico de Investigaciones Científicas-SINCHI: Bogotá, Colombia, 2015. [Google Scholar]
- Asociación de Reforestadores y Cultivadores de Caucho del Caquetá (ASOHECA). Estadísticas del Sector Cauchero en Caquetá; ASOHECA: Florencia, Colombia, 2020. [Google Scholar]
- Priyadarshan, P.M.; Gonçalves, P.S.; Omokhafe, K.O. Breeding Hevea rubber. Breed. Plant. Tree Crop. Trop. Species 2009, 469–522. [Google Scholar] [CrossRef]
- Goncalves, P.d.S.; Ortolani, A.A.; Cardoso, M. Melhoramento Genetico da Seringueira: Uma Revisao; Instituto Agronômico: Campinas, Brazil, 1997. [Google Scholar]
- Ahmad, B.; Idris, H.; Sulong, S.H. Early Selection of Promising High Yielding Hevea Progenies based on Selected Physiological and and Stomatal Characteristics. J. Rubber Res. 2009, 12, 140–150. [Google Scholar]
- Rodrigo, V.H.L. Ecophysiological factors underpinning productivity of Hevea brasiliensis. Braz. J. Plant Physiol. 2007, 19, 245–255. [Google Scholar] [CrossRef][Green Version]
- Holá, D.; Benešová, M.; Honnerová, J.; Hnilička, F.; Rothová, O.; Kočová, M.; Hniličková, H. The evaluation of photosynthetic parameters in maize inbred lines subjected to water deficiency: Can these parameters be used for the prediction of performance of hybrid progeny? Photosynthetica 2010, 48, 545–558. [Google Scholar] [CrossRef]
- Sterling, A.; Rodríguez, N.; Quiceno, E.; Trujillo, F.; Clavijo, A.; Suárez-Salazar, J.C. Dynamics of photosynthetic responses in 10 rubber tree (Hevea brasiliensis) clones in Colombian Amazon: Implications for breeding strategies. PLoS ONE 2019, 14, e0226254. [Google Scholar] [CrossRef]
- Granda, V.; Delatorre, C.; Cuesta, C.; Centeno, M.L.; Fernández, B.; Rodríguez, A.; Feito, I. Physiological and biochemical responses to severe drought stress of nine Eucalyptus globulus clones: A multivariate approach. Tree Physiol. 2014, 34, 778–786. [Google Scholar] [CrossRef]
- Kositsup, B.; Montpied, P.; Kasemsap, P.; Thaler, P.; Améglio, T.; Dreyer, E. Photosynthetic capacity and temperature responses of photosynthesis of rubber trees (Hevea brasiliensis Müll. Arg.) acclimate to changes in ambient temperatures. Trees Struct. Funct. 2009, 23, 357–365. [Google Scholar] [CrossRef]
- Nunes, S.; Santos, C.; Moutinho-Pereira, J.; Correia, C.; Oliveira, H.; Ferreira de Oliveira, J.M.; Pereira, V.T.; Almeida, T.; Marum, L.; Dias, M.C. Physiological characterization and true-to-typeness evaluation of in vitro and ex vitro seedlings of Pinus elliottii: A contribution to breeding programs. Plant Physiol. Biochem. 2016, 107, 222–227. [Google Scholar] [CrossRef]
- Zhao, X.; Li, Y.; Zheng, M.; Bian, X.; Liu, M.; Sun, Y.; Jiang, J.; Wang, F.; Li, S.; Cui, Y.; et al. Comparative analysis of growth and photosynthetic characteristics of (Populus simonii × P. nigra) × (P. nigra × P. simonii) hybrid clones of different ploidides. PLoS ONE 2015, 10, 1–16. [Google Scholar] [CrossRef]
- Espinoza, S.E.; Magni, C.R.; Rubilar, R.A.; Yañez, M.A.; Santelices, R.E.; Cabrera, A.M.; Ivković, M. Field performance of various Pinus radiata breeding families established on a drought-prone site in central Chile. N. Z. J. For. Sci. 2017, 47, 12. [Google Scholar] [CrossRef]
- Carr, M.K.V. The water relations of rubber (Hevea brasiliensis): A review. Exp. Agric. 2014, 48, 176–193. [Google Scholar] [CrossRef]
- Sterling, A.; Rodríguez, N.; Clavijo-Arias, E.A.; Claros-Loaiza, Y.P.; Salazar, J.C.S. Dynamics of water-use efficiency and status in promising Hevea brasiliensis genotypes: Implications for clonal selection. J. Rubber Res. 2021. [Google Scholar] [CrossRef]
- Sterling, A.; Martínez-Viuche, E.J.; Suárez-Córdoba, Y.D.; Agudelo-Sánchez, A.A.; Fonseca-Restrepo, J.A.; Andrade-Ramírez, T.K.; Virguez-Díaz, Y.R. Assessing growth, early yielding and resistance in rubber tree clones under low South American Leaf Blight pressure in the Amazon region, Colombia. Ind. Crops Prod. 2020, 158, 112958. [Google Scholar] [CrossRef]
- Sterling, A.; Rodríguez, C.H. Nuevos Clones de Caucho Natural Para la Amazonia Colombiana: Énfasis en la Resistencia al Mal Suramericano de las Hojas (Microcyclus ulei); Instituto Amazónico de Investigaciones Científicas-SINCHI: Bogotá, Colombia, 2011. [Google Scholar]
- Gouvêa, L.; Silva, G.; Verardi, C.; Silva, J.; Scaloppi-Junior, E.; Gonçalves, P. Temporal stability of vigor in rubber tree genotypes in the pre- and post-tapping phases using different methods. Euphytica 2012, 186, 625–634. [Google Scholar] [CrossRef]
- Junior, E.J.S.; de Gonçalves, P.S.; Aguiar, A.T.E.; Arantes, F.E. Seleção de progênies de seringueira a partir de caracteres de produção e vigor. In Proceedings of the Memórias I Congresso Brasileiro de Heveicultura; Borracha Natural. INCAPER: Guarapari, Brasil, 2007; p. 2. [Google Scholar]
- Gouvêa, L.R.L.; Silva, G.A.P.; Verardi, C.K.; de Oliveira, A.L.B.; Gonçalves, E.C.P.; Scaloppi-Junior, E.J.; de Moraes, M.L.T.; de Souza Gonçalves, P. Rubber tree early selection for yield stability in time and among locations. Euphytica 2013, 191, 365–373. [Google Scholar] [CrossRef]
- Marengo, J.; Souza, C., Jr. Climate Change: Impacts and Scenarios for the Amazon; University of São Paulo: São Paulo, Brasil, 2018. [Google Scholar]
- Wang, L. Physiological and Molecular Responses to Variation of Light Intensity in Rubber Tree (Hevea brasiliensis Muell. Arg.). PLoS ONE 2014, 9, e89514. [Google Scholar] [CrossRef]
- Razar, R.M.; Hamid, N.R.A.; Ghani, Z.A. GxE effect and stability analyses of selected rubber clones (Hevea brasiliensis) in Malaysia. J. Rubber Res. 2021. [Google Scholar] [CrossRef]
- Buckley, T.N.; Sack, L.; Farquhar, G.D. Optimal plant water economy. Plant Cell Environ. 2017, 40, 881–896. [Google Scholar] [CrossRef]
- Ullah, H.; Santiago-Arenas, R.; Ferdous, Z.; Attia, A.; Datta, A. Improving Water Use Efficiency, Nitrogen Use Efficiency, and Radiation Use Efficiency in Field Crops under Drought Stress: A Review, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; Volume 156. [Google Scholar]
- Sinclair, T.R.; Devi, J.; Shekoofa, A.; Choudhary, S.; Sadok, W.; Vadez, V.; Riar, M.; Rufty, T. Limited-transpiration response to high vapor pressure deficit in crop species. Plant Sci. 2017, 260, 109–118. [Google Scholar] [CrossRef]
- Tinoco-Ojanguren, C.; Pearcy, R.W. Stomatal dynamics and its importance to carbon gain in two rainforest Piper species-I. VPD effects on the transient stomatal response to lightflecks. Oecologia 1993, 94, 388–394. [Google Scholar] [CrossRef]
- Tambussi, E.A.; Bort, J.; Araus, J.L. Water use efficiency in C3 cereals under Mediterranean conditions: A review of physiological aspects. Ann. Appl. Biol. 2007, 150, 307–321. [Google Scholar] [CrossRef]
- Chaves, M.M.; Maroco, J.P.; Pereira, J.S. Understanding plant responses to drought—From genes to the whole plant. Funct. Plant Biol. 2003, 30, 239. [Google Scholar] [CrossRef]
- Tomás, M.; Medrano, H.; Escalona, J.M.; Martorell, S.; Pou, A.; Ribas-Carbó, M.; Flexas, J. Variability of water use efficiency in grapevines. Environ. Exp. Bot. 2014, 103, 148–157. [Google Scholar] [CrossRef]
- Zait, Y.; Shtein, I.; Schwartz, A. Long-term acclimation to drought, salinity and temperature in the thermophilic tree Ziziphus spina-christi: Revealing different tradeoffs between mesophyll and stomatal conductance. Tree Physiol. 2019, 39, 701–716. [Google Scholar] [CrossRef]
- Brodribb, T.J.; Holbrook, N.M. Stomatal closure during leaf dehydration, correlation with other leaf physiological traits. Plant Physiol. 2003, 132, 2166–2173. [Google Scholar] [CrossRef]
- Zhang, Z.X.; Cai, Z.Q.; Liu, G.Z.; Wang, H.; Huang, L.; Cai, C.T. Effects of fertilization on the growth, photosynthesis, and biomass accumulation in juvenile plants of three coffee (Coffea arabica L.) cultivars. Photosynthetica 2016, 55, 134–143. [Google Scholar] [CrossRef]
- Sinclair, T.R.; Hammer, G.L.; Van Oosterom, E.J. Potential yield and water-use efficiency benefits in sorghum from limited maximum transpiration rate. Funct. Plant Biol. 2005, 32, 945–952. [Google Scholar] [CrossRef]
- Taylor, H.M.; Jordan, W.R.; Sinclair, T.R. Limitations to Efficient Water Use in Crop Production; American Society of Agronomy: Madison, WI, USA, 1983. [Google Scholar]
- Gunderson, C.A.; O’hara, K.H.; Campion, C.M.; Walker, A.V.; Edwards, N.T. Thermal plasticity of photosynthesis: The role of acclimation in forest responses to a warming climate. Glob. Chang. Biol. 2010, 16, 2272–2286. [Google Scholar] [CrossRef]
- Kaiser, E.; Morales, A.; Harbinson, J.; Kromdijk, J.; Heuvelink, E.; Marcelis, L.F.M. Dynamic photosynthesis in different environmental conditions. J. Exp. Bot. 2015, 66, 2415–2426. [Google Scholar] [CrossRef]
- Dos Santos, J.O.; de Oliveira, L.E.M.; de Souza, T.; Lopes, G.M.; Coelho, V.T.; Gomes, M.P. Physiological mechanisms responsible for tolerance to, and recuperation from, drought conditions in four different rubber clones. Ind. Crops Prod. 2019, 141, 111714. [Google Scholar] [CrossRef]
- Blum, A. Effective use of water (EUW) and not water-use efficiency (WUE) is the target of crop yield improvement under drought stress. F. Crop. Res. 2009, 112, 119–123. [Google Scholar] [CrossRef]
- Morton, D.C.; Nagol, J.; Carabajal, C.C.; Rosette, J.; Palace, M.; Cook, B.D.; Vermote, E.F.; Harding, D.J.; North, P.R.J. Amazon forests maintain consistent canopy structure and greenness during the dry season. Nature 2014, 506, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Guan, K.; Pan, M.; Li, H.; Wolf, A.; Wu, J.; Medvigy, D.; Caylor, K.K.; Sheffield, J.; Wood, E.F.; Malhi, Y.; et al. Photosynthetic seasonality of global tropical forests constrained by hydroclimate. Nat. Geosci. 2015, 8, 284–289. [Google Scholar] [CrossRef]
- Nugawela, A.; Long, S.P.; Aluthhewage, R.K. Genotypic variation in non-steady state photosynthetic carbon dioxide assimilation of Hevea brasiliensis. J. Rubber Res. Inst. Sri Lanka 1995, 10, 266–275. [Google Scholar]
- Miguel, A.A.; de Oliveira, L.E.M.; Cairo, P.A.R.; de Oliveira, D.M. Photosynthetic behaviour during the leaf ontogeny of rubber tree clones [Hevea brasiliensis (Wild. ex. Adr. de Juss.) Muell. Arg.]. Ciênc. Agrotec. Lavras. 2007, 31, 91–97. [Google Scholar] [CrossRef]
- Martínez-Vilalta, J.; Poyatos, R.; Aguadé, D.; Retana, J.; Mencuccini, M. A new look at water transport regulation in plants. New Phytol. 2014, 204, 105–115. [Google Scholar] [CrossRef]
- Shahenshah; Isoda, A. Effects of water stress on leaf temperature and chlorophyll fluorescence parameters in cotton and peanut. Plant Prod. Sci. 2010, 13, 269–278. [Google Scholar] [CrossRef]
- Urban, J.; Ingwers, M.W.; McGuire, M.A.; Teskey, R.O. Increase in leaf temperature opens stomata and decouples net photosynthesis from stomatal conductance in Pinus taeda and Populus deltoides x nigra. J. Exp. Bot. 2017, 68, 1757–1767. [Google Scholar] [CrossRef]
- Dubey, R.; Pathak, H.; Chakrabarti, B.; Singh, S.; Gupta, D.K.; Harit, R.C. Impact of terminal heat stress on wheat yield in India and options for adaptation. Agric. Syst. 2020, 181, 102826. [Google Scholar] [CrossRef]
- Drake, B.G.; Gonzàlez-Meler, M.A.; Long, S.P. More efficient plants: A Consequence of Rising Atmospheric CO2? Annu. Rev. Plant Biol. 1997, 48, 609–639. [Google Scholar] [CrossRef]
- Nilkens, M.; Kress, E.; Lambrev, P.; Miloslavina, Y.; Müller, M.; Holzwarth, A.R.; Jahns, P. Identification of a slowly inducible zeaxanthin-dependent component of non-photochemical quenching of chlorophyll fluorescence generated under steady-state conditions in Arabidopsis. Biochim. Biophys. Acta 2010, 1797, 466–475. [Google Scholar] [CrossRef]
- Rodrigues, A.L.; da Silva Bertholdi, A.A.; Mantoan, L.P.B.; Franco, D.M.; Habermann, G.; de Almeida, L.F.R. Seasonal dynamics of the water relations and photochemical efficiency of Copaifera langsdorffii Desf. co-occurring in savanna and seasonal forest. Acta Physiol. Plant. 2019, 41, 22. [Google Scholar] [CrossRef]
- Martins, J.P.R.; Schimildt, E.R.; Alexandre, R.S.; Falqueto, A.R.; Otoni, W.C. Chlorophyll a fluorescence and growth of Neoregelia concentrica (Bromeliaceae) during acclimatization in response to light levels. Vitr. Cell. Dev. Biol. Plant 2015, 51, 471–481. [Google Scholar] [CrossRef]
- Adams, W.W., III; Zarter, C.R.; Mueh, K.E.; Amiard, V.; Demmig-Adams, B. Energy Dissipation and Photoinhibition: A Continuum of Photoprotection. In Photoprotection, Photoinhibition, Gene Regulation, and Environment. Advances in Photosynthesis and Respiration; Springer: Dordrecht, The Netherlands, 2008; pp. 49–64. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Adams, W.W. Xanthophyll cycle and light stress in nature: Uniform response to excess direct sunlight among higher plant species. Planta 1996, 198, 460–470. [Google Scholar] [CrossRef]
- Sterling, A.; Melgarejo, L.M. Photosynthetic performance of Hevea brasiliensis affected by South American Leaf Blight under field conditions. Eur. J. Plant Pathol. 2021. [Google Scholar] [CrossRef]
- Instituto Geográfico Agustin Codazzi (IGAC) Caquetá, Características Geográficas; Imprenta Nacional de Colombia: Bogotá, Colombia, 2010.
- Murad, C.A.; Pearse, J. Landsat study of deforestation in the Amazon region of Colombia: Departments of Caquetá and Putumayo. Remote Sens. Appl. Soc. Environ. 2018, 11, 161–171. [Google Scholar] [CrossRef]
- Feldmann, F.; Junqueira, N.T.; Meier, U. Phenological Growth Stages of the Rubber Tree Hevea Brasiliensis (Willd. ex Adr. de Juss.) Muell.-Arg.: Codification and Description According to the BBCH Scale; Embrapa: Cerrados, Brasil, 2005. [Google Scholar]
- Sterling, A.; Rodríguez, C.H. Valoración de Nuevos Clones de Hevea Brasiliensis con Proyección Para la Amazonia Colombiana: Fases de pre y Post-Sangría Temprana en el Caquetá; Instituto Amazónico de Investigaciones Científicas-Sinchi: Bogotá, Colombia, 2020. [Google Scholar]
- Confederación Cauchera Colombiana (CCC) Estado Actual del Gremio Cauchero Colombiano; CCC: Bogotá, Colombia, 2015.
- Sterling, A.; Rodriguez, O.L.; Rodriguez, C.H.; Martínez, O.; Bonilla, N.C.; Dussán, I. Variabilidad genética de genotipos élites de Hevea brasiliensis mediante el uso de descriptores morfológicos. Rev. Colomb. Amaz. 2011, 4, 129–142. [Google Scholar]
- Quintero, B.L.; Rodriguez, A.O.L.; Sterling, C.A.; Zapata, J.A.; Matínez, O. Caracterización morfológica y molecular de los nuevos clones de H. brasiliensis de origen franco. In Nuevos Clones de Caucho Natural Para la Amazonia Colombiana: Énfasis en la Resistencia al Mal Suramericano de las Hojas (Microcyclus Ulei); Sterling Cuéllar, A., Rodríguez León, C.H., Eds.; Instituto Amazónico de Investigaciones Científicas–SINCHI: Bogotá, Colombia, 2011; pp. 173–195. [Google Scholar]
- Sterling, A.; Galindo-Rodríguez, L.C.; Suárez-Córdoba, Y.D.; Velasco-Anacona, G.; Andrade-Ramírez, T.; Gómez-Torres, A.K. Early assessing performance and resistance of Colombian rubber tree genotypes under high South American Leaf Blight pressure in Amazon. Ind. Crops Prod. 2019, 141, 111775. [Google Scholar] [CrossRef]
- Sterling, A.; Melgarejo, L.M. Leaf gas exchange and chlorophyll a fluorescence in Hevea brasiliensis in response to Pseudocercospora ulei infection. Physiol. Mol. Plant Pathol. 2018, 103, 143–150. [Google Scholar] [CrossRef]
- Sterling, A.; Rodríguez, C.H. Valoración Inicial del Potencial Productivo de Hevea Brasiliensis en la Amazonia Colombiana Mediante la Evaluación de Nuevos Clones Promisorios Para la Región; Instituto Amazónico de Investigaciones Científicas-SINCHI: Bogotá, Colombia, 2020. [Google Scholar]
- Clément-Demange, A.; Nicolas, D.; Legnaté, H.; Rivano, F.; Le Guen, V.; Gnagne, M.; Chapuset, T. Hévéa: Stratégies de sélection. Plant. Rech. Développement 1995, 2, 5–14. [Google Scholar]
- Marino, G.; Aqil, M.; Shipley, B. The leaf economics spectrum and the prediction of photosynthetic light-response curves. Funct. Ecol. 2010, 24, 263–272. [Google Scholar] [CrossRef]
- Hallé, F.; Oldeman, R.A.A.; Tomlinson, P.B. Tropical Trees and Forest; Springer: Berlin/Heidelberg, Germany, 1978. [Google Scholar]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Baker, N.R. Chlorophyll Fluorescence: A Probe of Photosynthesis In Vivo. Annu. Rev. Plant Biol. 2008, 59, 89–113. [Google Scholar] [CrossRef]
- Verbeke, G.; Molenberghs, G. Linear Mixed Models for Longitudinal Data; Springer: New York, NY, USA, 2000. [Google Scholar]
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D. Nlme: Linear and Nonlinear Mixed Effects Models. R Package Version 3.1-131.1; The Comprehensive R Archive Network: Vienna, Austria, 2018. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Di Rienzo, J.A.; Casanoves, F.; Balzarini, M.G.; Gonzalez, L.; Tablada, M.; Robledo, C.W. InfoStat; Universidad Nacional de Córdoba: Córdoba, Argentina, 2020. [Google Scholar]
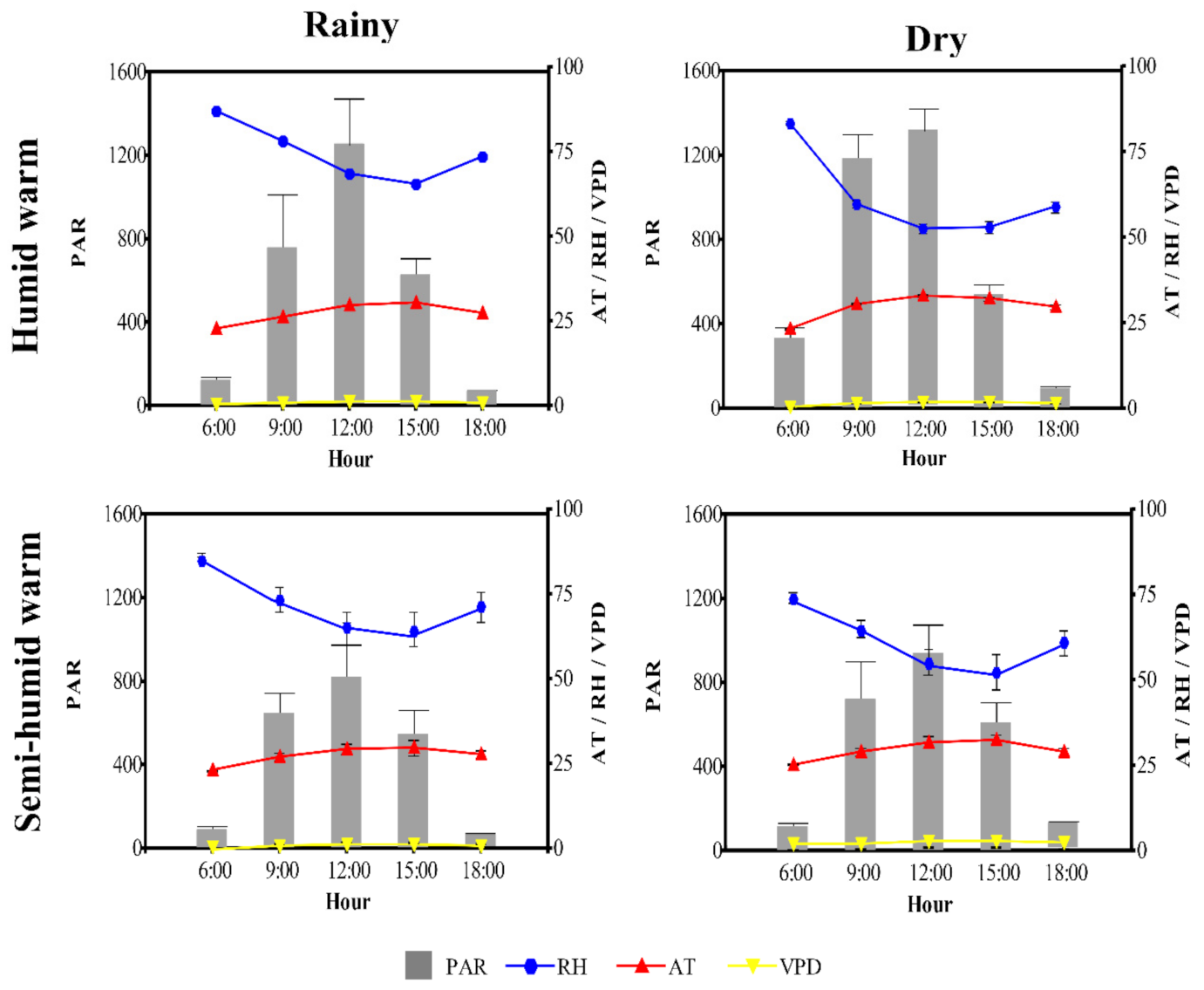
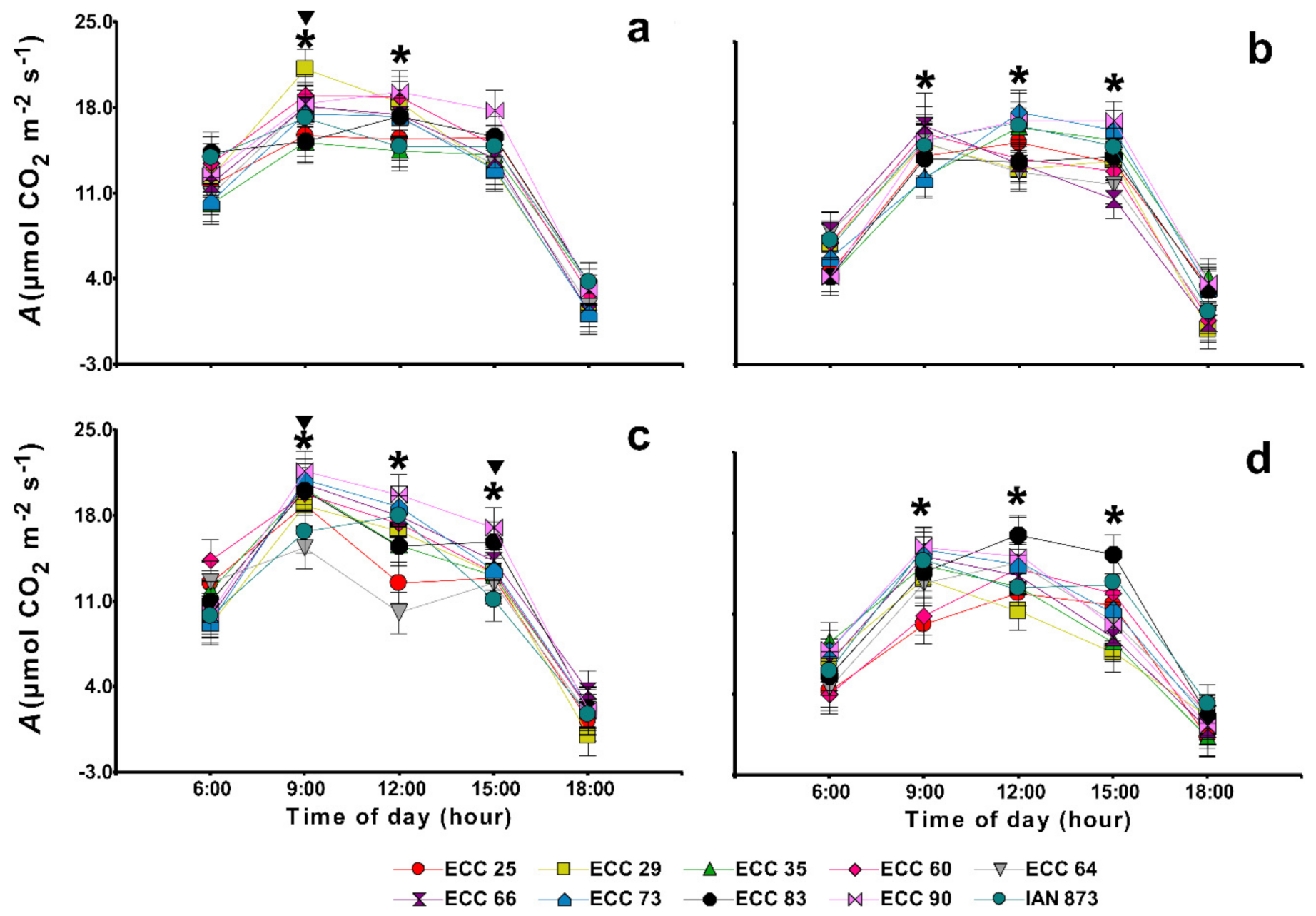

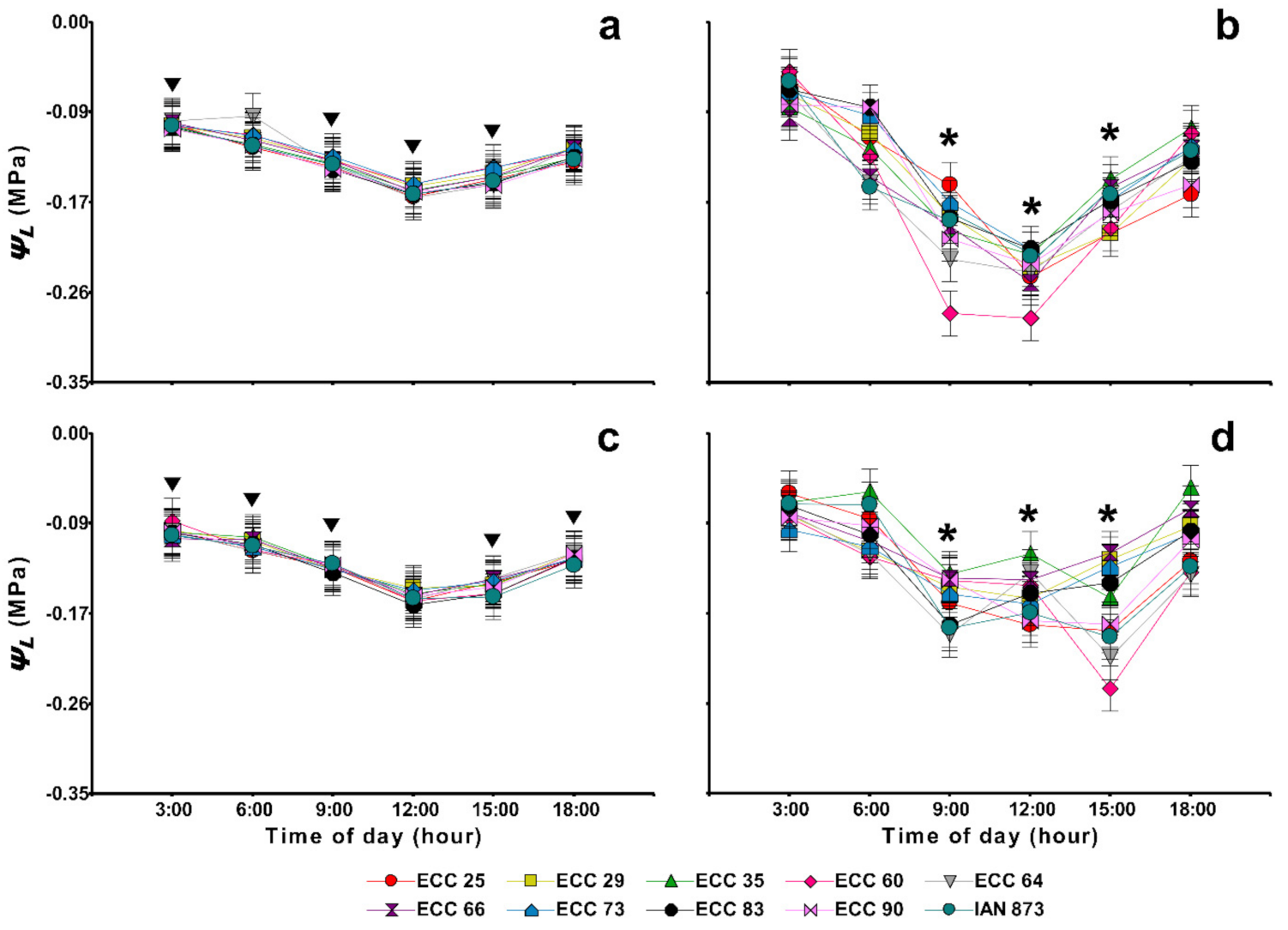
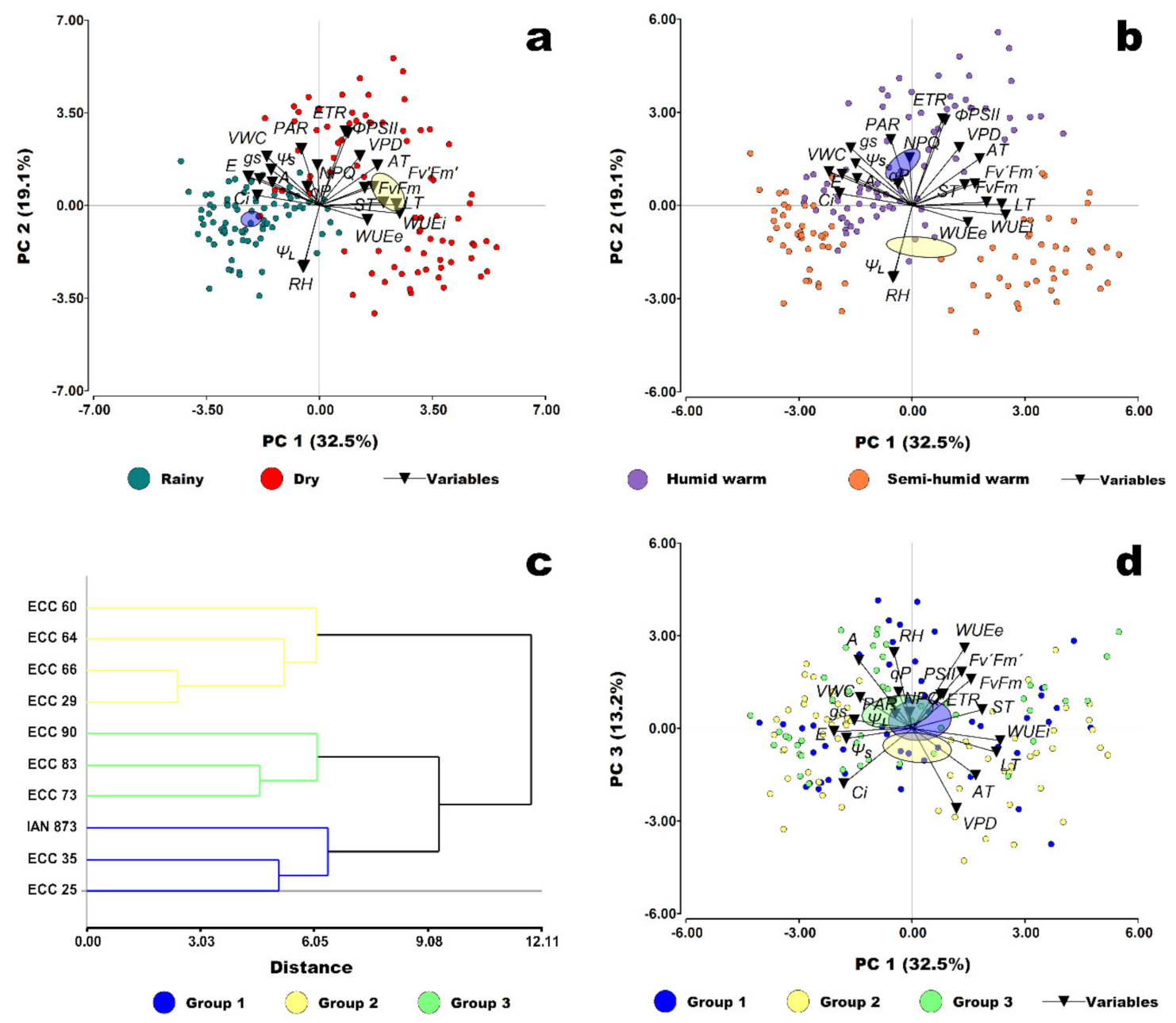
| Genotype | Amax | LCP | LSP | Rd | Aqe (×10−3) |
|---|---|---|---|---|---|
| ECC 25 | 21.30 ± 0.29 d | 47.80 ± 3.29 c | 1486.92 ± 16.69 b | −1.69 ± 0.13 a | 1.6 ± 0.1 e |
| ECC 29 | 24.83 ± 0.43 a | 45.64 ± 4.14 c | 1580.70 ± 20.70 a | −1.80 ± 0.19 b | 1.5 ± 0.1 e |
| ECC 35 | 16.79 ± 0.28 g | 126.73 ± 3.89 a | 1338.62 ± 22.85 c | −4.57 ± 0.16 d | 1.9 ± 0.1 c |
| ECC 60 | 15.25 ± 0.28 h | 48.03 ± 4.78 c | 900.84 ± 27.33 e | −2.11 ± 0.26 c | 2.7 ± 0.2 a |
| ECC 64 | 20.51 ± 0.21 e | 50.89 ± 2.50 c | 1262.78 ± 13.23 c | −2.01 ± 0.12 b | 1.9 ± 0.1 c |
| ECC 66 | 22.28 ± 0.29 b | 61.50 ± 2.99 b | 1500.62 ± 15.89 a | −2.20 ± 0.13 c | 1.6 ± 0.1 e |
| ECC 73 | 18.59 ± 0.60 f | 41.32 ± 8.49 d | 1192.61 ± 44.35 d | −1.58 ± 0.38 a | 2.0 ± 0.2 b |
| ECC 83 | 21.71 ± 0.21 c | 48.42 ± 2.35 c | 1402.88 ± 12.08 b | −1.82 ± 0.10 b | 1.7 ± 0.0 d |
| ECC 90 | 21.80 ± 0.36 c | 45.32 ± 4.28 c | 1257.21 ± 22.28 d | −1.96 ± 0.22 b | 1.9 ± 0.1 c |
| IAN 873 | 20.44 ± 0.25 e | 58.77 ± 3.39 b | 1210.06 ± 18.81 d | −2.30 ± 0.16 c | 2.0 ± 0.1 b |
| Variables | F Based p Values | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P | S | G | H | P × S | P × G | P × H | S × G | S × H | G × H | P × S × G | P × S × H | P × G × H | S × G × H | P × S × G × H | |
| A | <0.0001 | 0.0146 | 0.0107 | <0.0001 | 0.0572 | 0.3056 | <0.0001 | 0.6770 | 0.0011 | 0.1680 | 0.8396 | 0.0012 | 0.0349 | 0.0849 | 0.0005 |
| E | <0.0001 | 0.0032 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.3156 | 0.0079 | <0.0001 | 0.0045 | <0.0001 | <0.0001 | 0.0556 | 0.0245 | 0.0179 |
| gs | <0.0001 | 0.0006 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.8721 | <0.0001 | 0.0003 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Ci | <0.0001 | 0.0939 | 0.0003 | <0.0001 | <0.0001 | 0.0002 | <0.0001 | 0.0758 | <0.0001 | 0.4206 | <0.0001 | 0.0002 | <0.0001 | 0.0793 | <0.0001 |
| LT | <0.0001 | 0.0166 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| WUEe | 0.0011 | 0.0690 | <0.0001 | <0.0001 | <0.0001 | 0.1040 | <0.0001 | 0.0375 | <0.0001 | 0.0036 | <0.0001 | <0.0001 | 0.0399 | 0.0345 | <0.0001 |
| WUEi | <0.0001 | 0.0223 | 0.9444 | <0.0001 | 0.0001 | 0.9815 | <0.0001 | 0.9368 | <0.0001 | 0.0096 | 0.9566 | <0.0001 | 0.0001 | 0.0159 | 0.0018 |
| ΦPSII | <0.0001 | 0.0066 | 0.0889 | <0.0001 | 0.0611 | 0.0289 | <0.0001 | 0.7566 | <0.0001 | 0.0767 | 0.0253 | <0.0001 | 0.0216 | 0.1567 | 0.0737 |
| ETR | <0.0001 | 0.0062 | 0.0583 | <0.0001 | 0.0283 | 0.0259 | <0.0001 | 0.6949 | <0.0001 | 0.1168 | 0.0367 | <0.0001 | 0.0031 | 0.1494 | 0.0148 |
| Fv/Fm | <0.0001 | 0.0001 | 0.0010 | - | <0.0001 | 0.1665 | - | 0.0269 | - | - | 0.6266 | - | - | - | - |
| Fv´/Fm´ | <0.0001 | 0.0151 | 0.4053 | - | <0.0001 | 0.4018 | - | 0.2829 | - | - | 0.5865 | - | - | - | - |
| qP | 0.0174 | 0.0034 | 0.1801 | - | 0.0526 | 0.1899 | - | 0.0151 | - | - | 0.5708 | - | - | - | - |
| NPQ | 0.7772 | 0.0009 | 0.0511 | - | 0.4098 | 0.8606 | - | 0.2819 | - | - | 0.3618 | - | - | - | - |
| Ψl | 0.0060 | 0.0033 | 0.2741 | <0.0001 | 0.0081 | 0.6432 | <0.0001 | 0.7893 | <0.0001 | 0.1423 | 0.9707 | <0.0001 | 0.0642 | 0.2988 | 0.0112 |
| Effect | Level | Variables | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | E | gs | Ci | LT | WUEe | WUEi | ΦPSII | ETR | Fv/Fm | Fv´/Fm´ | qP | NPQ | ΨL | ||
| Period | Rainy | 12.63 ± 0.20 a | 4.36 ± 0.05 a | 372.37 ± 8.00 a | 304.28 ± 2.01 a | 28.09 ± 0.06 b | 2.79 ± 0.08 b | 0.013 ± 0.0025 b | 0.15 ± 0.0032 b | 82.64 ± 1.80 b | 0.75 ± 0.003 b | 0.72 ± 0.004 b | 0.92 ± 0.007 a | 0.018 ± 0.005 | −0.13 ± 0.0033 a |
| Dry | 10.47 ± 0.22 b | 3.27 ± 0.05 b | 331.59 ± 8.00 b | 290.76 ± 1.66 b | 29.53 ± 0.06 a | 3.11 ± 0.05 a | 0.039 ± 0.0014 a | 0.17 ± 0.0030 a | 94.33 ± 1.65 a | 0.79 ± 0.002a | 0.75 ± 0.003 a | 0.90 ± 0.005 b | 0.018 ± 0.001 | −0.14 ± 0.0021 b | |
| Site | Semi-humid warm | 11.08 ± 0.20 b | 3.62 ± 0.06 b | 309.28 ± 9.27 b | 294.72 ± 2.00 b | 28.98 ± 0.07 a | 3.05 ± 0.06 a | 0.031 ± 0.0023 a | 0.15 ± 0.0036 b | 82.63 ± 2.01 b | 0.76 ± 0.002 b | 0.73 ± 0.004 b | 0.89 ± 0.01 b | 0.016 ± 0.005 b | −0.13 ± 0.0025 a |
| Humid warm | 12.02 ± 0.20 a | 4.01 ± 0.06 a | 394.68 ± 9.27 a | 300.32 ± 2.00 a | 28.64 ± 0.07 b | 2.85 ± 0.06 b | 0.021 ± 0.0023 b | 0.17 ± 0.0036 a | 94.34 ± 2.01 a | 0.78 ± 0.003 a | 0.75 ± 0.005 a | 0.94 ± 0.01 a | 0.021 ± 0.001a | −0.14 ± 0.0025 b | |
| Genotype | ECC 25 | 10.71 ± 0.44 d | 3.20 ± 0.13 c | 302.00 ± 19.30 b | 290.52 ± 3.48 b | 28.72 ± 0.09 b | 3.29 ± 0.14 a | 0.027 ± 0.0038 | 0.15 ± 0.01 | 82.59 ± 3.42 | 0.77 ± 0.009 a,b,c | 0.74 ± 0.007 | 0.92 ± 0.01 | 0.020 ± 0.001 | −0.14 ± 0.0052 |
| ECC 29 | 10.95 ± 0.44 c | 3.76 ± 0.13 b | 303.92 ± 19.30 b | 300.37 ± 3.48 a | 28.94 ± 0.09 a | 2.68 ± 0.14 b | 0.027 ± 0.0038 | 0.16 ± 0.01 | 87.99 ± 3.42 | 0.77 ± 0.009 a,b,c | 0.74 ± 0.009 | 0.93 ± 0.01 | 0.015 ± 0.001 | −0.13 ± 0.0052 | |
| ECC 35 | 11.17 ± 0.44 bd | 3.45 ± 0.13 c | 317.30 ± 19.30 b | 294.21 ± 3.48 b | 28.49 ± 0.09 b | 3.02 ± 0.14 b | 0.024 ± 0.0038 | 0.16 ± 0.01 | 88.37 ± 3.42 | 0.75 ± 0.003 d | 0.73 ± 0.004 | 0.89 ± 0.01 | 0.021 ± 0.002 | −0.13 ± 0.0052 | |
| ECC 60 | 11.78 ± 0.44 ad | 4.25 ± 0.13 a | 386.77 ± 19.30 a | 305.70 ± 3.48 a | 29.09 ± 0.09 a | 2.71 ± 0.14 b | 0.025 ± 0.0038 | 0.16 ± 0.01 | 88.15 ± 3.42 | 0.77 ± 0.003 a,b,c | 0.73 ± 0.007 | 0.90 ± 0.01 | 0.018 ± 0.001 | −0.14 ± 0.0052 | |
| ECC 64 | 10.65 ± 0.44 d | 3.77 ± 0.13 b | 330.78 ± 19.30 b | 298.74 ± 3.48 a | 29.03 ± 0.09 a | 2.89 ± 0.14 b | 0.030 ± 0.0038 | 0.15 ± 0.01 | 81.99 ± 3.42 | 0.77 ± 0.003 b,c,d | 0.74 ± 0.005 | 0.91 ± 0.02 | 0.017 ± 0.001 | −0.14 ± 0.0052 | |
| ECC 66 | 11.45 ± 0.44 bd | 3.99 ± 0.13 a | 348.01 ± 19.30 b | 302.93 ± 3.48 a | 28.92 ± 0.09 a | 2.64 ± 0.14 b | 0.026 ± 0.0038 | 0.16 ± 0.01 | 87.57 ± 3.42 | 0.76 ± 0.004 b,c,d | 0.74 ± 0.008 | 0.92 ± 0.01 | 0.016 ± 0.001 | −0.13 ± 0.0052 | |
| ECC 73 | 12.20 ± 0.44 a,b | 3.66 ± 0.13 b | 342.09 ± 19.30 b | 286.78 ± 3.48 b | 29.06 ± 0.09 a | 3.32 ± 0.14 a | 0.028 ± 0.0038 | 0.18 ± 0.01 | 94.65 ± 3.42 | 0.78 ± 0.004 a | 0.74 ± 0.006 | 0.93 ± 0.01 | 0.020 ± 0.001 | −0.14 ± 0.0052 | |
| ECC 83 | 12.01 ± 0.44 ac | 3.78 ± 0.13 b | 368.37 ± 19.30 a | 292.55 ± 3.48 b | 28.71 ± 0.09 b | 3.31 ± 0.14 a | 0.027 ± 0.0038 | 0.17 ± 0.01 | 89.52 ± 3.42 | 0.77 ± 0.003 a,b | 0.74 ± 0.005 | 0.92 ± 0.01 | 0.018 ± 0.001 | −0.13 ± 0.0052 | |
| ECC 90 | 12.76 ± 0.44 a | 4.12 ± 0.13 a | 431.48 ± 19.30 a | 298.90 ± 3.48 a | 28.50 ± 0.09 b | 2.93 ± 0.14 b | 0.022 ± 0.0038 | 0.18 ± 0.01 | 96.24 ± 3.42 | 0.78 ± 0.003 b,c,d | 0.74 ± 0.006 | 0.91 ± 0.01 | 0.018 ± 0.001 | −0.14 ± 0.0052 | |
| IAN 873 | 11.81 ± 0.44 ad | 4.18 ± 0.13 a | 389.06 ± 19.30 a | 304.50 ± 3.48 a | 28.65 ± 0.09 b | 2.69 ± 0.14 b | 0.024 ± 0.0038 | 0.16 ± 0.01 | 87.80 ± 3.42 | 0.76 ± 0.003 c,d | 0.75 ± 0.006 | 0.90 ± 0.01 | 0.019 ± 0.001 | −0.13 ± 0.0052 | |
| Hour | 3 | - | - | - | - | - | - | - | - | - | - | - | - | - | −0.08 ± 0.0015 a |
| 6 | 9.02 ± 0.30 d | 3.81 ± 0.07 c | 323.17 ± 11.24 d | 309.52 ± 3.53 b | 28.06 ± 0.05 d | 2.49 ± 0.14 c | 0.021 ± 0.0015 b | 0.12 ± 0.0039 c | 65.84 ± 2.15 c | - | - | - | - | −0.11 ± 0.0038 b | |
| 9 | 16.84 ± 0.30 a | 4.72 ± 0.07 a | 500.75 ± 11.24 a | 283.76 ± 3.90 c | 28.67 ± 0.14 c | 3.65 ± 0.15 b | 0.024 ± 0.0024 b | 0.22 ± 0.0036 a | 119.25 ± 1.97 a | - | - | - | - | −0.16 ± 0.0035 c | |
| 12 | 16.08 ± 0.26 b | 4.42 ± 0.07 b | 420.35 ± 11.24 b | 269.62 ± 1.98 d | 29.76 ± 0.14 a | 3.85 ± 0.11 a | 0.033 ± 0.0018 a | 0.22 ± 0.0035 a | 120.99 ± 1.92 a | - | - | - | - | −0.18 ± 0.0036 d | |
| 15 | 13.64 ± 0.23 c | 4.00 ± 0.07 c | 377.68 ± 11.24 c | 273.44 ± 3.00 d | 29.06 ± 0.11 b | 3.92 ± 0.11 a | 0.036 ± 0.0019 a | 0.19 ± 0.0035 b | 102.89 ± 1.95 b | - | - | - | - | −0.16 ± 0.0036 c | |
| 18 | 2.17 ± 0.32 e | 2.12 ± 0.07 d | 137.95 ± 11.24 e | 351.25 ± 3.22 a | 28.49 ± 0.07 c | 0.83 ± 0.12 d | 0.017 ± 0.0019 c | 0.06 ± 0.0043 d | 33.46 ± 2.34 d | - | - | - | - | −0.12 ± 0.0037 b | |
| Genotype | Humid Warm | Semi-Humid Warm | Average |
|---|---|---|---|
| ECC 25 | 21.30 ± 0.62 b,c,d | 21.37 ± 0.71 b,c,d | 21.33 b,c |
| ECC 29 | 23.18 ± 0.81 a,b | 20.88 ± 0.82 d | 22.03 a,b |
| ECC 35 | 21.26 ± 0.65 b,c,d | 21.25 ± 0.57 b,c,d | 21.25 b,c |
| ECC 60 | 21.54 ± 0.78 b,c,d | 20.78 ± 0.75 c,d | 21.16 b,c |
| ECC 64 | 23.36 ± 0.52 a | 20.82 ± 0.78 b,c,d | 22.09 a,b |
| ECC 66 | 21.14 ± 0.78 b,c,d | 21.43 ± 0.47 b,c,d | 21.28 b,c |
| ECC 73 | 23.17 ± 0.51 a,b | 22.66 ± 0.56 a,b | 22.91 a,b |
| ECC 83 | 23.16 ± 0.32 a,b | 22.86 ± 0.36 a,b | 23.01 a |
| ECC 90 | 23.14 ± 0.48 a,b | 22.96 ± 0.96 a,b | 23.05 a |
| IAN 873 | 20.72 ± 0.88 c,d | 20.25 ± 0.86 c,d | 20.49 c |
| Mean | 22.20 a | 21.53 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sterling, A.; Guaca-Cruz, L.; Clavijo-Arias, E.A.; Rodríguez-Castillo, N.; Suárez, J.C. Photosynthesis-Related Responses of Colombian Elite Hevea brasiliensis Genotypes under Different Environmental Variations: Implications for New Germplasm Selection in the Amazon. Plants 2021, 10, 2320. https://doi.org/10.3390/plants10112320
Sterling A, Guaca-Cruz L, Clavijo-Arias EA, Rodríguez-Castillo N, Suárez JC. Photosynthesis-Related Responses of Colombian Elite Hevea brasiliensis Genotypes under Different Environmental Variations: Implications for New Germplasm Selection in the Amazon. Plants. 2021; 10(11):2320. https://doi.org/10.3390/plants10112320
Chicago/Turabian StyleSterling, Armando, Lised Guaca-Cruz, Edwin Andrés Clavijo-Arias, Natalia Rodríguez-Castillo, and Juan Carlos Suárez. 2021. "Photosynthesis-Related Responses of Colombian Elite Hevea brasiliensis Genotypes under Different Environmental Variations: Implications for New Germplasm Selection in the Amazon" Plants 10, no. 11: 2320. https://doi.org/10.3390/plants10112320
APA StyleSterling, A., Guaca-Cruz, L., Clavijo-Arias, E. A., Rodríguez-Castillo, N., & Suárez, J. C. (2021). Photosynthesis-Related Responses of Colombian Elite Hevea brasiliensis Genotypes under Different Environmental Variations: Implications for New Germplasm Selection in the Amazon. Plants, 10(11), 2320. https://doi.org/10.3390/plants10112320







