Transgenerational Effects of Maternal Water Condition on the Growth, C:N Stoichiometry and Seed Characteristics of the Desert Annual Atriplex aucheri
Abstract
:1. Introduction
2. Results
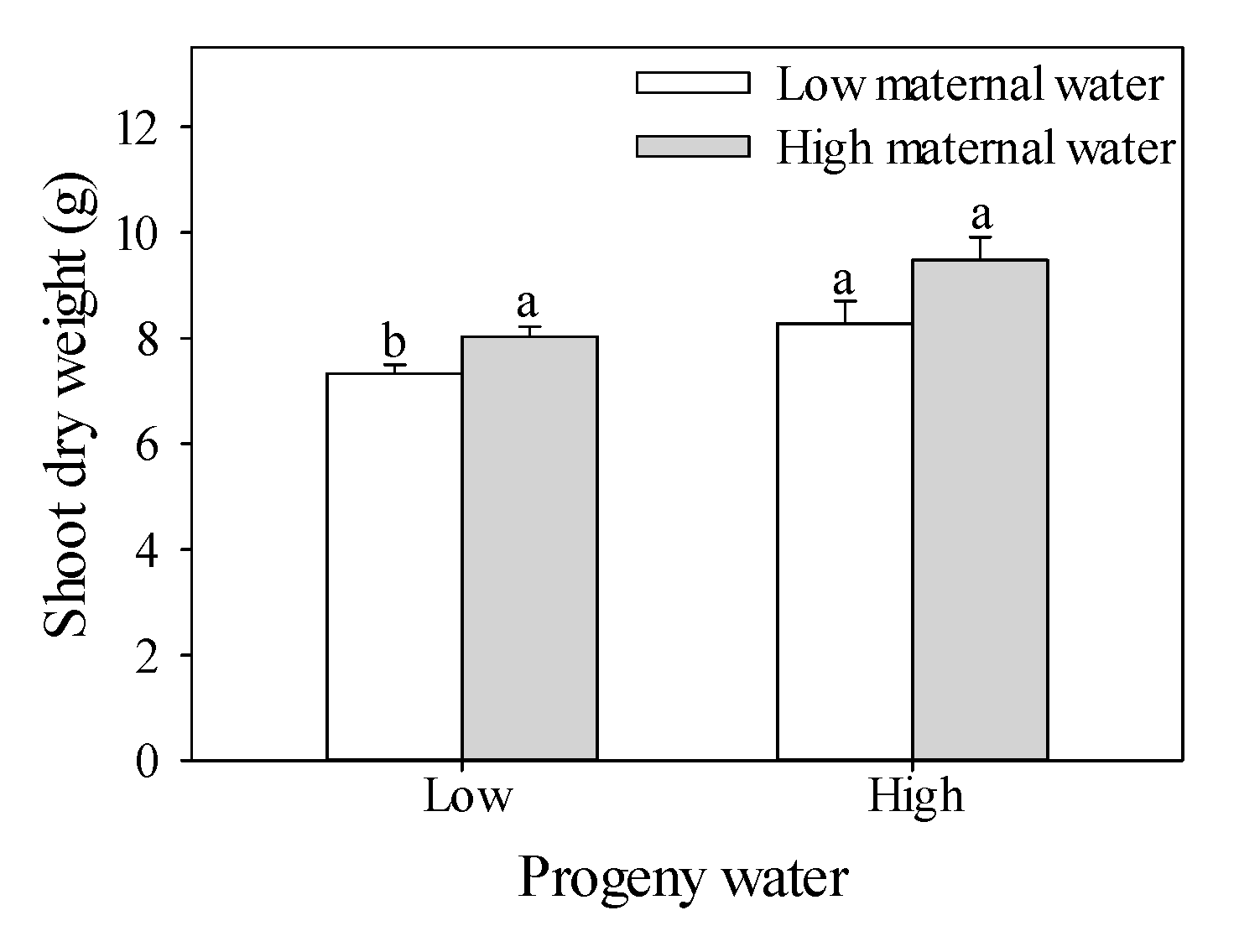
2.1. Shoot Dry Weight
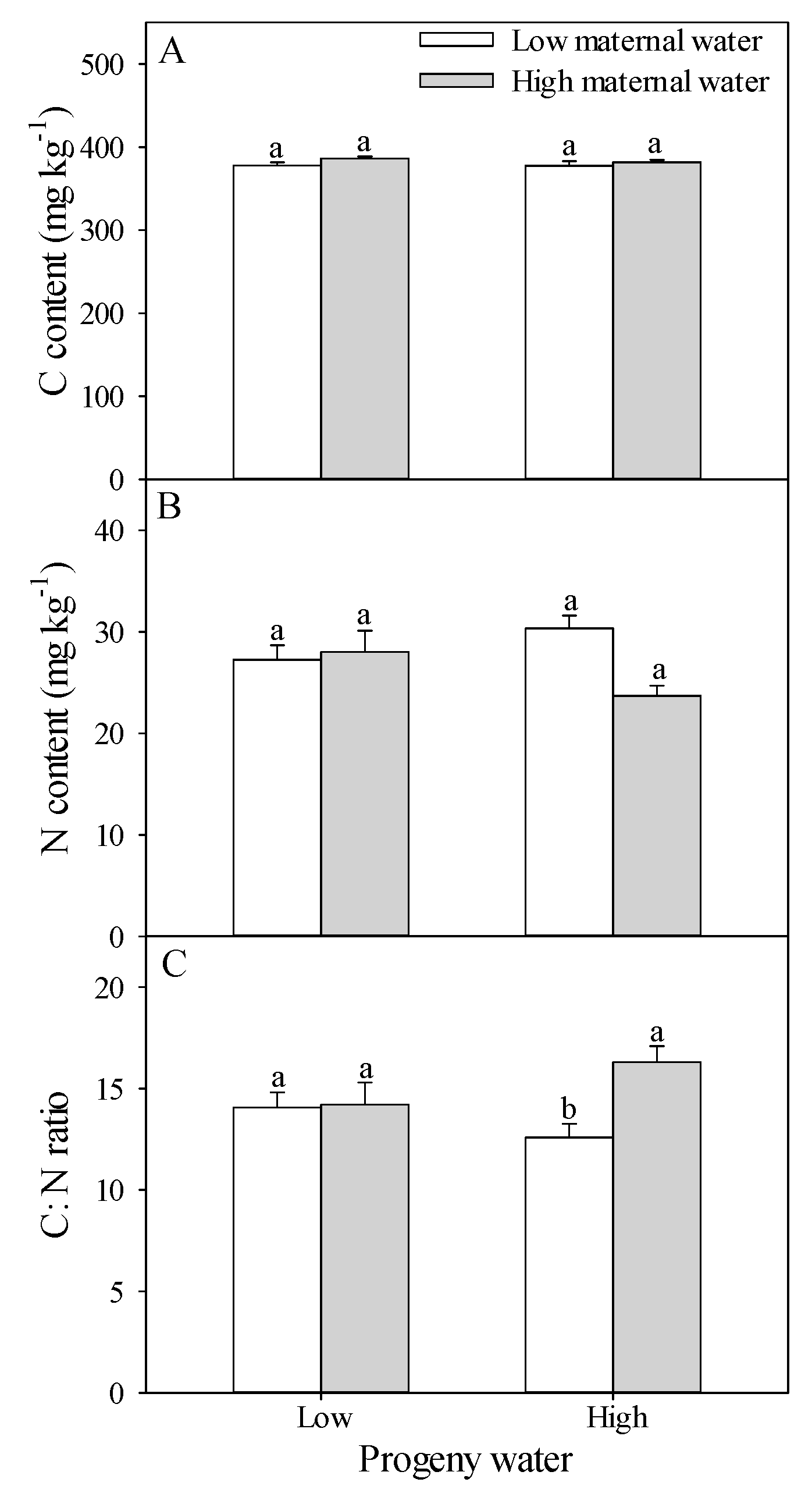
2.2. C Content, N Content and C:N Ratio
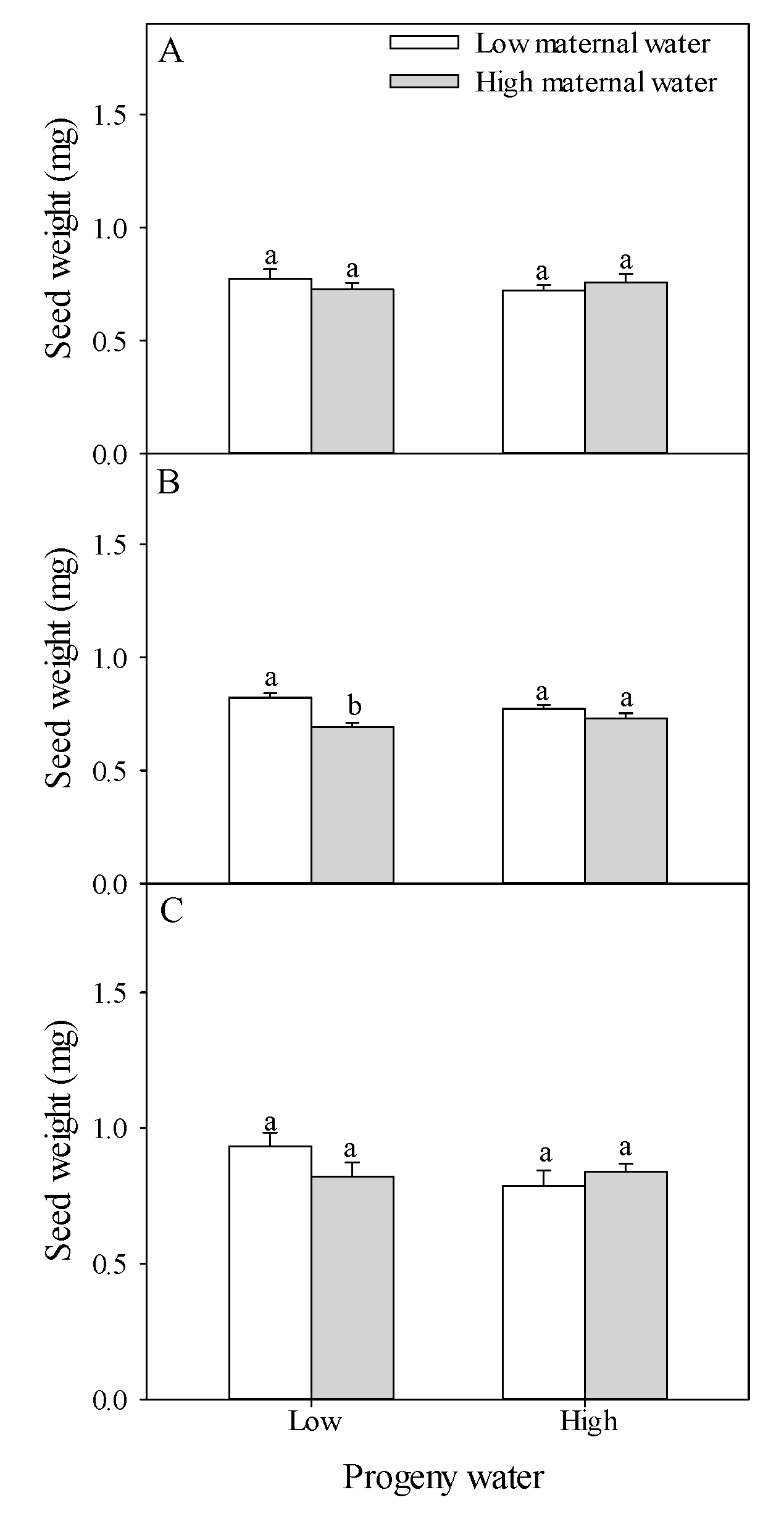
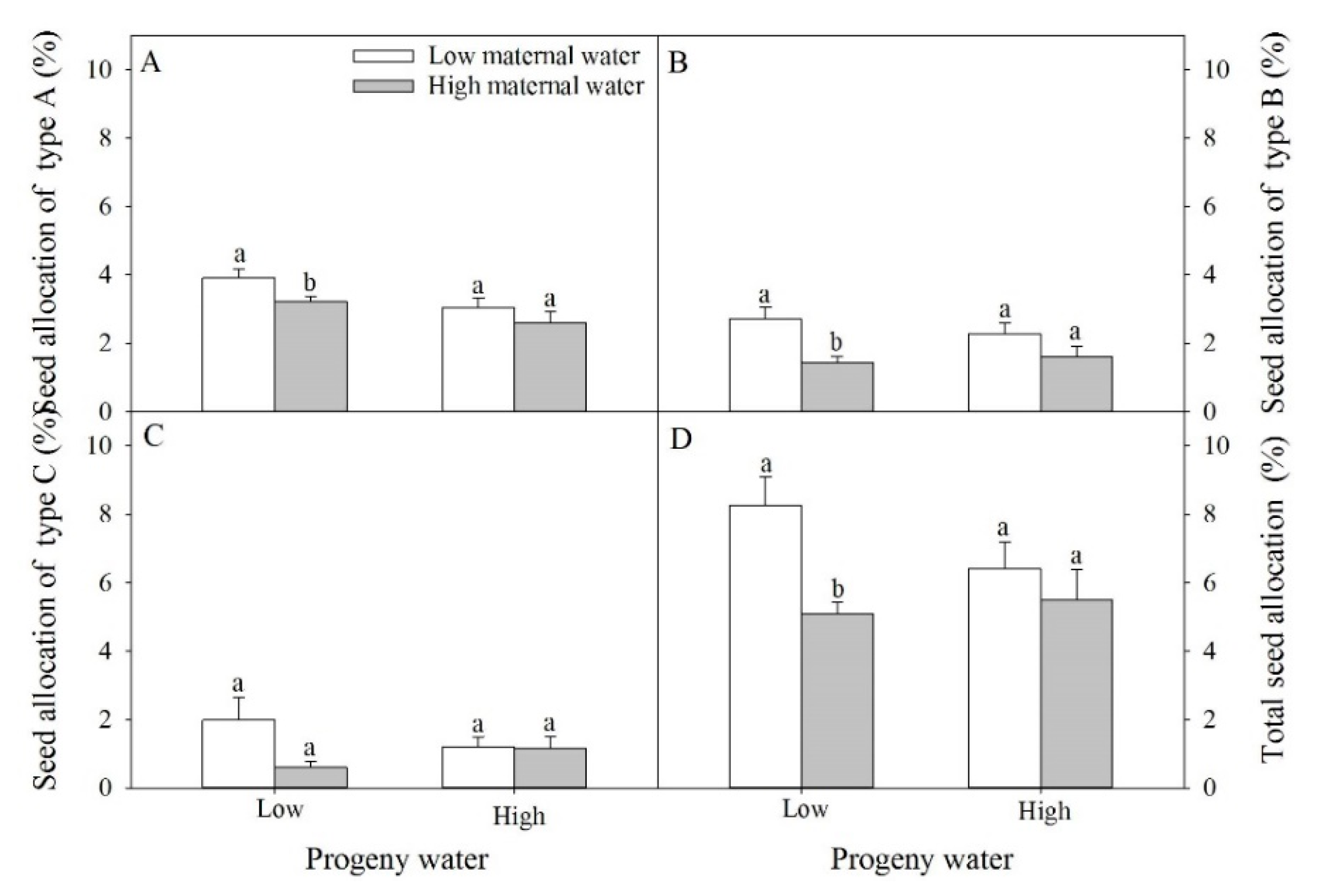
2.3. Seed Traits
3. Discussion
4. Conclusions
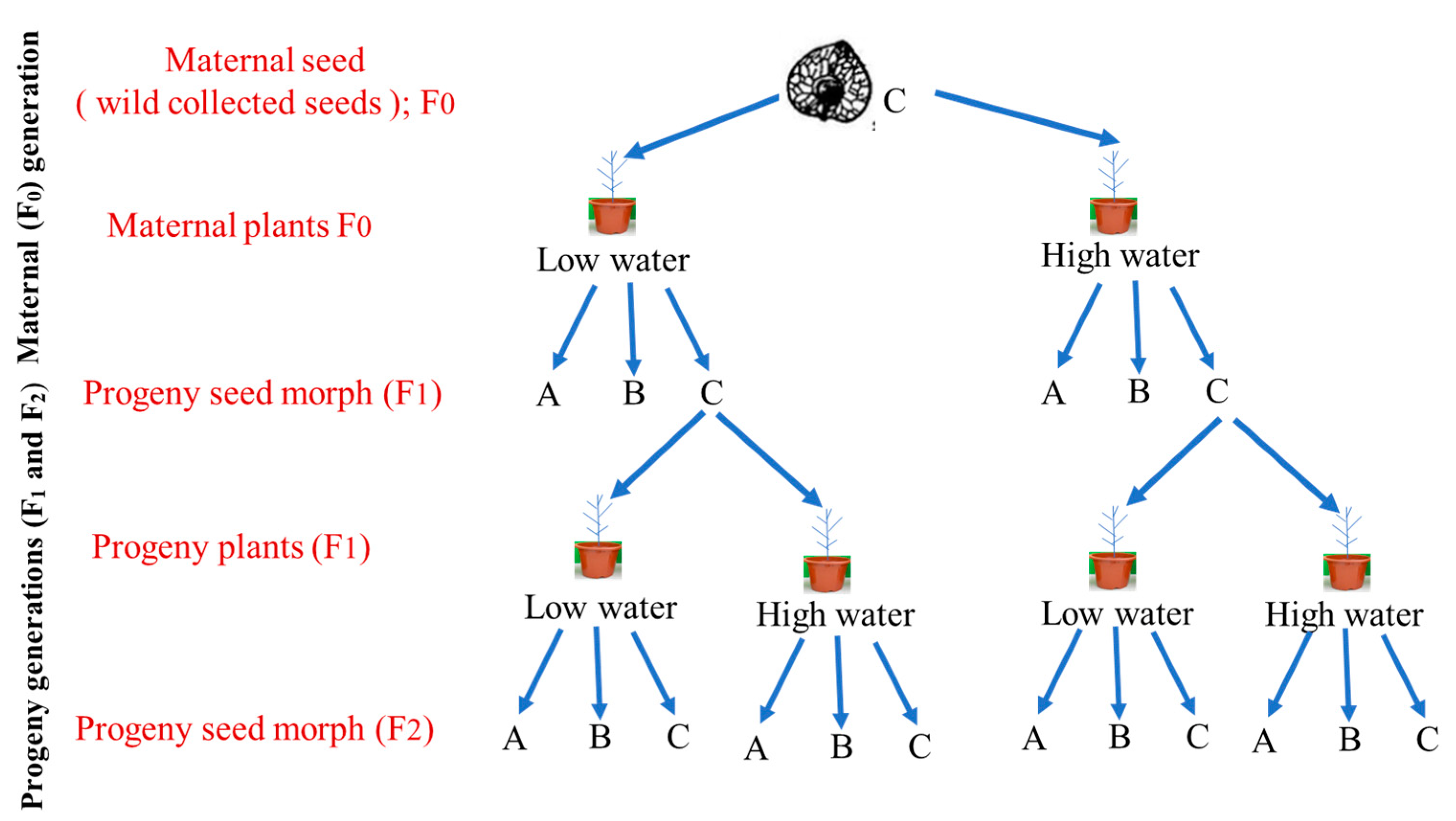
5. Materials and Methods
5.1. Seed Collection
5.2. Pot Experiment
5.3. Shoot Dry Weight
5.4. C Content, N Content and C:N Ratio
5.5. Seed Traits
5.6. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cano, L.; Fuertes-Mendizabal, T.; Garcia-Baquero, G.; Herrera, M.; Gonzalez-Moro, M.B. Plasticity to salinity and transgenerational effects in the nonnative shrub Baccharis halimifolia: Insights into an estuarine invasion. Am. J. Bot. 2016, 103, 808–820. [Google Scholar] [CrossRef] [Green Version]
- Hatzig, S.V.; Nuppenau, J.N.; Snowdon, R.J.; Schiessl, S.V. Drought stress has transgenerational effects on seeds and seedlings in winter oilseed rape (Brassica napus L.). BMC Plant Biol. 2018, 18, 297. [Google Scholar] [CrossRef]
- Li, K.-N.; Chen, J.-S.; Wei, Q.; Li, Q.; Lei, N.-F. Effects of transgenerational plasticity on morphological and physiological properties of stoloniferous herb Centella asiatica subjected to high/low light. Front. Plant Sci. 2018, 9, 1640. [Google Scholar] [CrossRef]
- Dong, B.-C.; Alpert, P.; Yu, F.-H. Transgenerational effects of herbivory and soil nutrients transmitted via vegetative reproduction in the clonal plant Alternanthera philoxeroides. Perspect. Plant Ecol. Evol. Sys. 2019, 41, 125498. [Google Scholar] [CrossRef]
- Lazaro-Lobo, A.; Herrera, M.; Campos, J.A.; Cano, L.; Goni, E.; Ervin, G.N. Influence of local adaptations, transgenerational effects and changes in offspring’s saline environment on Baccharis halimifolia L. under different salinity and light levels. Environ. Exp. Bot. 2020, 177, 104134. [Google Scholar] [CrossRef]
- Agrawal, A.A. Transgenerational consequences of plant responses to herbivory: An adaptive maternal effect? Am. Nat. 2001, 157, 555. [Google Scholar] [CrossRef] [PubMed]
- Gundel, P.E.; Rudgers, J.A.; Whitney, K.D. Vertically transmitted symbionts as mechanisms of transgenerational effects. Am. J. Bot. 2017, 104, 787–792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rottstock, T.; Kummer, V.; Fischer, M.; Joshi, J. Rapid transgenerational effects in Knautia arvensis in response to plant community diversity. J. Ecol. 2017, 105, 714–725. [Google Scholar] [CrossRef]
- Waterman, R.; Sultan, S.E. Transgenerational effects of parent plant competition on offspring development in contrasting conditions. Ecology 2021, e03531. [Google Scholar] [CrossRef]
- Nosalewicz, A.; Siecinska, J.; Smiech, M.; Nosalewicz, M.; Wiacek, D.; Pecio, A.; Wach, D. Transgenerational effects of temporal drought stress on spring barley morphology and functioning. Environ. Exp. Bot. 2016, 131, 120–127. [Google Scholar] [CrossRef]
- González, A.P.R.; Dumalasová, V.; Rosenthal, J.; Skuhrovec, J.; Latzel, V. The role of transgenerational effects in adaptation of clonal offspring of white clover (Trifolium repens) to drought and herbivory. Evol. Ecol. 2017, 31, 345–361. [Google Scholar] [CrossRef]
- Yin, J.-J.; Zhou, M.; Lin, Z.; Lin, Z.-R.; Li, Q.-S.; Zhang, Y.-Y. Transgenerational effects benefit offspring across diverse environments: A meta-analysis in plants and animals. Ecol. Lett. 2019, 22, 1976–1986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Able, A.J.; Able, J.A. Transgenerational effects of water-deficit and heat stress on germination and seedling vigour-new insights from durum wheat microRNAs. Plants 2020, 9, 189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walter, J.; Harter, D.E.V.; Beierkuhnlein, C.; Jentsch, A. Transgenerational effects of extreme weather: Perennial plant offspring show modified germination, growth and stoichiometry. J. Ecol. 2016, 104, 1032–1040. [Google Scholar] [CrossRef] [Green Version]
- Adrian-Kalchhauser, I.; Sultan, S.E.; Shama, L.N.S.; Spence-Jones, H.; Tiso, S.; Valsecchi, C.I.K.; Weissing, F.J. Understanding ‘Non-genetic’ Inheritance: Insights from molecular evolutionary crosstalk. Trends Ecol. Evol. 2020, 35, 1078–1089. [Google Scholar] [CrossRef] [PubMed]
- Merino-Martin, L.; Courtauld, C.; Commander, L.; Turner, S.; Lewandrowski, W.; Stevens, J. Interactions between seed functional traits and burial depth regulate germination and seedling emergence under water stress in species from semi-arid environments. J. Arid Environ. 2017, 147, 25–33. [Google Scholar] [CrossRef]
- Duncan, C.; Schultz, N.L.; Good, M.K.; Lewandrowski, W.; Cook, S. The risk-takers and -avoiders: Germination sensitivity to water stress in an arid zone with unpredictable rainfall. AoB Plants 2019, 11, plz066. [Google Scholar] [CrossRef]
- Lewandrowski, W.; Stevens, J.C.; Webber, B.L.; Dalziell, E.L.; Trudgen, M.S.; Bateman, A.M.; Erickson, T.E. Global change impacts on arid zone ecosystems: Seedling establishment processes are threatened by temperature and water stress. Ecol. Evol. 2021, 11, 8071–8084. [Google Scholar] [CrossRef]
- Zheng, X.; Chen, L.; Li, M.; Lou, Q.; Luo, L. Transgenerational variations in DNA methylation induced by drought stress in two rice varieties with distinguished difference to drought resistance. PLoS ONE 2013, 8, e80253. [Google Scholar] [CrossRef] [Green Version]
- Imbert, E. Ecological consequences and ontogeny of seed heteromorphism. Perspect Plant Ecol. Evol. Sys. 2002, 5, 13–36. [Google Scholar] [CrossRef]
- Mandák, B. Seed heteromorphism and the life cycle of plants: A literature review. Preslia 1997, 69, 129–159. [Google Scholar]
- Cheplick, G.P.; Quinn, J.A. The shift in aerial/subterranean fruit ratio in Amphicarpum purshii: Causes and significance. Oecologia 1983, 57, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Sadeh, A.; Guterman, H.; Gersani, M.; Ovadia, O. Plastic bet-hedging in an amphicarpic annual: An integrated strategy under variable conditions. Evol. Ecol. 2009, 23, 373–388. [Google Scholar] [CrossRef]
- Lu, J.-J.; Tan, D.-Y.; Baskin, J.M.; Baskin, C.C. Germination season and watering regime, but not seed morph, affect life history traits in a cold desert diaspore-heteromorphic annual. PLoS ONE 2014, 9, e102018. [Google Scholar] [CrossRef] [Green Version]
- Yang, F.; Yang, X.-J.; Baskin, J.M.; Baskin, C.C.; Cao, D.-C.; Huang, Z.-Y. Transgenerational plasticity provides ecological diversity for a seed heteromorphic species in response to environmental heterogeneity. Perspect. Plant Ecol. Evol. Sys. 2015, 17, 201–208. [Google Scholar] [CrossRef]
- Flora of China Editorial Committee. Flora of China; Science Press and Missouri Botanical Garden Press: Beijing, China, 2003; Volume 5. [Google Scholar]
- Wang, L.; Wang, H.-L.; Zhang, K.; Tian, C.-Y. Effects of maternal nutrient and water availability on seed production, size and germination of heterocarpic Atriplex aucheri. Seed Sci. Technol. 2015, 43, 71–79. [Google Scholar] [CrossRef]
- Huang, J.; Tian, C.-Y.; Bian, W.-G.; Yin, H.-L.; Ren, J.; Chen, C.-X. Response of growth of four halophyte species in oil-contaminated soil. Arid Zone Res. 2014, 31, 100–104, (In Chinese with English Abstract). [Google Scholar]
- Wei, Y.; Liu, P.-W.; An, S.-Z. Study on fruit polymorphism and germination measures of Atriplex aucheri Moq. Seeds. Arid Zone Res. 2007, 24, 835–839, (In Chinese with English Abstract). [Google Scholar]
- Wang, W.-H.; Jiang, L. Effects of four kinds of sodium salinity stress on seed germination and young seedling growth of Atriplex aucheri. Chin. J. Grassland 2020, 42, 23–29, (In Chinese with English Abstract). [Google Scholar]
- Chathurika, W.; Raja, R.K.; Jason, K.L.; Wei, G.; Nacer, B. Drought stress has transgenerational effects on soybean seed germination and seedling vigor. PLoS ONE 2019, 14, e0214977. [Google Scholar]
- Pias, B.; Matesanz, S.; Herrero, A.; Gimeno, T.E.; Escudero, A.; Valladares, F. Transgenerational effects of three global change drivers on an endemic Mediterranean plant. Oikos 2010, 119, 1435–1444. [Google Scholar] [CrossRef]
- Sardans, J.; Penuelas, J.; Estiarte, M.; Prieto, P. Warming and drought alter C and N concentration, allocation and accumulation in a Mediterranean shrubland. Glob. Chang. Biol. 2008, 14, 2304–2316. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.-L.; Zhao, G.-X.; Li, M.; Zhang, M.-T.; Zhang, L.-F.; Zhang, X.-F.; An, L.-Z.; Xu, S.-J. C:N:P stoichiometry and leaf traits of halophytes in an arid saline environment, Northwest China. PLoS ONE 2015, 10, e0119935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fenner, M.; Thompson, K. The Ecology of Seeds; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]


| Source | Progeny Water | Maternal Water | Progeny Water × Maternal Water |
|---|---|---|---|
| Shoot dry weight (n = 10) | 12.94 ** | 8.47 * | 0.58 |
| C content (n = 6) | 0.48 | 2.95 | 0.26 |
| N content (n = 6) | 0.01 | 3.87 | 6.04 * |
| C: N ratio (n = 6) | 0.01 | 5.32 * | 4.54 * |
| Seed number of type A (n = 10) | 0.12 | 0.75 | 0.15 |
| Seed number of type B (n = 10) | 0.94 | 3.10 | 0.38 |
| Seed number of type C (n = 10) | 1.31 | 2.61 | 1.48 |
| Total seed number (n = 10) | 1.06 | 3.22 | 0.87 |
| Seed weight of type A (n = 50) | 0.12 | 0.03 | 1.53 |
| Seed weight of type B (n = 50) | 0.06 | 17.62 *** | 4.75 * |
| Seed weight of type C (n = 50) | 1.73 | 0.37 | 2.85 |
| Seed yield of type A (n = 10) | 0.97 | 0.53 | 0.35 |
| Seed yield of type B (n = 10) | 0.22 | 5.56 * | 0.72 |
| Seed yield of type C (n = 10) | 0.97 | 1.70 | 1.50 |
| Total seed yield (n = 10) | 0.60 | 2.70 | 1.82 |
| Seed allocation of type A (n = 10) | 7.93 ** | 4.49 * | 0.24 |
| Seed allocation of type B (n = 10) | 0.19 | 10.28 ** | 0.95 |
| Seed allocation of type C (n = 10) | 0.15 | 1.39 | 2.73 |
| Total seed allocation (n = 10) | 0.92 | 7.23 * | 2.20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, L.; Wen, Z.; Zhang, Y.; Zhao, Z.; Tanveer, M.; Tian, C.; Wang, L. Transgenerational Effects of Maternal Water Condition on the Growth, C:N Stoichiometry and Seed Characteristics of the Desert Annual Atriplex aucheri. Plants 2021, 10, 2362. https://doi.org/10.3390/plants10112362
Jiang L, Wen Z, Zhang Y, Zhao Z, Tanveer M, Tian C, Wang L. Transgenerational Effects of Maternal Water Condition on the Growth, C:N Stoichiometry and Seed Characteristics of the Desert Annual Atriplex aucheri. Plants. 2021; 10(11):2362. https://doi.org/10.3390/plants10112362
Chicago/Turabian StyleJiang, Li, Zhibin Wen, Yunling Zhang, Zhenyong Zhao, Mohsin Tanveer, Changyan Tian, and Lei Wang. 2021. "Transgenerational Effects of Maternal Water Condition on the Growth, C:N Stoichiometry and Seed Characteristics of the Desert Annual Atriplex aucheri" Plants 10, no. 11: 2362. https://doi.org/10.3390/plants10112362







