Apiaceae Essential Oils: Boosters of Terbinafine Activity against Dermatophytes and Potent Anti-Inflammatory Effectors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Material
2.3. Essential Oil Extraction
2.4. Essential Oils Analysis
2.5. Microbial Strains
2.6. Antifungal Susceptibility Testing
2.7. Checkerboard Assay
2.8. Evaluation of Cytokine Production in Human Neutrophils
2.8.1. Neutrophils Isolation
2.8.2. Evaluation of Neutrophils Viability
2.8.3. Evaluation of IL-1β, IL-8 and TNF-α Secretion
2.9. Statistical Analyses
3. Results
3.1. Yield and Chemical Composition of Essential Oils
3.2. Antifungal Susceptibility Results
3.3. In Vitro Effects of Essential Oils/Main Constituents and Terbinafine Combinations against Dermatophytes
3.4. Effects on Cytokine Production in Human Neutrophils
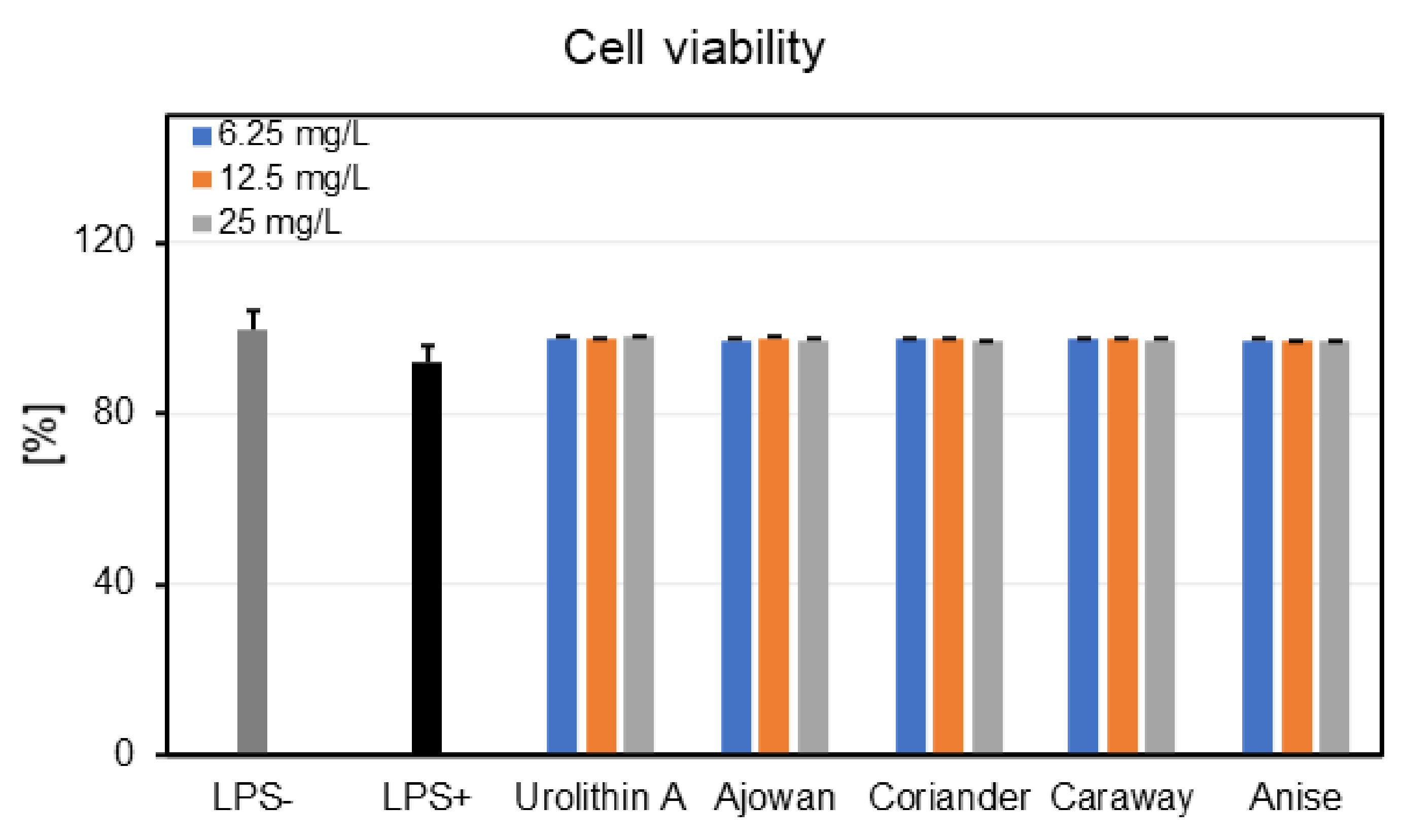
3.4.1. Effects on Neutrophils Viability
3.4.2. Effects on Pro-Inflammatory Cytokine Secretion
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Burstein, V.L.; Beccacece, I.; Guasconi, L.; Mena, C.J.; Cervi, L.; Chiapello, L.S. Skin immunity to dermatophytes: From experimental infection models to human disease. Front. Immunol. 2020, 11, 605644. [Google Scholar] [CrossRef]
- Gnat, S.; Łagowski, D.; Nowakiewicz, A. Major challenges and perspectives in the diagnostics and treatment of dermatophyte infections. J. Appl. Microbiol. 2020, 129, 212–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Hoog, G.S.; Dukik, K.; Monod, M.; Packeu, A.; Stubbe, D.; Hendrickx, M.; Kupsch, C.; Stielow, J.B.; Freeke, J.; Göker, M. Toward a novel multilocus phylogenetic taxonomy for the dermatophytes. Mycopathologia 2017, 182, 5–31. [Google Scholar] [CrossRef] [Green Version]
- Nenoff, P.; Verma, S.B.; Vasani, R.; Burmester, A.; Hipler, U.C.; Wittig, F.; Krüger, C.; Nenoff, K.; Wiegand, C.; Saraswat, A. The current Indian epidemic of superficial dermatophytosis due to Trichophyton mentagrophytes—A molecular study. Mycoses 2019, 62, 336–356. [Google Scholar] [CrossRef]
- Zhan, P.; Liang, G.; Liu, W. Dermatophytes and dermatophytic infections worldwide. In Dermatophytes and Dermatophytoses; Bouchara, J.P., Nenoff, P., Gupta, A.K., Chaturvedi, V., Eds.; Springer: Cham, Switzerland, 2021; pp. 15–40. [Google Scholar]
- Gupta, A.K.; Renaud, H.J.; Quinlan, E.M.; Shear, N.H.; Piguet, V. The growing problem of antifungal resistance in onychomycosis and other superficial mycoses. Am. J. Clin. Dermatol. 2021, 22, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Monod, M.; Feuermann, M.; Yamada, T. Terbinafine and itraconazole resistance in dermatophytes. In Dermatophytes and Dermatophytoses; Bouchara, J.P., Nenoff, P., Gupta, A.K., Chaturvedi, V., Eds.; Springer: Cham, Switzerland, 2021; pp. 415–429. [Google Scholar]
- Kramer, O.; Albrecht, J. Clinical presentation of terbinafine-induced severe liver injury and the value of laboratory monitoring: A Critically Appraised Topic. Brit. J. Dermatol. 2017, 177, 1279–1284. [Google Scholar] [CrossRef]
- Durdu, M.; Ilkit, M.; Tamadon, Y.; Tolooe, A.; Rafati, H.; Seyedmousavi, S. Topical and systemic antifungals in dermatology practice. Expert Rev. Clin. Pharmacol. 2017, 10, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Venkataraman, M.; Quinlan, E.M. New antifungal agents and new formulations against dermatophytes. In Dermatophytes and Dermatophytoses; Bouchara, J.P., Nenoff, P., Gupta, A.K., Chaturvedi, V., Eds.; Springer: Cham, Switzerland, 2021; pp. 433–471. [Google Scholar]
- Elewski, B.; Ghannoum, M.; Mayser, P.; Gupta, A.; Korting, H.C.; Shouey, R.; Baker, D.; Rich, P.; Ling, M.; Hugot, S. Efficacy, safety and tolerability of topical terbinafine nail solution in patients with mild to moderate toenail onychomycosis: Results from three randomized studies using double blind vehicle controlled and open label active controlled designs. J. Eur. Acad. Dermatol. Venereol. 2013, 27, 287–294. [Google Scholar] [CrossRef]
- Saunte, D.M.; Hare, R.K.; Jørgensen, K.M.; Jørgensen, R.; deleuran, M.; Zachariae, C.O.; Thomsen, S.F.; Bjørnskov-Halkier, L.; Kofoed, K.; Arendrup, M.C. Emerging terbinafine resistance in Trichophyton: Clinical characteristics, squalene epoxidase gene mutations, and a reliable EUCAST method for detection. Antimicrob. Agents Chemother. 2019, 63, e01126-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khurana, A.; Sardana, K.; Chowdhary, A. Antifungal resistance in dermatophytes: Recent trends and therapeutic implications. Fungal Genet. Biol. 2019, 132, 103255. [Google Scholar] [CrossRef]
- Lopes, G.; Pinto, E.; Salgueiro, L. Natural products: An alternative to conventional therapy for dermatophytosis? Mycopathologia 2017, 182, 143–167. [Google Scholar] [CrossRef] [PubMed]
- Brescini, L.; Fioriti, S.; Morroni, G.; Barchiesi, F. Antifungal combinations in dermatophytes. J. Fungi 2021, 7, 727. [Google Scholar] [CrossRef] [PubMed]
- Zuzarte, M.; Lopes, G.; Pinto, E.; Salgueiro, L. Are natural products an alternative therapy for dermatophytosis? In Dermatophytes and Dermatophytoses; Bouchara, J.P., Nenoff, P., Gupta, A.K., Chaturvedi, V., Eds.; Springer: Cham, Switzerland, 2021; pp. 473–519. [Google Scholar]
- Van Vuuren, S.; Viljoen, A. Plant-based antimicrobial studies–methods and approaches to study the interaction between natural products. Planta Med. 2011, 77, 1168–1182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adorjan, B.; Buchbauer, G. Biological properties of essential oils: An updated review. Flavour Fragr. J. 2010, 25, 407–426. [Google Scholar] [CrossRef]
- Hammer, K.A.; Carson, C.F. Antibacterial and antifungal activities of essential oils. In Lipids and Essential Oils as Antimicrobial Agents; Thormar, H., Ed.; John Wiley & Sons: Chichester, UK, 2011; pp. 255–295. [Google Scholar]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Bruneton, J. Pharmacognosy, Phytochemistry, Medicinal Plants, 2nd ed.; Lavoisier: Paris, France, 2008; p. 484. [Google Scholar]
- Trifan, A.; Luca, S.V.; Greige-Gerges, H.; Miron, A.; Gille, E.; Aprotosoaie, A.C. Recent advances in tackling microbial multidrug resistance with essential oils: Combinatorial and nano-based strategies. Crit. Rev. Microbiol. 2020, 46, 338–357. [Google Scholar] [CrossRef] [PubMed]
- Lopes, A.I.; Tavaria, F.K.; Pintado, M.E. Conventional and natural compounds for the treatment of dermatophytosis. Med. Mycol. 2020, 58, 707–720. [Google Scholar] [CrossRef]
- D’agostino, M.; Tesse, N.; Frippiat, J.P.; Machouart, M.; debourgogne, A. Essential oils and their natural active compounds presenting antifungal properties. Molecules 2019, 24, 3713. [Google Scholar] [CrossRef] [Green Version]
- Simpson, M.G. Plant Systematics; Elsevier Academic Press: Amsterdam, The Netherlands, 2019; p. 829. [Google Scholar]
- Aćimović, M. Nutraceutical potential of Apiaceae. In Bioactive Molecules in Food. Reference Series in Phytochemistry; Mérillon, J.M.., Ramawat, K., Eds.; Springer: Cham, Switzerland, 2017. [Google Scholar] [CrossRef]
- Heinrich, M.; Williamson, E.M.; Gibbons, S.; Barnes, J.; Prieto-Garcia, J. Fundamentals of Pharmacognosy and Phytotherapy, 2nd ed.; Elsevier: Edinbourg, UK, 2012; p. 34. [Google Scholar]
- Widelski, J.; Graikou, K.; Ganos, C.; Skalicka-Wozniak, K.; Chinou, I. Volatiles from selected Apiaceae species cultivated in Poland—Antimicrobial activities. Processes 2021, 9, 695. [Google Scholar] [CrossRef]
- Khalil, N.; Ashour, M.; Fikry, S.; Singab, A.N.; Salama, O. Chemical composition and antimicrobial activity of the essential oils of selected Apiaceous fruits. FJPS 2018, 4, 88–92. [Google Scholar] [CrossRef]
- Elshafie, H.S.; Camele, I. An overview of the biological effects of some mediterranean essential oils on human health. BioMed Res. Int. 2017, 2017. [Google Scholar] [CrossRef]
- Sayed-Ahmad, B.; Talou, T.; Saad, Z.; Hijazi, A.; Merah, O. The Apiaceae: Ethnomedicinal family as source for industrial uses. Ind. Crops Prod. 2017, 109, 661–671. [Google Scholar] [CrossRef] [Green Version]
- Trifan, A.; Bostănaru, A.-C.; Luca, S.V.; Grădinaru, A.C.; Jităreanu, A.; Aprotosoaie, A.C.; Miron, A.; Cioancă, O.; Hăncianu, M.; Ochiuz, L.; et al. Antifungal potential of Pimpinella anisum, Carum carvi and Coriandrum sativum extracts. A comparative study with focus on the phenolic composition. Farmacia 2020, 68, 22–27. [Google Scholar] [CrossRef]
- Grădinaru, A.; Trifan, A.; Şpac, A.; Brebu, M.; Miron, A.; Aprotosoaie, A. Antibacterial activity of traditional spices against lower respiratory tract pathogens: Combinatorial effects of Trachyspermum ammi essential oil with conventional antibiotics. Lett. Appl. Microbiol. 2018, 67, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Trifan, A.; Aprotosoaie, A.C.; Cioanca, O.; Hancianu, M.; Jitareanu, A.; Gille, E.; Miron, A. Antioxidant activity of essential oil from Carum carvi L. cultivated in North-eastern Romania. Med.-Surg. J. 2016, 120, 732–736. [Google Scholar]
- Trifan, A.; Miron, A.; Aprotosoaie, A.C.; Hancianu, M.; Cioanca, O.; Gille, E.; Stanescu, U. Phytotoxicity assessment of polyphenolic extracts from Carum carvi L. fruits. Farmacia 2013, 61, 12–19. [Google Scholar]
- Trifan, A.; Aprotosoaie, A.C.; Şpac, A.; Hăncianu, M.; Miron, A.; Stănescu, U. Contributions to the chemical study of the essential oil isolated from coriander (Omagiu cultivar) fruits. Farmacia 2012, 60, 177–183. [Google Scholar]
- Jain, N.; Sharma, M.; Joshi, S.; Kaushik, U. Chemical composition, toxicity and antidermatophytic activity of essential oil of Trachyspermum ammi. Indian J. Pharm. Sci. 2018, 80, 135–142. [Google Scholar] [CrossRef] [Green Version]
- Swamy, M.K.; Akhtar, M.S.; Sinniah, U.R. Antimicrobial properties of plant essential oils against human pathogens and their mode of action: An updated review. Evid.-Based Complementary Altern. Med. 2016, 2016. [Google Scholar] [CrossRef]
- Laribi, B.; Kouki, K.; M’Hamdi, M.; Bettaieb, T. Coriander (Coriandrum sativum L.) and its bioactive constituents. Fitoterapia 2015, 103, 9–26. [Google Scholar] [CrossRef]
- Bairwa, R.; Sodha, R.; Rajawat, B. Trachyspermum ammi. Pharmacogn. Rev. 2012, 6, 56. [Google Scholar] [CrossRef]
- Navarro-Rocha, J.; Andrés, M.F.; Díaz, C.E.; Burillo, J.; González-Coloma, A. Composition and biocidal properties of essential oil from pre-domesticated Spanish Satureja montana. Ind. Crops Prod. 2020, 145, 111958. [Google Scholar] [CrossRef]
- NIST Chemistry WebBook. NIST Standard Reference Database Number 69. Available online: https://webbook.nist.gov/chemistry (accessed on 20 January 2021).
- Arendrup, M.; Meletiadis, J.; Mouton, J.; Lagrou, K.; Hamal, P.; Guinea, J. Eucast Definitive Document E DEF. 9.3.1. Method for the Determination of Broth Dilution Minimum Inhibitory Concentrations of Antifungal Agents for Conidia Forming Moulds. London: European Committee on Antimicrobial Susceptibility Testing. Available online: https://www.eucast.org/ast_of_fungi/ (accessed on 4 June 2020).
- Markantonatou, A.-M.; Samaras, K.; Zachrou, E.; Vyzantiadis, T.-A. Comparison of four methods for the in vitro susceptibility testing of dermatophytes. Front. Microbiol. 2020, 11, 1593. [Google Scholar] [CrossRef]
- Verma, P. Methods for determining bactericidal activity and antimicrobial interactions: Synergy testing, time-kill curves, and population analysis. In Antimicrobial Susceptibility Testing Protocols; Schwalbe, R., Steele-Moore, L., Goodwin, A.C., Eds.; CRC Press: Boca Raton, FL, USA, 2007; pp. 275–298. [Google Scholar]
- Czerwińska, M.E.; Dudek, M.K.; Pawłowska, K.A.; Pruś, A.; Ziaja, M.; Granica, S. The influence of procyanidins isolated from small-leaved lime flowers (Tilia cordata Mill.) on human neutrophils. Fitoterapia 2018, 127, 115–122. [Google Scholar] [CrossRef]
- Trifan, A.; Skalicka-Woźniak, K.; Granica, S.; Czerwińska, M.E.; Kruk, A.; Marcourt, L.; Wolfender, J.-L.; Wolfram, E.; Esslinger, N.; Grubelnik, A. Symphytum officinale L.: Liquid-liquid chromatography isolation of caffeic acid oligomers and evaluation of their influence on pro-inflammatory cytokine release in LPS-stimulated neutrophils. J. Ethnopharmacol. 2020, 262, 113169. [Google Scholar] [CrossRef]
- Rønning, S.B.; Voldvik, V.; Bergum, S.K.; Aaby, K.; Borge, G.I.A. Ellagic acid and urolithin A modulate the immune response in LPS-stimulated U937 monocytic cells and THP-1 differentiated macrophages. Food Funct. 2020, 11, 7946–7959. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Gong, L.F.; Wu, Y.F.; Lin, Z.; Jiang, B.J.; Wu, L.; Yu, K.H. Urolithin A targets the PI3K/Akt/NF-κB pathways and prevents IL-1β-induced inflammatory response in human osteoarthritis: In vitro and in vivo studies. Food Funct. 2019, 10, 6135–6146. [Google Scholar] [CrossRef]
- Danielli, L.J.; Pippi, B.; Soares, K.D.; Duarte, J.A.; Maciel, A.J.; Machado, M.M.; Oliveira, L.F.S.; Bordignon, S.A.; Fuentefria, A.M.; Apel, M.A. Chemosensitization of filamentous fungi to antifungal agents using Nectandra Rol. ex Rottb. species essential oils. Ind. Crops Prod. 2017, 102, 7–15. [Google Scholar] [CrossRef]
- Danielli, L.J.; Pippi, B.; Duarte, J.A.; Maciel, A.J.; Lopes, W.; Machado, M.M.; Oliveira, L.F.S.; Vainstein, M.H.; Teixeira, M.L.; Bordignon, S.A. Antifungal mechanism of action of Schinus lentiscifolius Marchand essential oil and its synergistic effect in vitro with terbinafine and ciclopirox against dermatophytes. J. Pharm. Pharmacol. 2018, 70, 1216–1227. [Google Scholar] [CrossRef] [PubMed]
- Dias, N.; Dias, M.; Cavaleiro, C.; Sousa, M.; Lima, N.; Machado, M. Oxygenated monoterpenes-rich volatile oils as potential antifungal agents for dermatophytes. Nat. Prod. Res. 2017, 31, 460–464. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Lima, M.; de Medeiros, A.A.; Silva, K.S.; Cardoso, G.; de Oliveira Lima, E.; de Oliveira Pereira, F. Investigation of the antifungal potential of linalool against clinical isolates of fluconazole resistant Trichophyton rubrum. J. Mycol. Med. 2017, 27, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Ponte, H.A.S.; Lima, M.I.D.O.; Lima, E.D.O.; Pereira, F.D.O. Linalool modulates dermatophyte susceptibility to azole drugs. Med. Mycol. 2020, 58, 272–274. [Google Scholar] [CrossRef] [PubMed]
- Jain, N.; Sharma, M. Inhibitory effect of some selected essential oil terpenes on fungi causing superficial infection in human beings. J. Essent. Oil Bear. Plants 2020, 23, 862–869. [Google Scholar] [CrossRef]
- Obaid, A.J.; Al-Janabi, J.K.A.; Taj-Aldin, W.R. Chemical composition and bioactivity characteristics of Pimpinella anisum essential oil against Trichophyton rubrum. J. Glob. Pharma Technol. 2017, 8, 44–56. [Google Scholar]
- Pinto, E.; Gonçalves, M.-J.; Cavaleiro, C.; Salgueiro, L. Antifungal activity of Thapsia villosa essential oil against Candida, Cryptococcus, Malassezia, Aspergillus and dermatophyte species. Molecules 2017, 22, 1595. [Google Scholar] [CrossRef] [Green Version]
- Inouye, S.; Uchida, K.; Abe, S. Vapor activity of 72 essential oils against a Trichophyton mentagrophytes. J. Infect. Chemother. 2006, 12, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Shin, S.-W. Synergism in antifungal activity against Candida and Trichophyton species in combination with the essential oil of Coriandrum sativum L. and antibiotics. Nat. Prod. Sci. 2007, 13, 85–89. [Google Scholar]
- Gharib, R.; Auezova, L.; Charcosset, C.; Greige-Gerges, H. Effect of a series of essential oil molecules on DPPC membrane fluidity: A biophysical study. J. Iran. Chem. Soc. 2018, 15, 75–84. [Google Scholar] [CrossRef]
- Khan, M.S.A.; Ahmad, I.; Cameotra, S.S. Carum copticum and Thymus vulgaris oils inhibit virulence in Trichophyton rubrum and Aspergillus spp. Braz. J. Microbiol. 2014, 45, 523–531. [Google Scholar] [CrossRef] [Green Version]
- de Melo, J.O.; Bitencourt, T.A.; Fachin, A.L.; Cruz, E.M.O.; de Jesus, H.C.R.; Alves, P.B.; de Fátima Arrigoni-Blank, M.; de Castro Franca, S.; Beleboni, R.O.; Fernandes, R.P.M. Antidermatophytic and antileishmanial activities of essential oils from Lippia gracilis Schauer genotypes. Acta Trop. 2013, 128, 110–115. [Google Scholar] [CrossRef]
- Obaid, A.J.; Al-Janabi, J.; Taj-Aldin, W.R. Bioactivities of anethole, astragalin and cryptochlorogenic acid extracted from anise oil and Moringa oleifera on the keratinase gene expression of Trichophyton rubrum. J Pure Appl. Microbiol. 2020, 14, 615–626. [Google Scholar] [CrossRef] [Green Version]
- Flores, F.C.; Beck, R.C.; Da Silva, C.D.B. Essential oils for treatment for onychomycosis: A mini-review. Mycopathologia 2016, 181, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Vörös-Horváth, B.; Das, S.; Salem, A.; Nagy, S.; Böszörményi, A.; Kőszegi, T.; Pál, S.; Széchenyi, A. Formulation of tioconazole and Melaleuca alternifolia essential oil pickering emulsions for onychomycosis topical treatment. Molecules 2020, 25, 5544. [Google Scholar] [CrossRef] [PubMed]
- Christenson, J.K.; Peterson, G.M.; Naunton, M.; Bushell, M.; Kosari, S.; Baby, K.E.; Thomas, J. Challenges and opportunities in the management of onychomycosis. J. Fungi 2018, 4, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calderon, R.; Hay, R. Fungicidal activity of human neutrophils and monocytes on dermatophyte fungi, Trichophyton quinckeanum and Trichophyton rubrum. Immunology 1987, 61, 289. [Google Scholar] [PubMed]
- Van der Linden, M.; Meyaard, L. Fine-tuning neutrophil activation: Strategies and consequences. Immunol. Lett. 2016, 178, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Usui, M.L.; Lippman, S.I.; James, G.A.; Stewart, P.S.; Fleckman, P.; Olerud, J.E. Biofilms and inflammation in chronic wounds. Adv. Wound Care 2013, 2, 389–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romani, L. Immunity to fungal infections. Nat. Rev. Immunol. 2011, 11, 275–288. [Google Scholar] [CrossRef] [PubMed]
- Bahuguna, A.; Ramalingam, S.; Arumugam, A.; Natarajan, D.; Kim, M. Molecular and in silico evidences explain the anti-inflammatory effect of Trachyspermum ammi essential oil in lipopolysaccharide induced macrophages. Process Biochem. 2020, 96, 138–145. [Google Scholar] [CrossRef]
- Heidari, B.; Sajjadi, S.E.; Minaiyan, M. Effect of Coriandrum sativum hydroalcoholic extract and its essential oil on acetic acid-induced acute colitis in rats. Avicenna J. Phytomed. 2016, 6, 205. [Google Scholar]
- Ma, J.; Xu, H.; Wu, J.; Qu, C.; Sun, F.; Xu, S. Linalool inhibits cigarette smoke-induced lung inflammation by inhibiting NF-κB activation. Int. Immunopharmacol. 2015, 29, 708–713. [Google Scholar] [CrossRef] [PubMed]
- Huo, M.; Cui, X.; Xue, J.; Chi, G.; Gao, R.; deng, X.; Guan, S.; Wei, J.; Soromou, L.W.; Feng, H. Anti-inflammatory effects of linalool in RAW 264.7 macrophages and lipopolysaccharide-induced lung injury model. J. Surg. Res. 2013, 180, e47–e54. [Google Scholar] [CrossRef] [PubMed]
- Keshavarz, A.; Minaiyan, M.; Ghannadi, A.; Mahzouni, P. Effects of Carum carvi L.(caraway) extract and essential oil on TNBS-induced colitis in rats. Res. Pharm. Sci. 2013, 8, 1. [Google Scholar] [PubMed]
- Dadkhah, A.; Fatemi, F. Heart and kidney oxidative stress status in septic rats treated with caraway extracts. Pharm. Biol. 2011, 49, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Du, J. Anti-inflammatory and protective effects of D-carvone on lipopolysaccharide (LPS)-induced acute lung injury in mice. J. King Saud Univ. Sci. 2020, 32, 1592–1596. [Google Scholar] [CrossRef]
- Vieira, A.J.; Beserra, F.P.; Souza, M.; Totti, B.; Rozza, A. Limonene: Aroma of innovation in health and disease. Chem. Biol. Interact. 2018, 283, 97–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrade, L.N.; de Sousa, D.P. A review on anti-inflammatory activity of monoterpenes. Molecules 2013, 18, 1227–1254. [Google Scholar]
- Iannarelli, R.; Marinelli, O.; Morelli, M.B.; Santoni, G.; Amantini, C.; Nabissi, M.; Maggi, F. Aniseed (Pimpinella anisum L.) essential oil reduces pro-inflammatory cytokines and stimulates mucus secretion in primary airway bronchial and tracheal epithelial cell lines. Ind. Crops Prod. 2018, 114, 81–86. [Google Scholar] [CrossRef]
- Kim, K.Y.; Lee, H.S.; Seol, G.H. Anti-inflammatory effects of trans-anethole in a mouse model of chronic obstructive pulmonary disease. Biomed. Pharmacother. 2017, 91, 925–930. [Google Scholar] [CrossRef]
- Sebaaly, C.; Trifan, A.; Sieniawska, E.; Greige-Gerges, H. Chitosan-coating effect on the characteristics of liposomes: A focus on bioactive compounds and essential oils: A review. Processes 2021, 9, 445. [Google Scholar] [CrossRef]

| RI * | Compound | Ajowan (%) | Coriander (%) | Caraway (%) | Anise (%) |
|---|---|---|---|---|---|
| Monoterpene hydrocarbons | |||||
| 928 | α-Thujene | 0.54 | - | - | - |
| 931 | α-Pinene | 0.27 | 8.13 | 0.03 | - |
| 943 | Camphene | - | 1.02 | - | - |
| 960 | β-Phellandrene | - | - | 0.06 | - |
| 965 | Sabinene | - | 0.40 | - | - |
| 970 | β-Pinene | 2.48 | 0.72 | 0.02 | - |
| 979 | Myrcene | 0.62 | 1.80 | 0.41 | - |
| 1016 | m-Cymene | 21.09 | 0.92 | - | - |
| 1023 | β-Thujene | 0.18 | - | - | - |
| 1033 | Limonene | - | 2.36 | 42.74 | - |
| 1047 | γ-Terpinene | 23.18 | 5.77 | - | - |
| 1088 | α-Terpinolene | - | 0.57 | - | - |
| Oxygenated monoterpenes | |||||
| 1104 | Linalool | - | 67.87 | - | 0.32 |
| 1150 | Terpinen-4-ol | 0.17 | - | - | - |
| 1157 | Camphor | - | 3.82 | - | - |
| 1230 | Geraniol | - | 1.67 | - | - |
| 1238 | Carvone | - | - | 55.40 | 0.32 |
| 1242 | Perillaldehyde | - | - | 0.24 | - |
| 1275 | Thymol | 49.32 | - | - | - |
| 1278 | Carvacrol | 0.24 | - | - | - |
| 1323 | Methyl geranate | - | - | 0.12 | - |
| 1360 | Geranyl acetate | - | 3.71 | - | - |
| Sesquiterpene hydrocarbons | |||||
| 1480 | Germacrene D | - | - | - | 2.95 |
| 1490 | Zingiberene | - | - | - | 0.29 |
| 1499 | β-Himachalene | - | - | - | 0.14 |
| 1505 | β-Bisabolene | - | - | - | 0.26 |
| 1542 | σ-Himachalene | - | - | - | 0.19 |
| Oxygenated sesquiterpenes | |||||
| 1601 | Spathulenol | - | - | - | 0.09 |
| Phenylpropanoids | |||||
| 1180 | Estragole | - | - | - | 1.94 |
| 1249 | p-Anisaldehyde | - | - | - | 0.78 |
| 1280 | Anethole | - | - | - | 90.01 |
| 1831 | Isoeugenyl acetate | - | - | - | 1.19 |
| Monoterpene hydrocarbons | 48.36 | 21.69 | 43.26 | - | |
| Oxygenated monoterpenes | 49.73 | 77.07 | 55.76 | 0.64 | |
| Sesquiterpene hydrocarbons | - | - | - | 3.83 | |
| Oxygenated sesquiterpenes | - | - | - | 0.09 | |
| Phenylpropanoids | - | - | - | 93.92 | |
| Total | 98.09 | 98.76 | 99.02 | 98.48 | |
| Sample/Positive Control | Trichophyton rubrum ATCC 28188 | Trichophyton mentagrophytes ATCC 9533 | ||
|---|---|---|---|---|
| MIC | MFC | MIC | MFC | |
| Ajowan essential oil | 256 | 512 | 256 | 512 |
| Coriander essential oil | 512 | 1024 | 512 | 1024 |
| Caraway essential oil | 512 | 512 | 512 | 512 |
| Anise essential oil | 1024 | 2048 | 1024 | 2048 |
| Thymol | 1024 | 1024 | 1024 | 1024 |
| Linalool | 1024 | 1024 | 1024 | 2048 |
| Carvone | 1024 | 2048 | 1024 | 2048 |
| Limonene | 2048 | 2048 | 2048 | 2048 |
| Anethole | 2048 | 2048 | 2048 | 2048 |
| Terbinafine | 0.031 | 0.031 | 0.031 | 0.031 |
| Combination | Trichophyton rubrum ATCC 28188 | INT | Trichophyton mentagrophytes ATCC 9533 | INT | ||||
|---|---|---|---|---|---|---|---|---|
| MIC *in combination (mg/L) | FIC | FICI | MIC *in combination (mg/L) | FIC | FICI | |||
| Ajowan essential oil | 16 | 0.06 | 0.31 | S | 16 | 0.06 | 0.56 | Ad |
| Terbinafine | 0.007 | 0.25 | 0.015 | 0.50 | ||||
| Coriander essential oil | 8 | 0.01 | 0.26 | S | 32 | 0.06 | 0.56 | Ad |
| Terbinafine | 0.007 | 0.25 | 0.015 | 0.50 | ||||
| Caraway essential oil | 16 | 0.03 | 0.28 | S | 64 | 0.12 | 0.62 | Ad |
| Terbinafine | 0.007 | 0.25 | 0.015 | 0.50 | ||||
| Anise essential oil | 16 | 0.01 | 0.26 | S | 128 | 0.12 | 0.62 | Ad |
| Terbinafine | 0.007 | 0.25 | 0.015 | 0.50 | ||||
| Thymol | 64 | 0.06 | 0.56 | Ad | 128 | 0.12 | 0.62 | Ad |
| Terbinafine | 0.015 | 0.5 | 0.015 | 0.5 | ||||
| Linalool | 32 | 0.03 | 0.53 | Ad | 256 | 0.25 | 0.75 | Ad |
| Terbinafine | 0.015 | 0.5 | 0.015 | 0.5 | ||||
| Carvone | 32 | 0.03 | 0.53 | Ad | 256 | 0.25 | 0.75 | Ad |
| Terbinafine | 0.015 | 0.5 | 0.015 | 0.5 | ||||
| Limonene | 64 | 0.03 | 0.53 | Ad | 256 | 0.12 | 0.62 | Ad |
| Terbinafine | 0.015 | 0.5 | 0.015 | 0.5 | ||||
| Anethole | 128 | 0.06 | 0.56 | Ad | 512 | 0.25 | 0.75 | Ad |
| Terbinafine | 0.015 | 0.5 | 0.015 | 0.5 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trifan, A.; Luca, S.V.; Bostănaru, A.-C.; Brebu, M.; Jităreanu, A.; Cristina, R.-T.; Skalicka-Woźniak, K.; Granica, S.; Czerwińska, M.E.; Kruk, A.; et al. Apiaceae Essential Oils: Boosters of Terbinafine Activity against Dermatophytes and Potent Anti-Inflammatory Effectors. Plants 2021, 10, 2378. https://doi.org/10.3390/plants10112378
Trifan A, Luca SV, Bostănaru A-C, Brebu M, Jităreanu A, Cristina R-T, Skalicka-Woźniak K, Granica S, Czerwińska ME, Kruk A, et al. Apiaceae Essential Oils: Boosters of Terbinafine Activity against Dermatophytes and Potent Anti-Inflammatory Effectors. Plants. 2021; 10(11):2378. https://doi.org/10.3390/plants10112378
Chicago/Turabian StyleTrifan, Adriana, Simon Vlad Luca, Andra-Cristina Bostănaru, Mihai Brebu, Alexandra Jităreanu, Romeo-Teodor Cristina, Krystyna Skalicka-Woźniak, Sebastian Granica, Monika E. Czerwińska, Aleksandra Kruk, and et al. 2021. "Apiaceae Essential Oils: Boosters of Terbinafine Activity against Dermatophytes and Potent Anti-Inflammatory Effectors" Plants 10, no. 11: 2378. https://doi.org/10.3390/plants10112378
APA StyleTrifan, A., Luca, S. V., Bostănaru, A.-C., Brebu, M., Jităreanu, A., Cristina, R.-T., Skalicka-Woźniak, K., Granica, S., Czerwińska, M. E., Kruk, A., Greige-Gerges, H., Sieniawska, E., & Mareș, M. (2021). Apiaceae Essential Oils: Boosters of Terbinafine Activity against Dermatophytes and Potent Anti-Inflammatory Effectors. Plants, 10(11), 2378. https://doi.org/10.3390/plants10112378













