Is Your Moss Alive during Active Biomonitoring Study?
Abstract
:1. Introduction
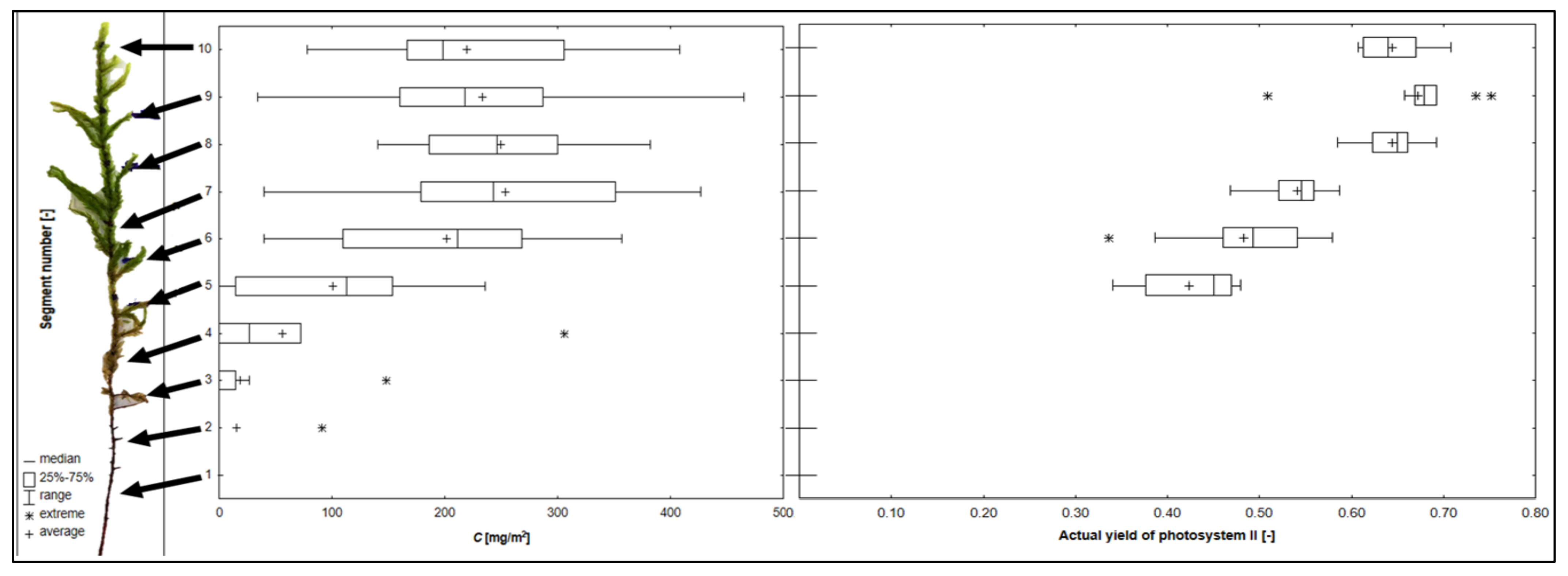
2. Results
3. Discussion
4. Materials and Methods
4.1. Material
4.2. Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Holt, E.A. Bioindicators: Using organisms to measure environmental impacts. Nat. Educ. Knowl. 2010, 3, 8. [Google Scholar]
- Plášek, V.; Nowak, A.; Nobis, M.; Kusza, G.; Kochanowska, K. Effect of 30 years of road traffic abandonment on epiphytic moss diversity. Environ. Monit. Assess. 2014, 186, 8943–8959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahapatra, B.; Dhal, N.K.; Dash, A.K.; Panda, B.P.; Panigrahi, K.C.S.; Pradhan, A. Perspective of mitigating atmospheric heavy metal pollution: Using mosses as biomonitoring and indicator organism. Environ. Sci. Pollut. Res. 2019, 26, 29620–29638. [Google Scholar] [CrossRef]
- Rajfur, M.; Świsłowski, P.; Nowainski, F.; Śmiechowicz, B. Mosses as Biomonitor of Air Pollution with Analytes Originating from Tobacco Smoke. Chem. Didact. Ecol. Metrol. 2018, 23, 127–136. [Google Scholar] [CrossRef] [Green Version]
- De Agostini, A.; Cortis, P.; Cogoni, A. Monitoring of Air Pollution by Moss Bags around an Oil Refinery: A Critical Evaluation over 16 Years. Atmosphere 2020, 11, 272. [Google Scholar] [CrossRef] [Green Version]
- Markert, B. From biomonitoring to integrated observation of the environment—The multi-markered bioindication concept. Ecol. Chem. Eng. S 2008, 15, 315–333. [Google Scholar]
- Ares, A.; Aboal, J.; Carballeira, A.; Giordano, S.; Adamo, P.; Fernández, J. Moss bag biomonitoring: A methodological review. Sci. Total Environ. 2012, 432, 143–158. [Google Scholar] [CrossRef] [PubMed]
- Świsłowski, P.; Banach, E.; Rajfur, M. Passive biomonitoring of influence of the communication traffic on deposition the pollution near the motorway. Ecol. Chem. Eng. A 2019, 26, 113–125. [Google Scholar] [CrossRef]
- Konopka, Z.; Świsłowski, P.; Rajfur, M. Biomonitoring of Atmospheric Aerosol with the use of Apis mellifera and Pleurozium schreberi. Chem. Didact. Ecol. Metrol. 2019, 24, 107–116. [Google Scholar] [CrossRef] [Green Version]
- Smodiš, B.; Parr, R.M. Biomonitoring of air pollution as exemplified by recent IAEA programs. Biol. Trace Elem. Res. 1999, 71–72, 257–266. [Google Scholar] [CrossRef]
- Aničić, M.; Tasić, M.; Frontasyeva, M.V.; Tomašević, M.; Rajšić, S.; Strelkova, L.P.; Popović, A.; Steinnes, E. Active biomonitoring with wet and dry moss: A case study in an urban area. Environ. Chem. Lett. 2009, 7, 55–60. [Google Scholar]
- Fernández, J.A.; Boquete, M.T.; Carballeira, A.; Aboal, J.R. A critical review of protocols for moss biomonitoring of atmospheric deposition: Sampling and sample preparation. Sci. Total Environ. 2015, 517, 132–150. [Google Scholar] [CrossRef] [PubMed]
- Fernández, J.A.; Aboal, J.R.; Carballeira, A. Testing differences in methods of preparing moss samples. Effect of washing on Pseudoscleropodium purum. Environ. Monit. Assess. 2010, 163, 669–684. [Google Scholar] [CrossRef] [PubMed]
- Świsłowski, P.; Kosior, G.; Rajfur, M. The influence of preparation methodology on the concentrations of heavy metals in Pleurozium schreberi moss samples prior to use in active biomonitoring studies. Environ. Sci. Pollut. Res. 2021, 28, 10068–10076. [Google Scholar] [CrossRef]
- Vukovic, G.; Uroševic, M.A. Is moss bag biomonitoring suitable method for assessment of intricate urban air pollution? In Biomonitoring of Air Pollution Using Mosses and Lichens: A Passive and Active Approach-State of the Art Research and Perspectives; University of Belgrade: Belgrade, Serbia, 2016; ISBN 9781536102123. [Google Scholar]
- Ciesielczuk, T.; Olszowski, T.; Prokop, M.; Kłos, A. Application of mosses to identification of emission sources of polycyclic aromatic hydrocarbons. Ecol. Chem. Eng. S 2012, 19, 585–595. [Google Scholar] [CrossRef] [Green Version]
- Saitanis, C.J.; Frontasyeva, M.V.; Steinnes, E.; Palmer, M.W.; Ostrovnaya, T.M.; Gundorina, S.F. Spatiotemporal distribution of airborne elements monitored with the moss bags technique in the Greater Thriasion Plain, Attica, Greece. Environ. Monit. Assess. 2013, 185, 955–968. [Google Scholar] [CrossRef] [PubMed]
- Adamo, P.; Crisafulli, P.; Giordano, S.; Minganti, V.; Modenesi, P.; Monaci, F.; Pittao, E.; Tretiach, M.; Bargagli, R. Lichen and moss bags as monitoring devices in urban areas. Part II: Trace element content in living and dead biomonitors and comparison with synthetic materials. Environ. Pollut. 2007, 146, 392–399. [Google Scholar] [CrossRef]
- Datt, B. A New Reflectance Index for Remote Sensing of Chlorophyll Content in Higher Plants: Tests using Eucalyptus Leaves. J. Plant Physiol. 1999, 154, 30–36. [Google Scholar] [CrossRef]
- Krupa, J. Anatomical structure of moss leaves and their photosynthetic activity. Acta Soc. Bot. Pol. 1984, 53, 43–51. [Google Scholar] [CrossRef] [Green Version]
- Fačkovcová, Z.; Vannini, A.; Monaci, F.; Grattacaso, M.; Paoli, L.; Loppi, S. Effects of wood distillate (pyroligneous acid) on sensitive bioindicators (lichen and moss). Ecotoxicol. Environ. Saf. 2020, 204, 111117. [Google Scholar] [CrossRef]
- Gaberščik, A.; Martinčič, A. Seasonal Dynamics of Net Photosynthesis and Productivity of Sphagnum papillosum. Lindbergia 1987, 13, 105–110. [Google Scholar]
- Tuba, Z.; Slack, N.G.; Stark, L.R.; Proctor, M.C.F.; Tuba, Z.; Slack, N.G.; Stark, L.R. Climatic responses and limits of Bryophytes: Comparisons and contrasts with vascular plants. In Bryophyte Ecology and Climate Change; Cambridge University Press: Cambridge, UK, 2012. [Google Scholar]
- Hyyryläinen, A.; Rautio, P.; Turunen, M.; Huttunen, S. Seasonal and inter-annual variation in the chlorophyll content of three co-existing Sphagnum species exceeds the effect of solar UV reduction in a subarctic peatland. SpringerPlus 2015, 4, 478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shakya, K.; Chettri, M.K.; Sawidis, T. Impact of Heavy Metals (Copper, Zinc, and Lead) on the Chlorophyll Content of Some Mosses. Arch. Environ. Contam. Toxicol. 2008, 54, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, H.; Itoh, K. Relationship between metal and pigment concentrations in the Fe-hyperaccumulator moss Scopelophila ligulata. J. Plant Res. 2017, 130, 135–141. [Google Scholar] [CrossRef]
- Verdoorn, F. Manual of Bryology; Martinus Nijhoff Publishers: The Hague, The Netherlands, 1932. [Google Scholar]
- Capozzi, F.; Sorrentino, M.C.; Di Palma, A.; Mele, F.; Arena, C.; Adamo, P.; Spagnuolo, V.; Giordano, S. Implication of vitality, seasonality and specific leaf area on PAH uptake in moss and lichen transplanted in bags. Ecol. Indic. 2020, 108, 105727. [Google Scholar] [CrossRef]
- González, A.; Pokrovsky, O. Metal adsorption on mosses: Toward a universal adsorption model. J. Colloid Interface Sci. 2014, 415, 169–178. [Google Scholar] [CrossRef] [Green Version]
- Kłos, A.; Gordzielik, E.; Jóźwiak, M.A.; Rajfur, M. Sorption of cadmium and zinc in selected species of epigeic mosses. Bull. Environ. Contam. Toxicol. 2014, 92, 323–328. [Google Scholar] [CrossRef] [Green Version]
- Bates, J.W. The relationship between physiological vitality and age in shoot segments of Pleurozium schreberi (Brid.) Mitt. J. Bryol. 1979, 10, 339–351. [Google Scholar] [CrossRef]
- Harmens, H.; Ilyin, I.; Mills, G.; Aboal, J.R.; Alber, R.; Blum, O.; Coşkun, M.; De Temmerman, L.; Fernández, J.A.; Figueira, R.; et al. Country-specific correlations across Europe between modelled atmospheric cadmium and lead deposition and concentrations in mosses. Environ. Pollut. 2012, 166, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Jägerbrand, A.K. Dead or alive? Testing the use of C:N ratios and chlorophyll fluorescence in vertical shoot profiles to determine depth of vitality and point of senescence in populations of bryophytes. Lindbergia 2015, 3, 4–13. [Google Scholar] [CrossRef]
- Parry, C.; Blonquist, J.M.; Bugbee, B. In situ measurement of leaf chlorophyll concentration: Analysis of the optical/absolute relationship. Plant Cell Environ. 2014, 37, 2508–2520. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and Carotenoids: Measurement and Characterization by UV-VIS Spectroscopy. Handb. Food Anal. Chem. 2005, 2, 171–178. [Google Scholar] [CrossRef]
- Nakajima, H.; Itoh, K.; Otake, H.; Fujimoto, K. Photoabsorption Study of Pigments in Mosses: Scopelophila ligulataHas an Abnormally High Formation Rate of Pheophytin. Chem. Lett. 2010, 39, 284–285. [Google Scholar] [CrossRef]
- Raven, J.A.; Edwards, D. Photosynthesis in Bryophytes and Early Land Plants. Diversif. Evol. Environ. 2014, 37, 29–58. [Google Scholar]
- Lichtenthaler, H.K.; Buschmann, C.; Rinderle, U.; Schmuck, G. Application of chlorophyll fluorescence in ecophysiology. Radiat. Environ. Biophys. 1986, 25, 297–308. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, X.; Yin, B.; Downing, A. Sensitivity of the xerophytic moss Syntrichia caninervis to prolonged simulated nitrogen deposition. Ann. Bot. 2016, 117, 1153–1161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zotz, G.; Kahler, H. A moss “canopy”-Small-scale differences in microclimate and physiological traits in Tortula ruralis. Flora Morphol. Distrib. Funct. Ecol. Plants 2007, 202, 661–666. [Google Scholar]
- Chen, Y.; Yuan, M.; Zhang, H.; Zeng, X.; Liu, H.; Du, X. Influences of cu and cr stress on antioxidant system and chlorophyll fluorescence in terrestrial moss taxiphyllum taxirameum. Fresenius Environ. Bull. 2015, 24, 2211–2219. [Google Scholar]
- Chen, Y.-E.; Cui, J.-M.; Yang, J.-C.; Zhang, Z.-W.; Yuan, M.; Song, C.; Yang, H.; Liu, H.-M.; Wang, C.-Q.; Zhang, H.-Y.; et al. Biomonitoring heavy metal contaminations by moss visible parameters. J. Hazard. Mater. 2015, 296, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Dołęgowska, S.; Migaszewski, Z.M. A first insight into the estimation of uncertainty associated with storage and physical preparation of forest moss samples for trace element analysis. Chemosphere 2020, 241, 125040. [Google Scholar] [CrossRef] [PubMed]
- Iodice, P.; Adamo, P.; Capozzi, F.; Di Palma, A.; Senatore, A.; Spagnuolo, V.; Giordano, S. Air pollution monitoring using emission inventories combined with the moss bag approach. Sci. Total Environ. 2016, 541, 1410–1419. [Google Scholar] [CrossRef] [PubMed]
- Arndt, J.; Planer-Friedrich, B. Moss bag monitoring as screening technique to estimate the relevance of methylated arsine emission. Sci. Total Environ. 2018, 610, 1590–1594. [Google Scholar] [CrossRef]
- Raskin, I.; Ensley, B.D.; Burt, D. Phytoremediation of Toxic Metals: Using Plants to Clean the Environment. J. Plant Biotechnol. 1999, 1, 304. [Google Scholar]
- Nagajyoti, P.C.; Lee, K.D.; Sreekanth, T.V.M. Heavy metals, occurrence and toxicity for plants: A review. Environ. Chem. Lett. 2010, 8, 199–216. [Google Scholar] [CrossRef]
- Rumyantsev, I.V.; Dunaev, A.M.; Frontasyeva, M.V.; Ostrovnaya, T.M. Interspecies comparison of elemental content in moss From Ivanovo region determined by NAA and AAS. In Proceedings of the XXI International Seminar on Interaction of Neutrons with Nuclei (Fundamental Interactions & Neutrons, Nuclear Structure, Ultracold Neutrons, Related Topics), Alushta, Ukraine, 20–25 May 2013. [Google Scholar]
- Kłos, A. Mchy W Biomonitoringu Środowiska; PWN: Warsaw, Poland, 2017; ISBN 9788301194345. [Google Scholar]
- Jozwiak, M.A.; Jozwiak, M. Bioindication as challenge in modern environmental protection. Ecol. Chem. Eng. S 2015, 21, 577–591. [Google Scholar]
- Zhuo, L.; Liang, Y.; Yang, H.; Li, X.; Zhang, Y.; Zhang, Y.; Guan, K.; Zhang, D. Thermal tolerance of dried shoots of the moss Bryum argenteum. J. Therm. Biol. 2020, 89, 102469. [Google Scholar] [CrossRef] [PubMed]
- Culicov, O.A.; Yurukova, L. Comparison of element accumulation of different moss- and lichen-bags, exposed in the city of Sofia (Bulgaria). J. Atmos. Chem. 2006, 55, 1–12. [Google Scholar] [CrossRef]
- Krzesłowska, M.; Lenartowska, M.; Mellerowicz, E.J.; Samardakiewicz, S.; Woźny, A. Pectinous cell wall thickenings for-mation—A response of moss protonemata cells to lead. Environ. Exp. Bot. 2009, 65, 119–131. [Google Scholar] [CrossRef]
- Stanković, J.D.; Sabovljević, A.D.; Sabovljević, M.S. Bryophytes and heavy metals: A review. Acta Bot. Croat. 2018, 77, 109–118. [Google Scholar] [CrossRef]
- Giordano, S.; Adamo, P.; Monaci, F.; Pittao, E.; Tretiach, M.; Bargagli, R. Bags with oven-dried moss for the active monitoring of airborne trace elements in urban areas. Environ. Pollut. 2009, 157, 2798–2805. [Google Scholar] [CrossRef] [PubMed]
- Cesa, M.; Bizzotto, A.; Ferraro, C.; Fumagalli, F.; Nimis, P.L. Oven-dried mosses as tools for trace element detection in polluted waters: A preliminary study under laboratory conditions. Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2011, 145, 832–840. [Google Scholar] [CrossRef]
- Debén, S.; Fernández, J.; Carballeira, A.; Aboal, J. Using devitalized moss for active biomonitoring of water pollution. Environ. Pollut. 2016, 210, 315–322. [Google Scholar] [CrossRef]
- Świsłowski, P.; Rajfur, M.; Wacławek, M. Influence of Heavy Metal Concentration on Chlorophyll Content in Pleurozium schreberi Mosses. Ecol. Chem. Eng. S 2020, 27, 591–601. [Google Scholar] [CrossRef]
- Aničić Urošević, M.; Krmar, M.; Radnović, D.; Jovanović, G.; Jakšić, T.; Vasić, P.; Popović, A. The use of moss as an indicator of rare earth element deposition over large area. Ecol. Indic. 2020, 109, 105828. [Google Scholar]
- Rojano, S.I.; Elustondo, D.; Ederra, A.; Lasheras, E.; Santamaría, C.; Santamaría, J.M. Pleurochaete squarrosa (Brid.) Lindb. as an alternative moss species for biomonitoring surveys of heavy metal, nitrogen deposition and δ15N signatures in a Mediterranean area. Ecol. Indic. 2016, 60, 1221–1228. [Google Scholar] [CrossRef]
- Tremper, A.H.; Agneta, M.; Burton, S.; Higgs, D.E.B. Field and Laboratory Exposures of Two Moss Species to Low Level Metal Pollution. J. Atmos. Chem. 2004, 49, 111–120. [Google Scholar] [CrossRef]
- Makholm, M.M.; Mladenoff, D.J. Efficacy of a Biomonitoring (Moss Bag) Technique for Determining Element Deposition Trends on a Mid-Range (375 Km) Scale. Environ. Monit. Assess. 2005, 104, 1–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tretiach, M.; Adamo, P.; Bargagli, R.; Baruffo, L.; Carletti, L.; Crisafulli, P.; Giordano, S.; Modenesi, P.; Orlando, S.; Pittao, E. Lichen and moss bags as monitoring devices in urban areas. Part I: Influence of exposure on sample vitality. Environ. Pollut. 2007, 146, 380–391. [Google Scholar] [CrossRef]
- Spagnuolo, V.; Zampella, M.; Giordano, S.; Adamo, P. Cytological stress and element uptake in moss and lichen exposed in bags in urban area. Ecotoxicol. Environ. Saf. 2011, 74, 1434–1443. [Google Scholar] [CrossRef]
- Bartels, D.; Lüttge, U.; Beck, E. Introduction. In Plant Desiccation Tolerance; Springer: Berlin/Heidelberg, Germany, 2011; pp. 3–8. [Google Scholar]
- Proctor, M.C.F.; Ligrone, R.; Duckett, J.G. Erratum: Desiccation tolerance in the moss Polytrichum formosum: Physiological and fine-structural changes during desiccation and recovery. Ann. Bot. 2007, 99, 1243. [Google Scholar] [CrossRef] [Green Version]
- Długosz-Lisiecka, M. Kinetics of 210Po accumulation in moss body profiles. Environ. Sci. Pollut. Res. 2017, 24, 20254–20260. [Google Scholar] [CrossRef] [Green Version]
- Kłos, A.; Rajfur, M.; Wacławek, M.; Wacławek, W. 137Cs transfer from local particulate matter to lichens and mosses. Nukleonika 2009, 54, 297–303. [Google Scholar]
- Jönsson, K.I.; Järemo, J. A model on the evolution of cryptobiosis. Ann. Zool. Fenn. 2003, 40, 331–340. [Google Scholar]
- Cannone, N.; Corinti, T.; Malfasi, F.; Gerola, P.; Vianelli, A.; Vanetti, I.; Zaccara, S.; Convey, P.; Guglielmin, M. Moss survival through in situ cryptobiosis after six centuries of glacier burial. Sci. Rep. 2017, 7, 4438. [Google Scholar] [CrossRef]
- ICP Vegetation. Heavy Metals, Nitrogen and Pops in European Mosses; 2020 Survey. 2020. Available online: https://icpvegetation.ceh.ac.uk/sites/default/files/ICP%20Vegetation%20moss%20monitoring%20manual%202020.pdf (accessed on 5 November 2021).
- Hájek, T.; Tuittila, E.-S.; Ilomets, M.; Laiho, R. Light responses of mire mosses—A key to survival after water-level drawdown? Oikos 2009, 118, 240–250. [Google Scholar] [CrossRef]
- Šraj Kržič, N.; Gaberščik, A. Photochemical efficiency of amphibious plants in an intermittent lake. Aquat. Bot. 2005, 83, 281–288. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef] [Green Version]
- Thermo Fisher Scientific Inc. iCE 3000 Series AA Spectrometers Operator’s Manual; Thermo Fisher Scientific Inc.: Waltham, MA, USA, 2011; Available online: http://photos.labwrench.com/equipmentManuals/9291-6306.pdf (accessed on 5 November 2021).
- Zinicovscaia, I.; Urošević, M.A.; Vergel, K.; Vieru, E.; Frontasyeva, M.; Povar, I.; Duca, G. Active Moss Biomonitoring of Trace Elements Air Pollution in Chisinau, Republic of Moldova. Ecol. Chem. Eng. S 2018, 25, 361–372. [Google Scholar] [CrossRef] [Green Version]
- Boquete, M.; Aboal, J.; Carballeira, A.; Fernández, J. Do mosses exist outside of Europe? A biomonitoring reflection. Sci. Total. Environ. 2017, 593–594, 567–570. [Google Scholar] [CrossRef] [PubMed]
- Szczepaniak, K.; Astel, A.; Simeonov, V.; Tsakovski, S.; Biziuk, M.; Bode, P.; Przyjazny, A. Comparison of dry and living Sphagnum palustre moss samples in determining their biocumulative capability as biomonitoring tools. J. Environ. Sci. Health Part A Toxic/Hazard. Subst. Environ. Eng. 2007, 42, 1101–1115. [Google Scholar] [CrossRef] [PubMed]


| Forest | Dry | Wet | ||
|---|---|---|---|---|
| Chl-a | min-max | 9.56–17.4 | 10.4–21.0 | 8.08–11.3 |
| average | 12.7 | 16.1 | 10.1 | |
| median | 11.9 | 16.4 | 10.5 | |
| SD | 3.38 | 3.57 | 1.38 | |
| Chl-b | min-max | 4.51–7.39 | 4.30–13.9 | 4.42–6.35 |
| average | 5.49 | 8.16 | 5.25 | |
| median | 5.03 | 7.55 | 5.11 | |
| SD | 1.33 | 3.25 | 0.977 | |
| Yield | min-max | 0.553–0.706 | 0.371–0.663 | 0.281–0.627 |
| average | 0.647 | 0.532 | 0.503 | |
| median | 0.658 | 0.524 | 0.573 | |
| SD | 0.044 | 0.116 | 0.149 | |
| Forest | Dry | Wet | ||
|---|---|---|---|---|
| Chl-a | forest | - | 0.175 | 0.196 |
| dry | 0.175 | - | <0.05 | |
| wet | 0.196 | <0.05 | - | |
| Chl-b | forest | - | 0.163 | 0.780 |
| dry | 0.163 | - | 0.125 | |
| wet | 0.780 | 0.125 | - | |
| ”0” | 1w | 4w | 8w | 12w | |
|---|---|---|---|---|---|
| Chl-a | 10.8 | 8.69 | 8.42 | 8.92 | 5.86 |
| Chl-b | 6.52 | 4.12 | 4.26 | 5.00 | 4.33 |
| Yield II | 0.532 | 0.332 | 0.350 | 0.354 | 0.240 |
| Metal | IDL | IQL |
|---|---|---|
| Ni | 0.0043 | 0.050 |
| Cu | 0.0045 | 0.033 |
| Zn | 0.0033 | 0.010 |
| Cd | 0.0028 | 0.013 |
| Pb | 0.0130 | 0.070 |
| Metal | BCR-482 lichen | AAS (n = 5) | Dev. ** | ||
|---|---|---|---|---|---|
| Concentration | Measurement Uncertainty | Average | ±SD * of the Concentrations | ||
| [mg/kg d.m.] | [%] | ||||
| Ni | 2.47 | 0.07 | 2.16 | 0.32 | −13.0 |
| Cu | 7.03 | 0.19 | 6.63 | 0.17 | −5.70 |
| Zn | 101 | 2.20 | 95.1 | 2.30 | −5.50 |
| Cd | 0.56 | 0.02 | 0.53 | 0.03 | −5.30 |
| Pb | 40.9 | 1.40 | 38.2 | 1.00 | −6.60 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Świsłowski, P.; Nowak, A.; Rajfur, M. Is Your Moss Alive during Active Biomonitoring Study? Plants 2021, 10, 2389. https://doi.org/10.3390/plants10112389
Świsłowski P, Nowak A, Rajfur M. Is Your Moss Alive during Active Biomonitoring Study? Plants. 2021; 10(11):2389. https://doi.org/10.3390/plants10112389
Chicago/Turabian StyleŚwisłowski, Paweł, Arkadiusz Nowak, and Małgorzata Rajfur. 2021. "Is Your Moss Alive during Active Biomonitoring Study?" Plants 10, no. 11: 2389. https://doi.org/10.3390/plants10112389








