Origin and Function of Structural Diversity in the Plant Specialized Metabolome
Abstract
:1. Introduction
2. The Four Major Specialized Metabolome Pathways
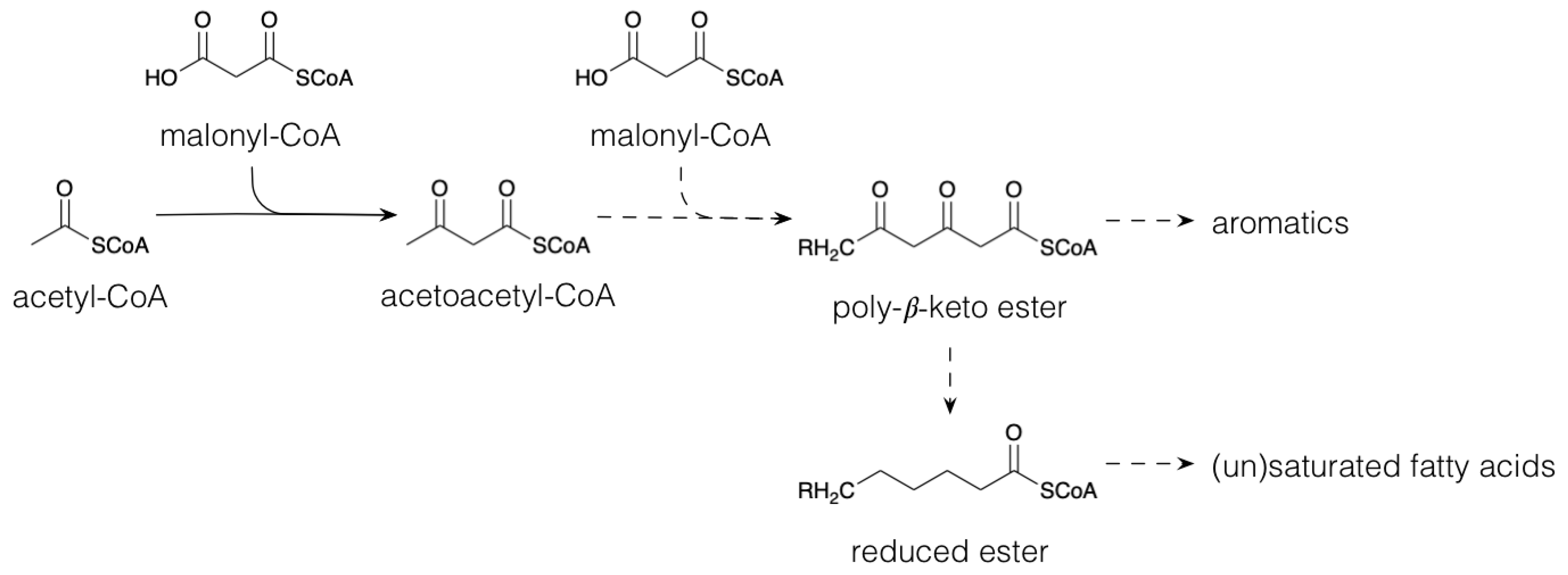
2.1. Polyketides and Fatty Acid Derivatives
2.2. Terpenoids
2.3. Alkaloids
2.4. Phenolics
3. Structure-Antioxidant Activity Relationships of Flavonoids
4. Decoration and Conjugation in Specialized Metabolism
4.1. Diversity of Decorations
4.2. High Molecular Weight Conjugates
4.3. Function of Decorations
4.4. Decoration Pathways
5. Plasticity and Promiscuity of the Plant Specialized Metabolism
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Nguyen, T.-K.; Marcelo, P.; Gontier, E.; Dauwe, R. Metabolic markers for the yield of lipophilic indole alkaloids in dried woad leaves (Isatis tinctoria L.). Phytochemistry 2019, 163, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.-K.; Jamali, A.; Grand, E.; Morreel, K.; Marcelo, P.; Gontier, E.; Dauwe, R. Phenylpropanoid profiling reveals a class of hydroxycinnamoyl glucaric acid conjugates in Isatis tinctoria leaves. Phytochemistry 2017, 144, 127–140. [Google Scholar] [CrossRef] [PubMed]
- Morreel, K.; Saeys, Y.; Dima, O.; Lu, F.; Van de Peer, Y.; Vanholme, R.; Ralph, J.; Vanholme, B.; Boerjan, W. Systematic Structural Characterization of Metabolites in Arabidopsis via Candidate Substrate-Product Pair Networks. Plant Cell 2014, 26, 929–945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dima, O.; Morreel, K.; Vanholme, B.; Kim, H.; Ralph, J.; Boerjan, W. Small Glycosylated Lignin Oligomers Are Stored in Arabidopsis Leaf Vacuoles. Plant Cell 2015, 27, 695–710. [Google Scholar] [CrossRef] [Green Version]
- Vanholme, R.; Sundin, L.; Seetso, K.C.; Kim, H.; Liu, X.; Li, J.; De Meester, B.; Hoengenaert, L.; Goeminne, G.; Morreel, K.; et al. COSY catalyses trans–cis isomerization and lactonization in the biosynthesis of coumarins. Nat. Plants 2019, 5, 1066–1075. [Google Scholar] [CrossRef] [PubMed]
- Desmet, S.; Saeys, Y.; Verstaen, K.; Dauwe, R.; Kim, H.; Niculaes, C.; Fukushima, A.; Goeminne, G.; Vanholme, R.; Ralph, J.; et al. Maize specialized metabolome networks reveal organ-preferential mixed glycosides. Comput. Struct. Biotechnol. J. 2021, 19, 1127–1144. [Google Scholar] [CrossRef]
- Van Acker, R.; Déjardin, A.; Desmet, S.; Hoengenaert, L.; Vanholme, R.; Morreel, K.; Laurans, F.; Kim, H.; Santoro, N.; Foster, C.; et al. Different Routes for Conifer- and Sinapaldehyde and Higher Saccharification upon Deficiency in the Dehydrogenase CAD1. Plant Physiol. 2017, 175, 1018–1039. [Google Scholar] [CrossRef]
- Morreel, K.; Dima, O.; Kim, H.; Lu, F.; Niculaes, C.; Vanholme, R.; Dauwe, R.; Goeminne, G.; Inzé, D.; Messens, E.; et al. Mass Spectrometry-Based Sequencing of Lignin Oligomers. Plant Physiol. 2010, 153, 1464–1478. [Google Scholar] [CrossRef] [Green Version]
- Castro-Moretti, F.R.; Gentzel, I.N.; Mackey, D.; Alonso, A.P. Metabolomics as an Emerging Tool for the Study of Plant–Pathogen Interactions. Metabolites 2020, 10, 52. [Google Scholar] [CrossRef] [Green Version]
- Williams, A.; Gamir, J.; Gravot, A.; Pétriacq, P. Untangling plant immune responses through metabolomics. In Advances in Botanical Research; Pétriacq, P., Bouchereau, A., Eds.; Academic Press: Cambridge, MA, USA, 2021. [Google Scholar]
- Berenbaum, M.R.; Zangerl, A.R. Facing the Future of Plant-Insect Interaction Research: Le Retour à la “Raison d’être”. Plant Physiol. 2008, 146, 804–811. [Google Scholar] [CrossRef] [Green Version]
- Vanhaelewyn, L.; Van Der Straeten, D.; De Coninck, B.; Vandenbussche, F. Ultraviolet radiation from a plant perspective: The plant-microorganism context. Front. Plant Sci. 2020, 11, 1984. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hartmann, T. Diversity and variability of plant secondary metabolism: A mechanistic view. In Proceedings of the 9th International Symposium on Insect-Plant Relationships, Gwatt, Switzerland, 24–30 June 1995; Städler, E., Rowell-Rahier, M., Bauer, R., Eds.; Springer: Dordrecht, The Netherlands, 1996; pp. 177–188. [Google Scholar]
- Kreis, W.; Munkert, J. Exploiting enzyme promiscuity to shape plant specialized metabolism. J. Exp. Bot. 2019, 70, 1435–1445. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.G.; Firn, R.D.; Malcolm, S.B. On the evolution of plant secondary chemical diversity [and discussion]. Philos. Trans. Biol. Sci. 1991, 333, 273–280. [Google Scholar]
- Firn, R.D.; Jones, C.G. Natural products–A simple model to explain chemical diversity. Nat. Prod. Rep. 2003, 20, 382–391. [Google Scholar] [CrossRef]
- Hadacek, F.; Bachmann, G.; Engelmeier, D.; Chobot, V. Hormesis and a chemical raison d’être for secondary plant metabolites. Dose Response Publ. Int. Hormesis Soc. 2010, 9, 79–116. [Google Scholar] [CrossRef] [PubMed]
- Dewick, P.M. The acetate pathway: Fatty acids and polyketides. In Medicinal Natural Products; John Wiley & Sons Ltd: Hoboken, NJ, USA, 2009; pp. 39–135. [Google Scholar]
- Flores-Sanchez, I.J.; Verpoorte, R. Plant polyketide synthases: A fascinating group of enzymes. Plant Physiol. Biochem. PPB 2009, 47, 167–174. [Google Scholar] [CrossRef]
- Blée, E. Phytooxylipins and plant defense reactions. Prog. Lipid Res. 1998, 37, 33–72. [Google Scholar] [CrossRef]
- Vincenti, S.; Mariani, M.; Alberti, J.-C.; Jacopini, S.; De Caraffa, V.B.-B.; Berti, L.; Maury, J. Biocatalytic Synthesis of Natural Green Leaf Volatiles Using the Lipoxygenase Metabolic Pathway. Catalysts 2019, 9, 873. [Google Scholar] [CrossRef] [Green Version]
- Wasternack, C. Jasmonates: An Update on Biosynthesis, Signal Transduction and Action in Plant Stress Response, Growth and Development. Ann. Bot. 2007, 100, 681–697. [Google Scholar] [CrossRef] [Green Version]
- Wasternack, C.; Hause, B. Jasmonates: Biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007 review in Annals of Botany. Ann. Bot. 2013, 111, 1021–1058. [Google Scholar] [CrossRef]
- Matsui, K. Green leaf volatiles: Hydroperoxide lyase pathway of oxylipin metabolism. Curr. Opin. Plant Biol. 2006, 9, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Matsui, K.; Koeduka, T. Green leaf volatiles in plant signaling and response. In Lipids in Plant and Algae Development; Nakamura, Y., Li-Beisson, Y., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 427–443. [Google Scholar]
- Frost, C.J.; Mescher, M.C.; Dervinis, C.; Davis, J.M.; Carlson, J.E.; De Moraes, C.M. Priming defense genes and metabolites in hybrid poplar by the green leaf volatile cis-3-hexenyl acetate. New Phytol. 2008, 180, 722–734. [Google Scholar] [CrossRef]
- McCormick, A.C.; Irmisch, S.; Reinecke, A.; Boeckler, G.A.; Veit, D.; Reichelt, M.; Hansson, B.S.; Gershenzon, J.; Köllner, T.; Unsicker, S.B. Herbivore-induced volatile emission in black poplar: Regulation and role in attracting herbivore enemies. Plant Cell Environ. 2014, 37, 1909–1923. [Google Scholar] [CrossRef]
- Scala, A.; Allmann, S.; Mirabella, R.; Haring, M.A.; Schuurink, R.C. Green Leaf Volatiles: A Plant’s Multifunctional Weapon against Herbivores and Pathogens. Int. J. Mol. Sci. 2013, 14, 17781–17811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zwenger, S.R.; Basu, C. Plant terpenoids: Applications and future potentials. Biotechnol. Mol. Biol. Rev. 2008, 3, 1–7. [Google Scholar]
- Zi, J.; Mafu, S.; Peters, R.J. To gibberellins and beyond! Surveying the evolution of (di)terpenoid metabolism. Annu. Rev. Plant Biol. 2014, 65, 259–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pichersky, E.; Raguso, R.A. Why do plants produce so many terpenoid compounds? New Phytol. 2018, 220, 692–702. [Google Scholar] [CrossRef]
- Dewick, P.M. The mevalonate and methylerythritol phosphate pathways: Terpenoids and steroids. In Medicinal Natural Products; John Wiley & Sons Ltd: Hoboken, NJ, USA, 2009; pp. 187–310. [Google Scholar]
- Lipko, A.; Swiezewska, E. Isoprenoid generating systems in plants—A handy toolbox how to assess contribution of the mevalonate and methylerythritol phosphate pathways to the biosynthetic process. Prog. Lipid Res. 2016, 63, 70–92. [Google Scholar] [CrossRef]
- Bohlmann, J.; Keeling, C.I. Terpenoid biomaterials. Plant J. Cell Mol. Biol. 2008, 54 4, 656–669. [Google Scholar] [CrossRef]
- Vranová, E.; Coman, D.; Gruissem, W. Structure and dynamics of the isoprenoid pathway network. Mol. Plant 2012, 5, 318–333. [Google Scholar] [CrossRef] [Green Version]
- Loreto, F.; Dicke, M.; Schnitzler, J.-P.; Turlings, T.C.J. Plant volatiles and the environment. Plant Cell Environ. 2014, 37, 1905–1908. [Google Scholar] [CrossRef] [PubMed]
- Tholl, D. Biosynthesis and biological functions of terpenoids in plants. Adv. Biochem. Eng. Biotechnol. 2015, 148, 63–106. [Google Scholar] [CrossRef]
- Gershenzon, J.; Dudareva, N. The function of terpene natural products in the natural world. Nat. Chem. Biol. 2007, 3, 408–414. [Google Scholar] [CrossRef]
- Block, A.K.; Vaughan, M.M.; Schmelz, E.A.; Christensen, S.A. Biosynthesis and function of terpenoid defense compounds in maize (Zea mays). Planta 2019, 249, 21–30. [Google Scholar] [CrossRef]
- Veenstra, A.; Moola, N.; Wighard, S.S.; Korsman, J.; Christensen, S.A.; Rafudeen, M.S.; Murray, S.L. Kauralexins and zealexins accumulate in sub-tropical maize lines and play a role in seedling resistance to Fusarium verticillioides. Eur. J. Plant Pathol. 2018, 153, 223–237. [Google Scholar] [CrossRef]
- Rasmann, S.; Köllner, T.G.; Degenhardt, J.; Hiltpold, I.; Toepfer, S.; Kuhlmann, U.; Gershenzon, J.; Turlings, T.C.J. Recruitment of entomopathogenic nematodes by insect-damaged maize roots. Nature 2005, 434, 732–737. [Google Scholar] [CrossRef]
- Irmisch, S.; Jiang, Y.; Chen, F.; Gershenzon, J.; Köllner, T.G. Terpene synthases and their contribution to herbivore-induced volatile emission in western balsam poplar (Populus trichocarpa). BMC Plant Biol. 2014, 14, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Lackus, N.D.; Lackner, S.; Gershenzon, J.; Unsicker, S.; Köllner, T.G. The occurrence and formation of monoterpenes in herbivore-damaged poplar roots. Sci. Rep. 2018, 8, 17936. [Google Scholar] [CrossRef]
- Dewick, P.M. Alkaloids. In Medicinal Natural Products; John Wiley & Sons: Hoboken, NJ, USA, 2009; pp. 311–420. [Google Scholar]
- Chen, D.; Shao, Q.; Yin, L.; Younis, A.; Zheng, B. Polyamine Function in Plants: Metabolism, Regulation on Development, and Roles in Abiotic Stress Responses. Front. Plant Sci. 2019, 9, 1945. [Google Scholar] [CrossRef] [PubMed]
- Azzeme, A.; Kamarul Zaman, M. Plant toxins: Alkaloids and their toxicities. GSC Biol. Pharm. Sci. 2019, 6, 21–29. [Google Scholar] [CrossRef] [Green Version]
- Matsuura, H.; Fett-Neto, A. Plant alkaloids: Main features, toxicity, and mechanisms of action. Plant Toxins 2017, 2, 243–261. [Google Scholar]
- Feng, G.; Chen, M.; Ye, H.-C.; Zhang, Z.-K.; Li, H.; Chen, L.-L.; Chen, X.-L.; Yan, C.; Zhang, J. Herbicidal activities of compounds isolated from the medicinal plant Piper sarmentosum. Ind. Crop. Prod. 2019, 132, 41–47. [Google Scholar] [CrossRef]
- Debnath, B.; Singh, W.S.; Das, M.; Goswami, S.; Singh, M.K.; Maiti, D.; Manna, K. Role of plant alkaloids on human health: A review of biological activities. Mater. Today Chem. 2018, 9, 56–72. [Google Scholar] [CrossRef]
- Zhou, S.; Richter, A.; Jander, G. Beyond defense: Multiple functions of benzoxazinoids in maize metabolism. Plant Cell Physiol. 2018, 59, 1528–1537. [Google Scholar] [CrossRef] [PubMed]
- Niemeyer, H.M. Hydroxamic acids derived from 2-hydroxy-2H-1,4-benzoxazin-3(4H)-one: Key defense chemicals of cereals. J. Agric. Food Chem. 2009, 57, 1677–1696. [Google Scholar] [CrossRef]
- Oikawa, A.; Ishihara, A.; Tanaka, C.; Mori, N.; Tsuda, M.; Iwamura, H. Accumulation of HDMBOA-Glc is induced by biotic stresses prior to the release of MBOA in maize leaves. Phytochemistry 2004, 65, 2995–3001. [Google Scholar] [CrossRef] [PubMed]
- Cheynier, V.; Comte, G.; Davies, K.M.; Lattanzio, V.; Martens, S. Plant phenolics: Recent advances on their biosynthesis, genetics, and ecophysiology. Plant Physiol. Biochem. PPB 2013, 72, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Dewick, P.M. The shikimate pathway: Aromatic amino acids and phenylpropanoids. In Medicinal Natural Products; John Wiley & Sons: Hoboken, NJ, USA, 2009; pp. 137–186. [Google Scholar]
- Maeda, H.; Dudareva, N. The Shikimate Pathway and Aromatic Amino Acid Biosynthesis in Plants. Annu. Rev. Plant Biol. 2012, 63, 73–105. [Google Scholar] [CrossRef]
- Grundhöfer, P.; Niemetz, R.; Schilling, G.; Gross, G.G. Biosynthesis and subcellular distribution of hydrolyzable tannins. Phytochemistry 2001, 57, 915–927. [Google Scholar] [CrossRef]
- Strobbe, S.; Van Der Straeten, D. Toward Eradication of B-Vitamin Deficiencies: Considerations for Crop Biofortification. Front. Plant Sci. 2018, 9, 443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strobbe, S.; Van Der Straeten, D. Folate biofortification in food crops. Curr. Opin. Biotechnol. 2017, 44, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, S.; Zhu, C.; Farre, G.; Ramessar, K.; Bassie, L.; Breitenbach, J.; Conesa, D.P.; Ros, G.; Sandmann, G.; Capell, T.; et al. Transgenic multivitamin corn through biofortification of endosperm with three vitamins representing three distinct metabolic pathways. Proc. Natl. Acad. Sci. USA 2009, 106, 7762–7767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallei, M.; Luschnig, C.; Friml, J. Auxin signalling in growth: Schrödinger’s cat out of the bag. Curr. Opin. Plant Biol. 2020, 53, 43–49. [Google Scholar] [CrossRef]
- Tzin, V.; Galili, G. New Insights into the Shikimate and Aromatic Amino Acids Biosynthesis Pathways in Plants. Mol. Plant 2010, 3, 956–972. [Google Scholar] [CrossRef]
- Yoo, H.; Widhalm, J.R.; Qian, Y.; Maeda, H.; Cooper, B.R.; Jannasch, A.S.; Gonda, I.; Lewinsohn, E.; Rhodes, D.; Dudareva, N. An alternative pathway contributes to phenylalanine biosynthesis in plants via a cytosolic tyrosine:phenylpyruvate aminotransferase. Nat. Commun. 2013, 4, 2833. [Google Scholar] [CrossRef] [Green Version]
- Vogt, T. Phenylpropanoid Biosynthesis. Mol. Plant 2010, 3, 2–20. [Google Scholar] [CrossRef] [Green Version]
- Widhalm, J.; Dudareva, N. A Familiar Ring to It: Biosynthesis of Plant Benzoic Acids. Mol. Plant 2015, 8, 83–97. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Zheng, Z.; Huang, J.; Lai, Z.; Fan, B. Biosynthesis of salicylic acid in plants. Plant Signal. Behav. 2009, 4, 493–496. [Google Scholar] [CrossRef]
- Mahdi, J.G. Biosynthesis and metabolism of β-d-salicin: A novel molecule that exerts biological function in humans and plants. Biotechnol. Rep. 2014, 4, 73–79. [Google Scholar] [CrossRef] [Green Version]
- Boeckler, G.A.; Gershenzon, J.; Unsicker, S.B. Phenolic glycosides of the Salicaceae and their role as anti-herbivore defenses. Phytochemistry 2011, 72, 1497–1509. [Google Scholar] [CrossRef]
- Vanholme, R.; De Meester, B.; Ralph, J.; Boerjan, W. Lignin biosynthesis and its integration into metabolism. Curr. Opin. Biotechnol. 2019, 56, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Boerjan, W.; Ralph, J.; Baucher, M. Lignin Biosynthesis. Annu. Rev. Plant Biol. 2003, 54, 519–546. [Google Scholar] [CrossRef]
- Niculaes, C.; Morreel, K.; Kim, H.; Lu, F.; McKee, L.S.; Ivens, B.; Haustraete, J.; Vanholme, B.; De Rycke, R.; Hertzberg, M.; et al. Phenylcoumaran Benzylic Ether Reductase Prevents Accumulation of Compounds Formed under Oxidative Conditions in Poplar Xylem. Plant Cell 2014, 26, 3775–3791. [Google Scholar] [CrossRef] [Green Version]
- Moss, G.P. Nomenclature of Lignans and Neolignans (IUPAC Recommendations 2000). Pure Appl. Chem. 2000, 72, 1493–1523. [Google Scholar] [CrossRef]
- Cong, L.H.; Dauwe, R.; Lequart, M.; Vinchon, S.; Renouard, S.; Fliniaux, O.; Colas, C.; Corbin, C.; Doussot, J.; Hano, C.; et al. Kinetics of glucosylated and non-glucosylated aryltetralin lignans in Linum hairy root cultures. Phytochemistry 2015, 115, 70–78. [Google Scholar] [CrossRef]
- Jullian-Pawlicki, N.; Lequart-Pillon, M.; Huynh-Cong, L.; Lesur, D.; Cailleu, D.; Mesnard, F.; Laberche, J.C.; Gontier, E.; Boitel-Conti, M. Arylnaphthalene and aryltetralin-type lignans in hairy root cultures ofLinum perenne, and the stereochemistry of 6-methoxypodophyllotoxin and one diastereoisomer by HPLC-MS and NMR spectroscopy. Phytochem. Anal. 2015, 26, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Ralph, J.; Lundquist, K.; Brunow, G.; Lu, F.; Kim, H.; Schatz, P.F.; Marita, J.M.; Hatfield, R.D.; Ralph, S.A.; Christensen, J.H.; et al. Lignins: Natural polymers from oxidative coupling of 4-hydroxyphenyl- propanoids. Phytochem. Rev. 2004, 3, 29–60. [Google Scholar] [CrossRef]
- Vanholme, R.; Ralph, J.; Akiyama, T.; Lu, F.; Pazo, J.R.; Kim, H.; Christensen, J.H.; Van Reusel, B.; Storme, V.; De Rycke, R.; et al. Engineering traditional monolignols out of lignin by concomitant up-regulation of F5H1 and down-regulation of COMT in Arabidopsis. Plant J. 2010, 64, 885–897. [Google Scholar] [CrossRef]
- Sundin, L.; Vanholme, R.; Geerinck, J.; Goeminne, G.; Höfer, R.; Kim, H.; Ralph, J.; Boerjan, W. Mutation of the Inducible ARABIDOPSIS THALIANA CYTOCHROME P450 REDUCTASE2 Alters Lignin Composition and Improves Saccharification. Plant Physiol. 2014, 166, 1956–1971. [Google Scholar] [CrossRef] [Green Version]
- Ralph, J.; Lapierre, C.; Boerjan, W. Lignin structure and its engineering. Curr. Opin. Biotechnol. 2019, 56, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Davies, K.M.; Albert, N.W.; Schwinn, K.E. From landing lights to mimicry: The molecular regulation of flower colouration and mechanisms for pigmentation patterning. Funct. Plant Biol. FPB 2012, 39, 619–638. [Google Scholar] [CrossRef]
- Khalid, M.; Rahman, S.U.; Bilal, M.; Huang, D.-F. Role of flavonoids in plant interactions with the environment and against human pathogens—A review. J. Integr. Agric. 2019, 18, 211–230. [Google Scholar] [CrossRef]
- Dixon, R.A.; Pasinetti, G. Flavonoids and Isoflavonoids: From Plant Biology to Agriculture and Neuroscience. Plant Physiol. 2010, 154, 453–457. [Google Scholar] [CrossRef] [Green Version]
- Brown, E.J.; Khodr, H.; Hider, C.R.; Rice-Evans, C.A. Structural dependence of flavonoid interactions with Cu2+ ions: Implications for their antioxidant properties. Biochem. J. 1998, 330, 1173–1178. [Google Scholar] [CrossRef]
- Mishra, A.; Kumar, S.; Pandey, A.K. Scientific validation of the medicinal efficacy of Tinospora cordifolia. Sci. World J. 2013, 2013, 292934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bors, W.; Heller, W.; Michel, C.; Saran, M. Flavonoids as antioxidants: Determination of radical-scavenging efficiencies. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1990; Volume 186, pp. 343–355. [Google Scholar]
- Mukai, K.; Nagai, S.; Ohara, K. Kinetic study of the quenching reaction of singlet oxygen by tea catechins in ethanol solution. Free. Radic. Biol. Med. 2005, 39, 752–761. [Google Scholar] [CrossRef]
- Cao, G.; Sofic, E.; Prior, R.L. Antioxidant and prooxidant behavior of flavonoids: Structure-activity relationships. Free. Radic. Biol. Med. 1997, 22, 749–760. [Google Scholar] [CrossRef]
- Metodiewa, D.; Jaiswal, A.K.; Cenas, N.; Dickancaité, E.; Segura-Aguilar, J. Quercetin may act as a cytotoxic prooxidant after its metabolic activation to semiquinone and quinoidal product. Free. Radic. Biol. Med. 1999, 26, 107–116. [Google Scholar] [CrossRef]
- Awad, H.M.; Boersma, M.G.; Boeren, S.; van Bladeren, P.J.; Vervoort, J.; Rietjens, I.M. Structure-activity study on the quinone/quinone methide chemistry of flavonoids. Chem. Res. Toxicol. 2001, 14, 398–408. [Google Scholar] [CrossRef] [PubMed]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Arroyo-Currás, N.; Rosas-García, V.M.; Videa, M. Substituent inductive effects on the electrochemical oxidation of flavonoids studied by square wave voltammetry and ab initio calculations. Molecules 2016, 21, 1422. [Google Scholar] [CrossRef]
- Heijnen, C.G.M.; Haenen, G.R.M.M.; Vekemans, J.A.J.M.; Bast, A. Peroxynitrite scavenging of flavonoids: Structure activity relationship. Environ. Toxicol. Pharmacol. 2001, 10, 199–206. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free. Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Jovanovic, S.V.; Steenken, S.; Tosic, M.; Marjanovic, B.; Simic, M.G. Flavonoids as Antioxidants. J. Am. Chem. Soc. 1994, 116, 4846–4851. [Google Scholar] [CrossRef]
- Han, R.-M.; Zhang, J.-P.; Skibsted, L.H. Reaction Dynamics of Flavonoids and Carotenoids as Antioxidants. Molecules 2012, 17, 2140–2160. [Google Scholar] [CrossRef] [Green Version]
- Nakanishi, I.; Miyazaki, K.; Shimada, T.; Ohkubo, K.; Urano, S.; Ikota, N.; Ozawa, T.; Fukuzumi, S.; Fukuhara, K. Effects of Metal Ions Distinguishing between One-Step Hydrogen-and Electron-Transfer Mechanisms for the Radical-Scavenging Reaction of (+)-Catechin. J. Phys. Chem. A 2002, 106, 11123–11126. [Google Scholar] [CrossRef]
- Erben-Russ, M.; Bors, W.; Saran, M. Reactions of linoleic acid peroxyl radicals with phenolic antioxidants: A pulse radiolysis study. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1987, 52, 393–412. [Google Scholar] [CrossRef]
- Jørgensen, L.V.; Cornett, C.; Justesen, U.; Skibsted, L.H.; Dragsted, L.O. Two-electron electrochemical oxidation of quercetin and kaempferol changes only the flavonoid C-ring. Free. Radic. Res. 1998, 29, 339–350. [Google Scholar] [CrossRef]
- Eklund, P.C.; Långvik, O.K.; Wärnå, J.P.; Salmi, T.O.; Willför, S.M.; Sjöholm, R.E. Chemical studies on antioxidant mechanisms and free radical scavenging properties of lignans. Org. Biomol. Chem. 2005, 3, 3336–3347. [Google Scholar] [CrossRef]
- Lan, W.; Lu, F.; Regner, M.; Zhu, Y.; Rencoret, J.; Ralph, S.A.; Zakai, U.I.; Morreel, K.; Boerjan, W.; Ralph, J. Tricin, a Flavonoid Monomer in Monocot Lignification. Plant Physiol. 2015, 167, 1284–1295. [Google Scholar] [CrossRef] [Green Version]
- Pannala, A.S.; Chan, T.S.; O’Brien, P.J.; Rice-Evans, C.A. Flavonoid B-ring chemistry and antioxidant activity: Fast reaction kinetics. Biochem. Biophys. Res. Commun. 2001, 282, 1161–1168. [Google Scholar] [CrossRef] [PubMed]
- Agati, G.; Azzarello, E.; Pollastri, S.; Tattini, M. Flavonoids as antioxidants in plants: Location and functional significance. Plant Sci. Int. J. Exp. Plant Biol. 2012, 196, 67–76. [Google Scholar] [CrossRef]
- Procházková, D.; Boušová, I.; Wilhelmová, N. Antioxidant and prooxidant properties of flavonoids. Fitoterapia 2011, 82, 513–523. [Google Scholar] [CrossRef]
- Yen, G.C.; Duh, P.D.; Tsai, H.L.; Huang, S.L. Pro-oxidative properties of flavonoids in human lymphocytes. Biosci. Biotechnol. Biochem. 2003, 67, 1215–1222. [Google Scholar] [CrossRef] [Green Version]
- Monks, T.J.; Hanzlik, R.P.; Cohen, G.M.; Ross, D.; Graham, D.G. Quinone chemistry and toxicity. Toxicol. Appl. Pharmacol. 1992, 112, 2–16. [Google Scholar] [CrossRef]
- Bors, W.; Michel, C. Antioxidant capacity of flavanols and gallate esters: Pulse radiolysis studies. Free. Radic. Biol. Med. 1999, 27, 1413–1426. [Google Scholar] [CrossRef]
- Bors, W.; Foo, L.Y.; Hertkorn, N.; Michel, C.; Stettmaier, K. Chemical studies of proanthocyanidins and hydrolyzable tannins. Antioxid. Redox Signal. 2001, 3, 995–1008. [Google Scholar] [CrossRef] [PubMed]
- Vennat, B.; Bos, M.A.; Pourrat, A.; Bastide, P. Procyanidins from tormentil: Fractionation and study of the anti-radical activity towards superoxide anion. Biol. Pharm. Bull. 1994, 17, 1613–1615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plumb, G.W.; De Pascual-Teresa, S.; Santos-Buelga, C.; Cheynier, V.; Williamson, G. Antioxidant properties of catechins and proanthocyanidins: Effect of polymerisation, galloylation and glycosylation. Free. Radic. Res. 1998, 29, 351–358. [Google Scholar] [CrossRef]
- Triantaphylidès, C.; Krischke, M.; Hoeberichts, F.A.; Ksas, B.; Gresser, G.; Havaux, M.; Van Breusegem, F.; Mueller, M.J. Singlet oxygen is the major reactive oxygen species involved in photooxidative damage to plants. Plant Physiol. 2008, 148, 960–968. [Google Scholar] [CrossRef] [Green Version]
- Tournaire, C.; Croux, S.; Maurette, M.-T.; Beck, I.; Hocquaux, M.; Braun, A.M.; Oliveros, E. Antioxidant activity of flavonoids: Efficiency of singlet oxygen (1Δg) quenching. J. Photochem. Photobiol. B Biol. 1993, 19, 205–215. [Google Scholar] [CrossRef]
- Pilkington, L.I.; Barker, D. Synthesis and biology of 1,4-benzodioxane lignan natural products. Nat. Prod. Rep. 2015, 32, 1369–1388. [Google Scholar] [CrossRef] [Green Version]
- Nyiredy, S.; Samu, Z.; Szücs, Z.; Gulácsi, K.; Kurtán, T.; Antus, S. New insight into the biosynthesis of flavanolignans in the white-flowered variant of Silybum marianum. J. Chromatogr. Sci. 2008, 46, 93–96. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, D.; Nel, R.J.J.; Bekker, R. 3.19-Condensed Tannins. In Comprehensive Natural Products Chemistry; Barton, S.D., Nakanishi, K., Meth-Cohn, O., Eds.; Pergamon: Oxford, UK, 1999; pp. 747–797. [Google Scholar]
- Weng, J.-K.; Noel, J.P. Chemodiversity in Selaginella: A reference system for parallel and convergent metabolic evolution in terrestrial plants. Front. Plant Sci. 2013, 4, 119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrade, A.W.L.; Machado, K.D.C.; Machado, K.D.C.; Figueiredo, D.D.R.; David, J.M.; Islam, M.T.; Uddin, S.J.; Shilpi, J.A.; Costa, J.P. In vitro antioxidant properties of the biflavonoid agathisflavone. Chem. Central J. 2018, 12, 75. [Google Scholar] [CrossRef] [PubMed]
- Gontijo, V.S.; Dos Santos, M.H.; Viegas, C., Jr. Biological and chemical aspects of natural biflavonoids from plants: A brief review. Mini Rev. Med. Chem. 2017, 17, 834–862. [Google Scholar] [CrossRef] [PubMed]
- Geiger, H.; Seeger, T. Triflavones and a biflavone from the moss Rhizogonium Distichum. Z. Naturforschung. 2000, 55, 870–873. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, F.J.; Boralle, N.; Silva, D.H.; Lopes, L.M. Bi- and tetraflavonoids from Aristolochia ridicula. Phytochemistry 2000, 55, 823–832. [Google Scholar] [CrossRef]
- Goossens, J.-F.; Goossens, L.; Bailly, C. Hinokiflavone and Related C–O–C-Type Biflavonoids as Anti-cancer Compounds: Properties and Mechanism of Action. Nat. Prod. Bioprospecting 2021, 11, 365–377. [Google Scholar] [CrossRef]
- Waterman, M.J.; Nugraha, A.S.; Hendra, R.; Ball, G.E.; Robinson, S.A.; Keller, P.A. Antarctic moss biflavonoids show high antioxidant and ultraviolet-screening activity. J. Nat. Prod. 2017, 80, 2224–2231. [Google Scholar] [CrossRef] [Green Version]
- Ye, Y.; Guo, Y.; Luo, Y.T.; Wang, Y.F. Isolation and free radical scavenging activities of a novel biflavonoid from the shells of Camellia oleifera Abel. Fitoterapia 2012, 83, 1585–1589. [Google Scholar] [CrossRef]
- Menezes, J.; Diederich, M.F. Bioactivity of natural biflavonoids in metabolism-related disease and cancer therapies. Pharmacol. Res. 2021, 167, 105525. [Google Scholar] [CrossRef]
- Silva, G.L.; Chai, H.; Gupta, M.P.; Farnsworth, N.R.; Cordell, G.A.; Pezzuto, J.M.; Beecher, C.W.W.; Douglas Kinghorn, A. Cytotoxic biflavonoids from Selaginella willdenowii. Phytochemistry 1995, 40, 129–134. [Google Scholar] [CrossRef]
- Effenberger, I.; Zhang, B.; Li, L.; Wang, Q.; Liu, Y.; Klaiber, I.; Pfannstiel, J.; Wang, Q.; Schaller, A. Dirigent proteins from cotton (Gossypium sp.) for the atropselective synthesis of gossypol. Angew. Chem. Int. Ed. 2015, 54, 14660–14663. [Google Scholar] [CrossRef] [PubMed]
- Paniagua, C.; Bilkova, A.; Jackson, P.; Dabravolski, S.; Riber, W.; Didi, V.; Houser, J.; Gigli-Bisceglia, N.; Wimmerova, M.; Budínská, E.; et al. Dirigent proteins in plants: Modulating cell wall metabolism during abiotic and biotic stress exposure. J. Exp. Bot. 2017, 68, 3287–3301. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Alseekh, S.; Fernie, A.R.; Luo, J. The structure and function of major plant metabolite modifications. Mol. Plant. 2019, 12, 899–919. [Google Scholar] [CrossRef]
- Thiombiano, B.; Gontier, E.; Molinié, R.; Marcelo, P.; Mesnard, F.; Dauwe, R. An untargeted liquid chromatography–mass spectrometry-based workflow for the structural characterization of plant polyesters. Plant J. 2020, 102, 1323–1339. [Google Scholar] [CrossRef]
- Luo, J.; Fuell, C.; Parr, A.; Hill, L.; Bailey, P.; Elliott, K.; Fairhurst, S.A.; Martin, C.; Michael, A.J. A Novel Polyamine Acyltransferase Responsible for the Accumulation of Spermidine Conjugates in Arabidopsis Seed. Plant Cell 2009, 21, 318–333. [Google Scholar] [CrossRef] [Green Version]
- Sagner, S.; Shen, Z.; Deus-Neumann, B.; Zenk, M.H. The biosynthesis of lunarine in seeds of Lunaria annua. Phytochemistry 1998, 47, 375–387. [Google Scholar] [CrossRef]
- Nezbedová, L.; Hesse, M.; Drandarov, K.; Bigler, L.; Werner, C. Phenol oxidative coupling in the biogenesis of the macrocyclic spermine alkaloids aphelandrine and orantine in Aphelandra sp. Planta 2001, 213, 411–417. [Google Scholar] [CrossRef]
- Baumert, A.; Milkowski, C.; Schmidt, J.; Nimtz, M.; Wray, V.; Strack, D. Formation of a complex pattern of sinapate esters in Brassica napus seeds, catalyzed by enzymes of a serine carboxypeptidase-like acyltransferase family? Phytochemistry 2005, 66, 1334–1345. [Google Scholar] [CrossRef]
- Bloor, S.J.; Falshaw, R. Covalently linked anthocyanin-flavonol pigments from blue Agapanthus flowers. Phytochemistry 2000, 53, 575–579. [Google Scholar] [CrossRef]
- Kolattukudy, P.E. Polyesters in higher plants. Adv. Biochem. Eng. Biotechnol. 2001, 71, 1–49. [Google Scholar]
- Alseekh, S.; Perez de Souza, L.; Benina, M.; Fernie, A.R. The style and substance of plant flavonoid decoration; towards defining both structure and function. Phytochemistry 2020, 174, 112347. [Google Scholar] [CrossRef]
- Zhao, J. Flavonoid transport mechanisms: How to go, and with whom. Trends Plant Sci. 2015, 20, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Huhman, D.; Shadle, G.; He, X.-Z.; Sumner, L.W.; Tang, Y.; Dixon, R.A. MATE2 Mediates Vacuolar Sequestration of Flavonoid Glycosides and Glycoside Malonates in Medicago truncatula. Plant Cell 2011, 23, 1536–1555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanholme, R.; Cesarino, I.; Rataj, K.; Xiao, Y.; Sundin, L.; Goeminne, G.; Kim, H.; Cross, J.; Morreel, K.; Araujo, P.; et al. Caffeoyl shikimate esterase (CSE) is an enzyme in the lignin biosynthetic pathway in Arabidopsis. Science 2013, 341, 1103–1106. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, L.; Maury, S.; Martz, F.; Geoffroy, P.; Legrand, M. Purification, cloning, and properties of an acyltransferase controlling shikimate and quinate ester intermediates in phenylpropanoid metabolism. J. Biol. Chem. 2003, 278, 95–103. [Google Scholar] [CrossRef] [Green Version]
- Schoch, G.; Goepfert, S.; Morant, M.; Hehn, A.; Meyer, D.; Ullmann, P.; Werck-Reichhart, D. CYP98A3 from Arabidopsis thaliana is a 3′-hydroxylase of phenolic esters, a missing link in the phenylpropanoid pathway. J. Biol. Chem. 2001, 276, 36566–36574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, C.L.; Chen, Z.J.; Bai, X.S.; Ding, C.; Long, T.J.; Wei, F.G.; Miao, K.R. Structure-activity relationships of anthocyanidin glycosylation. Mol. Divers. 2014, 18, 687–700. [Google Scholar] [CrossRef]
- Jakobek, L.; Seruga, M. Influence of anthocyanins, flavonols and phenolic acids on the antiradical activity of berries and small fruits. Int. J. Food 2012, 15, 122–133. [Google Scholar] [CrossRef] [Green Version]
- Thi, K.N.; Nguyen, N.; Pham, H.; Sawada, Y.; Hirai, M.Y.; Dauwe, R.; Dijoux-Franca, M. Development of a Pteris vittata L. compound database by widely targeted metabolomics profiling. Biomed. Chromatogr. 2021, 35, e5110. [Google Scholar] [CrossRef]
- Desmet, S.; Brouckaert, M.; Boerjan, W.; Morreel, K. Seeing the forest for the trees: Retrieving plant secondary biochemical pathways from metabolome networks. Comput. Struct. Biotechnol. J. 2021, 19, 72–85. [Google Scholar] [CrossRef] [PubMed]
- Roepke, J.; Bozzo, G.G. Arabidopsis thaliana β-glucosidase BGLU15 attacks flavonol 3-O-β-glucoside-7-O-α-rhamnosides. Phytochemistry 2015, 109, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Dauwe, R.; Roulard, R.; Ramos, M.; Thiombiano, B.; Mesnard, F.; Gontier, E.; Jamali, A. Etching of the seed cuticle by cold plasma shortens imbibitional leakage in Linum usitatissimum L. Ind. Crop. Prod. 2021, 167, 113536. [Google Scholar] [CrossRef]
- Nguyen, T.K.; Jamali, A.; Lanoue, A.; Gontier, E.; Dauwe, R. Unravelling the architecture and dynamics of tropane alkaloid biosynthesis pathways using metabolite correlation networks. Phytochemistry 2015, 116, 94–103. [Google Scholar] [CrossRef]
- Bar-Even, A.; Noor, E.; Savir, Y.; Liebermeister, W.; Davidi, D.; Tawfik, D.S.; Milo, R. The Moderately Efficient Enzyme: Evolutionary and Physicochemical Trends Shaping Enzyme Parameters. Biochemistry 2011, 50, 4402–4410. [Google Scholar] [CrossRef] [PubMed]
- Waki, T.; Takahashi, S.; Nakayama, T. Managing enzyme promiscuity in plant specialized metabolism: A lesson from flavonoid biosynthesis. BioEssays 2020, 43, e2000164. [Google Scholar] [CrossRef]
- Weng, J.-K.; Noel, J. The Remarkable Pliability and Promiscuity of Specialized Metabolism. Cold Spring Harb. Symp. Quant. Biol. 2012, 77, 309–320. [Google Scholar] [CrossRef] [Green Version]
- Landmann, C.; Hücherig, S.; Fink, B.; Hoffmann, T.; Dittlein, D.; Coiner, H.A.; Schwab, W. Substrate promiscuity of a rosmarinic acid synthase from lavender (Lavandula angustifolia L.). Planta 2011, 234, 305–320. [Google Scholar] [CrossRef]
- D’Auria, J. Acyltransferases in plants: A good time to be BAHD. Curr. Opin. Plant Biol. 2006, 9, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Moghe, G.D.; Leong, B.J.; Hurney, S.M.; Jones, A.D.; Last, R.L. Evolutionary routes to biochemical innovation revealed by integrative analysis of a plant-defense related specialized metabolic pathway. Elife 2017, 6, e28468. [Google Scholar] [CrossRef] [PubMed]
- Copley, S.D. Shining a light on enzyme promiscuity. Curr. Opin. Struct. Biol. 2017, 47, 167–175. [Google Scholar] [CrossRef]
- Hanson, A.D.; Henry, C.S.; Fiehn, O.; de Crécy-Lagard, V. Metabolite Damage and Metabolite Damage Control in Plants. Annu. Rev. Plant Biol. 2016, 67, 131–152. [Google Scholar] [CrossRef] [PubMed]
- Welchen, E.; Schmitz, J.; Fuchs, P. d-Lactate dehydrogenase links methylglyoxal degradation and electron transport through cytochrome c. Plant Physiol. 2016, 172, 901–912. [Google Scholar]
- Araújo, W.L.; Ishizaki, K.; Nunes-Nesi, A.; Larson, T.R.; Tohge, T.; Krahnert, I.; Witt, S.; Obata, T.; Schauer, N.; Graham, I.A.; et al. Identification of the 2-hydroxyglutarate and isovaleryl-CoA dehydrogenases as alternative electron donors linking lysine catabolism to the electron transport chain of Arabidopsis mitochondria. Plant Cell 2010, 22, 1549–1563. [Google Scholar] [CrossRef] [Green Version]
- Engqvist, M.K.M.; Kuhn, A.; Wienstroer, J.; Weber, K.; Jansen, E.E.W.; Jakobs, C.; Weber, A.P.M.; Maurino, V.G. Plant d-2-hydroxyglutarate dehydrogenase participates in the catabolism of lysine especially during senescence. J. Biol. Chem. 2011, 286, 11382–11390. [Google Scholar] [CrossRef] [Green Version]
- De Meester, B.; de Vries, L.; Özparpucu, M.; Gierlinger, N.; Corneillie, S.; Pallidis, A.; Goeminne, G.; Morreel, K.; De Bruyne, M.; De Rycke, R.; et al. Vessel-specific reintroduction of CINNAMOYL-COA REDUCTASE1 (CCR1) in dwarfed ccr1 mutants restores vessel and xylary fiber integrity and increases biomass. Plant Physiol. 2017, 176, 611–633. [Google Scholar] [CrossRef] [Green Version]
- Leong, B.J.; Last, R.L. Promiscuity, impersonation and accommodation: Evolution of plant specialized metabolism. Curr. Opin. Struct. Biol. 2017, 47, 105–112. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Desmet, S.; Morreel, K.; Dauwe, R. Origin and Function of Structural Diversity in the Plant Specialized Metabolome. Plants 2021, 10, 2393. https://doi.org/10.3390/plants10112393
Desmet S, Morreel K, Dauwe R. Origin and Function of Structural Diversity in the Plant Specialized Metabolome. Plants. 2021; 10(11):2393. https://doi.org/10.3390/plants10112393
Chicago/Turabian StyleDesmet, Sandrien, Kris Morreel, and Rebecca Dauwe. 2021. "Origin and Function of Structural Diversity in the Plant Specialized Metabolome" Plants 10, no. 11: 2393. https://doi.org/10.3390/plants10112393






