Effects of Irrigation and Fertilization on the Morphophysiological Traits of Populus sibirica Hort. Ex Tausch and Ulmus pumila L. in the Semiarid Steppe Region of Mongolia
Abstract
:1. Introduction
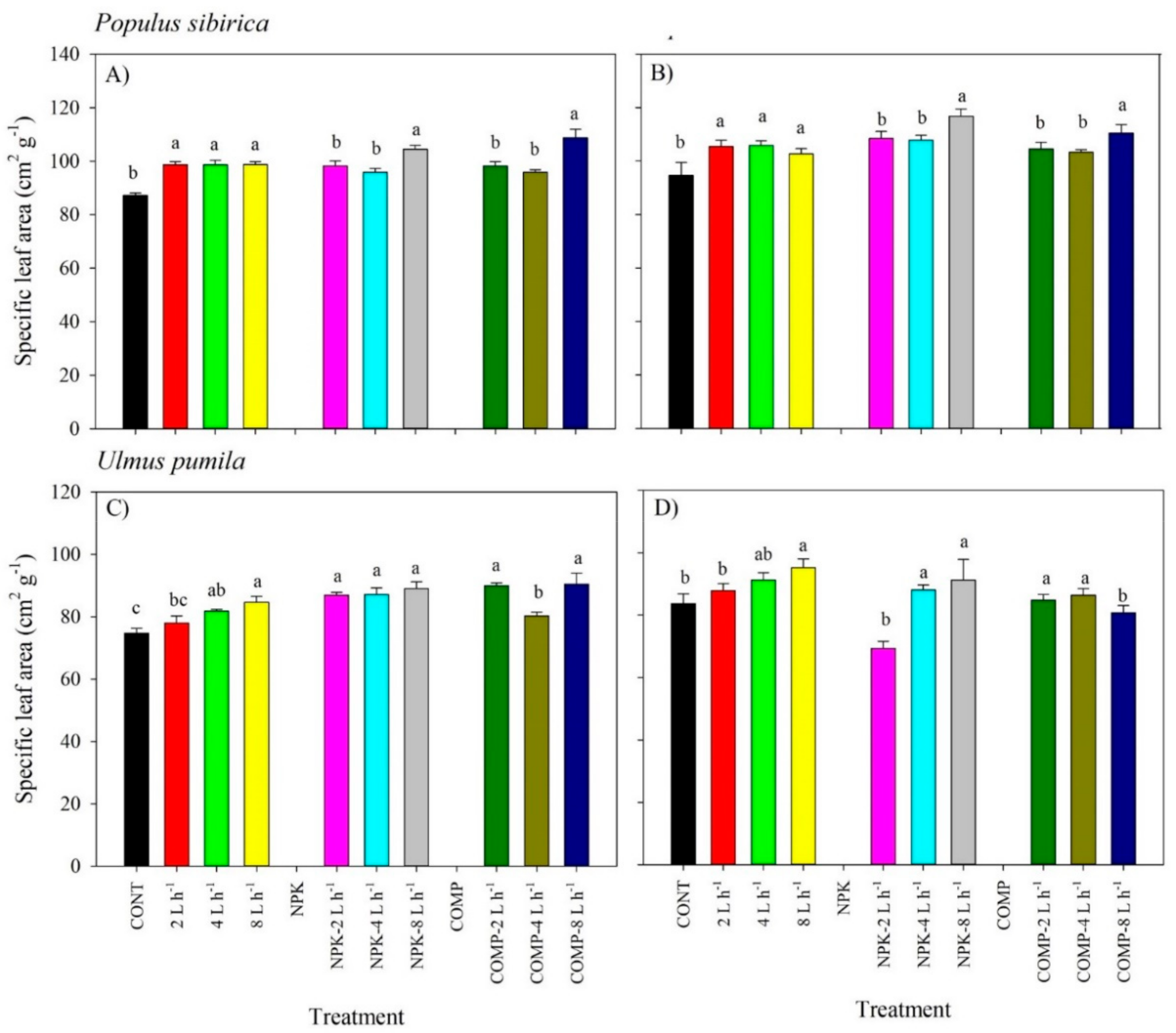
2. Results
3. Discussion
3.1. Effects of Irrigation and Fertilizer Treatments on Morphological Traits and Growth of P. sibirica and U. pumila
3.2. Effects of Irrigation and Fertilizer Treatments on Physiological Traits of P. sibirica and U. pumila
4. Materials and Methods
4.1. Site Description
4.2. Experimental Materials and Design
4.3. Measurements of Morphophysiological Traits
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, Z.; Yao, Z.; Huang, H.; Batjav, B.; Wang, R. Evaluation of extreme cold and drought over the Mongolian Plateau. Water 2019, 11, 74. [Google Scholar] [CrossRef] [Green Version]
- Lee, T.Y.; Je, S.M.; Kwak, M.J.; Akhmadi, K.; Tumurbaatar, E.; Khaine, I.; Lee, H.K.; Jang, J.H.; Kim, H.N.; Ahn, H.J.; et al. Physiological responses of Populus sibirica to different irrigation regimes for reforestation in arid area. S. Afr. J. Bot. 2017, 112, 329–335. [Google Scholar] [CrossRef]
- Nyam-Osor, B.; Byambadorj, S.; Park, B.B.; Terzaghi, M.; Gabriella, S.S.; Stanturf, J.A.; Chiatante, D.; Montagnoli, A. Root Biomass Distribution of Populus sibirica and Ulmus pumila Afforestation Stands Is Affected by Watering Regimes and Fertilization in the Mongolian Semi-arid Steppe. Front. Plant Sci. 2021, 12, 638828. [Google Scholar] [CrossRef]
- Byambadorj, S.-O.; Nyam-Osor, B.; Park, B.B.; Avirmed, T.; Scippa, G.S.; Chiatante, D.; Montagnoli, A.; Dimitrova, A. Afforestation of Mongolian steppe: Patterns of biomass partitioning in Populus sibirica and Ulmus pumila trees in response to management supporting measures. Plant Biosyst. 2021, 155, 1–13. [Google Scholar] [CrossRef]
- Cho, S.; Ser-Oddamba, B.; Batkhuu, N.-O.; Seok Kim, H. Comparison of water use efficiency and biomass production in 10-year-old Populus sibirica and Ulmus pumila plantations in Lun soum, Mongolia. For. Sci. Technol. 2019, 15, 147–158. [Google Scholar] [CrossRef] [Green Version]
- Fan, B.; Zhang, A.; Yang, Y.; Ma, Q.; Li, X.; Zhao, C. Long-Term Effects of Xerophytic Shrub Haloxylon ammodendron Plantations on Soil Properties and Vegetation Dynamics in Northwest China. PLoS ONE 2016, 11, e0168000. [Google Scholar] [CrossRef]
- Yan, Q.; Zhu, J.; Zheng, X.; Jin, C. Causal effects of shelter forests and water factors on desertification control during 2000–2010 at the Horqin Sandy Land region, China. J. For. Res. 2015, 26, 33–45. [Google Scholar] [CrossRef]
- Miyasaka, T.; Okuro, T.; Miyamori, E.; Zhao, X.; Takeuchi, K. Effects of different restoration measures and sand dune topography on short- and long-term vegetation restoration in northeast China. J. Arid. Environ. 2014, 111, 1–6. [Google Scholar] [CrossRef]
- Tsogtbaatar, J. Deforestation and reforestation needs in Mongolia. For. Ecol. Manag. 2004, 201, 57–63. [Google Scholar] [CrossRef]
- Wang, X.M.; Zhang, C.X.; Hasi, E.; Dong, Z.B. Has the Three Norths Forest Shelterbelt Program solved the desertification and dust storm problems in arid and semiarid China? J. Arid. Environ. 2010, 74, 13–22. [Google Scholar] [CrossRef]
- Byambadorj, S.; Park, B.B.; Hernandez, J.O.; Dulamsuren, N.; Sainbuyan, Z.; Altantugs, O.; Sharavdorj, K.; Seong, I.K. Optimal Irrigation Regime for Woody Species Potentially Suitable for Effective and Sustainable Afforestation in the Desert Region of Mongolia. Land 2021, 10, 212. [Google Scholar]
- Wang, N.; Fu, F.; Wang, H.; Wang, P.; He, S.; Shao, H.; Ni, Z.; Zhang, X. Effects of irrigation and nitrogen on chlorophyll content, dry matter and nitrogen accumulation in sugar beet (Beta vulgaris L.). Sci. Rep. 2021, 11, 16651. [Google Scholar] [CrossRef]
- Hernandez, J.O.; Quimado, M.O.; Fernando, E.S.; Province, B. Xerophytic characteristics of Tectona philippinensis Benth. & Hook. f. Philipp. J. Sci. 2016, 145, 259–269. [Google Scholar]
- Hasibeder, R.; Fuchslueger, L.; Richter, A.; Bahn, M. Summer drought alters carbon allocation to roots and root respiration in mountain grassland. New Phytol. 2015, 205, 1117–1127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Z.; Zhou, G.; Shimizu, H. Plant responses to drought and rewatering. Plant Signal. Behav. 2010, 5, 649–654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, C.; Wang, X.; Duan, B.; Luo, J.; Li, C. Early growth, dry matter allocation and water use efficiency of two sympatric Populus species as affected by water stress. Environ. Exp. Bot. 2005, 53, 315–322. [Google Scholar] [CrossRef]
- Guo, X.; Li, S.; Wang, D.; Huang, Z.; Sarwar, N.; Mubeen, K.; Shakeel, M.; Hussain, M. Effects of water and fertilizer coupling on the physiological characteristics and growth of rabbiteye blueberry. PLoS ONE 2021, 16, e0254013. [Google Scholar] [CrossRef]
- Basal, O.; Szabó, A. Physiomorphology of Soybean as Affected by Drought Stress and Nitrogen Application. Scientifica 2020, 2020, 6093836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guyot, G.; Scoffoni, C.; Sack, L. Combined impacts of irradiance and dehydration on leaf hydraulic conductance: Insights into vulnerability and stomatal control. Plant Cell Environ. 2012, 35, 857–871. [Google Scholar] [CrossRef] [PubMed]
- Maréchaux, I.; Bartlett, M.K.; Sack, L.; Baraloto, C.; Engel, J.; Joetzjer, E.; Chave, J. Drought tolerance as predicted by leaf water potential at turgor loss point varies strongly across species within an Amazonian forest. Funct. Ecol. 2015, 29, 1268–1277. [Google Scholar] [CrossRef]
- Kołodziejek, J.; Michlewska, S. Effect of soil moisture on morpho-anatomical leaf traits of Ranunculus acris (Ranunculaceae). Pol. J. Ecol. 2015, 63, 400–413. [Google Scholar] [CrossRef]
- Rad, M.H.; Assare, M.H.; Banakar, M.H.; Soltani, M. Effects of different soil moisture regimes on Leaf Area Index, Specific Leaf Area and Water Use Efficiency in Eucalyptus (Eucalyptus camaldulensis Dehnh) under dry climatic conditions. Asian J. Plant Sci. 2011, 10, 294–300. [Google Scholar]
- Hernandez, J.O.; Quimado, M.O.; Fernando, E.S.; Pulan, D.E.; Malabrigo, P.L.; Maldia, L.S.J. Functional traits of stem and leaf of Wrightia candollei S. Vidal. Philipp. J. Sci. 2019, 148, 307–314. [Google Scholar]
- Gómez-del-Campo, M.; Ruiz, C.; Lissarrague, J.R. Effect of water stress on leaf area development, photosynthesis, and productivity in Chardonnay and Airén grapevines. Am. J. Enol. Vitic. 2002, 53, 138–143. [Google Scholar]
- Cramer, M.D.; Hawkins, H.J.; Verboom, G.A. The importance of nutritional regulation of plant water flux. Oecologia 2009, 161, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.M.; Yu, Z.W.; Zhang, Y.L.; Xu, Z.Z. Changes in nitrogen accumulation, distribution, translocation and nitrogen use efficiency in different wheat cultivars under different irrigation conditions. Plant Nutr. Fertil. Sci. 2010, 16, 1041–1048. [Google Scholar]
- Chadha, A.; Florentine, S.K.; Chauhan, B.S.; Long, B.; Jayasundera, M. Influence of soil moisture regimes on growth, photosynthetic capacity, leaf biochemistry and reproductive capabilities of the invasive agronomic weed; Lactuca serriola. PLoS ONE 2018, 14, e0218191. [Google Scholar] [CrossRef]
- Jaleel, C.A.; Manivannan, P.; Wahid, A.; Farooq, M.; Al-Juburi, H.J.; Somasundaram, R.; Panneerselvam, R. Drought stress in plants: A review on morphological characteristics and pigments composition. Int. J. Agric. Biol. 2009, 11, 100–105. [Google Scholar]
- Rathore, V.S.; Nathawat, N.S.; Bhardwaj, S.; Yadav, B.M.; Kumar, M.; Santra, P.; Kumar, P.; Reager, M.L.; Yadava, N.D.; Yadav, O.P. Optimization of deficit irrigation and nitrogen fertilizer management for peanut production in an arid region. Sci. Rep. 2021, 11, 5456. [Google Scholar] [CrossRef] [PubMed]
- Piha, M.I. Optimizing fertilizer use and practical rainfall capture in a semi-arid environment with variable rainfall. Exp. Agric. 1993, 29, 405–415. [Google Scholar] [CrossRef]
- Zhang, C.; Xi, N. Precipitation Changes Regulate Plant and Soil Microbial Biomass Via Plasticity in Plant Biomass Allocation in Grasslands: A Meta-Analysis. Front. Plant Sci. 2021, 12, 614968. [Google Scholar] [CrossRef]
- Li, S.X.; Wang, Z.H.; Stewart, B.A. Responses of Crop Plants to Ammonium and Nitrate N. Adv. Agron. 2013, 118, 205–397. [Google Scholar] [CrossRef]
- Hassanzadeh, M.; Ebadi, A.; Panahyan-e-Kivi, M.; Eshghi, A.G.; Jamaati-e-Somarin, S.; Saeidi, M.; Zabihi-e-Mahmoodabad, R. Evaluation of Drought Stress on Relative Water Content and Chlorophyll Content of Sesame (Sesamum indicum L.) Genotypes at Early Flowering Stage. Res. J. Environ. Sci. 2009, 3, 345–350. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; He, N.; Hou, J.; Xu, L.; Liu, C.; Zhang, J.; Wang, Q.; Zhang, X.; Wu, X. Factors influencing leaf chlorophyll content in natural forests at the biome scale. Front. Ecol. Evol. 2018, 6, 64. [Google Scholar] [CrossRef] [Green Version]
- Zhou, G.S. Effect of water stress on photochemical activity of chloroplast from wheat. J. Beijing Agric. Coll. 2003, 18, 188–190. [Google Scholar] [CrossRef]
- Hailemichael, G.; Catalina, A.; González, M.R.; Martin, P. Relationships between water status, leaf chlorophyll content and photosynthetic performance in Tempranillo vineyards. S. Afr. J. Enol. Vitic. 2016, 37, 149–156. [Google Scholar] [CrossRef]
- Gholamin, R.; Khayatnezhad, M. The effect of end season drought stress on the chlorophyll content, chlorophyll fluorescence parameters and yield in maize cultivars. Sci. Res. Essays 2011, 6, 5351–5357. [Google Scholar] [CrossRef]
- Schlemmer, M.R.; Francis, D.D.; Shanahan, J.F.; Schepers, J.S. Remotely measuring chlorophyll content in corn leaves with differing nitrogen levels and relative water content. Agron. J. 2005, 97, 106–112. [Google Scholar] [CrossRef] [Green Version]
- Hamblin, J.; Stefanova, K.; Angessa, T.T. Variation in chlorophyll content per unit leaf area in spring wheat and implications for selection in segregating material. PLoS ONE 2014, 9, e92529. [Google Scholar] [CrossRef] [Green Version]
- Sanchez, R.A.; Hall, A.J.; Trapani, N. Effects of water stress on the chlorophyll content, nitrogen level and photosynthesis of leaves of two maize genotypes. Water 1983, 7, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Brougham, R.W. The Relationship between the Critical Leaf Area, Total Chlorophyll Content, and Maximum Growth-rate of some Pasture and Crop Plants. Ann. Bot. 1960, 24, 463–474. [Google Scholar] [CrossRef]
- Khajeddin, S.J.; Matinkhah, S.H.; Jafari, Z. A drought resistance index to select drought resistant plant species based on leaf water potential measurements. J. Arid. Land 2019, 11, 623–635. [Google Scholar] [CrossRef] [Green Version]
- Schneider, E.; Sanders, J.; Willert, D. Von Devil’s claw (Harpagophytum procumbens) from southern Africa: Sustainable use by cultivation combined with controlled harvesting in semi-wild populations. Frontis 2006, 17, 181–202. [Google Scholar] [CrossRef]
- Ulziykhutag, N. Overview of the Flora of Mongolia; State Publishing: Ulaanbaatar, Mongolia, 1989. [Google Scholar]
- Lavrenko, E.M.; Karamysheva, Z.V.; Nikulina, R.I. Steppes of Eurasia; Nauka: Leningrad, Russia, 1991. [Google Scholar]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2014, Update 2015. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; FAO: Rome, Italy, 2015; ISBN 9789251083697. [Google Scholar]
- Batkhishig, O. Soil classification of Mongolia. J. Mong. Soil Sci. 2016, 1, 18–31. [Google Scholar]
- NAMEM The National Agency for Meteorology and Environmental Monitoring of Mongolia. Weather Data. Available online: http://namem.gov.mn/eng/?p=56 (accessed on 2 October 2019).
- Byambadorj, S.O.; Chiatante, D.; Akhmadi, K.; Lunten, J.; Ochirbat, B.; Park, B.B.; Scippa, G.S.; Montagnoli, A.; Nyam-Osor, B. The effect of different watering regimes and fertilizer addition on the growth of tree species used to afforest the semi-arid steppe of Mongolia. Plant Biosyst. 2020, 155, 747–758. [Google Scholar] [CrossRef]
- Johnson, H.; Brandle, J. Shelterbelt Design. Available online: http://agriculture.vic.gov.au/agriculture/farm-management/soil-and-water/erosion/shelterbelt-design (accessed on 15 September 2019).
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Hernandez, J.O.; Maldia, L.S.; Pulan, D.E.; Buot, I.E.; Park, B.B. Leaf architecture and petiole anatomy of Philippine Dipterocarpus species (Dipterocarpaceae). Bangladesh J. Plant Taxon. 2020, 27, 1–14. [Google Scholar] [CrossRef]
- Li, Y.; Johnson, D.A.; Su, Y.; Cui, J.; Zhang, T. Specific leaf area and leaf dry matter content of plants growing in sand dunes. Bot. Bull. Acad. Sin. 2005, 46, 127–134. [Google Scholar]
- Kousar, M.; Suresh, G.B.; Lavanya, G.R.; Grard, A. Studies of chlorophyll content by different methods in Black Gram. Int. J. Agric. Res. 2007, 2, 651–654. [Google Scholar] [CrossRef]
- Wellburn, A.R. The Spectral Determination of Chlorophylls a and b, as well as Total Carotenoids, Using Various Solvents with Spectrophotometers of Different Resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Scholander, P.F.; Hammel, H.T.; Hemmingsen, E.; Bradstreet, E.D. Hydrostatic Pressure and Osmotic Potential in Leaves of Mangroves and Some Other Plants. Proc. Natl. Acad. Sci. USA 1964, 52, 119–125. [Google Scholar] [CrossRef] [Green Version]
- SAS Institute Inc SAS Software 9.4; SAS Inst. Inc.: Cary, NC, USA, 2014; pp. 1–25.







| Species | Treatment | June | July | August | |
|---|---|---|---|---|---|
| U. pumila | ψp | 0 L h−1 | −1.366 a | −1.133 a | −1.441 a |
| 2 L h−1 | −1.096 a | −1.096 a | −1.290 a | ||
| 4 L h−1 | −1.091 a | −1.091 a | −1.279 a | ||
| 8 L h−1 | −1.055 a | −1.055 a | −1.044 a | ||
| Ψm | 0 L h−1 | −1.486 a | −1.686 a | −2.270 c | |
| 2 L h−1 | −1.334 a | −1.559 a | −1.946 b | ||
| 4 L h−1 | −1.286 a | −1.534 a | −1.526 a | ||
| 8 L h−1 | −1.286 a | −1.478 a | −1.737 ab | ||
| P. sibirica | ψp | 0 L h−1 | −1.243 a | −1.110 a | −1.441 |
| 2 L h−1 | −1.143 a | −1.144 a | −1.291 | ||
| 4 L h−1 | −1.126 a | −1.126 a | −1.279 | ||
| 8 L h−1 | −1.155 a | −1.155 a | −1.044 | ||
| Ψm | 0 L h−1 | −1.977 c | −1.977 b | −2.271 b | |
| 2 L h−1 | −1.821 bc | −1.821 b | −1.946 ab | ||
| 4 L h−1 | −1.533 ab | −1.533 ab | −1.802 ab | ||
| 8 L h−1 | −1.453 a | −1.453 a | −1.711 a |
| Species | Treatment | June | July | August | |
|---|---|---|---|---|---|
| U. pumila | ψp | 0 L h−1 | −0.548 a | −0.823 b | −0.677 a |
| 2 L h−1 | −0.520 a | −0.808 b | −0.534 a | ||
| 4 L h−1 | −0.513 a | −0.789 b | −0.516 a | ||
| 8 L h−1 | −0.518 a | −0.619 a | −0.582 a | ||
| Ψm | 0 L h−1 | −1.071 a | −1.394 ab | −1.601 a | |
| 2 L h−1 | −1.026 a | −1.403 b | −1.570 a | ||
| 4 L h−1 | −1.197 a | −1.272 a | −1.572 a | ||
| 8 L h−1 | −1.145 a | −1.339 a | −1.524 a | ||
| P. sibirica | ψp | 0 L h−1 | −0.291 a | −0.312 a | −0.503 a |
| 2 L h−1 | −0.270 a | −0.245 a | −0.457 a | ||
| 4 L h−1 | −0.192 a | −0.331 a | −0.436 a | ||
| 8 L h−1 | −0.172 b | −0.297 a | −0.575 a | ||
| Ψm | 0 L h−1 | −1.457 b | −1.045 ab | −1.750 b | |
| 2 L h−1 | −1.369 b | −1.095 b | −1.620 ab | ||
| 4 L h−1 | −1.398 b | −0.988 a | −1.506 a | ||
| 8 L h−1 | −1.022 a | −1.014 ab | −1.550 ab |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Byambadorj, S.-O.; Park, B.B.; Hernandez, J.O.; Tsedensodnom, E.; Byambasuren, O.; Montagnoli, A.; Chiatante, D.; Nyam-Osor, B. Effects of Irrigation and Fertilization on the Morphophysiological Traits of Populus sibirica Hort. Ex Tausch and Ulmus pumila L. in the Semiarid Steppe Region of Mongolia. Plants 2021, 10, 2407. https://doi.org/10.3390/plants10112407
Byambadorj S-O, Park BB, Hernandez JO, Tsedensodnom E, Byambasuren O, Montagnoli A, Chiatante D, Nyam-Osor B. Effects of Irrigation and Fertilization on the Morphophysiological Traits of Populus sibirica Hort. Ex Tausch and Ulmus pumila L. in the Semiarid Steppe Region of Mongolia. Plants. 2021; 10(11):2407. https://doi.org/10.3390/plants10112407
Chicago/Turabian StyleByambadorj, Ser-Oddamba, Byung Bae Park, Jonathan O. Hernandez, Enkhchimeg Tsedensodnom, Otgonsaikhan Byambasuren, Antonio Montagnoli, Donato Chiatante, and Batkhuu Nyam-Osor. 2021. "Effects of Irrigation and Fertilization on the Morphophysiological Traits of Populus sibirica Hort. Ex Tausch and Ulmus pumila L. in the Semiarid Steppe Region of Mongolia" Plants 10, no. 11: 2407. https://doi.org/10.3390/plants10112407
APA StyleByambadorj, S.-O., Park, B. B., Hernandez, J. O., Tsedensodnom, E., Byambasuren, O., Montagnoli, A., Chiatante, D., & Nyam-Osor, B. (2021). Effects of Irrigation and Fertilization on the Morphophysiological Traits of Populus sibirica Hort. Ex Tausch and Ulmus pumila L. in the Semiarid Steppe Region of Mongolia. Plants, 10(11), 2407. https://doi.org/10.3390/plants10112407











