Root Trait Variation in Lentil (Lens culinaris Medikus) Germplasm under Drought Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Growth Conditions and Treatments
2.2. Root Traits Measurement
2.3. Statistical Analysis
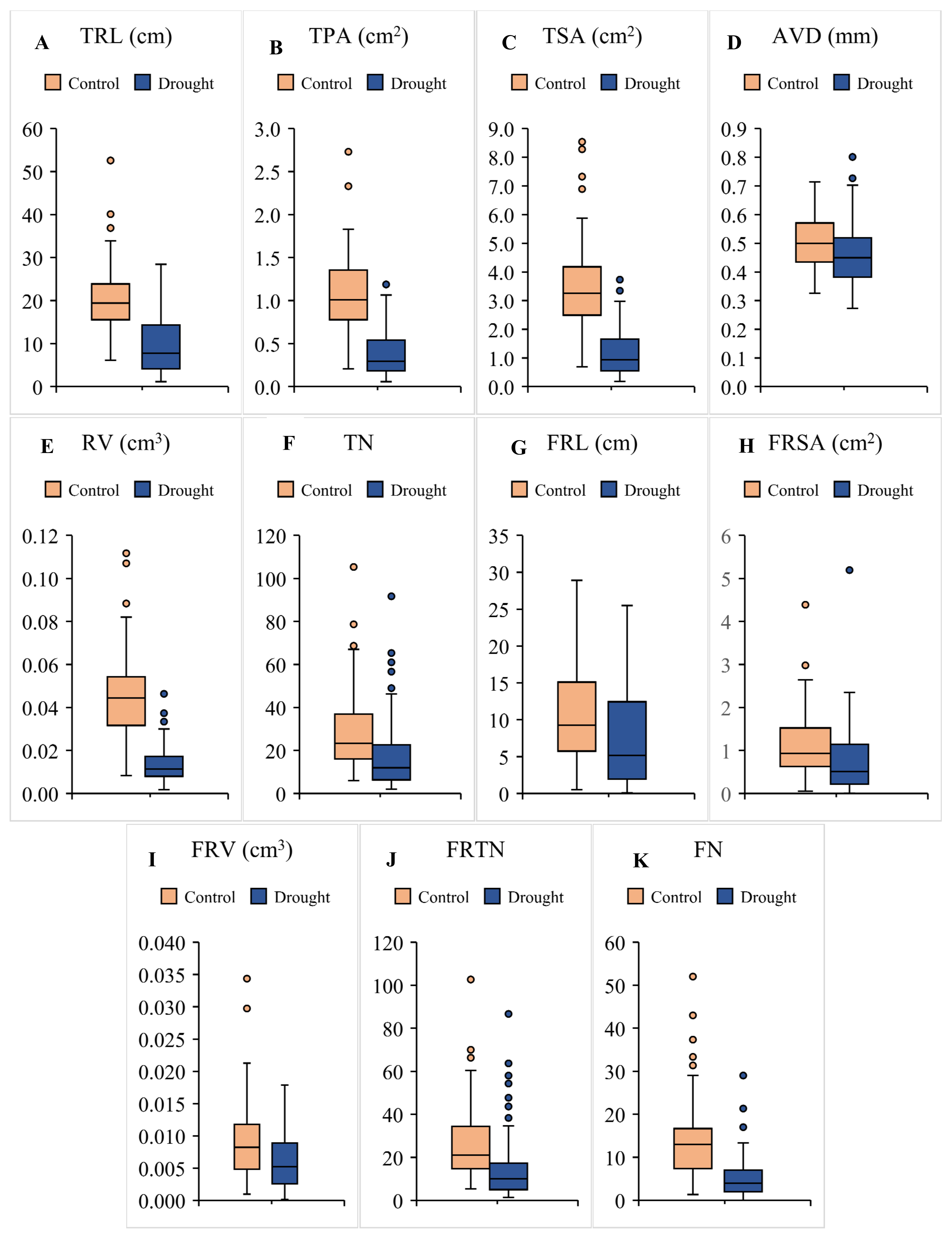
3. Results
3.1. Genetic Variation in Root Traits
3.2. Growth and Survival at Seedling Stage Drought Stress
3.3. Principal Traits and Correlation among Traits
3.4. Selection of Drought Tolerant Genotypes at Seedling Stage
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Masih, I.; Maskey, S.; Mussá, F.E.F.; Trambauer, P. A review of droughts on the African continent: A geospatial and long-term perspective. Hydrol. Earth Syst. Sci. 2014, 18, 3635–3649. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Yang, P.; Zhou, X. Identification of the driving forces of climate change using the longest instrumental temperature record. Sci. Rep. 2017, 7, 46091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naumann, G.; Alfieri, L.; Wyser, K.; Mentaschi, L.; Betts, R.A.; Carrao, H.; Spinoni, J.; Vogt, J.; Feyen, L. Global changes in drought conditions under different levels of warming. Geophys. Res. Lett. 2018, 45, 3285–3296. [Google Scholar] [CrossRef]
- Zhu, J.K. Salt and drought stress signal transduction in plants. Annu. Rev. Plant Biol. 2002, 53, 247–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salehi, M. The study of drought tolerance of lentil (Lens culinaris Medik) in seedling growth stages. Int. J. Agron. Plant Prod. 2012, 3, 38–41. [Google Scholar]
- Kumar, J.; Basu, P.S.; Srivastava, E.; Chaturvedi, S.K.; Nadarajan, N.; Kumar, S. Phenotyping of traits imparting drought tolerance in lentil. Crop Pasture Sci. 2012, 63, 547. [Google Scholar] [CrossRef]
- Mishra, B.K.; Srivastava, J.P.; Lal, J.P. Physiological and biochemical adaptations in lentil genotypes under drought stress. Russ. J. Plant Physiol. 2016, 63, 695–708. [Google Scholar] [CrossRef]
- Sehgal, A.; Sita, K.; Kumar, J.; Kumar, S.; Singh, S.; Siddique, K.H.M.; Nayyar, H. Effects of Drought, Heat and Their Interaction on the Growth, Yield and Photosynthetic Function of Lentil (Lens culinaris Medikus) Genotypes Varying in Heat and Drought Sensitivity. Front. Plant Sci. 2017, 8, 1776. [Google Scholar] [CrossRef] [Green Version]
- Wasson, A.P.; Richards, R.A.; Chatrath, R.; Misra, S.C.; Prasad, S.S.; Rebetzke, G.J.; Kirkegaard, J.A.; Wong, M.M.L.; Gujaria-Verma, N.; Ramsay, L.; et al. Classification and Characterization of Species within the Genus Lens Using Genotyping-by-Sequencing (GBS). PLoS ONE 2015, 10, e0122025. [Google Scholar]
- Fried, H.G.; Narayanan, S.; Fallen, B. Evaluation of soybean (Glycine max L. Merr.) genotypes for yield, water use efficiency, and root traits. PLoS ONE 2019, 14, e0212700. [Google Scholar] [CrossRef]
- Sofi, P.A.; Djanaguiraman, M.; Siddique, K.H.M.; Prasad, P.V.V. Reproductive fitness in common bean (Phaseolus vulgaris L.) under drought stress is associated with root length and volume. Indian J. Plant Physiol. 2018, 23, 796–809. [Google Scholar] [CrossRef]
- Ramamoorthy, P.; Lakshmanan, K.; Upadhyaya, H.D.; Vadez, V.; Varshney, R.K. Root traits confer grain yield advantages under terminal drought in chickpea (Cicer arietinum L.). Field Crops Res. 2017, 201, 146–161. [Google Scholar] [CrossRef] [Green Version]
- Wang, N.; Daun, J. Effects of variety and crude protein content on nutrients and anti-nutrients in lentils (Lens culinaris). Food Chem. 2006, 95, 493–502. [Google Scholar] [CrossRef]
- Hamadi, A.; Erskine, W. Reaction of wild species of the genus Lens to drought. Euphytica 1996, 91, 173–179. [Google Scholar] [CrossRef]
- Gorim, L.Y.; Vandenberg, A. Evaluation of wild lentil species as genetic resources to improve drought tolerance in cultivated lentil. Front. Plant Sci. 2017, 8, 1129. [Google Scholar] [CrossRef]
- Singh, D.; Dikshit, H.K.; Singh, R. A new phenotyping technique for screening for drought tolerance in lentil (Lens culinaris Medik.). Plant Breed. 2013, 132, 185–190. [Google Scholar] [CrossRef]
- Takahashi, H.; Pradal, C. Root phenotyping: Important and minimum information required for root modeling in crop plants. Breed. Sci. 2021, 71, 109–116. [Google Scholar] [CrossRef]
- Gorim, L.Y.; Rabani, E.M.; Barlow, B.; De Silva, D.; Vandenberg, A. Are artificial media valid for root analysis? A case study comparing root traits of five lentil genotypes in artificial media versus Soil. J. Soil Sci. Plant Health 2018, 2, 1. [Google Scholar]
- Singh, D.; Singh, C.K.; Taunk, J.; Tomar, R.S.S. Genetic analysis and molecular mapping of seedling survival drought tolerance gene in lentil (Lens culinaris Medikus). Mol. Breed. 2016, 36, 58. [Google Scholar] [CrossRef]
- Fernandez, G.C. Effective selection criteria for assessing plant stress tolerance. In Proceedings of the International Symposium on Adaptation of Vegetables and Other Food Crops in Temperature and Water Stress, Taiwan, 13–18 August 1992. [Google Scholar]
- Malamy, J.E. Intrinsic and environmental response pathways that regulate root system architecture. Plant Cell Environ. 2005, 28, 67–77. [Google Scholar] [CrossRef]
- Kell, D.B. Breeding crop plants with deep roots: Their role in sustainable carbon, nutrient and water sequestration. Ann. Bot. 2011, 108, 407–418. [Google Scholar] [CrossRef] [Green Version]
- Idrissi, O.; Udupa, S.M.; De Keyser, E.; McGee, R.J.; Coyne, C.J.; Saha, G.C.; Muehlbauer, F.J.; Van Damme, P.; De Riek, J. Identification of Quantitative Trait Loci controlling root and shoot traits associated with drought tolerance in a lentil (Lens culinaris Medik.) recombinant inbred line population. Front. Plant Sci. 2016, 7, 1174. [Google Scholar] [CrossRef] [Green Version]
- Lou, Q.; Chen, L.; Mei, H.; Xu, K.; Wei, H.; Feng, F.; Li, T.; Pang, X.; Shi, C.; Luo, L.; et al. Root Transcriptomic analysis revealing the importance of energy metabolism to the development of deep roots in rice (Oryza sativa L.). Front. Plant Sci. 2017, 8, 1314. [Google Scholar] [CrossRef] [Green Version]
- Tracy, S.R.; Nagel, K.A.; Postma, J.A.; Fassbender, H.; Wasson, A.; Watt, M. Crop improvement from phenotyping roots: Highlights reveal expanding opportunities. Trends Plant Sci. 2020, 25, 105–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhasi, A.; Senalik, D.; Simon, P.W.; Kumar, B.; Manikandan, V.; Philip, P.; Senapathy, P. RoBuST: An integrated genomics resource for the root and bulb crop families Apiaceae and Alliaceae. BMC Plant Biol. 2010, 10, 161. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Zhou, Q.; He, M.; Zhong, X.; Guixiang, T. Wilting index and root morphological characteristics used as drought-tolerance variety selection at the seedling stage in soybean (Glycine max L.). Plant Growth Regul. 2020, 92, 29–42. [Google Scholar] [CrossRef]
- Canales, F.J.; Nagel, K.A.; Müller, C.; Rispail, N.; Prats, E. Deciphering root architectural traits involved to cope with water deficit in Oat. Front. Plant Sci. 2019, 10, 1558. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Iqbal, M. Breeding for drought tolerance in wheat (Triticum aestivum L.): Constraints and future prospects. Front. Agric. China 2011, 5, 31–34. [Google Scholar] [CrossRef]
- Prince, S.J.; Valliyodan, B.; Ye, H.; Yang, M.; Tai, S.; Hu, W.; Murphy, M.; Durnell, L.A.; Song, L.; Joshi, T.; et al. Understanding genetic control of root system architecture in soybean: Insights into the genetic basis of lateral root number. Plant Cell Environ. 2019, 42, 212–229. [Google Scholar] [CrossRef] [Green Version]
- Clark, L.J.; Whalley, W.R.; Barraclough, P.B. How do roots penetrate strong soil? Plant Soil 2003, 255, 93–104. [Google Scholar] [CrossRef]
- Vanhees, D.J.; Loades, K.W.; Bengough, A.G.; Mooney, S.J.; Lynch, J.P. Root anatomical traits contribute to deeper rooting of maize under compacted field conditions. J. Exp. Bot. 2020, 71, 4243–4257. [Google Scholar] [CrossRef] [PubMed]
- Prince, S.J.; Murphy, M.; Mutava, R.N.; Durnell, L.A.; Valliyodan, B.; Shannon, J.G.; Nguyen, H.T. Root xylem plasticity to improve water use and yield in water-stressed soybean. J. Exp. Bot. 2017, 68, 2027–2036. [Google Scholar] [CrossRef] [Green Version]
- Gorim, L.; Vandenberg, A. Variation in total root length and root diameter of wild and cultivated lentil grown under drought and re-watered conditions. Plant Genet. Resour. Charact. Util. 2019, 17, 45–53. [Google Scholar] [CrossRef]
- Reddy, V.R.P.; Aski, M.S.; Mishra, G.P.; Dikshit, H.K.; Singh, A.; Pandey, R.; Singh, M.P.; Gayacharan; Ramtekey, V.; Priti; et al. Genetic variation for root architectural traits in response to phosphorus deficiency in mungbean at the seedling stage. PLoS ONE 2020, 15, e0221008. [Google Scholar] [CrossRef]
- Muscolo, A.; Sidari, M.; Anastasi, U.; Santonoceto, C.; Maggio, A. Effect of PEG-induced drought stress on seed germination of four lentil genotypes. J. Plant Interact. 2014, 9, 354–363. [Google Scholar] [CrossRef]
- Sallam, A.; Mourad, A.M.I.; Hussain, W.; Baenziger, P.S. Genetic variation in drought tolerance at seedling stage and grain yield in low rainfall environments in wheat (Triticum aestivum L.). Euphytica 2018, 214, 169. [Google Scholar] [CrossRef]
- Strock, C.F.; Burridge, J.; Massas, A.S.F.; Beaver, J.; Beebe, S.; Camilo, S.A.; Fourie, D.; Jochua, C.; Miguel, M.; Miklas, P.N.; et al. Seedling root architecture and its relationship with seed yield across diverse environments in Phaseolus vulgaris. Field Crops Res. 2019, 237, 53–64. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Priya, S.; Bansal, R.; Kumar, G.; Dikshit, H.K.; Kumari, J.; Pandey, R.; Singh, A.K.; Tripathi, K.; Singh, N.; Kumari, N.K.P.; et al. Root Trait Variation in Lentil (Lens culinaris Medikus) Germplasm under Drought Stress. Plants 2021, 10, 2410. https://doi.org/10.3390/plants10112410
Priya S, Bansal R, Kumar G, Dikshit HK, Kumari J, Pandey R, Singh AK, Tripathi K, Singh N, Kumari NKP, et al. Root Trait Variation in Lentil (Lens culinaris Medikus) Germplasm under Drought Stress. Plants. 2021; 10(11):2410. https://doi.org/10.3390/plants10112410
Chicago/Turabian StylePriya, Swati, Ruchi Bansal, Gaurav Kumar, Harsh Kumar Dikshit, Jyoti Kumari, Rakesh Pandey, Amit Kumar Singh, Kuldeep Tripathi, Narender Singh, N. K. Prasanna Kumari, and et al. 2021. "Root Trait Variation in Lentil (Lens culinaris Medikus) Germplasm under Drought Stress" Plants 10, no. 11: 2410. https://doi.org/10.3390/plants10112410





