Legume-Based Mobile Green Manure Can Increase Soil Nitrogen Availability and Yield of Organic Greenhouse Tomatoes
Abstract
:1. Introduction
2. Results
2.1. Legume Biomass, BNF and Soil N Supply
2.2. Soil Measurements
2.3. Tomato Growth, Yield and Yield Components
2.4. Tomato Tissue Analysis and N Partitioning to Fruit
3. Discussion
3.1. Faba Bean Aboveground Biomass, N Accumulation and BNF
3.2. Soil Nutrient Availability
3.3. Tomato Growth and Yield
4. Materials and Methods
4.1. Plant Material, Growth Conditions, and Treatments
4.2. Growth, Mineral Analysis, and N Fixation by Legumes
4.3. Tomato Tissue Sampling and Mineral Analysis
4.4. Soil Analysis
4.5. Tomato Yield and N Fruit Uptake
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gatsios, A.; Ntatsi, G.; Tampakaki, A.; Celi, L.; Savvas, D. Assessing the possibility to use legume plants as cover crops or intercrops in organic tomato production to optimize NUE. Acta Hortic. 2020, 1286, 83–89. [Google Scholar] [CrossRef]
- Sainju, U.M.; Whitehead, W.F.; Singh, B.P. Cover crops and nitrogen fertilization effects on soil aggregation and carbon and nitrogen pools. Can. J. Soil Sci. 2003, 83, 155–165. [Google Scholar] [CrossRef]
- Gianquinto, G.; Muñoz, P.; Pardossi, A.; Ramazzotti, S.; Savvas, D. Soil fertility and plant nutrition. In Good Agricultural Practices for Greenhouse Vegetable Crops. Principles for Mediterranean Climate Areas; FAO: Rome, Italy, 2013; pp. 205–269. ISBN 9789251076491. [Google Scholar]
- Voogt, W.; de Visser, P.H.E.; van Winkel, A.; Cuijpers, W.J.M.; Van de Burgt, G.J.H.M. Nutrient management in organic greenhouse production: Navigation between constraints. Acta Hortic. 2011, 915, 75–82. [Google Scholar] [CrossRef] [Green Version]
- The Commission of the European Communities. Commission Regulation (EC) No 889/2008. Off. J. Eur. Union 2008, 250, 1–84. [Google Scholar]
- Regulation (EU) 2018/848 of the European Parliament and of the Council of 30 May 2018 on organic production and labelling of organic products and repealing Council Regulation (EC) No 834/2007. Off. J. Eur. Union 2018, 150, 1–92.
- Baldwin, K.R. Soil Fertility on Organic Farms; North Carolina Cooperative Extension: Raleigh, NC, USA, 2006; pp. 1–32. [Google Scholar]
- Nair, A.; Delate, K. Composting, crop rotation, and cover crop practices in organic vegetable production. In Sustainable Development and Biodiversity; Springer: Berlin/Heidelberg, Germany, 2016; pp. 231–257. [Google Scholar]
- Tittarelli, F.; Båth, B.; Ceglie, F.G.; del Carmen Garcia, M.; Möller, K.; Reents, H.J.; Védie, H.; Voogt, W. Soil Fertility Management in Organic Greenhouses in Europe; BioGreenhouse: Wageningen, The Netherlands, 2016; pp. 1–48. ISBN 9789462575363. [Google Scholar]
- Reckling, M.; Bergkvist, G.; Watson, C.A.; Stoddard, F.L.; Zander, P.M.; Walker, R.L.; Pristeri, A.; Toncea, I.; Bachinger, J. Trade-Offs between Economic and Environmental Impacts of Introducing Legumes into Cropping Systems. Front. Plant Sci. 2016, 7, 669. [Google Scholar] [CrossRef]
- Peoples, M.B.; Brockwell, J.; Herridge, D.F.; Rochester, I.J.; Alves, B.J.R.; Urquiaga, S.; Boddey, R.M.; Dakora, F.D.; Bhattarai, S.; Maskey, S.L.; et al. The contributions of nitrogen-fixing crop legumes to the productivity of agricultural systems. Symbiosis 2009, 48, 1–17. [Google Scholar] [CrossRef]
- Stagnari, F.; Maggio, A.; Galieni, A.; Pisante, M. Multiple benefits of legumes for agriculture sustainability: An overview. Chem. Biol. Technol. Agric. 2017, 4, 2. [Google Scholar] [CrossRef] [Green Version]
- Thönnissen, C.; Midmore, D.J.; Ladha, J.K.; Holmer, R.J.; Schmidhalter, U. Tomato crop response to short-duration legume green manures in tropical vegetable systems. Agron. J. 2000, 92, 245–253. [Google Scholar] [CrossRef]
- Campiglia, E.; Mancinelli, R.; Radicetti, E. Influence of no-tillage and organic mulching on tomato (Solanum lycopersicum L.) production and nitrogen use in the mediterranean environment of central Italy. Sci. Hortic. 2011, 130, 588–598. [Google Scholar] [CrossRef]
- Sugihara, Y.; Ueno, H.; Hirata, T.; Araki, H. Uptake and distribution of nitrogen derived from hairy vetch used as a cover crop by tomato plant. J. Jpn. Soc. Hortic. Sci. 2013, 82, 30–38. [Google Scholar] [CrossRef] [Green Version]
- Araki, H. Tomato Production with Cover Crops in Greenhouse. In Alternative Crops and Cropping Systems; Intechopen: London, UK, 2016; p. 87. [Google Scholar] [CrossRef] [Green Version]
- Gatsios, A.; Ntatsi, G.; Celi, L.; Said-Pullicino, D.; Tampakaki, A.; Giannakou, I.; Savvas, D. Nitrogen Nutrition Optimization in Organic Greenhouse Tomato Through the Use of Legume Plants as Green Manure or Intercrops. Agronomy 2019, 9, 766. [Google Scholar] [CrossRef] [Green Version]
- Gatsios, A.; Ntatsi, G.; Celi, L.; Said-Pullicino, D.; Tampakaki, A.; Savvas, D. Impact of legumes as a pre-crop on nitrogen nutrition and yield in organic greenhouse tomato. Plants 2021, 10, 468. [Google Scholar] [CrossRef]
- Van der Burgt, G.J.; Rietema, C.; Bus, M. Planty Organic 5 Year: Evaluation of Soil Fertility, Nitrogen Dynamics and Production; Louis Bolk Institute: Bunnik, The Netherlands, 2018; pp. 1–38. [Google Scholar]
- Palomba, I. Effects of C: N Ratio in Cut-and-Carry Green Manure and Nitrogen Application Rate in Organic Potato Production. Master’s Thesis, Wageningen University, Wageningen, The Netherlands, 2016. [Google Scholar]
- Sorensen, J.N.; Grevsen, K. Strategies for cut-and-carry green manure production. Acta Hortic. 2016, 1137, 39–45. [Google Scholar] [CrossRef]
- Barker, A.V. Science and Technology of Organic Farming; CRC Press: Boca Raton, FL, USA, 2016; pp. 1–225. ISBN 1439882134. [Google Scholar]
- Unkovich, M.; Herridge, D.; Peoples, M.; Cadisch, G.; Boddey, B.; Giller, K.; Alves, B.; Chalk, P. Biological nitrogen fixation. In Measuring Plant-Associated Nitrogen Fixation in Agricultural Systems; ACIAR: Canberra, Australia, 2008; pp. 9–20. ISBN 978-1-921531-26-2. [Google Scholar]
- Kaniszewski, S.; Babik, I.; Babik, J. New Pelleted Plant-Based Fertilizers for Sustainable Onion Production. Univers. J. Agric. Res. 2019, 7, 210–220. [Google Scholar] [CrossRef] [Green Version]
- Qian, P.; Schoenau, J.J.; King, T.; Fatteicher, C. Effect of soil amendment with alfalfa powders and distillers grains on nutrition and growth of canola. J. Plant Nutr. 2011, 34, 1403–1417. [Google Scholar] [CrossRef]
- Karavidas, I.; Ntatsi, G.; Ntanasi, T.; Vlachos, I.; Tampakaki, A.; Iannetta, P.P.M.; Savvas, D. Comparative Assessment of Different Crop Rotation Schemes for Organic Common Bean Production. Agronomy 2020, 10, 1269. [Google Scholar] [CrossRef]
- Berry, P.M.; Sylvester-Bradley, R.; Philipps, L.; Hatch, D.J.; Cuttle, S.P.; Rayns, F.W.; Gosling, P. Is the productivity of organic farms restricted by the supply of available nitrogen? Soil Use Manag. 2002, 18, 248–255. [Google Scholar] [CrossRef]
- Dahlin, S.; Kirchmann, H.; Kätterer, T.; Gunnarsson, S.; Bergström, L. Possibilities for improving nitrogen use from organic materials in agricultural cropping systems. Ambio 2005, 34, 288–295. [Google Scholar] [CrossRef]
- Watson, C.A.; Atkinson, D.; Gosling, P.; Jackson, L.R.; Rayns, F.W. Managing soil fertility in organic farming systems. Soil Use Manag. 2002, 18, 239–247. [Google Scholar] [CrossRef] [Green Version]
- De Neve, S. Organic Matter Mineralization as a Source of Nitrogen. In Advances in Research on Fertilization Management of Vegetable Crops; Tei, F., Nicola, S., Benincasa, P., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 65–83. ISBN 978-3-319-53626-2. [Google Scholar]
- Watson, C.; Stockdale, E.; Philipps, L. Laboratory Mineral Soil Analysis and Soil Mineral Management in Organic Farming; Institute of Organic Training & Advice: Craven Arms, UK, 2008. [Google Scholar]
- Clark, M.S.; Horwath, W.R.; Shennan, C.; Scow, K.M.; Lantni, W.T.; Ferris, H. Nitrogen, weeds and water as yield-limiting factors in conventional, low-input, and organic tomato systems. Agric. Ecosyst. Environ. 1999, 73, 257–270. [Google Scholar] [CrossRef]
- Lenzi, A.; Antichi, D.; Bigongiali, F.; Mazzoncini, M.; Migliorini, P.; Tesi, R. Effect of different cover crops on organic tomato production. Renew. Agric. Food Syst. 2009, 24, 92–101. [Google Scholar] [CrossRef]
- Ren, T.; Christie, P.; Wang, J.; Chen, Q.; Zhang, F. Root zone soil nitrogen management to maintain high tomato yields and minimum nitrogen losses to the environment. Sci. Hortic. 2010, 125, 25–33. [Google Scholar] [CrossRef] [Green Version]
- Sainju, U.M.; Singh, B.P.; Whitehead, W.F. Comparison of the effects of cover crops and nitrogen fertilization on tomato yield, root growth, and soil properties. Sci. Hortic. 2001, 91, 201–214. [Google Scholar] [CrossRef]
- Sainju, U.M.; Dris, R.; Singh, B. Mineral nutrition of tomato. Food Agric. Environ. 2003, 1, 176–184. [Google Scholar]
- Van Eysinga, J.R. Fertilization of Tomatoes with Nitrogen; Pudoc: Wageningen, The Netherlands, 1971; p. 17. [Google Scholar]
- Thompson, R.B.; Tremblay, N.; Fink, M.; Gallardo, M.; Padilla, F.M. Tools and Strategies for Sustainable Nitrogen Fertilisation of Vegetable Crops. In Advances in Research on Fertilization Management of Vegetable Crops; Tei, F., Nicola, S., Benincasa, P., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 11–63. ISBN 978-3-319-53626-2. [Google Scholar]
- Herridge, D.F.; Peoples, M.B.; Boddey, R.M. Global inputs of biological nitrogen fixation in agricultural systems. Plant Soil 2008, 311, 1–18. [Google Scholar] [CrossRef]
- Ntatsi, G.; Karkanis, A.; Yfantopoulos, D.; Pappa, V.; Konosonoka, I.H.; Travlos, I.; Bilalis, D.; Bebeli, P.; Savvas, D. Evaluation of the field performance, nitrogen fixation efficiency and competitive ability of pea landraces grown under organic and conventional farming systems. Arch. Agron. Soil Sci. 2019, 65, 294–307. [Google Scholar] [CrossRef]
- Ruisi, P.; Amato, G.; Badagliacca, G.; Frenda, A.S.; Giambalvo, D.; Di Miceli, G. Agro-ecological benefits of faba bean for rainfed mediterranean cropping systems. Ital. J. Agron. 2017, 12, 233–245. [Google Scholar] [CrossRef] [Green Version]
- Peoples, M.B.; Herridge, D.F.; Ladha, J.K. Biological nitrogen fixation: An efficient source of nitrogen for sustainable agricultural production. In Management of Biological Nitrogen Fixation for the Development of More Productive and Sustainable Agricultural Systems; Springer: Berlin, Germany, 1995; pp. 3–28. [Google Scholar]
- Bryson, G.M.; Barker, A.V. Determination of optimal fertilizer concentration range for tomatoes grown in peat-based medium. Commun. Soil Sci. Plant Anal. 2002, 33, 759–777. [Google Scholar] [CrossRef]
- Barnard, R.; Leadley, P.W.; Hungate, B.A. Global change, nitrification, and denitrification: A review. Glob. Biogeochem. Cycles 2005, 19, 1–13. [Google Scholar] [CrossRef]
- Bonanomi, G.; Sarker, T.C.; Zotti, M.; Cesarano, G.; Allevato, E.; Mazzoleni, S. Predicting nitrogen mineralization from organic amendments: Beyond C/N ratio by 13 C-CPMAS NMR approach. Plant Soil 2019, 441, 129–146. [Google Scholar] [CrossRef]
- Li, Z.; Zeng, Z.; Tian, D.; Wang, J.; Fu, Z.; Zhang, F.; Zhang, R.; Chen, W.; Luo, Y.; Niu, S. Global patterns and controlling factors of soil nitrification rate. Glob. Chang. Biol. 2020, 26, 4147–4157. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, D.; Kobashi, Y.; Kai, T.; Kawagoe, T.; Kubota, K.; Araki, K.S.; Kubo, M. Suitable Soil Conditions for Tomato Cultivation under an Organic Farming System. J. Agric. Chem. Environ. 2018, 7, 117–132. [Google Scholar] [CrossRef] [Green Version]
- Sullivan, D.M.; Andrews, N.D. Estimating plant available nitrogen release from cover crops. Pac. Northwest Ext. Publ. 2012, 636, 1–23. [Google Scholar]
- Thorup-Kristensen, K.; Magid, J.; Jensen, L.S. Catch crops and green manures as biological tools in nitrogen management in temperate zones. Adv. Agron. 2003, 79, 227–302. [Google Scholar] [CrossRef]
- Bénard, C.; Gautier, H.; Bourgaud, F.; Grasselly, D.; Navez, B.; Caris-Veyrat, C.; Weiss, M.; Génard, M. Effects of low nitrogen supply on tomato (Solanum lycopersicum) fruit yield and quality with special emphasis on sugars, acids, ascorbate, carotenoids, and phenolic compounds. J. Agric. Food Chem. 2009, 57, 4112–4123. [Google Scholar] [CrossRef]
- Zotarelli, L.; Dukes, M.D.; Scholberg, J.M.S.; Muñoz-Carpena, R.; Icerman, J. Tomato nitrogen accumulation and fertilizer use efficiency on a sandy soil, as affected by nitrogen rate and irrigation scheduling. Agric. Water Manag. 2009, 96, 1247–1258. [Google Scholar] [CrossRef]
- Papadopoulos, A.P. Growing Greenhouse Tomatoes in Soil and in Soilless Media; Communications Branch, Agriculture Canada: Ottawa, ON, Canada, 1991; p. 79. ISBN 0662188594. [Google Scholar]
- Askegaard, M.; Olesen, J.E.; Rasmussen, I.A.; Kristensen, K. Nitrate leaching from organic arable crop rotations is mostly determined by autumn field management. Agric. Ecosyst. Environ. 2011, 142, 149–160. [Google Scholar] [CrossRef]
- Bustamante, S.C.; Hartz, T.K. Nitrogen management in organic processing tomato production: Nitrogen sufficiency prediction through early-season soil and plant monitoring. HortScience 2015, 50, 1055–1063. [Google Scholar] [CrossRef] [Green Version]
- Benincasa, P.; Tosti, G.; Guiducci, M.; Farneselli, M.; Tei, F. Crop rotation as a system approach for soil fertility management in vegetables. In Advances in Research on Fertilization Management of Vegetable Crops; Springer: Cham, Switzerland, 2017; pp. 115–148. [Google Scholar] [CrossRef]
- Briggs, S. Nitrogen Supply and Mmanagement in Organic Farming; Report; Institute of Organic Training and Advice (IOTA): Shropshire, UK, 2008; pp. 1–30. [Google Scholar]
- Sorensen, J.N.; Thorup-Kristensen, K. Plant-based fertilizers for organic vegetable production. J. Plant Nutr. Soil Sci. 2011, 174, 321–332. [Google Scholar] [CrossRef]
- Colla, G.; Mitchell, J.P.; Poudel, D.D.; Temple, S.R. Changes of Tomato Yield and Fruit Elemental Composition in Conventional, Low Input, and Organic Systems. J. Sustain. Agric. 2002, 20, 53–67. [Google Scholar] [CrossRef]
- Berry, P.M.; Stockdale, E.A.; Sylvester-Bradley, R.; Philipps, L.; Smith, K.A.; Lord, E.I.; Watson, C.A.; Fortune, S. N, P and K budgets for crop rotations on nine organic farms in the UK. Soil Use Manag. 2003, 19, 112–118. [Google Scholar] [CrossRef]
- Efstathiadou, E.; Savvas, D.; Tampakaki, A.P. Genetic diversity and phylogeny of indigenous rhizobia nodulating faba bean (Vicia faba L.) in Greece. Syst. Appl. Microbiol. 2020, 43, 126149. [Google Scholar] [CrossRef] [PubMed]
- Olsen, R.; Sommers, L.E.; Phosphorus, A.L.; Page, R.H.; Miller, D.R.K.E. Methods of Soil Analysis. In Part 2 Chemical and Microbiological Properties; ASA: Madison, WI, USA, 1982; pp. 403–427. [Google Scholar]
- Ntatsi, G.; Karkanis, A.; Yfantopoulos, D.; Olle, M.; Travlos, I.; Thanopoulos, R.; Bilalis, D.; Bebeli, P.; Savvas, D. Impact of variety and farming practices on growth, yield, weed flora and symbiotic nitrogen fixation in faba bean cultivated for fresh seed production. Acta Agric. Scand. Sect. B—Soil Plant Sci. 2018, 68, 619–630. [Google Scholar] [CrossRef]
- Unkovich, M.J.; Herridge, D.; Peoples, M.; Cadish, G.; Boddey, R.; Giller, K.; Alves, B.; Chalk, P. 15N natural abundance method. In Measuring Plant Associated Nitrogen Fixation in Agricultural Systems; ACIAR: Canberra, Australia, 2008; pp. 131–162. ISBN 978-1-921531-26-2. [Google Scholar]
- Miller, R.O.; Gavlak, R.; Horneck, D. Soil, plant and water reference methods for the western region. In WREP-125, 4th ed.; Colorado State University: Fort Collins, CO, USA, 2013; p. 155. [Google Scholar]
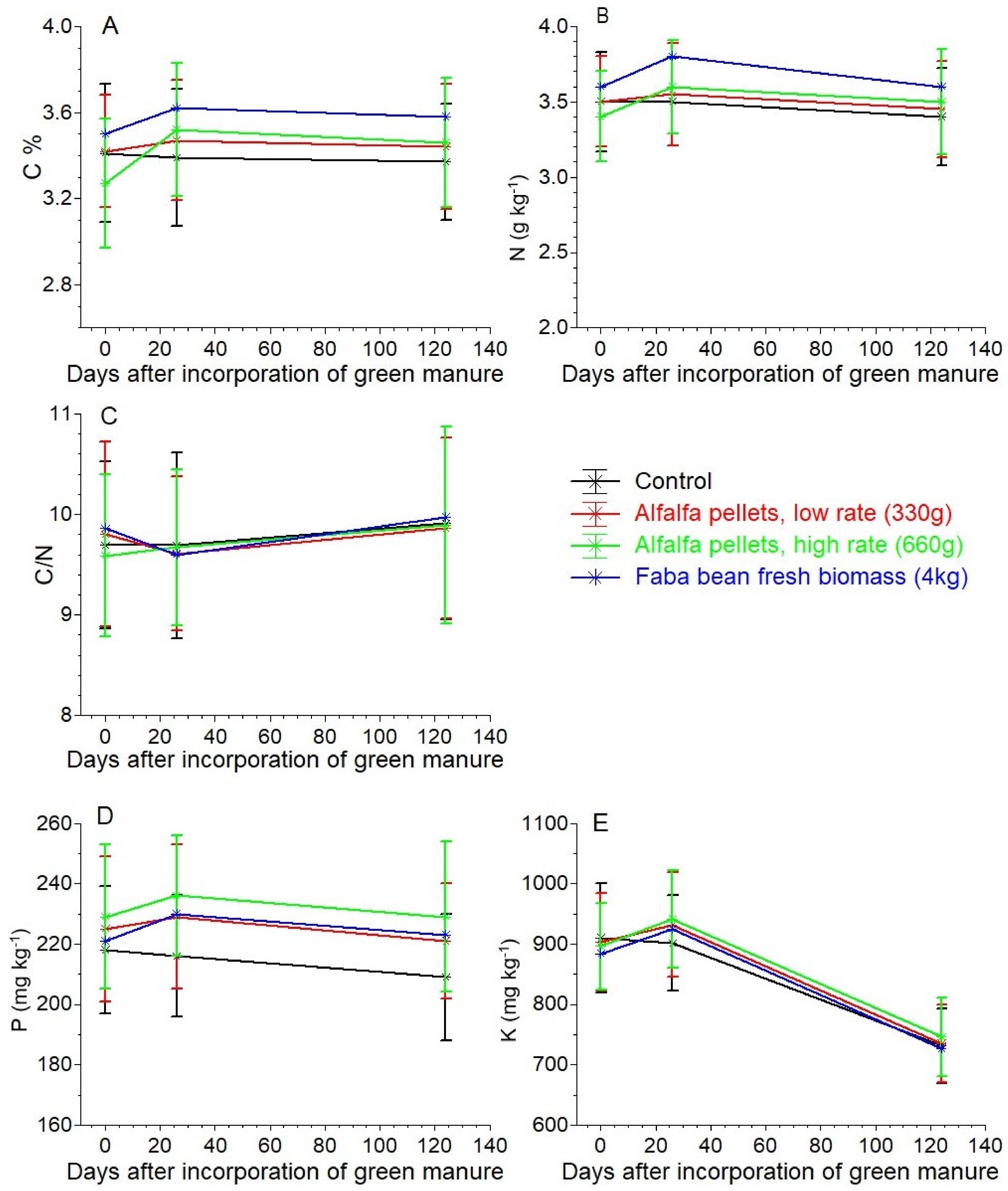
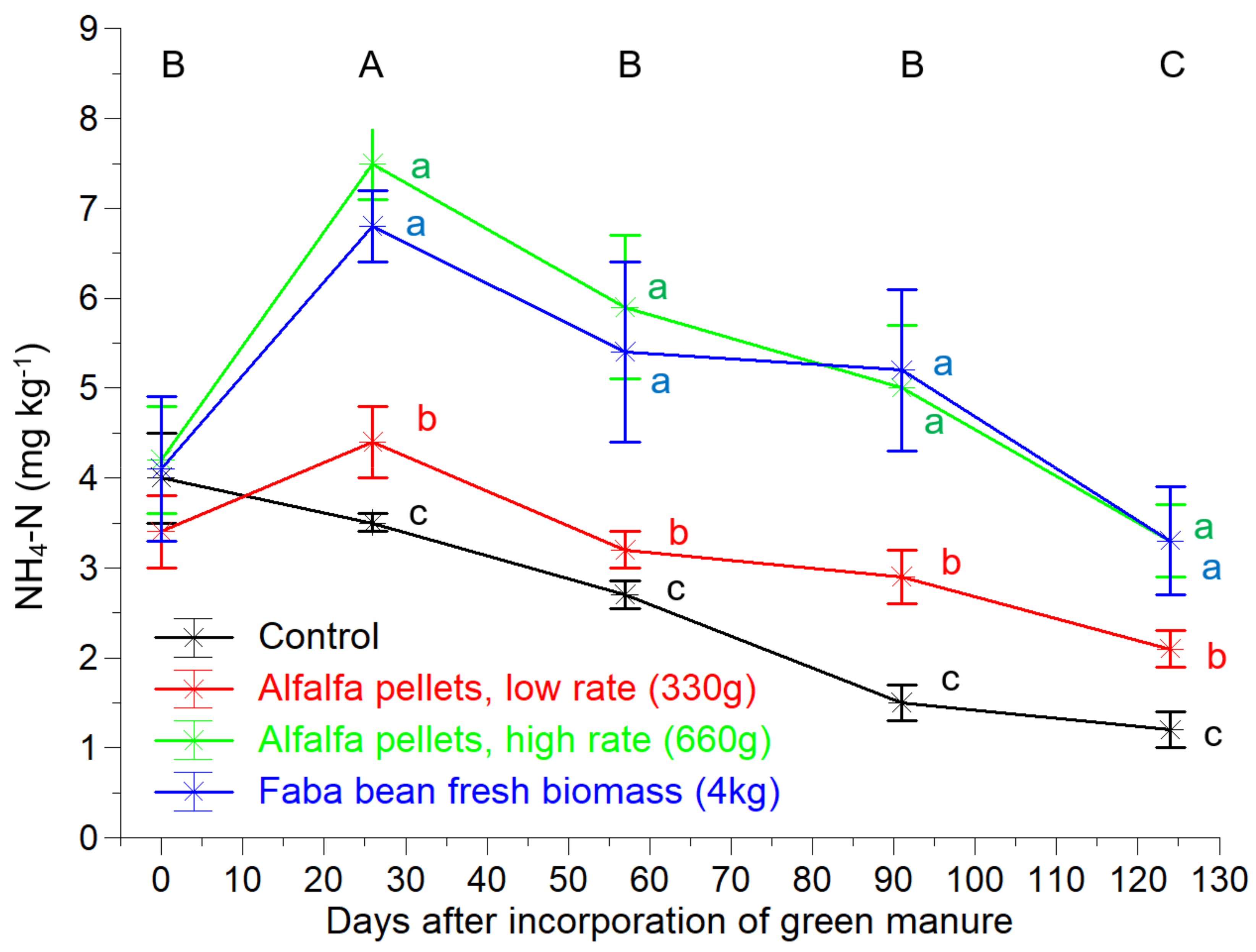
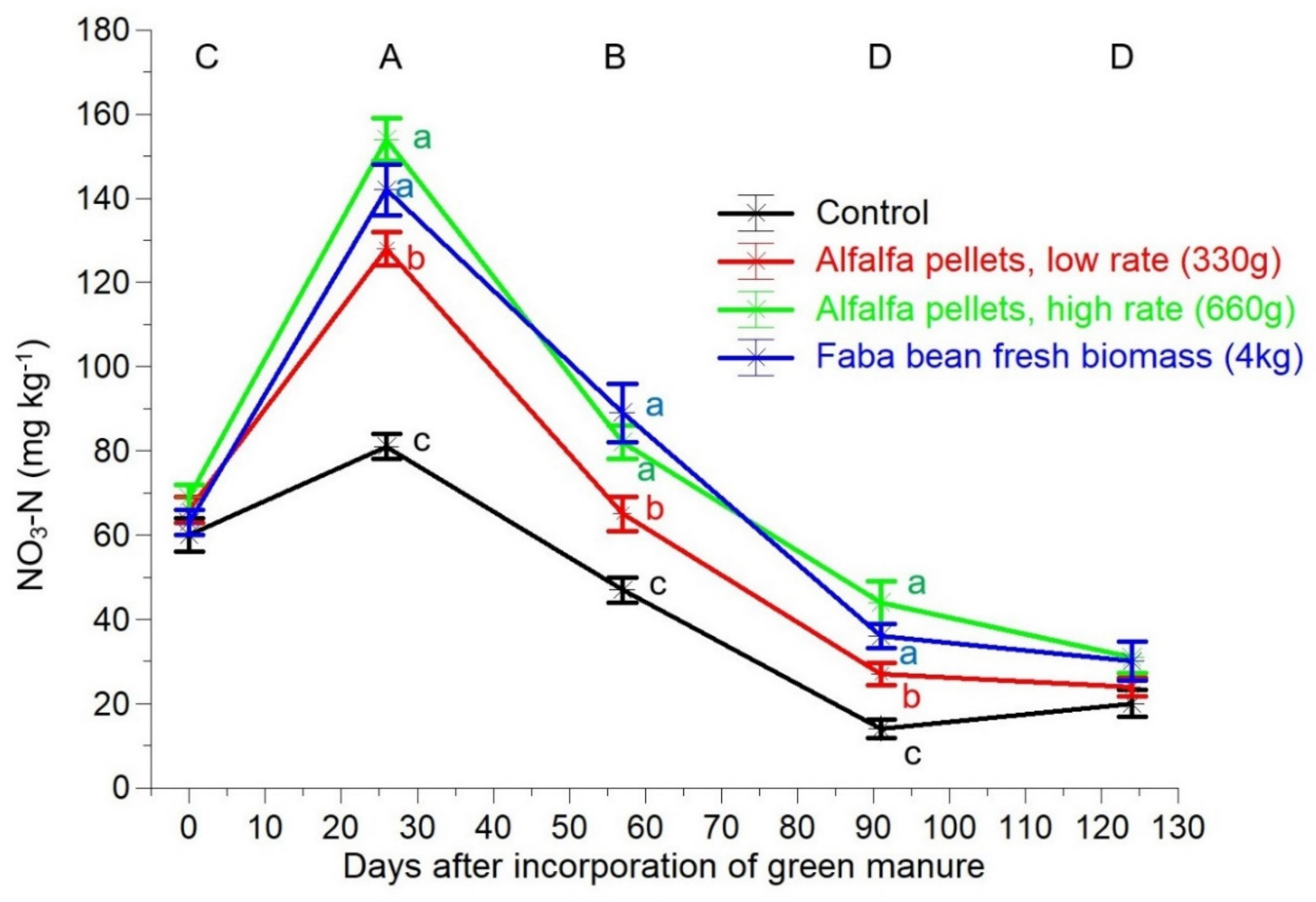

| Treatment Short Name | Treatment Description | Dry Biomass g m−2 | N % | C % | C/N Ratio | N Input g m−2 |
|---|---|---|---|---|---|---|
| CON | Control | - | - | - | - | |
| AAL | Alfalfa pellets, low rate (330 g m−2) | 297 | 3.37 | 41.8 | 12.4 | 10 |
| AAH | Alfalfa pellets, high rate (660 g m−2) | 594 | 3.37 | 41.8 | 12.4 | 20 |
| FAB | Faba bean fresh biomass (4 kg m−2) | 382 | 3.93 | 40.4 | 10.3 | 15 |
| Treatment | Yield kg m−2 | Fruit Number per Plant | Mean Fruit Weight |
|---|---|---|---|
| Control | 9.8 c | 20.2 b | 228 b |
| Alfalfa pellets, low rate (330 g m−2) | 11.7 b | 23.7 a | 231 b |
| Alfalfa pellets, high rate (660 g m−2) | 13.0 a | 24.0 a | 254 a |
| Faba bean fresh biomass (4 kg m−2) | 13.3 a | 25.7 a | 244 a |
| Significance of differences | *** | ** | ** |
| Treatment | N mg g−1 | P mg g−1 | K mg g−1 |
|---|---|---|---|
| Control | 23.9 c | 2.78 | 78 |
| Alfalfa pellets, low rate (330 g m−2) | 26.6 b | 2.80 | 81 |
| Alfalfa pellets, high rate (660 g m−2) | 28.1 a | 2.99 | 86 |
| Faba bean fresh biomass (4 kg m−2) | 28.8 a | 2.90 | 84 |
| Significance of differences | *** | ns | ns |
| Treatment | N mg g−1 | P mg g−1 | K mg g−1 | N Removal through Fruit g m−2 |
|---|---|---|---|---|
| Control | 15.2 | 2.81 | 81 | 7.6 c |
| Alfalfa pellets, low rate (330 g m−2) | 15.5 | 2.61 | 84 | 8.9 b |
| Alfalfa pellets, high rate (660 g m−2) | 16.2 | 2.84 | 81 | 10.7 a |
| Faba bean fresh biomass (4 kg m−2) | 16.8 | 2.65 | 75 | 11.2 a |
| Significance of differences | ns | ns | ns | *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gatsios, A.; Ntatsi, G.; Celi, L.; Said-Pullicino, D.; Tampakaki, A.; Savvas, D. Legume-Based Mobile Green Manure Can Increase Soil Nitrogen Availability and Yield of Organic Greenhouse Tomatoes. Plants 2021, 10, 2419. https://doi.org/10.3390/plants10112419
Gatsios A, Ntatsi G, Celi L, Said-Pullicino D, Tampakaki A, Savvas D. Legume-Based Mobile Green Manure Can Increase Soil Nitrogen Availability and Yield of Organic Greenhouse Tomatoes. Plants. 2021; 10(11):2419. https://doi.org/10.3390/plants10112419
Chicago/Turabian StyleGatsios, Anastasios, Georgia Ntatsi, Luisella Celi, Daniel Said-Pullicino, Anastasia Tampakaki, and Dimitrios Savvas. 2021. "Legume-Based Mobile Green Manure Can Increase Soil Nitrogen Availability and Yield of Organic Greenhouse Tomatoes" Plants 10, no. 11: 2419. https://doi.org/10.3390/plants10112419
APA StyleGatsios, A., Ntatsi, G., Celi, L., Said-Pullicino, D., Tampakaki, A., & Savvas, D. (2021). Legume-Based Mobile Green Manure Can Increase Soil Nitrogen Availability and Yield of Organic Greenhouse Tomatoes. Plants, 10(11), 2419. https://doi.org/10.3390/plants10112419









