Chemical Diversity between Three Graminoid Plants Found in Western Kenya Analyzed by Headspace Solid-Phase Microextraction Gas Chromatography–Mass Spectrometry (HS-SPME-GC-MS)
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Composition of Headspace Samples from C. dactylon, C. exaltatus, and P. repens
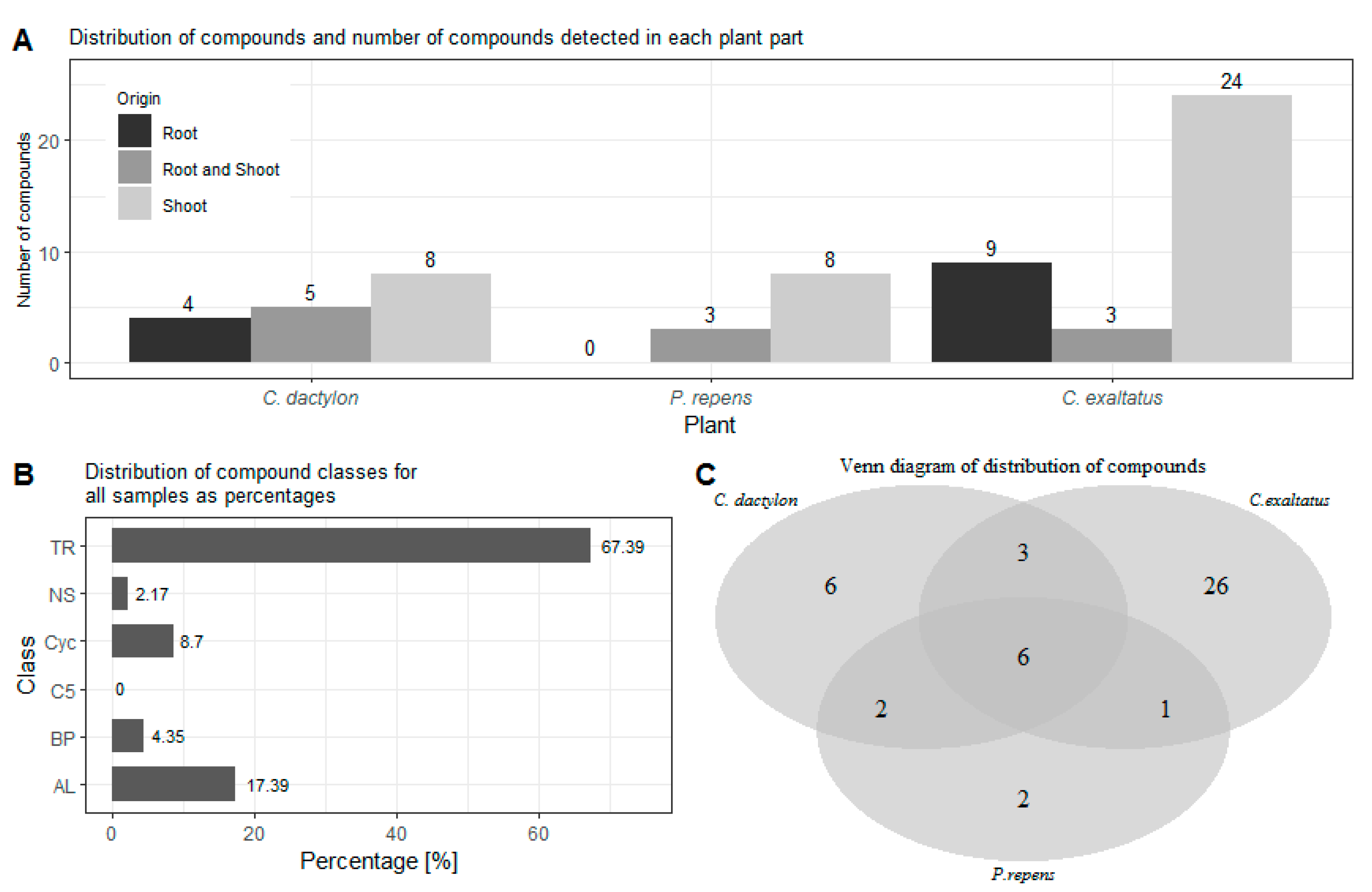
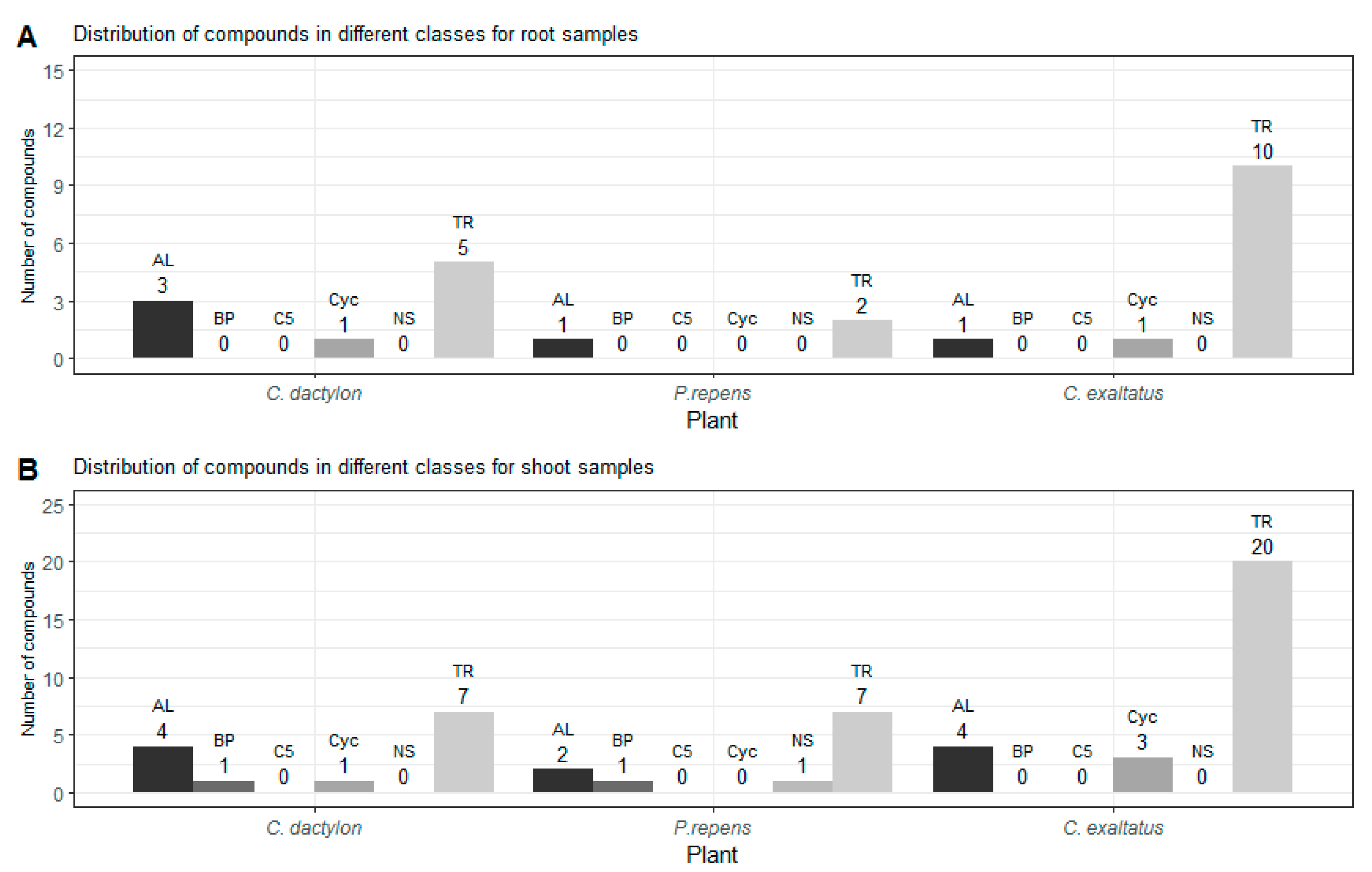
2.2. Statistical Evaluation of Results
2.3. Relating Findings to the Olfactometric Results
3. Materials and Methods
3.1. Solid-Phase Micro Extraction
3.2. Gas Chromatography–Mass Spectrometry Parameters
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. World Malaria Report 2020: 20 Years of Global Progress and Challenges; WHO: Geneva, Switzerland, 2020; pp. 22, 60, 62. [Google Scholar]
- World Health Organization. Global Technical Strategy for Malaria 2016–2030; WHO: Geneva, Switzerland, 2015. [Google Scholar]
- Ranson, H.; Lissenden, N. Insecticide Resistance in African Anopheles Mosquitoes: A Worsening Situation that Needs Urgent Action to Maintain Malaria Control. Trends Parasitol. 2016, 32, 187–196. [Google Scholar] [CrossRef]
- Bentley, M.D.; Day, J.F. Chemical ecology and behavioral aspects of mosquito oviposition. Annu. Rev. Entomol. 1989, 34, 401–421. [Google Scholar] [CrossRef]
- Takken, W.; Knols, B.G.J. Odor-mediated behaviour of afrotropical malaria mosquitoes. Annu. Rev. Entomol. 1999, 44, 131–157. [Google Scholar] [CrossRef]
- Zwiebel, L.J.; Takken, W. Olfactory regulation of mosquito–host interactions. Insect Biochem. Mol. Biol. 2004, 34, 645–652. [Google Scholar] [CrossRef] [Green Version]
- Govella, N.J.; Ferguson, H.M. Why use of interventions targeting outdoor biting mosquitoes will be necessary to achieve malaria elimination. Front. Physiol. 2012, 3, 5. [Google Scholar] [CrossRef] [Green Version]
- Ferguson, H.; Dornhaus, A.; Beeche, A.; Borgemeister, C.; Gottlieb, M.; Mulla, M.S.; Gimnig, J.E.; Fish, D.; Killeen, G.F. Ecology: A prerequisite for malaria elimination and eradication. PLoS Med. 2010, 7, e1000303. [Google Scholar] [CrossRef] [Green Version]
- Bokore, G.E.; Ouma, P.; Onyango, P.O.; Bukhari, T.; Fillinger, U. A cross-sectional observational study investigating the association between sedges (swamp grasses, Cyperaceae) and the prevalence of immature malaria vectors in aquatic habitats along the shore of Lake Victoria, western Kenya. F1000Research 2020, 9, 1032. [Google Scholar] [CrossRef]
- Abo-Altmene, R.A.; Al-Shammari, A.M.; Shawkat, M.S. GC-MS analysis and chemical composition identification of Cyperus rotundus L. from Iraq. Energy Procedia 2019, 157, 1462–1474. [Google Scholar] [CrossRef]
- Janaki, S.; Zandi-Sohani, N.; Ramezani, L.; Szumny, A. Chemical composition and insecticidal efficacy of Cyperus rotundus essential oil against three stored product pests. Int. Biodeterior. Biodegrad. 2018, 133, 93–98. [Google Scholar] [CrossRef]
- Yagi, S.; Babiker, R.; Tzanova, T.; Schohn, H. Chemical composition, antiproliferative, antioxidant and antibacterial activities of essential oils from aromatic plants growing in Sudan. Asian Pac. J. Trop. Med. 2016, 9, 763–770. [Google Scholar] [CrossRef] [Green Version]
- Poyraz, I.E.; Demirci, B.; Kucuk, S. Volatiles of Turkish Cyperus rotundus L. Roots. Rec. Nat. Prod. 2017, 12, 222–228. [Google Scholar] [CrossRef]
- Kamala, A.; Middha, S.K.; Karigar, C.S. Plants in traditional medicine with special reference to Cyperus rotundus L.: A review. 3 Biotech 2018, 8, 11. [Google Scholar] [CrossRef] [PubMed]
- Eneh, L.K.; Saijo, H.; Borg-Karlson, A.-K.; Lindh, J.M.; Rajarao, G.K. Cedrol, a malaria mosquito oviposition attractant is produced by fungi isolated from rhizomes of the grass cyperus rotundus. Malar. J. 2016, 15, 478–482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eneh, L.K.; Okal, M.N.; Borg-Karlson, A.K.; Fillinger, U.; Lindh, J.M. Gravid Anopheles gambiae sensu stricto avoid ovipositing in Bermuda grass hay infusion and it’s volatiles in two choice egg-count bioassays. Malar. J. 2016, 15, 276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G.; Scheffer, J.J.C. Factors affecting secondary metabolite production in plants: Volatile components and essential oils. Flavour Fragr. J. 2008, 23, 213–226. [Google Scholar] [CrossRef]
- Knudsen, J.T. Variation in floral scent composition within and between populations of Geonoma macrostachys (Arecaceae) in the Western Amazon. Am. J. Bot. 2002, 89, 1772–1778. [Google Scholar] [CrossRef] [PubMed]
- Lawal, O.A.; Oyedeji, A.O. Chemical Composition of the Essential Oils of Cyperus rotundus L. from South Africa. Molecules 2009, 14, 2909–2917. [Google Scholar] [CrossRef] [PubMed]
- Bokore, G.E.; Svenberg, L.; Tamre, R.; Onyango, P.; Bukhari, T.; Emmer, Å.; Fillinger, U. Grass-like plants release general volatile cues attractive for gravid Anopheles gambiae sensu stricto mosquitoes. Parasit. Vectors 2021, 14, 552. [Google Scholar] [CrossRef]
- Tholl, D.; Boland, W.; Hansel, A.; Loreto, F.; Röse, U.S.; Schnitzler, J.-P. Practical approaches to plant volatile analysis. Plant J. 2006, 45, 540–560. [Google Scholar] [CrossRef] [PubMed]
- Baek, Y.-S.; Ramya, M.; An, H.-R.; Park, P.-M.; Lee, S.-Y.; Baek, N.-I.; Park, P.-H. Volatiles Profile of the Floral Organs of a New Hybrid Cymbidium, ‘Sunny Bell’ Using Headspace Solid-Phase Microextraction Gas Chromatography-Mass Spectrometry Analysis. Plants 2019, 8, 251. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.-G.; Choi, W.-S.; Yang, S.-O.; Hwang-Bo, J.; Kim, H.-G.; Fang, M.; Yi, T.-H.; Kang, S.C.; Lee, Y.-H.; Baek, N.-I. Volatile Profiles of Five Variants of Abeliophyllum distichum Flowers Using Headspace Solid-Phase Microextraction Gas Chromatography–Mass Spectrometry (HS-SPME-GC-MS) Analysis. Plants 2021, 10, 224. [Google Scholar] [CrossRef]
- Knudsen, J.T.; Eriksson, R.; Gershenzon, J.; Ståhl, B. Diversity and Distribution of Floral Scent. Bot. Rev. 2006, 72, 1. [Google Scholar] [CrossRef]
- Knudsen, J.T.; Tollstein, L.; Bergström, L.G. Floral scents—A checklist of voaltile compounds isolated by headspace techniques. Phytochemistry 1993, 33, 253–280. [Google Scholar] [CrossRef]
- Peñuelas, J.; Llusiá, J. The complexity of factors driving volatile organic compound emissions by plants. Biol. Plant. 2001, 44, 481–487. [Google Scholar] [CrossRef]
- Dudareva, N.; Pichersky, E.; Gershenzon, J. Biochemistry of plant volatiles. Plant Physiol. 2004, 135, 1893–1902. [Google Scholar] [CrossRef] [Green Version]
- Vivaldo, G.; Masi, E.; Taiti, C.; Caldarelli, G.; Mancuso, S. The network of plants volatile organic compounds. Sci. Rep. 2017, 7, 18. [Google Scholar] [CrossRef] [Green Version]
- Wondwosen, B.; Birgersson, G.; Seyoum, E.; Tekie, H.; Torto, B.; Fillinger, U.; Hill, S.R.; Ignell, R. Rice volatiles lure gravid malaria mosquitoes, Anopheles arabiensis. Sci. Rep. 2016, 6, 37930. [Google Scholar] [CrossRef] [Green Version]
- Wooding, M.; Naude, Y.; Rohwer, E.; Bouwer, M. Controlling mosquitoes with semiochemicals: A review. Parasit. Vectors 2020, 13, 80. [Google Scholar] [CrossRef] [PubMed]
- Bruce, T.J.; Pickett, J.A. Perception of plant volatile blends by herbivorous insects—Finding the right mix. Phytochemistry 2011, 72, 1605–1611. [Google Scholar] [CrossRef]
- Gfeller, V.; Huber, M.; Förster, C.; Huang, W.; Köllner, T.; Erb, M. Root volatiles in plant–plant interactions I: High root sesquiterpene release is associated with increased germination and growth of plant neighbours. Plant Cell Environ. 2019, 42, 1950–1963. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.; Gfeller, A.; Erb, M. Root volatiles in plant–plant interactions II: Root volatiles alter root chemistry and plant–herbivore interactions of neighbouring plants. Plant Cell Environ. 2019, 42, 1964–1973. [Google Scholar] [CrossRef] [Green Version]
- Svenberg, L.; Emmer, Å.; Lindh, J. Analysis of six secondary metabolites from Cyperus rotundus—Comparing different methods for determining volatile compounds in laboratory and field settings. 2021. Submited. [Google Scholar]
| S | Relative Composition % [Peak Area/Total Area] ± Standard Error | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C. dactylon | C. exaltatus | P. repens | ||||||||
| No. | Compound | RT | RI | Class | Root | Shoot | Root | Shoot | Root | Shoot |
| Alcohols | ||||||||||
| 1 | 2-octyn-1-ol | 7.17 | 979 | AL | - | 0.47 a | - | - | - | - |
| 2 | phenylethyl alcohol | 9.60 | 1121 | BP | - | - | - | - | - | 8.72 ± 3.32 |
| 3 | 2,6-nonadien-1-ol | 10.41 | 1171 | AL | - | - | - | 0.16 a | - | - |
| 4 | citronellol | 11.37 | 1231 | TR | - | 2.51 ± 0.93 | - | 0.2 a | - | 5.35 ± 2.47 |
| Aldehydes | ||||||||||
| 5 | citral | 8.94 | 1082 | TR | - | - | - | 0.51 ± 0.29 | - | - |
| 6 | nonanal | 9.23 | 1097 | AL | 1.37 ± 0.79 | - | - | 0.32 ± 0.19 | - | - |
| 7 | 2,6-nonadienal | 10.19 | 1158 | AL | - | - | - | 0.57 ± 0.33 | - | - |
| 8 | 2-nonenal | 10.29 | 1164 | AL | - | - | - | 1.25 ± 0.47 | - | - |
| Amines | ||||||||||
| 9 | Myrtanylamine b | 9.52 | 1116 | NS | - | - | - | - | - | 0.43 a |
| Aromatics | ||||||||||
| 10 | Mesitylene b | 7.53 | 998 | BP | - | 4.21 ± 1.46 | - | - | - | - |
| Epoxides | ||||||||||
| 11 | caryophyllene oxide | 16.71 | 1609 | TR | - | - | - | 0.16 a | - | - |
| Furans | ||||||||||
| 12 | 2-pentyl-furan | 7.47 | 994 | Cyc | 4.04 ± 2.33 | - | - | 4.49 ± 1.18 | - | - |
| 13 | 2-(2-pentenyl)furan | 7.63 | 1004 | Cyc | - | - | - | 3.2 ± 0.76 | - | - |
| Hydrocarbons | ||||||||||
| 14 | α-pinene | 6.52 | 943 | TR | 8.03 ± 3.64 | 3.77 ± 1.35 | 8.73 ± 5.06 | - | 2.99 a | 14.42 ± 4.63 |
| 15 | β-pinene | 7.26 | 984 | TR | 9.34 ± 3.3 | 16.38 ± 1.89 | 16.69 ± 1.61 | 0.41 ± 0.26 | 38.7 ± 2.22 | 16.35 ± 2.45 |
| 16 | β-myrcene | 7.46 | 994 | TR | - | 8.61 ± 1.98 | 0.83 ± 0.51 | - | - | 12.05 ± 1.85 |
| 17 | decane | 7.59 | 1001 | AL | 1.24 ± 0.74 | 3.3 ± 1.13 | - | - | - | 1.44 ± 0.95 |
| 18 | 3-carene | 7.84 | 1017 | TR | - | - | 0.73 ± 0.42 | - | - | 1.28 ± 0.75 |
| 19 | limonene | 8.15 | 1036 | TR | 2.96 ± 1.71 | 8.05 ± 1.6 | 1.99 ± 1.19 | - | - | 4.67 ± 2.7 |
| 20 | 1-undecyne | 8.81 | 1074 | AL | - | 0.88 a | - | - | - | - |
| 21 | α-copaene | 13.80 | 1392 | TR | - | - | - | 3.51 ± 0.25 | - | - |
| 22 | β-cubebene | 13.89 | 1398 | TR | - | - | - | 0.98 ± 0.34 | - | - |
| 23 | β-elemene | 14.01 | 1406 | TR | - | - | - | 22.92 ± 5.87 | - | - |
| 24 | cyperene | 14.22 | 1422 | TR | - | - | 10.34 ± 0.93 | 4.72 ± 0.33 | - | - |
| 25 | γ-elemene | 14.35 | 1432 | TR | - | - | 3.85 ± 3.02 | - | - | - |
| 26 | α-bergamotene | 14.38 | 1434 | TR | 1.17 ± 0.68 | - | - | - | - | - |
| 27 | α-cedrene | 14.40 | 1435 | TR | - | - | 3.76 ± 1.55 | - | - | - |
| 28 | caryophyllene | 14.48 | 1441 | TR | - | - | - | 8.68 ± 0.44 | - | - |
| 29 | β-gurjunene | 14.60 | 1450 | TR | - | - | 3.5 ± 2.02 | - | - | - |
| 30 | α-gurjunene | 14.83 | 1467 | TR | - | - | - | 0.17 a | - | - |
| 31 | germacrene D | 14.87 | 1469 | TR | 21.67 ± 7.05 | - | - | - | - | - |
| 32 | humulene | 14.95 | 1475 | TR | - | - | - | 6.61 ± 0.4 | - | - |
| 33 | β-selinene | 15.05 | 1483 | TR | - | - | 11.81 ± 4.3 | 1.33 ± 0.11 | - | - |
| 34 | α-selinene | 15.20 | 1493 | TR | - | - | - | 4.9 ± 0.25 | - | - |
| 35 | valencene | 15.26 | 1497 | TR | - | - | - | 1.17 ± 0.06 | - | - |
| 36 | α-bulnesene | 15.41 | 1509 | TR | - | - | - | 10.18 ± 1.86 | - | - |
| 37 | α-farnesene | 15.46 | 1512 | TR | - | - | - | 1.37 ± 0.12 | - | - |
| 38 | 4,11-selinadiene | 15.52 | 1517 | TR | - | - | - | 9.22 ± 1.94 | - | - |
| 39 | δ-cadinene | 15.83 | 1542 | TR | - | - | - | 1.03 ± 0.36 | - | - |
| 40 | bi-1-cycloocten-1-yl b | 15.89 | 1546 | Cyc | - | - | 1.2 ± 0.7 | - | - | - |
| Ketones | ||||||||||
| 41 | 3-octanone | 7.35 | 988 | AL | 14.65 ± 1.21 | 11.01 ± 1.83 | 15.57 ± 2.94 | - | 9.95 ± 5.75 | 13.83 ± 4.25 |
| 42 | sulcatone | 7.35 | 989 | TR | - | - | - | 1.95 ± 0.38 | - | - |
| 43 | 2,2,6-trimethylcyclohexanone | 8.24 | 1042 | Cyc | - | 0.91 ± 0.53 | - | 0.21 a | - | - |
| 44 | α-isophorone | 8.66 | 1066 | TR | - | 0.41a | - | - | - | - |
| 45 | geranyl acetone | 14.70 | 1458 | TR | - | - | - | 0.76 ± 0.03 | - | - |
| 46 | β-ionone | 15.26 | 1497 | TR | - | 1.79 ± 1.04 | - | - | - | 1.26 ± 0.79 |
| 95 % Confidence Interval | |||||||
|---|---|---|---|---|---|---|---|
| Compound | (I) Plant | (II) Plant | Mean Difference | Lower | Upper | p-Value | Significantly Different |
| α-pinene | |||||||
| P. repens | C. dactylon | 6.058835 | −2.923069 | 15.04074 | 0.161362 | No | |
| β -pinene | |||||||
| P. repens | C. dactylon | 12.830001 | 0.4544081 | 25.20559 | 0.0414622 | Yes | |
| P. repens | C. exaltatus | 16.122119 | 3.2082078 | 29.03603 | 0.0134851 | Yes | |
| C. exaltatus | C. dactylon | −3.292118 | −16.5954810 | 10.01124 | 0.8048685 | No | |
| 3-octanone | |||||||
| P. repens | C. dactylon | 3.0250021 | −4.699573 | 10.749577 | 0.5777641 | No | |
| P. repens | C. exaltatus | 0.2862853 | −8.946348 | 9.518918 | 0.9964308 | No | |
| C. exaltatus | C. dactylon | 2.7387168 | −6.020128 | 11.497562 | 0.7014438 | No | |
| β-myrcene | |||||||
| P. repens | C. dactylon | 3.447685 | −3.19232 | 10.08769 | 0.250943 | No | |
| citronellol | |||||||
| P. repens | C. dactylon | 3.7893436 | −3.091271 | 10.66996 | 0.2009792 | No | |
| 95 % Confidence Interval | ||||||||
|---|---|---|---|---|---|---|---|---|
| Plant | Compound | (I) Part | (II) Part | Mean Difference | Lower | Upper | p-Value | Significantly Different |
| C. dactylon | ||||||||
| α-pinene | ||||||||
| Shoot | Root | −4.265665 | −13.76537 | 5.234041 | 0.3140036 | No | ||
| β-pinene | ||||||||
| Shoot | Root | 7.038193 | −2.267427 | 16.34381 | 0.113681 | No | ||
| 3-octanone | ||||||||
| Shoot | Root | −3.63632 | −9.012898 | 1.740259 | 0.1490245 | No | ||
| decane | ||||||||
| Shoot | Root | 2.052726 | −1.2501 | 5.355553 | 0.1791336 | No | ||
| limonene | ||||||||
| Shoot | Root | 5.093832 | −0.63325 | 10.82091 | 0.0724245 | No | ||
| C. exaltatus | ||||||||
| β-pinene | ||||||||
| Shoot | Root | −16.27886 | −20.27341 | −12.28431 | 0.0000588 | Yes | ||
| cyperene | ||||||||
| Shoot | Root | −5.619737 | −8.031604 | −3.20787 | 0.0012586 | Yes | ||
| β-selinene | ||||||||
| Shoot | Root | −10.47628 | −21.00256 | 0.05001014 | 0.050796 | No | ||
| P. repens | ||||||||
| β-pinene | ||||||||
| Shoot | Root | −22.34587 | −30.42966 | −14.26208 | 0.0005098 | Yes | ||
| 3-octanone | ||||||||
| Shoot | Root | 3.887919 | −13.6089 | 21.38473 | 0.6062301 | No | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Svenberg, L.; Emmer, Å. Chemical Diversity between Three Graminoid Plants Found in Western Kenya Analyzed by Headspace Solid-Phase Microextraction Gas Chromatography–Mass Spectrometry (HS-SPME-GC-MS). Plants 2021, 10, 2423. https://doi.org/10.3390/plants10112423
Svenberg L, Emmer Å. Chemical Diversity between Three Graminoid Plants Found in Western Kenya Analyzed by Headspace Solid-Phase Microextraction Gas Chromatography–Mass Spectrometry (HS-SPME-GC-MS). Plants. 2021; 10(11):2423. https://doi.org/10.3390/plants10112423
Chicago/Turabian StyleSvenberg, Linus, and Åsa Emmer. 2021. "Chemical Diversity between Three Graminoid Plants Found in Western Kenya Analyzed by Headspace Solid-Phase Microextraction Gas Chromatography–Mass Spectrometry (HS-SPME-GC-MS)" Plants 10, no. 11: 2423. https://doi.org/10.3390/plants10112423
APA StyleSvenberg, L., & Emmer, Å. (2021). Chemical Diversity between Three Graminoid Plants Found in Western Kenya Analyzed by Headspace Solid-Phase Microextraction Gas Chromatography–Mass Spectrometry (HS-SPME-GC-MS). Plants, 10(11), 2423. https://doi.org/10.3390/plants10112423






