The Effect of Hydrothermal Treatment on Metabolite Composition of Hass Avocados Stored in a Controlled Atmosphere
Abstract
:1. Introduction
2. Results
2.1. Differences at Harvest in Primary and Secondary Metabolites and Antioxidant Activity between Early and Middle Harvest Fruit
2.2. Changes in Primary and Secondary Metabolites and Antioxidant Activity after Postharvest Treatments and at Edible Ripeness for Early and Middle Harvest Fruit
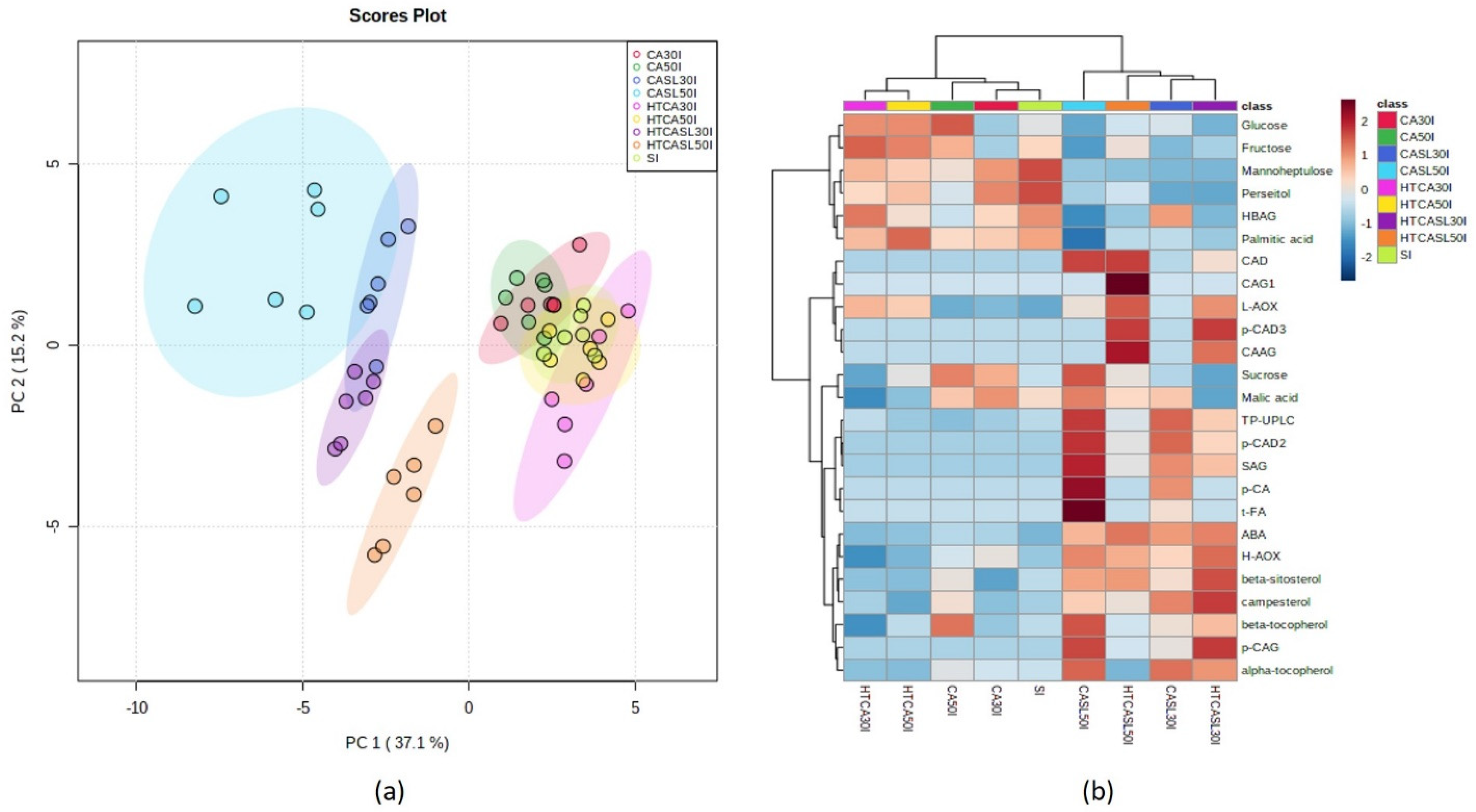
2.3. Multivariate Analysis of Primary and Secondary Metabolites and Antioxidant Activities According to Harvest Stages and Postharvest Treatments
3. Discussion
3.1. Differences at Harvest in Primary and Secondary Metabolites and Antioxidant Activity between Early and Middle Harvest Fruit
3.2. Changes in Primary and Secondary Metabolites and Antioxidant Activity after Postharvest Treatments and at Edible Ripeness for Early and Middle Harvest Fruit
4. Materials and Methods
4.1. Fruit Material
4.2. Postharvest Treatments and Storage Conditions
4.3. Determination of Relevant Primary and Secondary Metabolites
4.3.1. Determination of Dry Matter and Oil Content
4.3.2. Determination of Sugars and Organic Acids
4.3.3. Fatty Acid Content and Profile
4.3.4. Tocopherol Content and Profile
4.3.5. Phytosterol Content and Profile
4.3.6. Determination of Hydrophilic and Lipophilic Antioxidant Activity
4.3.7. Abscisic Acid Content by UPLC-PDA
4.3.8. UPLC-MS-MS/PDA/-qToF Analysis of Phenolic Compounds Content and Profile
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Villa-Rodríguez, J.A.; Yahia, E.M.; González-León, A.; Ifie, I.; Robles-Zepeda, R.E.; Domínguez-Ávila, J.A.; González-Aguilar, G.A. Ripening of ‘Hass’ avocado mesocarp alters its phytochemical profile and the in vitro cytotoxic activity of its methanolic extracts. S. Afr. J. Bot. 2020, 128, 1–8. [Google Scholar] [CrossRef]
- Landahl, S.; Meyer, M.D.; Terry, L.A. Spatial and temporal analysis of textural and biochemical changes of imported avocado cv. Hass during fruit ripening. J. Agric. Food Chem. 2009, 57, 7039–7047. [Google Scholar] [CrossRef] [PubMed]
- Dreher, M.L.; Davenport, A.J. Hass Avocado Composition and Potential Health Effects. Crit. Rev. Food Sci. Nutr. 2013, 53, 738–750. [Google Scholar] [CrossRef] [Green Version]
- Villa-Rodríguez, J.; Molina-Corral, J.; Ayala-Zavala, F.; Olivas, G.; González-Aguilar, G. Effect of maturity stage on the content of fatty acids and antioxidant activity of ‘Hass’ avocado. Food Res. Int. 2011, 44, 1231–1237. [Google Scholar] [CrossRef]
- Campos, D.; Terán-Hilares, F.; Chirinos, R.; Aguilar-Galvez, A.; García-Ríos, D.; Pacheco-Ávalos, A.; Pedreschi, R. Bioactive compounds and antioxidant activity from harvest to edible ripeness of avocado cv. Hass (Persea americana) throughout the harvest seasons. Int. J. Food Sci. Technol. 2020, 55, 2208–2218. [Google Scholar] [CrossRef]
- Weschenfelder, C.; dos Santos, J.L.; Lopes de Souza, P.A.; Paiva de Campos, V.; Marcadenti, A. Avocado and Cardiovascular Health. Open J. Endocr. Metab. Dis. 2015, 5, 77–83. [Google Scholar] [CrossRef] [Green Version]
- MINAGRI (Ministerio de Agricultura y Riego). La Situación del Mercado Internacional de la Palta; MINAGRI (Ministerio de Agricultura y Riego): Lima, Peru, 2019; p. 40. (In Spanish) [Google Scholar]
- Donetti, M.; Terry, L. Biochemical markers defining growing area and ripening stage of imported avocado fruit cv. Hass. J. Food Compos. Anal. 2014, 34, 90–98. [Google Scholar] [CrossRef]
- Mpai, S.; Sivakumar, D. Influence of growing seasons on metabolic composition, and fruit quality of avocado cultivars at ‘ready-to-eat stage’. Sci. Hortic. 2020, 265, 109159. [Google Scholar] [CrossRef]
- Blakey, R.; Bower, J. The feasibility of a hot water treatment for South African avocados (Persea americana [Mill] cv. Hass). S. Afr. Avocado Grow. Assoc. Yearb. 2007, 30, 66–68. [Google Scholar]
- Blakey, R.; Bower, J.; Bertling, I. Influence of water and ABA supply on the ripening pattern of avocado (Persea americana Mill.) fruit and the prediction of water content using near infrared spectroscopy. Postharvest Biol. Technol. 2009, 53, 72–76. [Google Scholar] [CrossRef]
- Hernández, I.; Fuentealba, C.; Olaeta, J.; Poblete-Echeverríab, C.; Defilippi, B.; González-Agüeroc, M.; Campos-Vargas, R.; Lurie, S.; Pedreschi, R. Effects of heat shock and nitrogen shock pre-treatments on ripening heterogeneity of Hass avocados stored in controlled atmosphere. Sci. Hortic. 2017, 225, 408–415. [Google Scholar] [CrossRef]
- Hernández, I.; Uarrota, V.; Paredes, D.; Fuentealba, C.; Defilippi, B.; Campos-Vargas, R.; Meneses, C.; Hertog, M.; Pedreschi, R. Can metabolites at harvest be used as physiological markers for modelling the softening behaviour of Chilean Hass avocados destined to local and distant markets? Postharvest Biol. Technol. 2021, 174, 111457. [Google Scholar] [CrossRef]
- Pedreschi, R.; Hollaka, S.; Harkema, H.; Otma, E.; Robledo, P.; Westra, E.; Somhorst, D.; Ferreyra, R.; Defilippi, B.G. Impact of postharvest ripening strategies on ‘Hass’ avocado fatty acid profiles. S. Afr. J. Bot. 2016, 103, 32–35. [Google Scholar] [CrossRef]
- Lurie, S.; Pedreschi, R. Fundamental aspects of heat treatments. Hortic. Res. 2014, 1, 14030. [Google Scholar] [CrossRef] [Green Version]
- Uarrota, V.; Fuentealba, C.; Hernández, I.; Defilippi, B.; Meneses, C.; Campos-Vargas, R.; Hertog, M.; Nicolai, B.; Carpentier, S.; Poblete-Echeverría, C.; et al. Integration of proteomics and metabolomics data of early and middle season Hass avocados under heat treatment. Food Chem. 2019, 289, 512–521. [Google Scholar] [CrossRef]
- Coria-Ávalos, V.M. Cosecha. In Tecnología Para la Producción de Aguacate en Mexico; Coria-Avalos, V.M., Ed.; SAGARPA-INIFAP: Michoacán, Mexico, 2008; pp. 167–178. (In Spanish) [Google Scholar]
- Blakey, R.J.; Tesfay, S.Z.; Bertling, I.; Bower, J.P. Changes in sugars, total protein, and oil in ’Hass’ avocado (Persea americana Mill.) fruit during ripening. J. Hortic. Sci. Biotechnol. 2012, 87, 381–387. [Google Scholar] [CrossRef]
- Ozdemir, F.; Topuz, A. Changes in dry matter, oil content and fatty acids composition of avocado during harvesting time and post-harvesting ripening period. Food Chem. 2004, 86, 79–83. [Google Scholar] [CrossRef]
- Pedreschi, R.; Uarrota, V.; Fuentealba, C.; Alvaro, J.; Olmedo, P.; Defilippi, B.; Meneses, C.; Campos-Vargas, R. Primary metabolism in avocado fruit. Front. Plant Sci. 2019, 10, 795. [Google Scholar] [CrossRef]
- Contreras-Gutiérrez, P.; Hurtado-Fernández, E.; Gómez-Romero, M.; Ignacio, J.; Carrasco-Pancorbo, A.; Fernández-Gutiérrez, A. Determination of changes in the metabolic profile of avocado fruits (Persea americana) by two CE-MS approaches (targeted and non-targeted). Electrophoresis 2013, 34, 2928–2942. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.; Terry, L. Development of a rapid method for the sequential extraction and subsequent quantification of fatty acids and sugars from avocado mesocarp tissue. J. Agric. Food Chem. 2008, 56, 7439–7445. [Google Scholar] [CrossRef] [PubMed]
- Cowan, K. Metabolic control of avocado fruit growth: 3-hydroxy-3- methylglutaryl coenzyme a reductase, active oxygen species and the role of C7 sugars. S. Afr. J. Bot. 2004, 70, 75–82. [Google Scholar] [CrossRef] [Green Version]
- Marsh, K.B.; Bolding, H.L.; Shilton, R.S.; Laing, W.A. Changes in quinic acid metabolism during fruit development in three kiwifruit species. Funct. Plant Biol. 2009, 36, 463–470. [Google Scholar] [CrossRef]
- Lu, Q.; Zhang, Y.; Wang, Y.; Wang, D.; Lee, R.; Gao, K.; Byrns, R.; Heber, D. California Hass Avocado: Profiling of Carotenoids, Tocopherol, Fatty Acid, and Fat Content during Maturation and from Different Growing Areas. J. Agric. Food Chem. 2009, 57, 10408–10413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Sievert, J.; Arpaia, M.; Madore, M. Postulated Physiological Roles of the Seven-carbon Sugars, Mannoheptulose, and Perseitol in Avocado. J. Am. Soc. Hortic. Sci. 2002, 127, 108–114. [Google Scholar] [CrossRef] [Green Version]
- Mirdehghan, S.S.; Rahemi, M.; Serrano, M.; Guillén, F.; Martínez-Romero, D.; Valero, D. Prestorage Heat Treatment To Maintain Nutritive and Functional Properties during Postharvest Cold Storage of Pomegranate. J. Agric. Food Chem. 2006, 54, 8495–8500. [Google Scholar] [CrossRef]
- Defilippi, B.; Robledo, P.; Rivera, S.; Ferreyra, R. Heterogeneidad de la calidad y condición de paltas. In Optimización de la Calidad de Palta ‘Hass’; Defilippi, B., Ferreyra, R., Rivera, S., Eds.; Boletín INIA 307: La Cruz, Chile, 2015; pp. 43–68. (In Spanish) [Google Scholar]
- Chernys, J.T.; Zeevaart, J.A.D. Characterization of the 9-cis-epoxycarotenoid dioxygenase gene family and the regulation of abscisic acid biosynthesis in avocado. Plant Physiol. 2000, 124, 343–353. [Google Scholar] [CrossRef] [Green Version]
- Cutting, J.; Hofman, P.; Lishman, A.; Wolstenholme, B. Abscisic acid, isopentenyladenine and isopentenyladenosine concentrations in ripening fruit as determined by radioimmunoassay. Acta Hortic. 1986, 179, 793–800. [Google Scholar] [CrossRef]
- Hurtado-Fernández, E.; Fernández-Gutiérrez, A.; Carrasco-Pancorbo, A. Avocado fruit-Persea americana. In Exotic Fruits Reference Guide; Rodrigues, S., Silva, E.O., de Brito, E.S., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 37–47. [Google Scholar]
- Di Stefano, V.; Avellone, G.; Bongiorno, D.; Indelicato, S.; Massenti, R.; Bianco, R.L. Quantitative evaluation of the phenolic profile in fruits of six avocado (Persea americana) cultivars by ultra-high-performance liquid chromatography-heated electrospray-mass spectrometry. Int. J. Food Prop. 2017, 20, 1302–1312. [Google Scholar] [CrossRef] [Green Version]
- Rivero, R.M.; Ruiz, J.M.; García, P.C.; López-Lefebre, L.R.; Sánchez, E.; Romero, L. Resistance to cold and heat stress: Accumulation of phenolic compounds in tomato and watermelon plants. Plant Sci. 2001, 160, 315–321. [Google Scholar] [CrossRef]
- AOAC (Association of Official Analytical Chemists). Official Methods of Analysis of AOAC International, 18th ed.; Horwitz, W., Ed.; AOAC: Washington, DC, USA, 2007. [Google Scholar]
- Hatoum, D.; Hertog, M.L.A.T.M.; Geeraerd, A.H.; Nicolai, B.M. Effect of browning related pre- and postharvest factors on the "Braeburn" apple metabolome during CA storage. Postharvest Biol. Technol. 2016, 111, 106–116. [Google Scholar] [CrossRef]
- Meurens, M.; Baeten, V.; Yan, S.H.; Mignolet, E.; Larondelle, Y. Determination of the conjugated linoleic acids in cow’s milk fat by Fourier transform Raman spectroscopy. J. Agric. Food Chem. 2005, 53, 5831–5835. [Google Scholar] [CrossRef] [PubMed]
- Amaral, J.S.; Alves, M.R.; Seabra, R.M.; Oliveira, B.P.P. Vitamin E composition of walnuts (Juglans regia L.): A 3-year comparative study of different cultivars. J. Agric. Food Chem. 2005, 53, 5467–5472. [Google Scholar] [CrossRef] [PubMed]
- Chirinos, R.; Zuloeta, G.; Pedreschi, R.; Mignolet, E.; Larondelle, Y.; Campos, D. Sacha inchi (Plukenetia volubilis): A seed source of polyunsaturated fatty acids, tocopherols, phytosterols, phenolic compounds and antioxidant capacity. Food Chem. 2013, 141, 1732–1739. [Google Scholar] [CrossRef]
- Arnao, M.; Cano, A.; Acosta, M. The hydrophilic and lipophilic contribution to total antioxidant capacity. Food Chem. 2001, 73, 239–244. [Google Scholar] [CrossRef]


| Hydrothermal Treatment Followed by Controlled Atmosphere | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Compound/ Harvest | Initial (0 day) | Storage (day) CA | Shelf-Life—Edible Ripeness CASL | Storage (day) HTCA | Shelf-Life—Edible Ripeness HTCASL | ||||
| 30 | 50 | 30 * | 50 * | 30 | 50 | 30 * | 50 * | ||
| Dry matter (g/100 g DM) | |||||||||
| Early (I) | 20.08 ± 0.77 aB | 22.15 ± 1.22 aB | 22.23 ± 1.00 aB | 22.42 ± 3.14 aA | 21.01 ± 2.09 aA | 20.26 ± 1.68 aB | 21.51 ± 1.64 aA | 21.58 ± 1.45 aB | 20.50 ± 1.37 aA |
| Middle (II) | 22.95 ± 1.00 aA | 24.13 ± 1.21 aA | 23.62 ± 0.64 aA | 22.88 ± 1.59 aA | 22.93 ± 1.79 aA | 24.35 ± 1.37 aA | 24.94 ± 2.11 aA | 22.92 ± 1.08 aAB | 21.98 ± 0.79 aA |
| Oil (g/100 g DM) | |||||||||
| Early (I) | 10.47 ± 0.97 aB | 11.99 ± 0.57 aB | 12.72 ± 1.29 aB | 13.20 ± 2.03 aA | 12.24 ± 2.21 aA | 10.59 ± 1.64 aB | 11.18 ± 1.29 aB | 12.96 ± 1.22 aB | 11.26 ± 1.99 aA |
| Middle (II) | 13.31 ± 1.49 aA | 14.47 ± 0.96 aA | 14.57 ± 0.73 aA | 14.87 ± 1.58 aA | 14.45 ± 1.44 aAA | 15.33 ± 0.37 aA | 15.84 ± 1.06 aA | 14.66 ± 1.05 aAB | 14.57 ± 1.05 aAB |
| Sugars (g/kg DM) | |||||||||
| Mannoheptulose | |||||||||
| Early (I) | 87.40 ± 15.89 aA | 66.61 ± 18.72 abA | 37.84 ± 1.16 cA | 1.09 ± 0.37 eA | 7.28 ± 6.38 dA | 56.31 ± 14.14 bA | 48.31 ± 18.04 bA | 0.52 ± 0.18 eB | 4.13 ± 1.73 dAB |
| Middle (II) | 54.08 ± 18.70 aB | 34.47 ± 10.02 abB | 23.50 ± 2.08 abA | 1.52 ± 0.13 dA | 3.24 ± 1.74 dA | 33.72 ± 5.45 abB | 31.18 ± 13.40 abAB | 8.05 ± 1.19 cA | 6.58 ± 0.72 cA |
| Perseitol | |||||||||
| Early (I) | 32.62 ± 7.06 aA | 26.99 ± 8.28 abA | 11.57 ± 1.73 cA | 0.41 ± 0.01 eA | 6.22 ± 2.73 dA | 17.52 ± 5.46 bcA | 20.41 ± 2.98 bA | 0.25 ± 0.10 efB | 10.47 ± 1.90 cA |
| Middle (II) | 22.03 ± 6.57 aAB | 20.20 ± 6.73 aA | 9.06 ± 0.71 cA | 3.46 ± 2.44 dB | 4.45 ± 0.21 dA | 18.15 ± 1.42 aA | 16.40 ± 3.54 abAB | 7.30 ± 0.88 cA | 7.60 ± 2.04 cAB |
| Sucrose | |||||||||
| Early (I) | 2.27 ± 0.76 cAB | 5.12 ± 1.13 abB | 6.13 ± 1.23 aB | 1.84 ± 0.68 cB | 7.03 ± 3.00 aA | ND | 3.06 ± 1.00 bcA | ND | 3.26 ± 0.83 bcA |
| Middle (II) | 3.59 ± 0.42 dA | 11.58 ± 0.65 aA | 9.93 ± 0.51 bA | 9.68 ± 5.46 abA | 7.57 ± 1.37 bcA | 3.89 ± 0.23 d | 2.81 ± 0.66 deA | 3.92 ± 0.05 d | 4.21 ± 0.13 dA |
| Glucose | |||||||||
| Early (I) | 2.34 ± 0.58 b | 1.30 ± 0.43 cdA | 4.93 ± 0.94 aA | 2.15 ± 0.59 bc | 0.55 ± 0.34 d | 4.23 ± 0.91 aA | 4.28 ± 0.53 aA | 0.73 ± 0.13 dA | 2.03 ± 0.22 bA |
| Middle (II) | ND | 1.06 ± 1.04 abA | 2.58 ± 0.81 abB | ND | ND | 3.74 ± 0.69 aA | 2.18 ± 0.75 abB | 0.78 ± 0.18 bcA | 0.80 ± 0.08 cB |
| Fructose | |||||||||
| Early (I) | 3.37 ± 0.83 bA | 1.84 ± 0.44 cA | 3.98 ± 0.16 bA | 1.38 ± 0.16 cd | 0.87 ± 0.20 d | 5.05 ± 0.76 aA | 4.65 ± 0.41 abA | 1.88 ± 0.33 cA | 2.99 ± 0.29 bA |
| Middle (II) | 1.06 ± 0.46 cB | 1.37 ± 0.40 cA | 1.15 ± 0.09 cB | ND | ND | 3.75 ± 0.75 aB | 2.35 ± 0.06 bB | 1.67 ± 0.26 cAB | 1.90 ± 0.23 cB |
| Organic acids (g/kg DM) | |||||||||
| Malic acid | |||||||||
| Early (I) | 7.43 ± 0.97 aA | 8.70 ± 1.20 aA | 7.90 ± 0.72 aA | 7.90 ± 1.50 aA | 8.98 ± 0.94 aA | 4.69 ± 0.83 bA | 5.64 ± 0.35 abA | 5.13 ± 1.43 abA | 7.56 ± 1.24 aA |
| Middle (II) | 6.88 ± 0.02 bA | 7.62 ± 0.29 abA | 7.92 ± 0.17 abA | 9.19 ± 0.61 aA | 8.79 ± 0.19 aA | 6.90 ± 0.17 abA | 6.03 ± 1.11 abA | 5.45 ± 0.06 bA | 8.35 ± 1.77 aA |
| Quinic acid | |||||||||
| Early (I) | 2.14 ± 0.61 abA | 2.05 ± 0.61 abA | 1.78 ± 0.35 abA | 2.68 ± 0.86 aA | 2.27 ± 0.71 abA | 1.93 ± 0.73 aA | 1.83 ± 0.35 aA | 2.10 ± 0.40 aA | 2.41 ± 0.24 aA |
| Middle (II) | 0.68 ± 0.02 bB | 1.24 ± 0.08 aA | 1.29 ± 0.04 aA | 1.54 ± 0.14 aA | 1.53 ± 0.22 aA | 0.58 ± 0.08 bB | 0.84 ± 0.13 bB | 0.77 ± 0.05 bB | 1.29 ± 0.36 abB |
| Controlled Atmosphere | Hydrothermal Treatment Followed by Controlled Atmosphere | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Compound/ Harvest | Initial (0 day) | Storage (day) CA | Shelf-Life—Edible Ripeness CASL | Storage (day) HTCA | Shelf-Life—Edible Ripeness HTCASL | ||||
| 30 | 50 | 30 * | 50 * | 30 | 50 | 30 * | 50 * | ||
| Fatty acids (%) | |||||||||
| Palmitic | |||||||||
| Early (I) | 30.09 ± 1.05 aA | 29.51 ± 1.91 aA | 29.30 ± 1.16 aA | 28.32 ± 0.81 abA | 26.50 ± 2.22 bA | 29.77 ± 1.85 abA | 30.76 ± 1.67 aA | 27.88 ± 1.33 bA | 28.26 ± 1.53 abA |
| Middle (I) | 29.35 ± 0.83 aA | 29.15 ± 2.22 aA | 28.66 ± 0.69 aA | 27.36 ± 2.26 aA | 26.93 ± 3.25 aA | 27.52 ± 0.88 abAB | 26.79 ± 1.26 bB | 27.40 ± 1.89 abA | 26.96 ± 1.84 abAB |
| Palmitoleic | |||||||||
| Early (I) | 14.38 ± 0.65 aA | 15.23 ± 1.09 aA | 15.80 ± 0.72 aA | 14.07 ± 1.06 aA | 14.74 ± 2.13 aA | 14.41 ± 1.99 aA | 14.84 ± 0.62 aA | 14.43 ± 1.62 aA | 14.57 ± 1.76 aAB |
| Middle (II) | 15.34 ± 1.09 aA | 15.24 ± 1.19 aA | 15.94 ± 1.05 aA | 14.53 ± 0.96 aA | 15.09 ± 2.41 aA | 15.23 ± 1.48 aA | 15.02 ± 2.09 aA | 15.34 ± 1.19 aA | 15.75 ± 1.06 aA |
| Oleic | |||||||||
| Early (I) | 40.01 ± 1.17 aA | 39.55 ± 1.85 aA | 37.87 ± 1.83 aA | 41.37 ± 1.42 aA | 41.98 ± 4.65 aA | 41.66 ± 3.75 aA | 39.22 ± 1.03 aAB | 41.86 ± 2.53 aA | 40.26 ± 2.02 aB |
| Middle (II) | 40.25 ± 2.26 aA | 41.40 ± 4.47 aA | 39.80 ± 1.84 aA | 42.76 ± 2.53 aA | 41.20 ± 5.19 aA | 42.66 ± 1.89 aA | 43.31 ± 2.53 aA | 41.84 ± 2.22 aA | 42.22 ± 2.68 aB |
| Linoleic | |||||||||
| Early (I) | 14.83 ± 1.37 aA | 14.99 ± 1.50 aA | 16.28 ± 1.09 aA | 15.36 ± 1.05 aA | 15.87 ± 1.83 aA | 14.80 ± 1.79 aA | 14.51 ± 1.22 aA | 15.02 ± 1.86 aA | 15.99 ± 1.61 aA |
| Middle (II) | 14.38 ± 1.18 abA | 13.59 ± 1.63 abA | 14.96 ± 0.82 abAB | 14.58 ± 1.65 abA | 15.99 ± 1.27 aA | 14.02 ± 0.79 aAB | 14.26 ± 0.99 aA | 14.73 ± 0.81 aA | 14.40 ± 1.49 aAB |
| α-linolenic | |||||||||
| Early (I) | 0.77 ± 0.06 aA | 0.70 ± 0.09 aA | 0.72 ± 0.07 aA | 0.86 ± 0.19 aA | 0.88 ± 0.18 aA | 0.73 ± 0.17 abA | 0.67 ± 0.07 bA | 0.82 ± 0.14 abA | 0.93 ± 0.18 aA |
| Middle (II) | 0.67 ± 0.08 aA | 0.60 ± 0.05 aAB | 0.62 ± 0.04 aA | 0.75 ± 0.10 aA | 0.77 ± 0.13 aA | 0.57 ± 0.05 aAB | 0.63 ± 0.06 aA | 0.69 ± 0.07 aAB | 0.69 ± 0.07 aB |
| Tocopherols (mg/kg DM) | |||||||||
| α-tocopherol | |||||||||
| Early (I) | 61.06 ± 10.79 bcB | 61.78 ± 6.80 bcB | 64.89 ± 8.46 bcB | 81.37 ± 4.68 aA | 83.45 ± 11.92 aA | 54.03 ± 7.15 cB | 53.22 ± 5.52 cB | 78.13 ± 8.90 aA | 52.57 ± 3.00 cB |
| Middle (II) | 72.56 ± 5.74 bAB | 70.71 ± 24.87 abAB | 67.59 ± 11.70 abA | 85.39 ± 6.14 aA | 87.43 ± 9.07 aA | 52.35 ± 3.66 cB | 59.59 ± 5.67 cAB | 76.40 ± 9.32 bA | 77.36 ± 5.18 bA |
| β-tocopherol | |||||||||
| Early (I) | 20.74 ± 2.35 bcA | 19.15 ± 3.80 cB | 29.01 ± 4.96 abA | 23.35 ± 2.70 ab cAB | 27.56 ± 7.68 abA | 16.31 ± 2.46 cB | 20.83 ± 1.52 bcB | 25.54 ± 3.42 abA | 21.50 ± 3.19 bcB |
| Middle (II) | 21.57 ± 4.05 bA | 23.54 ± 10.16 abAB | 27.69 ± 3.89 abA | 27.85 ± 4.39 abA | 32.23 ± 6.70 aA | 22.58 ± 3.64 bAB | 28.93 ± 3.54 abA | 29.32 ± 2.61 abA | 32.18 ± 1.90 aA |
| Phytosterols (g/kg DM) | |||||||||
| β-sitosterol | |||||||||
| Early (I) | 155.67 ± 15.05 abA | 141.87 ± 10.55 bA | 163.38 ± 10.31 abA | 166.38 ± 12.93 abA | 177.72 ± 32.90 aA | 148.30 ± 14.35 abB | 147.06 ± 10.93 abB | 190.44 ± 20.11 aB | 179.57 ± 10.97 aAB |
| Middle (II) | 150.08 ± 11.06 bA | 142.55 ± 15.02 bA | 149.68 ± 10.91 abAB | 173.52 ± 15.12 aA | 163.10 ± 17.55 abA | 179.05 ± 4.83 aA | 182.01 ± 10. 68 aA | 198.10 ± 3.80 aA | 198.54 ± 9.15 aA |
| Campesterol | |||||||||
| Early (I) | 24.78 ± 1.71 aA | 24.41 ± 1.49 aA | 26.19 ± 1.86 aAB | 27.95 ± 2.80 aA | 26.71 ± 5.02 aA | 24.82 ± 0.93 bB | 23.90 ± 1.52 bB | 28.98 ± 1.63 aA | 26.06 ± 1.76 bAB |
| Middle (II) | 25.43 ± 1.90 aA | 24.06 ± 1.72 aA | 25.99 ± 2.02 abAB | 27.40 ± 1.47 aA | 25.99 ± 2.02 aA | 28.31 ± 1.03 aA | 28.39 ± 0.92 aA | 29.33 ± 1.70 aA | 30.13 ± 0.97 aA |
| Abscisic acid (mg/kg DM) | |||||||||
| Early (I) | 1.79 ± 0.40 cA | 6.01 ± 0.97 bA | 7.13 ± 0.23 bA | 22.36 ± 3.84 aA | 19.64 ± 4.03 aA | 2.52 ± 0.10 bA | 3.17 ± 1.66 bA | 24.41 ± 3.21 aA | 25.49 ± 0.05 aA |
| Middle (II) | 2.37 ± 0.30 cAB | 2.16 ± 0.06 cB | 2.13 ± 0.19 cB | 24.36 ± 9.86 aA | 20.72 ± 5.25 aA | 2.47 ± 1.39 bcA | 3.92 ± 1.02 bA | 26.76 ± 1.56 aA | 19.73 ± 1.03 aB |
| H-Antioxidant activity (µmol/g TE DM) | |||||||||
| Early (I) | 16.96 ± 2.12 bA | 18.74 ± 1.79 abA | 18.18 ± 1.60 abA | 19.50 ± 2.27 abA | 21.25 ± 3.44 aA | 15.47 ± 2.30 bA | 16.47 ± 2.25 bA | 21.75 ± 2.95 aA | 20.47 ± 2.63 abA |
| Middle (II) | 14.60 ± 1.97 bA | 16.60 ± 2.67 bA | 15.77 ± 1.04 bB | 20.50 ± 4.23 abA | 25.37 ± 3.22 aA | 15.13 ± 1.74 bA | 15.10 ± 1.3 bA | 20.74 ± 2.88 aA | 19.85 ± 2.44 aA |
| L-Antioxidant activity (µmol/g TE DM) | |||||||||
| Early (I) | 2.44 ± 0.14 bA | 2.59 ± 0.15 bA | 2.46 ± 0.40 bA | 3.10 ± 0.44 abA | 3.45 ± 0.56 aA | 3.98 ± 0.60 abA | 3.77 ± 0.58 abA | 4.28 ± 0.45 abA | 4.63 ± 0.58 aA |
| Middle (II) | 2.85 ± 0.51 bA | 2.18 ± 0.18 bA | 2.58 ± 0.25 bA | 2.39 ± 0.30 bB | 2.47 ± 0.51 bB | 3.18 ± 0.10 abB | 3.11 ± 0.31 abB | 4.27 ± 0.36 aA | 4.11 ± 0.67 aAB |
| Controlled Atmosphere | Hydrothermal Treatment Followed by Controlled Atmosphere | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Peak N° | Phenolic Compound (mg/kg DM)/ Harvest Stage | Initial (0 day) | Storage (day) CA | Shelf-Life—Edible Ripeness CASL | Storage (day) HTCA | Shelf-Life—Edible Ripeness HTCASL | ||||
| 30 | 50 | 30 * | 50 * | 30 | 50 | 30 * | 50 * | |||
| 1 | Dihydroxybenzoic acid glycoside (DHBAG) | |||||||||
| Early (I) | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | Tr | Tr | |
| Middle (II) | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 7.28 ± 1.37 a | 1.12 ± 0.09 b | |
| 2 | Syringic acid glycoside (SAG) | |||||||||
| Early (I) | Tr | Tr | Tr | 10.53 ± 5.40 aA | 16.20 ± 0.61 aA | Tr | Tr | 7.76 ± 0.33 bB | 4.33 ± 0.92 cB | |
| Middle (II) | 0.0 | 0.0 | 0.0 | 35.68 ± 3.95 aB | 16.82 ± 3.45 cA | Tr | Tr | 22.20 ± 0.61 bA | 25.04 ± 1.09 bA | |
| 3 | Caffeic acid glycoside 1 (CAG1) | |||||||||
| Early (I) | 0.0 | 0.0 | 0.0 | Tr | Tr | 0.0 | 0.0 | 0.34 ± 0.11 bB | 2.47 ± 0.52 aB | |
| Middle (II) | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 21.57 ± 1.88 aA | 22.54 ± 1.37 aA | |
| 4 | Caffeic acid glycoside 2 (CAG2) | |||||||||
| Early (I) | 0.0 | 0.0 | 0.0 | Tr | Tr | 0.0 | 0.0 | Tr | Tr | |
| Middle (II) | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 6.97 ± 1.23 a | 8.77 ± 0.29 a | |
| 5 | Hydroxybenzoic acid glycoside (HBAG) | |||||||||
| Early (I) | 102.73 ± 1.86 bA | 85.77 ± 13.34 cA | 71.23 ± 2.09 cdA | 100.63 ± 3.44 bA | 43.49 ± 1.63 eB | 107.88 ± 0.71 aA | 82.45 ± 5.67 cA | 54.77 ± 3.41 e | 59.65 ± 3.66 e | |
| Middle (II) | 56.18 ± 14.13 cB | 68.66 ± 11.96 bcA | 88.62 ± 1.08 bA | 117.64 ± 18.57 aA | 115.02 ± 5.60 aA | 73.01 ± 1.18 cB | 74.54 ± 11.81 cA | Tr | Tr | |
| 6 | p-Coumaric acid glycoside (p-CAG) | |||||||||
| Early (I) | 0.0 | Tr | Tr | 12.19 ± 0.71 bB | 41.44 ± 0.42 aA | 0.0 | 0.0 | 43.11 ± 1.84 aB | 7.22 ± 1.77 cB | |
| Middle (II) | 0.0 | 0.0 | 0.0 | 55.79 ± 5.84 bA | 31.57 ± 1.73 cA | 0.0 | 0.0 | 81.98 ± 4.54 aA | 86.17 ± 6.10 aA | |
| 7 | Caffeic acid derivative (CAD) | |||||||||
| Early (I) | 0.0 | 0.0 | 0.0 | 0.30 ± 0.11 cB | 8.67 ± 1.92 aA | 0.0 | 0.0 | 3.13 ± 0.12 bB | 9.34 ± 0.96 aB | |
| Middle (II) | 0.0 | 0.0 | 0.0 | 14.73 ± 5.92 bA | 2.51 ± 1.44 cB | 0.0 | 0.0 | 27.31 ± 0.59 aA | 17.92 ± 1.89 bA | |
| 8 | p-Coumaric acid derivative (p-CAD1) | |||||||||
| Early (I) | 0.0 | 0.0 | 0.0 | Tr | Tr | 0.0 | 0.0 | 0.0 | 0.0 | |
| Middle (II) | 0.0 | 0.0 | 0.0 | 0.59 ± 0.30 c | 0.10 ± 0.00 d | 0.0 | 0.0 | 5.88 ± 0.30 a | 1.13 ± 0.19 b | |
| 9 | Caffeic acid acetilglycoside (CAAG) | |||||||||
| Early (I) | 0.0 | 0.0 | 0.0 | Tr | Tr | 0.0 | 0.0 | 3.16 ± 0.66 abB | 4.46 ± 1.05 aB | |
| Middle (II) | 0.0 | 0.0 | 0.0 | 0.28 ± 0.08 b | 0.11 ± 0.01 b | 0.0 | 0.0 | 13.81 ± 1.12 aA | 13.06 ± 2.37 aA | |
| 10 | p-Coumaric acid (p-CA) | |||||||||
| Early (I) | Tr | Tr | Tr | 12.87 ± 1.13 bB | 23.39 ± 3.50 aA | Tr | Tr | 0.63 ± 0.10 cB | 0.09 ± 0.00 dB | |
| Middle (II) | Tr | Tr | Tr | 27.51 ± 4.14 aA | 19.94 ± 0.11 abAB | Tr | Tr | 25.46 ± 1.80 aA | 21.26 ± 3.75 aA | |
| 11 | p-Coumaric acid acetylglycoside (p-CAAG) | |||||||||
| Early (I) | 0.0 | 0.0 | Tr | 0.0 | Tr | 0.0 | 0.0 | Tr | Tr | |
| Middle (II) | 0.0 | 0.0 | 0.0 | 5.13 ± 1.50 b | 1.67 ± 0.77 c | 0.0 | 0.0 | 7.29 ± 0.51 a | 5.59 ± 0.37 b | |
| 12 | p-Coumaric acid derivative (p-CAD2) | |||||||||
| Early (I) | 0.0 | 0.0 | 0.0 | 105.38 ± 10.93 abB | 125.31 ± 22.26 aB | 0.0 | 0.0 | 56.00 ± 3.79 cB | 36.22 ± 145 dB | |
| Middle (II) | 0.0 | 0.0 | 0.0 | 188.02 ± 22.21 bA | 181.98 ± 15.08 bA | 0.0 | 0.0 | 262.10 ± 3.26 aA | 184.75 ± 19.59 bA | |
| 13 | trans-Ferulic acid (t-FA) | |||||||||
| Early (I) | 0.0 | Tr | Tr | 1.57 ± 0.54 bB | 9.01 ± 2.33 aA | 0.0 | 0.0 | Tr | Tr | |
| Middle (II) | 0.0 | Tr | Tr | 5.24 ± 1.73 bA | 9.49 ± 2.35 aA | 0.0 | 0.0 | 3.90 ± 0.31 b | Tr | |
| 14 | p-Coumaric acid derivative (p-CAD3) | |||||||||
| Early (I) | 0.0 | 0.0 | 0.0 | Tr | Tr | 0.0 | 0.0 | 3.32 ± 0.57 aB | 3.01 ± 0.41 aB | |
| Middle (II) | 0.0 | 0.0 | 0.0 | 1.51 ± 0.00 d | 1.91 ± 1.00 c | 0.0 | 0.0 | 30.01 ± 0.44 aA | 5.43 ± 1.45 bAB | |
| Total phenolic compounds | ||||||||||
| Early (I) | 102.73 ± 1.86 dA | 85.77 ± 13.34 eA | 71.23 ± 2.09 efA | 243.46 ± 20.80 aB | 267.52 ± 28.01 aB | 107.88 ± 0.71 dA | 82.45 ± 5.67 eA | 172.22 ± 9.58 bB | 126.69 ± 9.66 cB | |
| Middle (II) | 56.18 ± 14.13 eB | 68.66 ± 11.96 deA | 88.62 ± 1.09 cA | 454.62 ± 67.72 abA | 381.97 ± 27.32 bA | 73.01 ± 1.18 dB | 74.54 ± 11.81 dA | 515.76 ± 9.44 aA | 392.72 ± 4.21 bA | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chirinos, R.; Campos, D.; Martínez, S.; Llanos, S.; Betalleluz-Pallardel, I.; García-Ríos, D.; Pedreschi, R. The Effect of Hydrothermal Treatment on Metabolite Composition of Hass Avocados Stored in a Controlled Atmosphere. Plants 2021, 10, 2427. https://doi.org/10.3390/plants10112427
Chirinos R, Campos D, Martínez S, Llanos S, Betalleluz-Pallardel I, García-Ríos D, Pedreschi R. The Effect of Hydrothermal Treatment on Metabolite Composition of Hass Avocados Stored in a Controlled Atmosphere. Plants. 2021; 10(11):2427. https://doi.org/10.3390/plants10112427
Chicago/Turabian StyleChirinos, Rosana, David Campos, Sofía Martínez, Sílfida Llanos, Indira Betalleluz-Pallardel, Diego García-Ríos, and Romina Pedreschi. 2021. "The Effect of Hydrothermal Treatment on Metabolite Composition of Hass Avocados Stored in a Controlled Atmosphere" Plants 10, no. 11: 2427. https://doi.org/10.3390/plants10112427
APA StyleChirinos, R., Campos, D., Martínez, S., Llanos, S., Betalleluz-Pallardel, I., García-Ríos, D., & Pedreschi, R. (2021). The Effect of Hydrothermal Treatment on Metabolite Composition of Hass Avocados Stored in a Controlled Atmosphere. Plants, 10(11), 2427. https://doi.org/10.3390/plants10112427






