Photosynthetic Characteristics of Three Cohabitated Macroalgae in the Daya Bay, and Their Responses to Temperature Rises
Abstract
:1. Introduction
2. Results
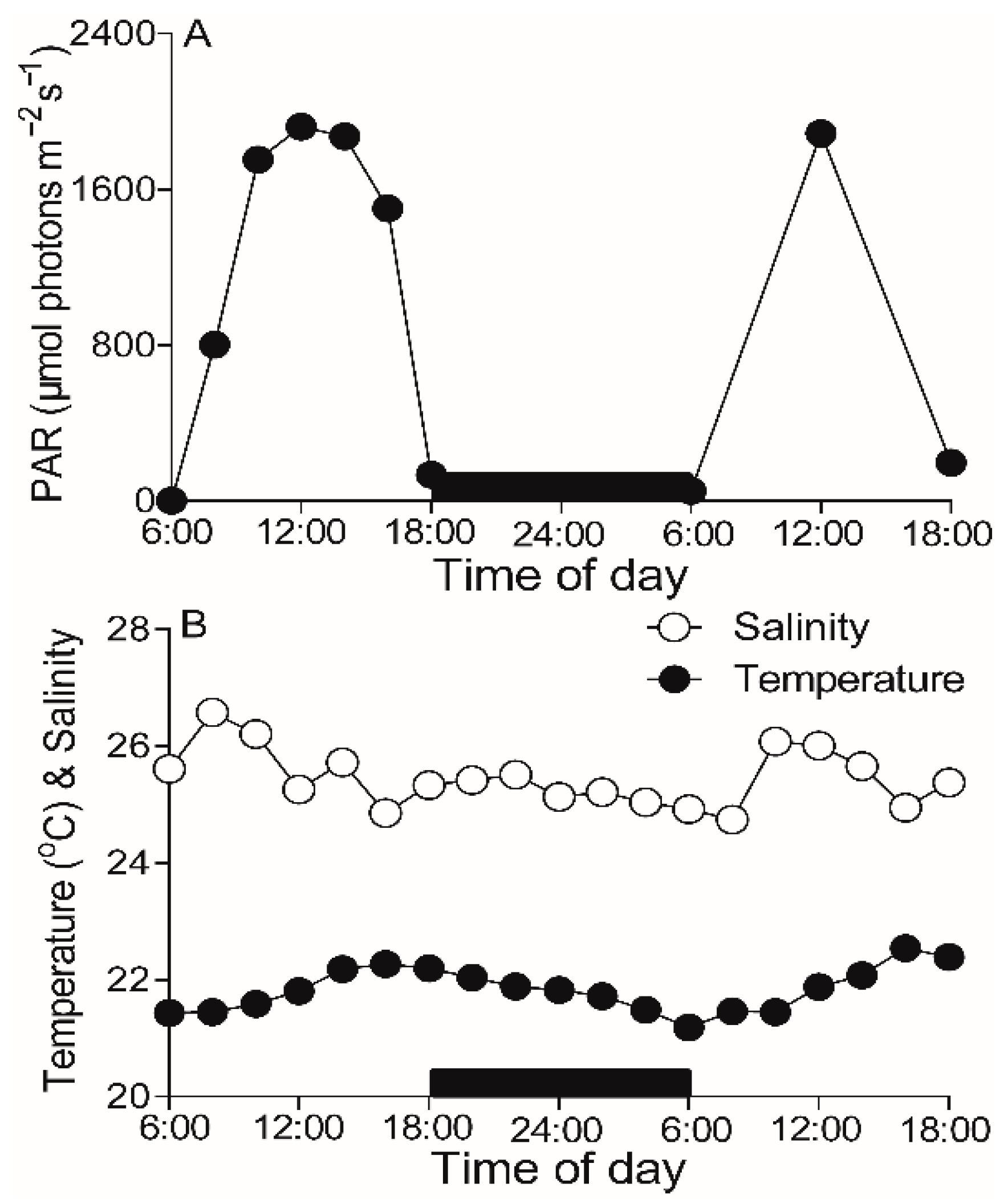
2.1. Field Environments
2.2. Cell Compositions
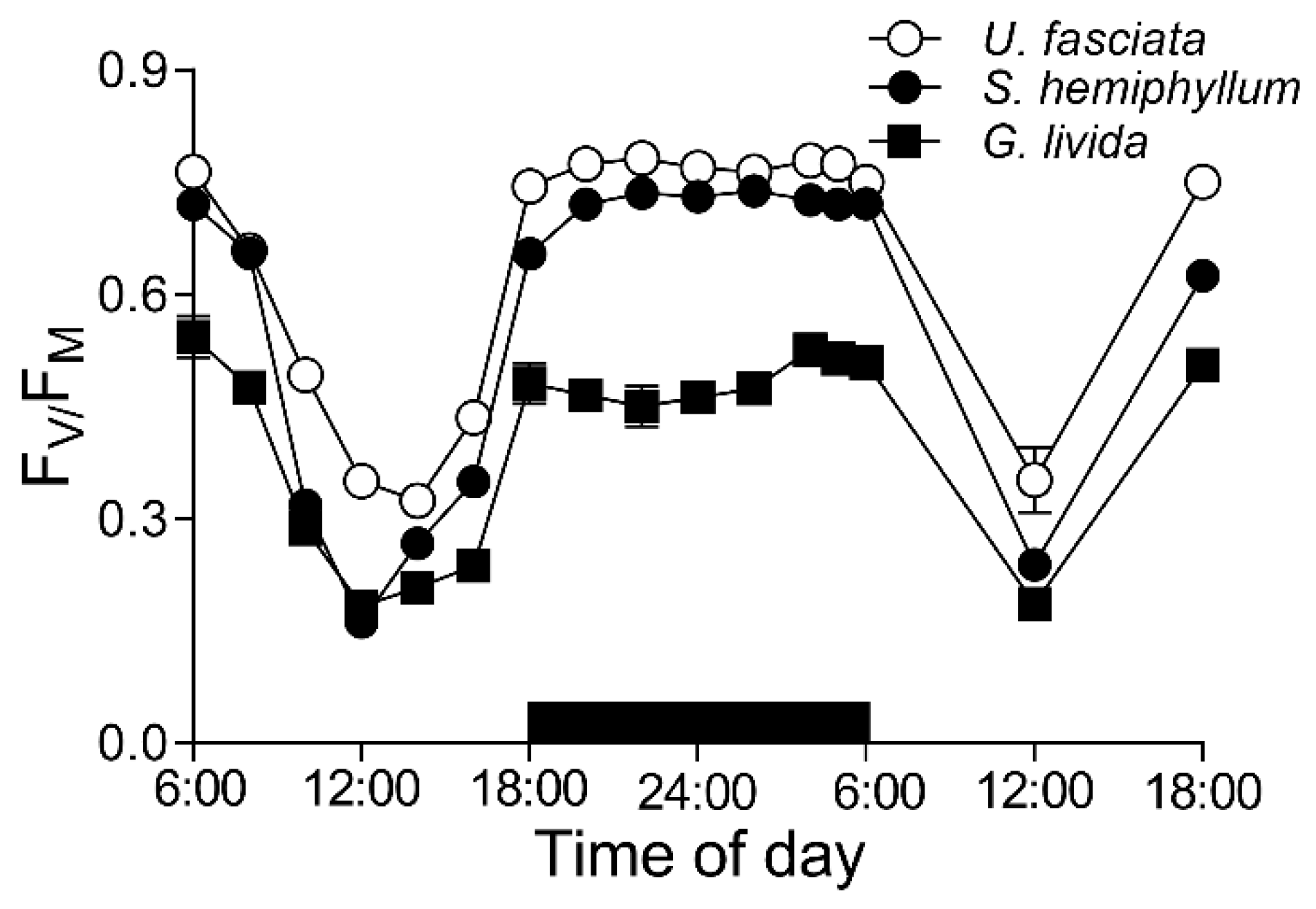
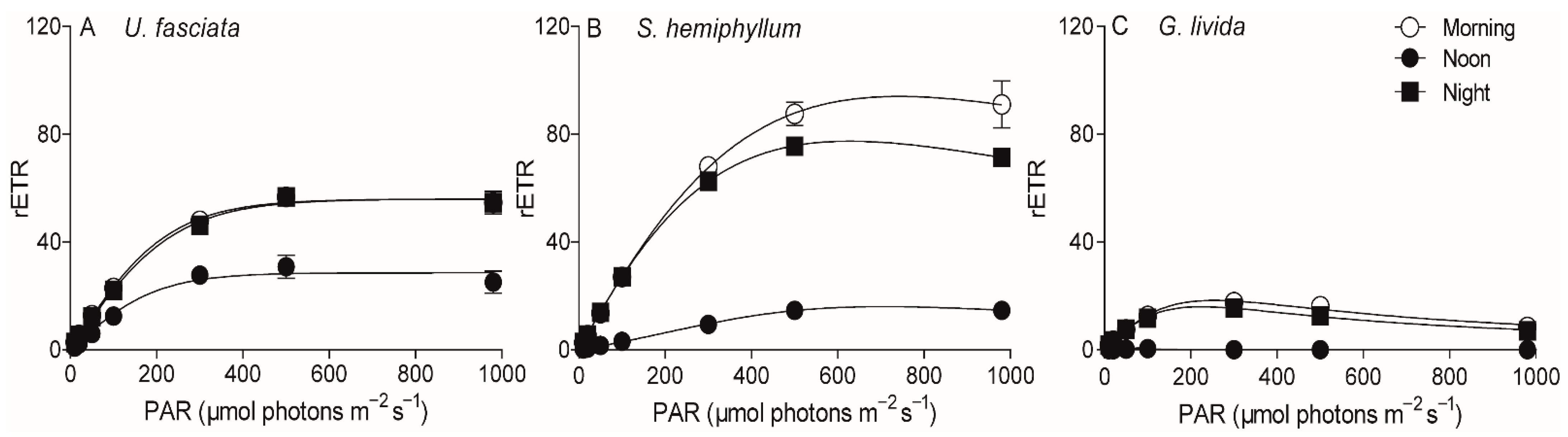
2.3. Chlorophyll Fluorescence
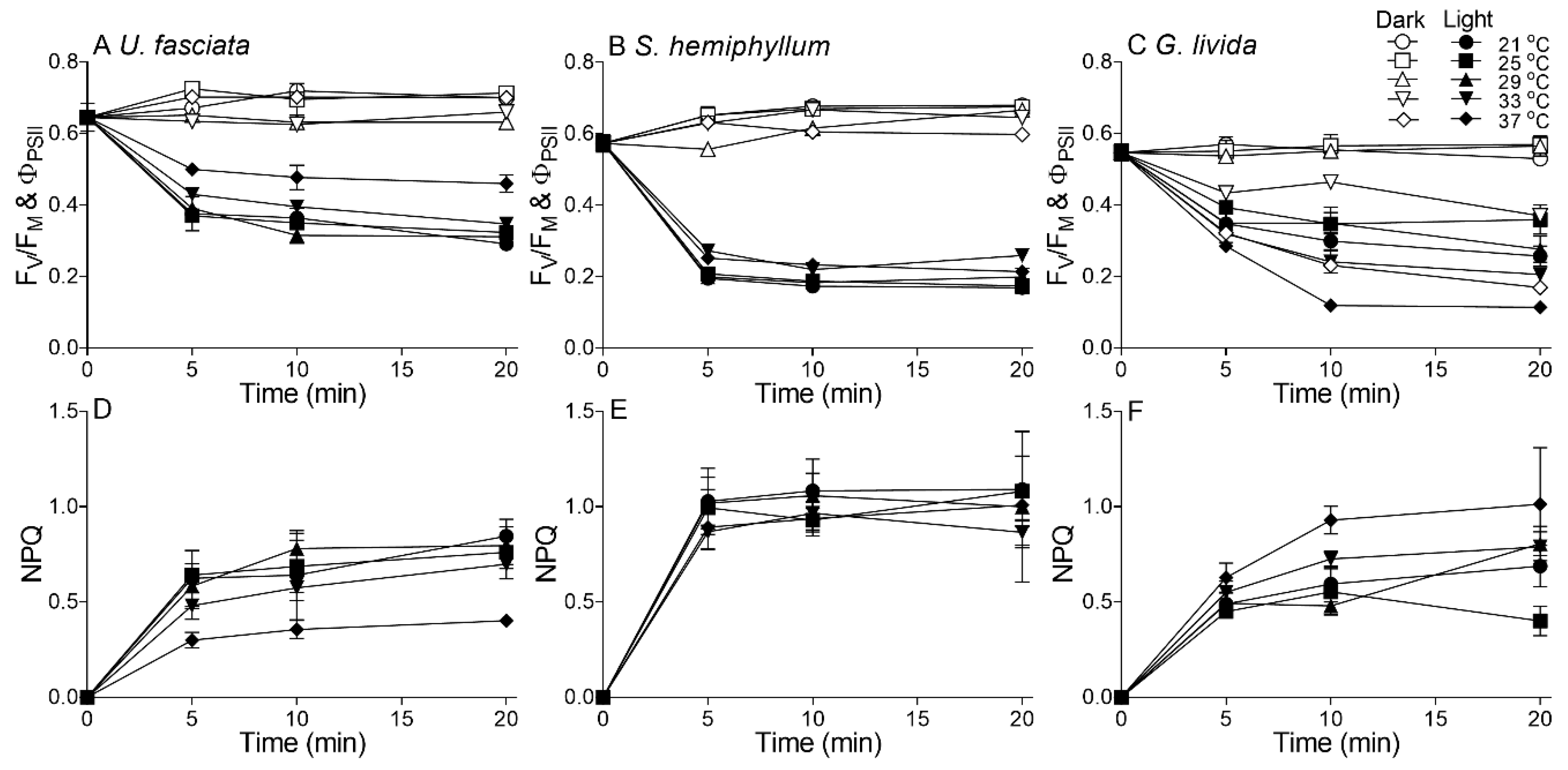
2.4. Effects of Temperature Rise
3. Discussion
4. Materials and Methods
4.1. Study Area and Experimental Protocol
4.2. Environmental Factors Measurements
4.3. Chlorophyll Fluorescence Measurements
4.4. Cellular Compositions Measurements
4.5. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ware, C.; Dijkstra, J.A.; Mello, K.; Stevens, A.; O’Brien, B.; Ikedo, W. A novel three-dimensional analysis of functional architecture that describes the properties of macroalgae as a refuge. Mar. Ecol. Prog. Ser. 2019, 608, 93–103. [Google Scholar] [CrossRef] [Green Version]
- Krause-Jensen, D.; Duarte, C.M. Substantial role of macroalgae in marine carbon sequestration. Nat. Geosci. 2016, 9, 737–742. [Google Scholar] [CrossRef]
- Neori, A.; Chopin, T.; Troell, M.; Buschmann, A.H.; Kraemer, G.; Halling, C.; Shpigel, M.; Yarish, C. Integrated aquaculture: Rationale, evolution and state of the art emphasizing seaweed biofiltration in modern aquaculture. Aquaculture 2004, 231, 361–391. [Google Scholar] [CrossRef]
- Yang, Y.; Chai, Z.; Wang, Q.; Chen, W.; He, Z.; Jiang, S. Cultivation of seaweed Gracilaria in Chinese coastal waters and its contribution to environmental improvements. Algal Res. 2015, 9, 236–244. [Google Scholar] [CrossRef]
- Yang, L.; Lu, Q.; Brodie, J. A review of the bladed Bangiales (Rhodophyta) in China: History, culture, and taxonomy. Eur. J. Phycol. 2017, 52, 251–263. [Google Scholar] [CrossRef]
- Falkenberg, M.; Nakano, E.; Zambotti-Villela, L.; Zatelli, G.A.; Philippus, A.C.; Imamura, K.B.; Velasquez, A.M.A.; Freitas, R.F.; Tallarico, L.F.; Colepicolo, P.; et al. Bioactive compounds against neglected diseases isolated from macroalgae: A review. J. Appl. Phycol. 2019, 31, 797–823. [Google Scholar] [CrossRef] [Green Version]
- Gao, G.; Burgess, G.; Wu, M.; Wang, S.; Gao, K. Using macroalgae as biofuel: Current opportunities and challenges. Bot. Mar. 2020, 63, 355–371. [Google Scholar] [CrossRef]
- Messyasz, B.; Michalak, I.; Łęska, B.; Schroeder, G.; Górka, B.; Korzeniowska, K.; Lipok, J.; Wieczorek, P.; Rój, E.; Wilk, R.; et al. Valuable natural products from marine and freshwater macroalgae obtained from supercritical fluid extracts. J. Appl. Phycol. 2018, 30, 591–603. [Google Scholar] [CrossRef]
- Wells, E.; Wilkinson, M.; Wood, P.; Scanlan, C. The use of macroalgal species richness and composition on intertidal rocky seashores in the assessment of ecological quality under the European Water Framework Directive. Mar. Pollut. Bull. 2007, 55, 151–161. [Google Scholar] [CrossRef]
- Li, G.; Qin, Z.; Zhang, J.; Lin, Q.; Ni, G.; Tan, Y.; Zou, D. Algal density mediates the photosynthetic responses of a marine macroalga Ulva conglobata (Chlorophyta) to temperature and pH changes. Algal Res. 2020, 46, 101797. [Google Scholar] [CrossRef]
- Necchi, O. Photosynthetic responses to temperature in tropical lotic macroalgae. Phycol. Res. 2004, 52, 140–148. [Google Scholar] [CrossRef]
- Iñiguez, C.; Galmés, J.; Gordillo, F.J.L. Rubisco carboxylation kinetics and inorganic carbon utilization in polar versus cold-temperate seaweeds. J. Exp. Bot. 2019, 70, 1283–1297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nejrup, L.B.; Staehr, P.A.; Thomsen, M.S. Temperature- and light-dependent growth and metabolism of the invasive red algae Gracilaria vermiculophylla—A comparison with two native macroalgae. Eur. J. Phycol. 2013, 48, 295–308. [Google Scholar] [CrossRef] [Green Version]
- Rautenberger, R.; Huovinen, P.; Gómez, I. Effects of increased seawater temperature on UV tolerance of Antarctic marine macroalgae. Mar. Biol. 2015, 162, 1087–1097. [Google Scholar] [CrossRef]
- IPCC. Summary for Policymakers: Climate change 2013—The physical science basis. In Working Group 1 Contribution to the IPCC Fifth Assessment Report; Stocker, T.F.S.T.G., Qin, D., Plattner, G.-K., Tignor, M., Allen, S.K., Boschung, J., Eds.; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar]
- Von Schuckmann, K.; Cheng, L.; Palmer, M.D.; Hansen, J.; Tassone, C.; Aich, V.; Adusumilli, S.; Beltrami, H.; Boyer, T.; Cuesta-Valero, F.J.; et al. Heat stored in the Earth system: Where does the energy go? Earth Syst. Sci. Data 2020, 12, 2013–2041. [Google Scholar] [CrossRef]
- Tokarska, K.B.; Hegerl, G.C.; Schurer, A.P.; Forster, P.M.; Marvel, K. Observational constraints on the effective climate sensitivity from the historical period. Environ. Res. Lett. 2020, 15, 034043. [Google Scholar] [CrossRef] [Green Version]
- Thomsen, M.S.; Mondardini, L.; Alestra, T.; Gerrity, S.; Tait, L.; South, P.M.; Lilley, S.A.; Schiel, D.R. Local extinction of bull kelp (Durvillaea spp.) due to a marine heatwave. Front. Mar. Sci. 2019, 6, 84. [Google Scholar] [CrossRef] [Green Version]
- Hao, Y.; Tang, D.; Boicenco, L.; Wang, S. Environmental ecological response to increasing water temperature in the Daya Bay, Southern China in 1982–2012. Nat. Res. 2016, 7, 184–192. [Google Scholar] [CrossRef] [Green Version]
- Ren, L.; Song, X.; He, D.; Wang, J.; Tan, M.; Xia, X.; Li, G.; Tan, Y.; Wu, Q. Bacterioplankton metacommunity processes across thermal gradients: Weaker species sorting but stronger niche segregation in summer than in winter in a subtropical bay. Appl. Environ. Microb. 2019, 85, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Li, T.; Liu, S.; Huang, L.; Huang, H.; Lian, J.; Yan, Y.; Lin, S. Diatom to dinoflagellate shift in the summer phytoplankton community in a bay impacted by nuclear power plant thermal effluent. Mar. Ecol. Prog. Ser. 2011, 424, 75–85. [Google Scholar] [CrossRef]
- Wang, Y.; Lou, Z.; Sun, C.; Sun, S. Ecological environment changes in Daya Bay, China, from 1982 to 2004. Mar. Pollut. Bull. 2008, 56, 1871–1879. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Lin, Q.; Lin, Y.; Jia, X.; Li, C.; Wang, W. Spatial-temporal variation of large macrobenthic animals in cage culture sea area in Daya Bay. Chin Environ. Sci. 2005, 25, 412–416. [Google Scholar]
- Wu, M.; Wang, Y. Using chemometrics to evaluate anthropogenic effects in Daya Bay, China. Estuar. Coast Shelf Sci. 2007, 72, 732–742. [Google Scholar] [CrossRef]
- Xu, G.; Liu, J.; Song, X.; Tan, M.; Ren, H.; Li, D.; Tan, Y.; Huang, L.; Li, G. Diel rhythm in photosynthetic performance of phytoplankton assemblages is predicted to be light-dependent from in situ and mesocosm chlorophyll fluorescence. J. Coast. Res. 2020, 104, 445–454. [Google Scholar] [CrossRef]
- Song, X.; Li, Y.; Xiang, C.; Su, X.; Xu, G.; Tan, M.; Huang, Y.; Liu, J.; Ma, Z.; Huang, L.; et al. Nitrogen and phosphorus enrichments alter the dynamics of plankton community in Daya Bay, northern South China Sea: Results of mesocosm studies. Mar. Freshw. Res. 2021, 72, 1632–1642. [Google Scholar] [CrossRef]
- Luo, D.; Chen, X.; Li, T.; Yi, J. Production of TM satellite image distribution map of macroalgae in Daya Bay. Remote Sens. Inf. 1990, 4, 30–32. [Google Scholar]
- Yang, Y. Coastal Environmental Bioremediation and Seaweed Resource Utilization; Science Press: Beijing, China, 2016; pp. 1–364. [Google Scholar]
- Kok, B. On the inhibition of photosynthesis by intense light. Biochim. Biophys. Acta 1956, 21, 234–244. [Google Scholar] [CrossRef]
- Wijnen, H.; Young, M.W. Interplay of circadian clocks and metabolic rhythms. Annu. Rev. Genet. 2006, 40, 409–448. [Google Scholar] [CrossRef]
- Zhang, S.; Xiang, C.; Zhou, X.; Liu, S.; Cheng, X.; Wang, K. Photosynthetic fluorescence characteristics of six macroalgae species seaweed beds of Gouqi Island, Zhejiang, China. Chin. J. Appl. Ecol. 2018, 29, 3441–3448. [Google Scholar]
- Zhang, D.; Beer, S.; Li, H.; Gao, K. Photosystems I and II in Ulva lactuca are well protected from high incident sunlight. Algal Res. 2020, 52, 102094. [Google Scholar] [CrossRef]
- Cruces, E.; Rautenberger, R.; Cubillos, V.M.; Ramírez-Kushel, E.; Rojas-Lillo, Y.; Lara, C.; Montory, J.A.; Gómez, I. Interaction of photoprotective and acclimation mechanisms in Ulva rigida (Chlorophyta) in response to diurnal changes in solar radiation in Southern Chile. J. Phycol. 2019, 55, 1011–1027. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Badger, M.R. Photoprotection in plants: A new light on photosystem II damage. Trends Plant Sci. 2011, 16, 53–60. [Google Scholar] [CrossRef]
- Dudgeon, S.R.; Johnson, A.S. Thick vs. thin: Thallus morphology and tissue mechanics influence differential drag and dislodgement of two co-dominant seaweeds. J. Exp. Mar. Biol. Ecol. 1992, 165, 23–43. [Google Scholar] [CrossRef]
- Bajjouk, T.; Guillaumont, B.; Populus, J. Application of airborne imaging spectrometry system data to intertidal seaweed classification and mapping. Hydrobiologia 1996, 326, 463–471. [Google Scholar] [CrossRef]
- Chao-Rodríguez, Y.; Domínguez-Gómez, J.A.; Sánchez-Carnero, N.; Rodríguez-Pérez, D. A comparison of spectral macroalgae taxa separability methods using an extensive spectral library. Algal Res. 2017, 26, 463–473. [Google Scholar] [CrossRef]
- Häder, D.-P.; Lebert, M.; Figueroa, F.L.; Jiménez, C.; Viñegla, B.; Perez-Rodriguez, E. Photoinhibition in Mediterranean macroalgae by solar radiation measured on site by PAM fluorescence. Aquat. Bot. 1998, 61, 225–236. [Google Scholar] [CrossRef]
- Foyer, C.H.; Lelandais, M.; Kunert, K.J. Photooxidative stress in plants. Physiol. Plant 1994, 92, 696–717. [Google Scholar] [CrossRef]
- Bischof, K.; Peralta, G.; Kräbs, G.; van de Poll, W.H.; Pérez-Lloréns, J.L.; Breeman, A.M. Effects of solar UV-B radiation on canopy structure of Ulva communities from Southern Spain. J. Exp. Bot. 2002, 53, 2411–2421. [Google Scholar] [CrossRef] [Green Version]
- Gómez, I.; Huovinen, P. Morpho-functional patterns and zonation of South Chilean seaweeds: The importance of photosynthetic and bio-optical traits. Mar. Ecol. Prog. Ser. 2011, 422, 77–91. [Google Scholar] [CrossRef] [Green Version]
- Cruces, E.; Flores-Molina, M.R.; Díaz, M.J.; Huovinen, P.; Gómez, I. Phenolics as photoprotective mechanism against combined action of UV radiation and temperature in the red alga Gracilaria chilensis? J. Appl. Phycol. 2018, 30, 1247–1257. [Google Scholar] [CrossRef]
- Johansson, G.; Snoeijs, P. Macroalgal photosynthetic responses to light in relation to thallus morphology and depth zonation. Mar. Ecol. Prog. Ser. 2002, 244, 63–72. [Google Scholar] [CrossRef] [Green Version]
- Gómez, I.; Ulloa, N.; Orostegui, M. Morpho-functional patterns of photosynthesis and UV sensitivity in the kelp Lessonia nigrescens (Laminariales, Phaeophyta). Mar. Biol. 2005, 148, 231–240. [Google Scholar] [CrossRef]
- Müller, P.; Li, X.; Niyogi, K.K. Non-photochemical quenching. A response to excess light energy. Plant Physiol. 2001, 125, 1558–1566. [Google Scholar] [CrossRef] [Green Version]
- Häder, D.-P.; Lebert, M.; Sinha, R.P.; Barbieri, E.S.; Helbling, E.W. Role of protective and repair mechanisms in the inhibition of photosynthesis in marine macroalgae. Photochem. Photobiol. Sci. 2002, 1, 809–814. [Google Scholar] [CrossRef] [PubMed]
- Häder, D.-P.; Figueroa, F.L. Photoecophysiology of marine macroalgae. Photochem. Photobiol. 1997, 66, 1–14. [Google Scholar] [CrossRef]
- Niyogi, K.K.; Grossman, A.R.; Björkman, O. Arabidopsis mutants define a central role for the xanthophyll cycle in the regulation of photosynthetic energy conversion. Plant Cell 1998, 10, 1121–1134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, C.Y.; Teoh, M.L.; Phang, S.M.; Lim, P.E.; Beardall, J. Interactive effects of temperature and UV radiation on photosynthesis of Chlorella strains from polar, temperate and tropical environments: Differential impacts on damage and repair. PLoS ONE 2015, 10, e139469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, J.; Tang, D.; Wang, S.; Lian, J.; Wang, Y. Changes of water temperature and harmful algal bloom in the Daya Bay in the northern South China Sea. Mar. Sci. Bull. 2007, 9, 25–33. [Google Scholar]
- Hamid, S.S.; Wakayama, M.; Ichihara, K.; Sakurai, K.; Ashino, Y.; Kadowaki, R.; Soga, T.; Tomita, M. Metabolome profiling of various seaweed species discriminates between brown, red, and green algae. Planta 2019, 249, 1921–1947. [Google Scholar] [CrossRef]
- Rautenberger, R.; Bischof, K. Impact of temperature on UV-susceptibility of two Ulva (Chlorophyta) species from Antarctic and Subantarctic regions. Polar Biol. 2006, 29, 988–996. [Google Scholar] [CrossRef] [Green Version]
- Liang, Y.; Feng, L.; Tian, C. Effect of temperature stress on the chlorophyll fluorescence of Isochrysis galbana 3011 and 8701. J. Fish Chin. 2009, 33, 37–44. [Google Scholar]
- Xu, G. Environments and Resources of Daya Bay; Anhui Press of Science and Technology: Hefei, China, 1989. [Google Scholar]
- Li, G.; Gao, K. Variation in UV irradiance related to stratospheric ozone levels affects photosynthetic carbon fixation of winter phytoplankton assemblages in the South China Sea. Mar. Biol. Res. 2012, 8, 670–676. [Google Scholar] [CrossRef] [Green Version]
- Genty, B.; Briantais, J.M.; Baker, N.R. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta 1989, 990, 87–92. [Google Scholar] [CrossRef]
- Eilers, P.H.C.; Peeters, J.C.H. A model for the relationship between light intensity and the rate of photosynthesis in phytoplankton. Ecol. Modell. 1988, 42, 199–215. [Google Scholar] [CrossRef]
- Wellburn, A.R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Beer, S.; Eshel, A. Determining phycoerythrin and phycocyanin concentrations in aqueous crude extracts of red algae. Mar. Freshw. Res. 1985, 36, 785–792. [Google Scholar] [CrossRef]
- Laurentin, A.; Edwards, C.A. A microtiter modification of the anthrone-sulfuric acid colorimetric assay for glucose-based carbohydrates. Anal. Biochem. 2003, 315, 143–145. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]

| Cell Compositions | U. fasciata | S. hemiphyllum | G. livida |
|---|---|---|---|
| Water (%) | 88.8 ± 2.39 a | 96.8 ± 1.16 b | 87.9 ± 2.27 a |
| Chl a (mg g−1 FW) | 1.00 ± 0.15 a | 0.62 ± 0.02 b | 0.34 ± 0.02 c |
| Car (mg g−1 FW) | 0.57 ± 0.08 a | 0.19 ± 0.01 b | 0.12 ± 0.01 c |
| PE (mg g−1 FW) | -- | -- | 0.16 ± 0.004 |
| PC (mg g−1 FW) | -- | -- | 0.02 ± 0.006 |
| Carbohydrate (mg g−1 FW) | 20.3 ± 0.07 a | 5.19 ± 0.67 b | 9.45 ± 0.09 c |
| Protein (mg g−1 FW) | 3.19 ± 0.18 a | 3.04 ± 0.16 a | 1.20 ± 0.20 b |
| SOD (U g−1 FW) | 54.2 ± 5.30 a | 68.4 ± 3.58 b | 61.7 ± 0.50 c |
| CAT (U g−1 FW) | 0.57 ± 0.16 a | 0.88 ± 0.05 b | 1.46 ± 0.08 c |
| Parameters | U. fasciata | S. hemiphyllum | G. livida | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Morning | Noon | Evening | Morning | Noon | Evening | Morning | Noon | Evening | |
| α | 0.28 ± 0.003 a | 0.13 ± 0.015 b | 0.26 ± 0.007 c | 0.29 ± 0.014 a | 0.03 ± 0.002 d | 0.29 ± 0.011 a | 0.16 ± 0.011 e | -- | 0.16 ± 0.026 e |
| EK | 210 ± 11.0 a | 239 ± 33.4 a | 223 ± 5.46 a | 331 ± 37.9 b | 607 ± 83.4 c | 264 ± 6.2 d | 116 ± 21.6 e | -- | 99.0 ± 19.4 e |
| rETRmax | 58.0 ± 2.96 a | 31.2 ± 3.88 b | 57.7 ± 0.08 a | 94.4 ± 7.62 d | 16.3 ± 1.17 e | 77.7 ± 1.74 f | 18.3 ± 2.46 e | -- | 15.9 ± 0.61 e |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, X.; Zou, D.; Hu, S.; Mai, G.; Ma, Z.; Li, G. Photosynthetic Characteristics of Three Cohabitated Macroalgae in the Daya Bay, and Their Responses to Temperature Rises. Plants 2021, 10, 2441. https://doi.org/10.3390/plants10112441
Shi X, Zou D, Hu S, Mai G, Ma Z, Li G. Photosynthetic Characteristics of Three Cohabitated Macroalgae in the Daya Bay, and Their Responses to Temperature Rises. Plants. 2021; 10(11):2441. https://doi.org/10.3390/plants10112441
Chicago/Turabian StyleShi, Xiaohan, Dinghui Zou, Shanshan Hu, Guangming Mai, Zengling Ma, and Gang Li. 2021. "Photosynthetic Characteristics of Three Cohabitated Macroalgae in the Daya Bay, and Their Responses to Temperature Rises" Plants 10, no. 11: 2441. https://doi.org/10.3390/plants10112441
APA StyleShi, X., Zou, D., Hu, S., Mai, G., Ma, Z., & Li, G. (2021). Photosynthetic Characteristics of Three Cohabitated Macroalgae in the Daya Bay, and Their Responses to Temperature Rises. Plants, 10(11), 2441. https://doi.org/10.3390/plants10112441







