Phytochemical Composition, Anti-Inflammatory and ER Stress-Reducing Potential of Sambucus ebulus L. Fruit Extract
Abstract
:1. Introduction
2. Results
2.1. Phytochemical Content and Composition
2.1.1. Polar Compounds
2.1.2. Polyphenolic Content
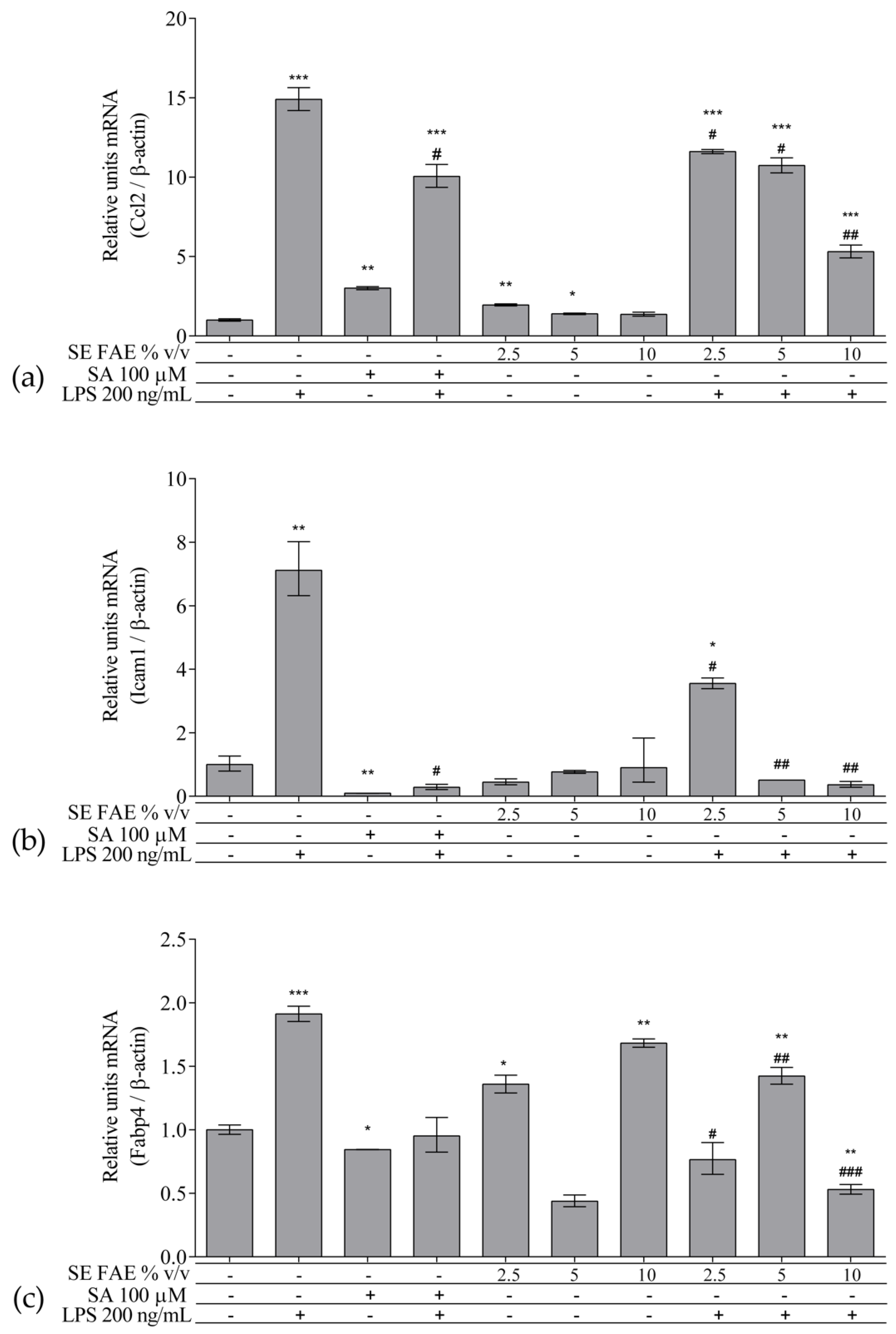
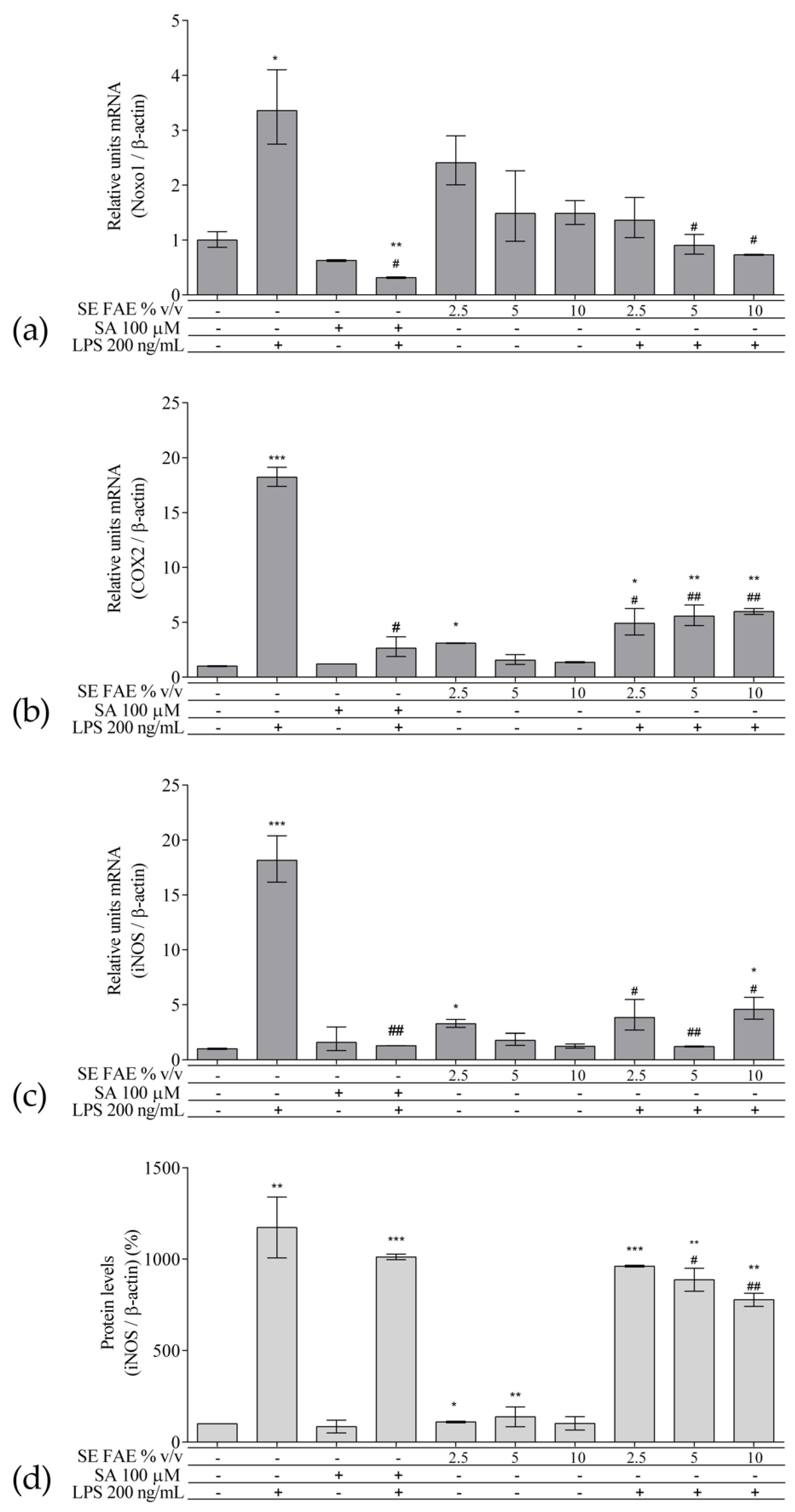
2.2. Investigation of Inflammation Related Biomarkers in a Model of LPS-Stimulated J774A.1 Macrophages
2.2.1. The Effect of LPS-Stimulation on Inflammation Related Biomarkers in J774A.1 Macrophages
2.2.2. The Effect of SE FAE on Inflammation-Related Biomarkers in Non-Stimulated J774A.1 Macrophages
2.2.3. The effect of SE FAE on Inflammation-Related Biomarkers in LPS-Stimulated J774A.1 Macrophages
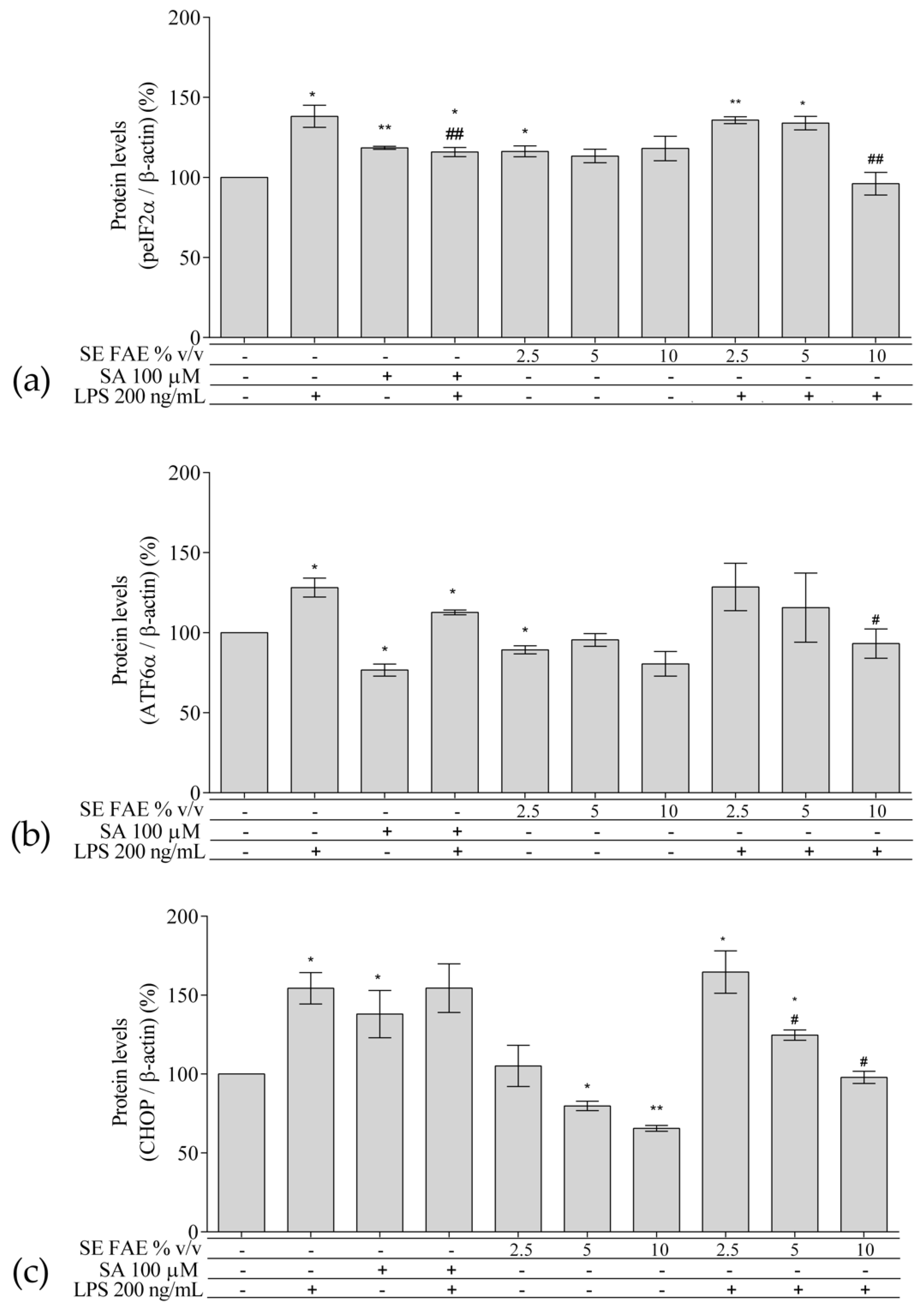
2.3. Investigation of ER Stress-Related Biomarkers in a Model of LPS-Stimulated J744A.1 Macrophages
2.3.1. The Effect of LPS-Stimulation on ER Stress-Related Biomarkers in J774A.1 Macrophages
2.3.2. The Effect of SE FAE on ER Stress-Related Biomarkers in Non-Stimulated J744A.1 Macrophages
2.3.3. The Effect of SE FAE on ER Stress-Related Biomarkers in LPS-Stimulated J744A.1 Macrophages
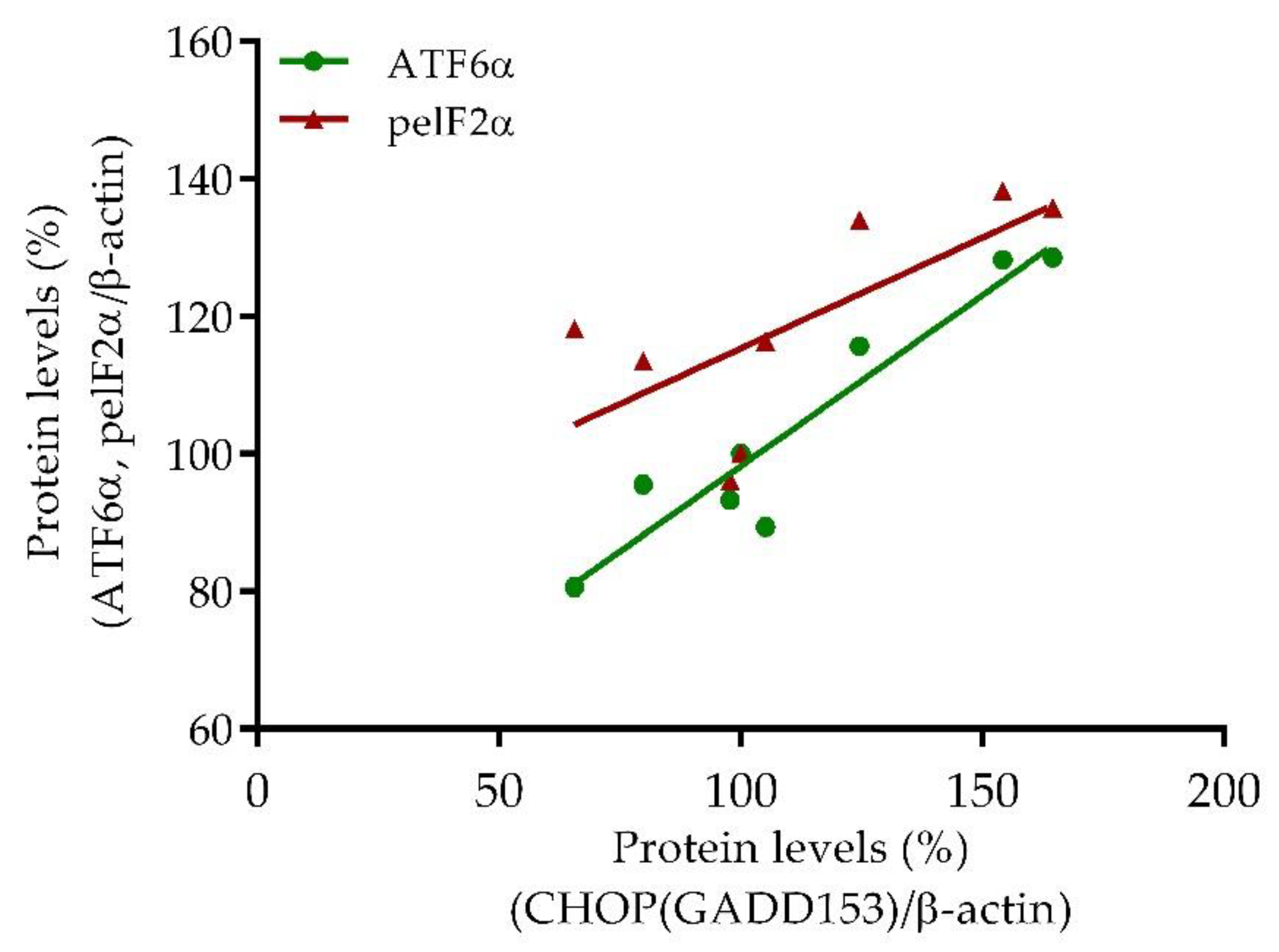
2.3.4. Correlation Analyzes of ER Stress-Related Biomarkers
3. Discussion
3.1. Newly Detected Phytochemicals in SE Fruit Aqueous Extract
3.1.1. Amino Acids
3.1.2. Organic Acids
3.1.3. Sugar Acids and Alcohols
3.1.4. Saccharides
3.1.5. Fatty Acids and Fatty Esters
3.1.6. Phenolic Compounds
3.2. SE FAE Modulates mRNA and Protein Levels of Inflammation-Related Biomarkers in LPS-Challenged J774A.1 Macrophages
3.3. SE FAE Modulates Levels of ER Stress-Related Proteins in LPS-Challenged J774A.1 Macrophages
4. Materials and Methods
4.1. Plant Material
4.2. Phytochemical Analysis
4.2.1. Extraction
4.2.2. Analysis of Polar Fraction (A)
4.2.3. Analysis of Fractions B and C
4.2.4. Identification and Quantitative Analysis
4.3. Cell Culture
4.4. Study Design
4.5. Gene Expression Analysis
4.5.1. RNA Extraction and cDNA Synthesis
4.5.2. qPCR Analysis
4.6. Protein Expression Analysis
4.6.1. Protein Extraction and Quantification
4.6.2. Western Blotting
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kültür, Ş. Medicinal plants used in Kırklareli Province (Turkey). J. Ethnopharmacol. 2007, 111, 341–364. [Google Scholar] [CrossRef] [PubMed]
- Tasinov, O.; Kiselova-Kaneva, Y.; Ivanova, D. Sambucus ebulus from traditional medicine to recent studies. Scr. Sci. Med. 2013, 45, 36–43. [Google Scholar] [CrossRef] [Green Version]
- Ebrahimzadeh, M.A.; Pourmorad, F.; Bekhradnia, A.R. Iron chelating activity, phenol and flavonoid content of some medicinal plants from Iran. Afr. J. Biotechnol. 2008, 7, 3188–3192. [Google Scholar] [CrossRef]
- Šarić-Kundalić, B.; Dobeš, C.; Klatte-Asselmeyer, V.; Saukel, J. Ethnobotanical study on medicinal use of wild and cultivated plants in middle, south and west Bosnia and Herzegovina. J. Ethnopharmacol. 2010, 131. [Google Scholar] [CrossRef] [PubMed]
- Chirigiu, L.; Chirigiu, R.G.; Tircomnicu, V.; Bubulica, M.V. GC-MS analysis of chemical composition of Sambucus ebulus leaves. Chem. Nat. Compd. 2011, 47, 126–127. [Google Scholar] [CrossRef]
- Marc, E.B.; Nelly, A.; Annick, D.D.; Frederic, D. Plants used as remedies antirheumatic and antineuralgic in the traditional medicine of Lebanon. J. Ethnopharmacol. 2008, 120, 315–334. [Google Scholar] [CrossRef]
- Tasinov, O.; Kiselova-Kaneva, Y.; Ivanova, D. Antioxidant activity, total polyphenol content and anthocyanins content of Sambucus ebulus L. aqueous and aqueous–ethanolic extracts depend on the type and concentration of extragent. Sci. Technol. 2012, II, 37–41. [Google Scholar]
- Ebrahimzadeh, M.A.; Nabavi, S.F.; Nabavi, S.M.; Pourmorad, F. Nitric oxide radical scavenging potential of some Elburz medicinal plants. Afr. J. Biotechnol. 2010, 9, 5212–5217. [Google Scholar] [CrossRef]
- Tasinov, O.B.; Kiselova-Kaneva, Y.D.; Nazifova-Tasinova, N.F.; Todorova, M.N.; Trendafilova, A.B.; Ivanova, D.G. Chemical composition and cytoprotective and anti-inflammatory potential of Sambucus ebulus fruit ethyl acetate fraction. Bulg. Chem. Commun. 2020, 52, 100–106. [Google Scholar]
- Pribela, A.; Durcanska, J.; Piry, J.; Karovicova, J. Volatile substances of dwarf elder (Sambucus ebulus) fruits. Biol. Ser. C 1992, 47, 225–230. [Google Scholar]
- Bubulica, M.V.; Chirigiu, L.; Popescu, M.; Simionescu, A.; Anoaica, G.; Popescu, A. Analysis of sterol compounds from Sambucus ebulus. Chem. Nat. Compd. 2012, 48, 520–521. [Google Scholar] [CrossRef]
- Zahmanov, G.; Alipieva, K.; Denev, P.; Todorov, D.; Hinkov, A.; Shishkov, S.; Simova, S.; Georgiev, M.I. Flavonoid glycosides profiling in dwarf elder fruits (Sambucus ebulus L.) and evaluation of their antioxidant and anti-herpes simplex activities. Ind. Crops Prod. 2015, 63, 58–64. [Google Scholar] [CrossRef]
- Zahmanov, G.; Alipieva, K.; Simova, S.; Georgiev, M.I. Metabolic differentiations of dwarf elder by NMR-based metabolomics. Phytochem. Lett. 2015, 11, 404–409. [Google Scholar] [CrossRef]
- Mikulic-Petkovsek, M.; Schmitzer, V.; Slatnar, A.; Todorovic, B.; Veberic, R.; Stampar, F.; Ivancic, A. Investigation of anthocyanin profile of four elderberry species and interspecific hybrids. J. Agric. Food Chem. 2014, 62, 5573–5580. [Google Scholar] [CrossRef]
- Mikulic-Petkovsek, M.; Ivancic, A.; Todorovic, B.; Veberic, R.; Stampar, F. Fruit Phenolic Composition of Different Elderberry Species and Hybrids. J. Food Sci. 2015, 80, C2180–C2190. [Google Scholar] [CrossRef]
- Vankova, D.V.; Todorova, M.N.; Kisselova-Kaneva, Y.D.; Galunska, B.T. Development of new and robust LC-MS method for simultaneous quantification of polyphenols from Sambucus ebulus fruits. J. Liq. Chromatogr. Relat. Technol. 2019, 42, 408–416. [Google Scholar] [CrossRef]
- Tasinov, O.; Kiselova-Kaneva, Y.; Ivanova, D. Sambucus ebulus L. fruit aqueous infusion modulates GCL and GPX4 gene expression. Bulg. J. Agric. Sci. 2013, 19, 143–146. [Google Scholar]
- Tasinov, O.B.; Kiselova-Kaneva, Y.D.; Ivanova, D.G. Effects of dwarf elder fruit infusion on nuclear factor kappa B and glutathione metabolism-related genes transcription in a model of lipopolysaccharides challenged macrophages. Bulg. Chem. Commun. 2020, 52, 68–74. [Google Scholar]
- Todorova, M.; Tasinov, O.; Pasheva, M.; Vankova, D.; Ivanova, D.; Galunska, B.; Kiselova-Kaneva, Y. Cytoprotective activity of Sambucus ebulus fruit extracts in conditions of oxidative tert-buthyl-hydroperoxyde induced cell toxicity. Bulg. Chem. Commun. 2019, 51, 125–130. [Google Scholar]
- Ebrahimzadeh, M.A.; Mahmoudi, M.; Karami, M.; Saeedi, S.; Ahmadi, A.H.; Salimi, E. Separation of active and toxic portions in Sambucus ebulus. Pak. J. Biol. Sci. 2007, 10, 4171–4173. [Google Scholar] [CrossRef] [Green Version]
- Fathi, H.; Ebrahimzadeh, M.A.; Ziar, A.; Mohammadi, H. Oxidative damage induced by retching; antiemetic and neuroprotective role of Sambucus ebulus L. Cell Biol. Toxicol. 2015, 31, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, D.; Tasinov, O.; Kiselova-Kaneva, Y. Improved lipid profile and increased serum antioxidant capacity in healthy volunteers after Sambucus ebulus L. fruit infusion consumption. Int. J. Food Sci. Nutr. 2014, 65, 740–744. [Google Scholar] [CrossRef]
- Ivanova, D.; Nashar, M.; Kiselova-Kaneva, Y.; Tasinov, O.; Vankova, D.; Nazifova-Tasinova, N. The impact of human intervention studies on the evaluation of medicinal plant antioxidant and anti-inflammatory activities. In Human Health and Nutrition: New Research; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2015; pp. 145–166. ISBN 9781634828536. [Google Scholar]
- Ebrahimzadeh, M.A.; Rafati, M.R.; Damchi, M.; Golpur, M.; Fathiazad, F. Treatment of paederus dermatitis with Sambucus ebulus lotion. Iran. J. Pharm. Res. 2014, 13, 1065–1072. [Google Scholar] [CrossRef] [PubMed]
- Olefsky, J.M.; Glass, C.K. Macrophages, Inflammation, and Insulin Resistance. Annu. Rev. Physiol. 2010, 72, 219–246. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S.; Arner, P.; Caro, J.F.; Atkinson, R.L.; Spiegelman, B.M. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J. Clin. Investig. 1995, 95, 2409–2415. [Google Scholar] [CrossRef]
- Hirosumi, J.; Tuncman, G.; Chang, L.; Görgün, C.Z.; Uysal, K.T.; Maeda, K.; Karin, M.; Hotamisligil, G.S. A central, role for JNK in obesity and insulin resistance. Nature 2002, 420, 333–336. [Google Scholar] [CrossRef]
- Bandyopadhyay, G.K.; Yu, J.G.; Ofrecio, J.; Olefsky, J.M. Increased p85/55/50 expression and decreased phosphotidylinositol 3-kinase activity in insulin-resistant human skeletal muscle. Diabetes 2005, 54, 2351–2359. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, M.T.A.; Favelyukis, S.; Nguyen, A.K.; Reichart, D.; Scott, P.A.; Jenn, A.; Liu-Bryan, R.; Glass, C.K.; Neels, J.G.; Olefsky, J.M. A subpopulation of macrophages infiltrates hypertrophic adipose tissue and is activated by free fatty acids via toll-like receptors 2 and 4 and JNK-dependent pathways. J. Biol. Chem. 2007, 282, 35279–35292. [Google Scholar] [CrossRef] [Green Version]
- Boden, G. Insulin Resistance and Inflammation: Links between Obesity and Cardiovascular Disease. In Glucose Intake and Utilization in Pre-Diabetes and Diabetes: Implications for Cardiovascular Disease; Academic Press: Amsterdam, The Netherlands, 2015; pp. 95–101. ISBN 9780128005798. [Google Scholar]
- Özcan, U.; Cao, Q.; Yilmaz, E.; Lee, A.H.; Iwakoshi, N.N.; Özdelen, E.; Tuncman, G.; Görgün, C.; Glimcher, L.H.; Hotamisligil, G.S. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 2004, 306, 457–461. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Kaufman, R.J. From endoplasmic-reticulum stress to the inflammatory response. Nature 2008, 454, 455–462. [Google Scholar] [CrossRef] [Green Version]
- Anthony, T.G.; Wek, R.C. TXNIP switches tracks toward a terminal UPR. Cell Metab. 2012, 16, 135–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, M.; Wang, H.; Liu, Z.; Lin, L.; Wang, L.; Xie, M.; Li, D.; Zhang, J.; Zhang, R. Endoplasmic reticulum stress-dependent activation of iNOS/NO-NF-κB signaling and NLRP3 inflammasome contributes to endothelial inflammation and apoptosis associated with microgravity. FASEB J. 2020, 34, 10835–10849. [Google Scholar] [CrossRef]
- Sun, Z.M.; Guan, P.; Luo, L.F.; Qin, L.Y.; Wang, N.; Zhao, Y.S.; Ji, E.S. Resveratrol protects against CIH-induced myocardial injury by targeting Nrf2 and blocking NLRP3 inflammasome activation. Life Sci. 2020, 245, 117362. [Google Scholar] [CrossRef]
- Li, Y.; Xu, S.; Giles, A.; Nakamura, K.; Lee, J.W.; Hou, X.; Donmez, G.; Li, J.; Luo, Z.; Walsh, K.; et al. Hepatic overexpression of SIRT1 in mice attenuates endoplasmic reticulum stress and insulin resistance in the liver. FASEB J. 2011, 25, 1664–1679. [Google Scholar] [CrossRef] [Green Version]
- Karthikeyan, B.; Harini, L.; Krishnakumar, V.; Kannan, V.R.; Sundar, K.; Kathiresan, T. Insights on the involvement of (–)-epigallocatechin gallate in ER stress-mediated apoptosis in age-related macular degeneration. Apoptosis 2017, 22, 72–85. [Google Scholar] [CrossRef]
- Fu, K.; Chen, L.; Miao, L.; Guo, Y.; Zhang, W.; Bai, Y. Grape Seed Proanthocyanidins Protect N2a Cells against Ischemic Injury via Endoplasmic Reticulum Stress and Mitochondrial-associated Pathways. CNS Neurol. Disord.—Drug Targets 2019, 18, 334–341. [Google Scholar] [CrossRef] [PubMed]
- NCI Thesaurus. Available online: https://ncit.nci.nih.gov/ncitbrowser/ConceptReport.jsp?dictionary=NCI_Thesaurus&ns=NCI_Thesaurus&code=C82933 (accessed on 14 August 2021).
- Veberic, R.; Jakopic, J.; Stampar, F.; Schmitzer, V. European elderberry (Sambucus nigra L.) rich in sugars, organic acids, anthocyanins and selected polyphenols. Food Chem. 2009, 114, 511–515. [Google Scholar] [CrossRef]
- Washüttl, J.; Riederer, P.; Bancher, E. A qualitative and quantitative study of sugar-alcohols in several foods. J. Food Sci. 1973, 38, 1262–1263. [Google Scholar] [CrossRef]
- Chakravarti, B.; Maurya, R.; Siddiqui, J.A.; Kumar Bid, H.; Rajendran, S.M.; Yadav, P.P.; Konwar, R. In vitro anti-breast cancer activity of ethanolic extract of Wrightia tomentosa: Role of pro-apoptotic effects of oleanolic acid and urosolic acid. J. Ethnopharmacol. 2012, 142, 72–79. [Google Scholar] [CrossRef]
- Juan, M.E.; Planas, J.M.; Ruiz-Gutierrez, V.; Daniel, H.; Wenzel, U. Antiproliferative and apoptosis-inducing effects of maslinic and oleanolic acids, two pentacyclic triterpenes from olives, on HT-29 colon cancer cells. Br. J. Nutr. 2008, 100, 36–43. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.-J.; Lu, J.; Wu, D.-M.; Zheng, Z.H.; Zheng, Y.-L.; Wang, X.-H.; Ruan, J.; Sun, X.; Shan, Q.; Zhang, Z.-F. Ursolic acid attenuates lipopolysaccharide-induced cognitive deficits in mouse brain through suppressing p38/NF-κB mediated inflammatory pathways. Neurobiol. Learn. Mem. 2011, 96, 156–165. [Google Scholar] [CrossRef]
- Kaewthawee, N.; Brimson, S. The effects of ursolic acid on cytokine production via the MAPK pathways in leukemic T-cells. EXCLI J. 2013, 12, 102–114. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Wu, D.-M.; Zheng, Y.-L.; Hu, B.; Cheng, W.; Zhang, Z.-F.; Shan, Q. Ursolic acid improves high fat diet-induced cognitive impairments by blocking endoplasmic reticulum stress and IκB kinase β/nuclear factor-κB-mediated inflammatory pathways in mice. Brain. Behav. Immun. 2011, 25, 1658–1667. [Google Scholar] [CrossRef]
- Zhang, W.; Hong, D.; Zhou, Y.; Zhang, Y.; Shen, Q.; Li, J.Y.; Hu, L.H.; Li, J. Ursolic acid and its derivative inhibit protein tyrosine phosphatase 1B, enhancing insulin receptor phosphorylation and stimulating glucose uptake. Biochim. Biophys. Acta Gen. Subj. 2006, 1760, 1505–1512. [Google Scholar] [CrossRef]
- Jayaprakasam, B.; Olson, L.K.; Schutzki, R.E.; Tai, M.H.; Nair, M.G. Amelioration of obesity and glucose intolerance in high-fat-fed C57BL/6 mice by anthocyanins and ursolic acid in cornelian cherry (Cornus mas). J. Agric. Food Chem. 2006, 54, 243–248. [Google Scholar] [CrossRef]
- Best, M.M.; Duncan, C.H.; van Loon, E.J.; Wathen, J.D. Lowering of serum cholesterol by the administration of a plant sterol. Circulation 1954, 10, 201–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kassis, A.N.; Vanstone, C.A.; AbuMweis, S.S.; Jones, P.J.H. Efficacy of plant sterols is not influenced by dietary cholesterol intake in hypercholesterolemic individuals. Metabolism 2008, 57, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.Y.; Ma, K.Y.; Liang, Y.; Peng, C.; Zuo, Y. Role and classification of cholesterol-lowering functional foods. J. Funct. Foods 2011, 3, 61–69. [Google Scholar] [CrossRef]
- Jesch, E.D.; Seo, J.M.; Carr, T.P.; Lee, J.Y. Sitosterol reduces messenger RNA and protein expression levels of Niemann-Pick C1-like 1 in FHs 74 Int cells. Nutr. Res. 2009, 29, 859–866. [Google Scholar] [CrossRef]
- Jones, P.J.H.; MacDougall, D.E.; Ntanios, F.; Vanstone, C.A. Dietary phytosterols as cholesterol-lowering agents in humans. Can. J. Physiol. Pharmacol. 1997, 75, 217–227. [Google Scholar] [CrossRef]
- Liang, Z.; Liang, H.; Guo, Y.; Yang, D. Cyanidin 3-o-galactoside: A natural compound with multiple health benefits. Int. J. Mol. Sci. 2021, 22, 2261. [Google Scholar] [CrossRef]
- Kiselova-Kaneva, Y.; Galunska, B.; Nikolova, M.; Dincheva, I.; Badjakov, I. High resolution LC-MS/MS characterization of polyphenolic composition and evaluation of antioxidant activity of Sambucus ebulus fruit tea traditionally used in Bulgaria as a functional food. Food Chem. 2021, 367, 130759. [Google Scholar] [CrossRef]
- Gu, L.; Kelm, M.A.; Hammerstone, J.F.; Beecher, G.; Holden, J.; Haytowitz, D.; Gebhardt, S.; Prior, R.L. Concentrations of Proanthocyanidins in Common Foods and Estimations of Normal Consumption. J. Nutr. 2004, 134, 613–617. [Google Scholar] [CrossRef]
- Rivière, C.; Pawlus, A.D.; Mérillon, J.M. Natural stilbenoids: Distribution in the plant kingdom and chemotaxonomic interest in Vitaceae. Nat. Prod. Rep. 2012, 29, 1317–1333. [Google Scholar] [CrossRef] [PubMed]
- Timmers, S.; Konings, E.; Bilet, L.; Houtkooper, R.H.; Van De Weijer, T.; Goossens, G.H.; Hoeks, J.; Van Der Krieken, S.; Ryu, D.; Kersten, S.; et al. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab. 2011, 14, 612–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Espinoza, J.L.; Trung, L.Q.; Inaoka, P.T.; Yamada, K.; An, D.T.; Mizuno, S.; Nakao, S.; Takami, A. The Repeated Administration of Resveratrol Has Measurable Effects on Circulating T-Cell Subsets in Humans. Oxid. Med. Cell. Longev. 2017, 2017, 6781872. [Google Scholar] [CrossRef]
- Yiu, E.M.; Tai, G.; Peverill, R.E.; Lee, K.J.; Croft, K.D.; Mori, T.A.; Scheiber-Mojdehkar, B.; Sturm, B.; Praschberger, M.; Vogel, A.P.; et al. An open-label trial in Friedreich ataxia suggests clinical benefit with high-dose resveratrol, without effect on frataxin levels. J. Neurol. 2015, 262, 1344–1353. [Google Scholar] [CrossRef] [PubMed]
- Crandall, J.P.; Oram, V.; Trandafirescu, G.; Reid, M.; Kishore, P.; Hawkins, M.; Cohen, H.W.; Barzilai, N. Pilot study of resveratrol in older adults with impaired glucose tolerance. Journals Gerontol. Ser. A Biol. Sci. Med. Sci. 2012, 67, 1307–1312. [Google Scholar] [CrossRef] [Green Version]
- Most, J.; Timmers, S.; Warnke, I.; Jocken, J.W.; Van Boekschoten, M.; De Groot, P.; Bendik, I.; Schrauwen, P.; Goossens, G.H.; Blaak, E.E. Combined epigallocatechin-3-gallate and resveratrol supplementation for 12 wk increases mitochondrial capacity and fat oxidation, but not insulin sensitivity, in obese humans: A randomized controlled trial. Am. J. Clin. Nutr. 2016, 104, 215–227. [Google Scholar] [CrossRef] [Green Version]
- Herrmann, K. Occurrence and content of hydroxycinnamic and hydroxybenzoic acid compounds in foods. Crit. Rev. Food Sci. Nutr. 1989, 28, 315–347. [Google Scholar] [CrossRef]
- Weng, C.J.; Yen, G.C. Chemopreventive effects of dietary phytochemicals against cancer invasion and metastasis: Phenolic acids, monophenol, polyphenol, and their derivatives. Cancer Treat. Rev. 2012, 38, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.O.; Min, K.J.; Kwon, T.K.; Um, B.H.; Moreau, R.A.; Choi, S.W. Anti-inflammatory activity of hydroxycinnamic acid derivatives isolated from corn bran in lipopolysaccharide-stimulated Raw 264.7 macrophages. Food Chem. Toxicol. 2012, 50, 1309–1316. [Google Scholar] [CrossRef]
- Hamauzu, Y.; Yasui, H.; Inno, T.; Kume, C.; Omanyuda, M. Phenolic profile, antioxidant property, and anti-influenza viral activity of Chinese quince (Pseudocydonia sinensis Schneid.), quince (Cydonia oblonga Mill.), and apple (Malus domestica Mill.) fruits. J. Agric. Food Chem. 2005, 53, 928–934. [Google Scholar] [CrossRef] [PubMed]
- Iwai, K.; Kishimoto, N.; Kakino, Y.; Mochida, K.; Fujita, T. In vitro antioxidative effects and tyrosinase inhibitory activities of seven hydroxycinnamoyl derivatives in green coffee beans. J. Agric. Food Chem. 2004, 52, 4893–4898. [Google Scholar] [CrossRef]
- Liu, S.L.; Peng, B.J.; Zhong, Y.L.; Liu, Y.L.; Song, Z.; Wang, Z. Effect of 5-caffeoylquinic acid on the NF-κB signaling pathway, peroxisome proliferator-activated receptor gamma 2, and macrophage infiltration in high-fat diet-fed Sprague-Dawley rat adipose tissue. Food Funct. 2015, 6, 2779–2786. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Lu, Y.; Wei, D. Antioxidant activity of a flavonoid-rich extract of Hypericum perforatum L. in vitro. J. Agric. Food Chem. 2004, 52, 5032–5039. [Google Scholar] [CrossRef]
- Mansour, S.; Djebli, N.; Ozkan, E.E.; Mat, A. In vivo antiinflammatory activity and chemical composition of Hypericum scabroides. Asian Pac. J. Trop. Med. 2014, 7, S514–S520. [Google Scholar] [CrossRef] [Green Version]
- Ahn, H.; Lee, G.S. Isorhamnetin and hyperoside derived from water dropwort inhibits inflammasome activation. Phytomedicine 2017, 24, 77–86. [Google Scholar] [CrossRef]
- Li, L.; Zhang, X.H.; Liu, G.R.; Liu, C.; Dong, Y.M. Isoquercitrin suppresses the expression of histamine and pro-inflammatory cytokines by inhibiting the activation of MAP Kinases and NF-κB in human KU812 cells. Chin. J. Nat. Med. 2016, 14, 407–412. [Google Scholar] [CrossRef]
- Chua, L.S. A review on plant-based rutin extraction methods and its pharmacological activities. J. Ethnopharmacol. 2013, 150, 805–817. [Google Scholar] [CrossRef]
- Wang, W.; Sun, C.; Mao, L.; Ma, P.; Liu, F.; Yang, J.; Gao, Y. The biological activities, chemical stability, metabolism and delivery systems of quercetin: A review. Trends Food Sci. Technol. 2016, 56, 21–38. [Google Scholar] [CrossRef]
- Suganthy, N.; Devi, K.P.; Nabavi, S.F.; Braidy, N.; Nabavi, S.M. Bioactive effects of quercetin in the central nervous system: Focusing on the mechanisms of actions. Biomed. Pharmacother. 2016, 84, 892–908. [Google Scholar] [CrossRef] [PubMed]
- Pellegatta, F.; Bertelli, A.A.E.; Staels, B.; Duhem, C.; Fulgenzi, A.; Ferrero, M.E. Different short- and long-term effects of resveratrol on nuclear factor-κB phosphorylation and nuclear appearance in human endothelial cells. Am. J. Clin. Nutr. 2003, 77, 1220–1228. [Google Scholar] [CrossRef] [Green Version]
- Norata, G.D.; Marchesi, P.; Passamonti, S.; Pirillo, A.; Violi, F.; Catapano, A.L. Anti-inflammatory and anti-atherogenic effects of cathechin, caffeic acid and trans-resveratrol in apolipoprotein E deficient mice. Atherosclerosis 2007, 191, 265–271. [Google Scholar] [CrossRef]
- Hou, D.X.; Yanagita, T.; Uto, T.; Masuzaki, S.; Fujii, M. Anthocyanidins inhibit cyclooxygenase-2 expression in LPS-evoked macrophages: Structure-activity relationship and molecular mechanisms involved. Biochem. Pharmacol. 2005, 70, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Pergola, C.; Rossi, A.; Dugo, P.; Cuzzocrea, S.; Sautebin, L. Inhibition of nitric oxide biosynthesis by anthocyanin fraction of blackberry extract. Nitric Oxide—Biol. Chem. 2006, 15, 30–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karlsen, A.; Retterstøl, L.; Laake, P.; Paur, I.; Bøhn, S.K.; Sandvik, L.; Blomhoff, R. Anthocyanins inhibit nuclear factor-kappaB activation in monocytes and reduce plasma concentrations of pro-inflammatory mediators in healthy adults. J. Nutr. 2007, 137, 1951–1954. [Google Scholar] [CrossRef]
- Kim, J.; Kim, H.; Choi, H.; Jo, A.; Kang, H.; Yun, H.; Im, S.; Choi, C. Anti-inflammatory effects of a stauntonia hexaphylla fruit extract in lipopolysaccharide-activated RAW-264.7 macrophages and rats by carrageenan-induced hind paw swelling. Nutrients 2018, 10, 110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwaiger, S.; Zeller, I.; Pölzelbauer, P.; Frotschnig, S.; Laufer, G.; Messner, B.; Pieri, V.; Stuppner, H.; Bernhard, D. Identification and pharmacological characterization of the anti-inflammatory principal of the leaves of dwarf elder (Sambucus ebulus L.). J. Ethnopharmacol. 2011, 133, 704–709. [Google Scholar] [CrossRef] [Green Version]
- van de Stolpe, A.; Caldenhoven, E.; Stade, B.G.; Koenderman, L.; Raaijmakers, J.A.; Johnson, J.P.; van der Saag, P.T. 12-O-tetradecanoylphorbol-13-acetate- and tumor necrosis factor alpha-mediated induction of intercellular adhesion molecule-1 is inhibited by dexamethasone. Functional analysis of the human intercellular adhesion molecular-1 promoter. J. Biol. Chem. 1994, 269, 6185–6192. [Google Scholar] [CrossRef]
- Ueda, A.; Okuda, K.; Ohno, S.; Shirai, A.; Igarashi, T.; Matsunaga, K.; Fukushima, J.; Kawamoto, S.; Ishigatsubo, Y.; Okubo, T. NF-kappa B and Sp1 regulate transcription of the human monocyte chemoattractant protein-1 gene. J. Immunol. 1994, 153, 2052–2063. [Google Scholar] [CrossRef] [Green Version]
- Hsieh, I.-N.; Chang, A.S.-Y.; Teng, C.-M.; Chen, C.-C.; Yang, C.-R. Aciculatin inhibits lipopolysaccharide-mediated inducible nitric oxide synthase and cyclooxygenase-2 expression via suppressing NF-κB and JNK/p38 MAPK activation pathways. J. Biomed. Sci. 2011, 18, 28. [Google Scholar] [CrossRef] [Green Version]
- Tan, K.S.; Qian, L.; Rosado, R.; Flood, P.M.; Cooper, L.F. The role of titanium surface topography on J774A.1 macrophage inflammatory cytokines and nitric oxide production. Biomaterials 2006, 27, 5170–5177. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Park, S.B.; Suk, K.; Kim, I.K.; Kim, S.Y.; Kim, J.A.; Lee, S.H.; Kim, S.H. Gallotannin isolated from Euphorbia species, 1,2,6-tri-O-galloyl-β-D- allose, decreases nitric oxide production through inhibition of nuclear factor- κB and downstream inducible nitric oxide synthase expression in macrophages. Biol. Pharm. Bull. 2009, 32, 1053–1056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maraslioglu, M.; Oppermann, E.; Blattner, C.; Weber, R.; Henrich, D.; Jobin, C.; Schleucher, E.; Marzi, I.; Lehnert, M. Chronic ethanol feeding modulates inflammatory mediators, activation of nuclear factor- B, and responsiveness to endotoxin in murine kupffer cells and circulating leukocytes. Mediat. Inflamm. 2014, 2014, 808695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Y.; Zhu, M.; Lance, P. INOS signaling interacts with COX-2 pathway in colonic fibroblasts. Exp. Cell Res. 2012, 318, 2116–2127. [Google Scholar] [CrossRef]
- Barak, V.; Halperin, T.; Kalickman, I. The effect of Sambucol, a black elderberry-based, natural product, on the production of human cytokines: I. Inflammatory cytokines. Eur. Cytokine Netw. 2001, 12, 290–296. [Google Scholar]
- Drummond, G.R.; Selemidis, S.; Griendling, K.K.; Sobey, C.G. Combating oxidative stress in vascular disease: NADPH oxidases as therapeutic targets. Nat. Rev. Drug Discov. 2011, 10, 453–471. [Google Scholar] [CrossRef] [Green Version]
- Blough, N.V.; Zafiriou, O.C. Reaction of Superoxide with Nitric Oxide to Form Peroxonitrite in Alkaline Aqueous Solution. Inorg. Chem. 1985, 24, 3502–3504. [Google Scholar] [CrossRef]
- Gryglewski, R.J.; Palmer, R.M.J.; Moncada, S. Superoxide anion is involved in the breakdown of endothelium-derived vascular relaxing factor. Nature 1986, 320, 454–456. [Google Scholar] [CrossRef]
- Leto, T.L.; Geiszt, M. Role of Nox family NADPH oxidases in host defense. Antioxidants Redox Signal. 2006, 8, 1549–1561. [Google Scholar] [CrossRef] [Green Version]
- Guzik, T.J.; Harrison, D.G. Vascular NADPH oxidases as drug targets for novel antioxidant strategies. Drug Discov. Today 2006, 11, 524–533. [Google Scholar] [CrossRef] [PubMed]
- Abo, A.; Pick, E.; Hall, A.; Totty, N.; Teahan, C.G.; Segal, A.W. Activation of the NADPH oxidase involves the small GTP-binding protein p21rac1. Nature 1991, 353, 668–670. [Google Scholar] [CrossRef] [PubMed]
- Potenza, M.A.; Marasciulo, F.L.; Tarquinio, M.; Tiravanti, E.; Colantuono, G.; Federici, A.; Kim, J.A.; Quon, M.J.; Montagnani, M. EGCG, a green tea polyphenol, improves endothelial function and insulin sensitivity, reduces blood pressure, and protects against myocardial I/R injury in SHR. Am. J. Physiol.—Endocrinol. Metab. 2007, 292, E1378–E1387. [Google Scholar] [CrossRef] [PubMed]
- Galindo, P.; González-Manzano, S.; Zarzuelo, M.J.; Gómez-Guzmán, M.; Quintela, A.M.; González-Paramás, A.; Santos-Buelga, C.; Pérez-Vizcaíno, F.; Duarte, J.; Jiménez, R. Different cardiovascular protective effects of quercetin administered orally or intraperitoneally in spontaneously hypertensive rats. Food Funct. 2012, 3, 643–650. [Google Scholar] [CrossRef]
- Sánchez, M.; Galisteo, M.; Vera, R.; Villar, I.C.; Zarzuelo, A.; Tamargo, J.; Pérez-Vizcaíno, F.; Duarte, J. Quercetin downregulates NADPH oxidase, increases eNOS activity and prevents endothelial dysfunction in spontaneously hypertensive rats. J. Hypertens. 2006, 24, 75–84. [Google Scholar] [CrossRef] [Green Version]
- Sanchez, M.; Lodi, F.; Vera, R.; Villar, I.C.; Cogolludo, A.; Jimenez, R.; Moreno, L.; Romero, M.; Tamargo, J.; Perez-Vizcaino, F.; et al. Quercetin and isorhamnetin prevent endothelial dysfunction, superoxide production, and overexpression of p47phox induced by angiotensin II in rat aorta. J. Nutr. 2007, 137, 910–915. [Google Scholar] [CrossRef] [Green Version]
- Ihm, S.H.; Lee, J.O.; Kim, S.J.; Seung, K.B.; Schini-Kerth, V.B.; Chang, K.; Oak, M.H. Catechin prevents endothelial dysfunction in the prediabetic stage of OLETF rats by reducing vascular NADPH oxidase activity and expression. Atherosclerosis 2009, 206, 47–53. [Google Scholar] [CrossRef]
- Schilder, Y.D.C.; Heiss, E.H.; Schachner, D.; Ziegler, J.; Reznicek, G.; Sorescu, D.; Dirsch, V.M. NADPH oxidases 1 and 4 mediate cellular senescence induced by resveratrol in human endothelial cells. Free Radic. Biol. Med. 2009, 46, 1598–1606. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Xu, Y.C.; Wu, R.F.; Nwariaku, F.E.; Souza, R.F.; Flores, S.C.; Terada, L.S. p47phox participates in activation of RelA in endothelial cells. J. Biol. Chem. 2003, 278, 17210–17217. [Google Scholar] [CrossRef]
- Makowski, L.; Boord, J.B.; Maeda, K.; Babaev, V.R.; Uysal, K.T.; Morgan, M.A.; Parker, R.A.; Suttles, J.; Fazio, S.; Hotamisligil, G.S.; et al. Lack of macrophage fatty-acid-binding protein aP2 protects mice deficient in apolipoprotein E against atherosclerosis. Nat. Med. 2001, 7, 699–705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kazemi, M.R.; McDonald, C.M.; Shigenaga, J.K.; Grunfeld, C.; Feingold, K.R. Adipocyte fatty acid-binding protein expression and lipid accumulation are increased during activation of murine macrophages by toll-like receptor agonists. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1220–1224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furuhashi, M.; Fucho, R.; Görgün, C.Z.; Tuncman, G.; Cao, H.; Hotamisligil, G.S. Adipocyte/macrophage fatty acid–binding proteins contribute to metabolic deterioration through actions in both macrophages and adipocytes in mice. J. Clin. Investig. 2008, 118, 2640–2650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furuhashi, M.; Tuncman, G.; Görgün, C.Z.; Makowski, L.; Atsumi, G.; Vaillancourt, E.; Kono, K.; Babaev, V.R.; Fazio, S.; Linton, M.F.; et al. Treatment of diabetes and atherosclerosis by inhibiting fatty-acid-binding protein aP2. Nature 2007, 447, 959–965. [Google Scholar] [CrossRef] [Green Version]
- Milne, J.C.; Lambert, P.D.; Schenk, S.; Carney, D.P.; Smith, J.J.; Gagne, D.J.; Jin, L.; Boss, O.; Perni, R.B.; Vu, C.B.; et al. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature 2007, 450, 712–716. [Google Scholar] [CrossRef] [Green Version]
- Csiszar, A.; Labinskyy, N.; Podlutsky, A.; Kaminski, P.M.; Wolin, M.S.; Zhang, C.; Mukhopadhyay, P.; Pacher, P.; Hu, F.; De Cabo, R.; et al. Vasoprotective effects of resveratrol and SIRT1: Attenuation of cigarette smoke-induced oxidative stress and proinflammatory phenotypic alterations. Am. J. Physiol.—Heart Circ. Physiol. 2008, 294, 2721–2735. [Google Scholar] [CrossRef] [Green Version]
- Yoshizaki, T.; Milne, J.C.; Imamura, T.; Schenk, S.; Sonoda, N.; Babendure, J.L.; Lu, J.-C.; Smith, J.J.; Jirousek, M.R.; Olefsky, J.M. SIRT1 Exerts Anti-Inflammatory Effects and Improves Insulin Sensitivity in Adipocytes. Mol. Cell. Biol. 2009, 29, 1363–1374. [Google Scholar] [CrossRef] [Green Version]
- Yu, C.; Chen, Y.; Cline, G.W.; Zhang, D.; Zong, H.; Wang, Y.; Bergeron, R.; Kim, J.K.; Cushman, S.W.; Cooney, G.J.; et al. Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 3-kinase activity in muscle. J. Biol. Chem. 2002, 277, 50230–50236. [Google Scholar] [CrossRef] [Green Version]
- Bettigole, S.E.; Glimcher, L.H. Endoplasmic reticulum stress in immunity. Annu. Rev. Immunol. 2015, 33, 107–138. [Google Scholar] [CrossRef]
- Li, S.; Ye, L.; Yu, X.; Xu, B.; Li, K.; Zhu, X.; Liu, H.; Wu, X.; Kong, L. Hepatitis C virus NS4B induces unfolded protein response and endoplasmic reticulum overload response-dependent NF-κB activation. Virology 2009, 391, 257–264. [Google Scholar] [CrossRef] [Green Version]
- Randjelović, P.; Veljković, S.; Stojiljković, N.; Sokolović, D.; Ilić, I.; Laketić, D.; Randjelović, D.; Randjelović, N. The beneficial biological properties of salicylic acid. Acta Fac. Med. Naissensis 2015, 32, 259–265. [Google Scholar] [CrossRef]
- Yoshiuchi, K.; Kaneto, H.; Matsuoka, T.A.; Kohno, K.; Iwawaki, T.; Nakatani, Y.; Yamasaki, Y.; Hori, M.; Matsuhisa, M. Direct monitoring of in vivo ER stress during the development of insulin resistance with ER stress-activated indicator transgenic mice. Biochem. Biophys. Res. Commun. 2008, 366, 545–550. [Google Scholar] [CrossRef]
- Boden, G.; Duan, X.; Homko, C.; Molina, E.J.; Song, W.; Perez, O.; Cheung, P.; Merali, S. Increase in endoplasmic reticulum stress-related proteins and genes in adipose tissue of obese, insulin-resistant individuals. Diabetes 2008, 57, 2438–2444. [Google Scholar] [CrossRef] [Green Version]
- Sharma, N.K.; Das, S.K.; Mondal, A.K.; Hackney, O.G.; Chu, W.S.; Kern, P.A.; Rasouli, N.; Spencer, H.J.; Yao-Borengasser, A.; Elbein, S.C. Endoplasmic reticulum stress markers are associated with obesity in nondiabetic subjects. J. Clin. Endocrinol. Metab. 2008, 93, 4532–4541. [Google Scholar] [CrossRef] [PubMed]
- Eizirik, D.L.; Cardozo, A.K.; Cnop, M. The role for endoplasmic reticulum stress in diabetes mellitus. Endocr. Rev. 2008, 29, 42–61. [Google Scholar] [CrossRef] [Green Version]
- Lin, J.H.; Walter, P.; Yen, T.S.B. Endoplasmic reticulum stress in disease pathogenesis. Annu. Rev. Pathol. Mech. Dis. 2008, 3, 399–425. [Google Scholar] [CrossRef] [PubMed]
- Ron, D.; Hubbard, S.R. How IRE1 Reacts to ER Stress. Cell 2008, 132, 24–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ron, D.; Walter, P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell Biol. 2007, 8, 519–529. [Google Scholar] [CrossRef]
- Tsukano, H.; Gotoh, T.; Endo, M.; Miyata, K.; Tazume, H.; Kadomatsu, T.; Yano, M.; Iwawaki, T.; Kohno, K.; Araki, K.; et al. The endoplasmic reticulum stress-C/EBP homologous protein pathway-mediated apoptosis in macrophages contributes to the instability of atherosclerotic plaques. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 1925–1932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, G.; Scull, C.; Ozcan, L.; Tabas, I. NADPH oxidase links endoplasmic reticulum stress, oxidative stress, and PKR activation to induce apoptosis. J. Cell Biol. 2010, 191, 1113–1125. [Google Scholar] [CrossRef] [Green Version]
- Pahl, H.L.; Baeuerle, P.A. Activation of NF-κB by ER stress requires both ca2+ and reactive oxygen intermediates as messengers. FEBS Lett. 1996, 392, 129–136. [Google Scholar] [CrossRef] [Green Version]
- Deng, J.; Lu, P.D.; Zhang, Y.; Scheuner, D.; Kaufman, R.J.; Sonenberg, N.; Harding, H.P.; Ron, D. Translational Repression Mediates Activation of Nuclear Factor Kappa B by Phosphorylated Translation Initiation Factor 2. Mol. Cell. Biol. 2004, 24, 10161–10168. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Sánchez, N.; Seoane-Collazo, P.; Contreras, C.; Varela, L.; Villarroya, J.; Rial-Pensado, E.; Buqué, X.; Aurrekoetxea, I.; Delgado, T.C.; Vázquez-Martínez, R.; et al. Hypothalamic AMPK-ER Stress-JNK1 Axis Mediates the Central Actions of Thyroid Hormones on Energy Balance. Cell Metab. 2017, 26, 212–229.e12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González-García, I.; Contreras, C.; Estévez-Salguero, Á.; Ruíz-Pino, F.; Colsh, B.; Pensado, I.; Liñares-Pose, L.; Rial-Pensado, E.; Martínez de Morentin, P.B.; Fernø, J.; et al. Estradiol Regulates Energy Balance by Ameliorating Hypothalamic Ceramide-Induced ER Stress. Cell Rep. 2018, 25, 413–423.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Compound | Content, µg/mL |
|---|---|
| Amino Acids | |
| L-Valine | 3.02 ± 0.21 |
| L-Leucine | 8.06 ± 0.56 |
| L-Isoleucine | 8.48 ± 0.59 |
| L-Proline | 20.01 ± 1.40 |
| L-Threonine | 3.89 ± 0.27 |
| L-Phenylalanine | 10.25 ± 0.72 |
| L-Lysine | 4.37 ± 0.31 |
| Glycine | 3.78 ± 0.26 |
| Serine | 2.59 ± 0.18 |
| L-Aspartic acid | 16.32 ± 1.14 |
| L-Asparagine | 6.19 ± 0.43 |
| L-Glutamic acid | 1.34 ± 0.09 |
| L-Glutamine | 22.99 ± 1.61 |
| DL-Ornithine | 12.36 ± 0.86 |
| L-Tyrosine | 2.66 ± 0.19 |
| Organic Acids | |
| Succinic acid | 12.64 ± 0.88 |
| Fumaric acid | 6.61 ± 0.46 |
| Malic acid | 9.22 ± 0.65 |
| Pyroglutamic acid (5-oxoproline) | 33.63 ± 2.35 |
| 4-Aminobutyric acid | 5.69 ± 0.40 |
| 2-Hydroxyglutaric acid | 4.07 ± 0.29 |
| 2-Ketoglutaric acid | 8.02 ± 0.56 |
| Phenylpyruvic acid | 2.18 ± 0.15 |
| 2,3-Dihydroxybutanedioic acid | 10.49 ± 0.73 |
| Isocitric acid | 18.12 ± 1.27 |
| Sugar Acids and Alcohols | |
| Glycerol | 36.12 ± 2.53 |
| Digalactosylglycerol | 6.99 ± 0.63 |
| Glyceric acid | 17.05 ± 1.19 |
| Threitol | 7.66 ± 0.54 |
| Erythreol | 2.09 ± 0.15 |
| Erithreonic acid | 2.65 ± 0.19 |
| Threonic acid | 8.40 ± 0.59 |
| Xylitol | 4.20 ± 0.29 |
| Arabinitol | 34.65 ± 2.43 |
| Pentonic acid | 7.69 ± 0.54 |
| L-Glycerol-3-phosphate | 17.72 ± 1.24 |
| Ribonic acid | 4.76 ± 0.33 |
| Manitol | 2.98 ± 0.21 |
| Sorbitol | 49.26 ± 3.45 |
| Glucuronic acid isomer | 8.49 ± 0.59 |
| Galactitol | 1.91 ± 0.13 |
| Galacturonic acid isomer | 15.91 ± 1.11 |
| Glucuronic acid isomer | 13.03 ± 0.91 |
| Gluconic acid isomer | 1.78 ± 0.12 |
| Galacturonic acid isomer | 2.89 ± 0.20 |
| Glucuronic acid isomer | 3.87 ± 0.27 |
| Galactonic acid | 6.33 ± 0.44 |
| Gluconic acid isomer | 3.71 ± 0.26 |
| Glucaric acid | 14.00 ± 0.98 |
| Galactaric acid | 3.38 ± 0.24 |
| Myo-inositol | 6.71 ± 0.47 |
| Galactosylglycerol | 22.50 ± 1.58 |
| Sorbitol-6-phosphate | 43.32 ± 3.03 |
| myo-Inositol-1-phosphate isomer | 5.64 ± 0.39 |
| myo-Inositol-2-phosphate isomer | 7.43 ± 0.52 |
| Gluconic acid-6-phosphate | 1.54 ± 0.11 |
| myo-Inositol-1-phosphate isomer | 3.30 ± 0.23 |
| myo-Inositol-2-phosphate isomer | 6.87 ± 0.48 |
| Maltitol; alpha-D-Glc-(1,4)-D-sorbitol | 4.90 ± 0.34 |
| Galactinol isomer; alpha-D-Gal-(1,3)-myo-Inositol | 0.69 ± 0.05 |
| Galactinol isomer; alpha-D-Gal-(1,3)-myo-Inositol | 3.67 ± 0.26 |
| Saccharides (mono-, di-, and tri-) | |
| Xylose methoxyamine | 5.94 ± 0.42 |
| Arabinose methoxyamine | 12.65 ± 0.89 |
| Fructose isomer | 14.31 ± 1.00 |
| Fructose isomer | 18.89 ± 1.32 |
| Sorbose isomer | 28.11 ± 1.97 |
| Sorbose isomer | 21.35 ± 1.49 |
| Galactose isomer | 35.19 ± 2.46 |
| Galactose isomer | 13.86 ± 0.97 |
| Glucose isomer | 17.34 ± 1.21 |
| Glucose isomer | 13.59 ± 0.95 |
| Fructose-6-phosphate isomer | 16.20 ± 1.13 |
| Mannose-6-phosphate isomer | 3.47 ± 0.24 |
| Galactose-6-phosphate isomer | 18.79 ± 1.32 |
| Glucose-6-phosphate isomer | 30.27 ± 2.12 |
| Fructose-6-phosphate isomer | 5.81 ± 0.41 |
| Galactose-6-phosphate isomer | 3.32 ± 0.23 |
| Glucose-6-phosphate isomer | 4.52 ± 0.32 |
| Sucrose; alpha-D-Glc-(1,2)-beta-D-Fru isomer | 24.81 ± 1.74 |
| Trehalose; alpha-D-Glc-(1,1)-alpha-D-Glc isomer | 10.10 ± 0.71 |
| Melibiose isomer; alpha-D-Gal-(1,6)-D-Glc isomer | 18.59 ± 1.30 |
| Melibiose isomer; alpha-D-Gal-(1,6)-D-Glc isomer | 18.80 ± 1.32 |
| Sucrose; alpha-D-Glc-(1,2)-beta-D-Fru isomer | 20.55 ± 1.44 |
| Trehalose; alpha-D-Glc-(1,1)-alpha-D-Glc isomer | 16.13 ± 1.13 |
| Raffinose; alpha-D-Gal-(1,6)-alpha-D-Glc-(1,2)-beta-D-Fru isomer | 12.91 ± 0.90 |
| Raffinose; alpha-D-Gal-(1,6)-alpha-D-Glc-(1,2)-beta-D-Fru isomer | 25.61 ± 1.79 |
| Saturated, unsaturated acids and esters | |
| 9-(E)-Hexadecenoic acid | 8.52 ± 0.77 |
| 9-(Z)-Hexadecenoic acid | 6.57 ± 0.59 |
| Heptadecanoic acid | 7.56 ± 0.68 |
| Hexadecatrienoic acid | 4.85 ± 0.44 |
| Hexadecanoic acid (Palmitic acid) | 6.56 ± 0.59 |
| Heptadecanoic acid | 6.06 ± 0.55 |
| 9,12-(Z,Z)-Octadecadienoic acid (Linoleic acid) | 9.69 ± 0.87 |
| 9,12,15-(Z,Z,Z)-Octadecatrienoic acid (Linolenic acid) | 8.42 ± 0.76 |
| Octadecanoic acid (Stearic acid) | 11.12 ± 1.00 |
| (2E,4E)-2,4-Octadecadienoic acid | 15.65 ± 1.41 |
| 1-Monopalmitin | 13.80 ± 1.24 |
| Monooctadecanoylglycerol | 8.62 ± 0.78 |
| beta-Sitosterol | 15.22 ± 1.37 |
| Compound | Content, µg/mL |
|---|---|
| Anthocyanins | |
| Cyanidin-3-O-galactoside (idaein) | 382.15 ± 13.19 |
| Cyanidin-3-O-glucoside (chrysanthemin) | 31.07 ± 1.10 |
| Cyanidin-3-O-arabinoside | 85.87 ± 2.80 |
| Cyanidin-3-O-xyloside | 14.35 ± 0.53 |
| Proanthocyanidin monomers | |
| Catechin | 40.19 ± 1.33 |
| Epicatechin | 322.37 ± 11.75 |
| Proanthocyanidin dimers | |
| EC→EC (1) | 171.40 ± 6.23 |
| EC→EC (2) | 169.24 ± 6.15 |
| EC→EC (3) | 189.86 ± 6.90 |
| EC→EC (4) | 157.91 ± 5.74 |
| Proanthocyanidin trimers | |
| EC→EC→EC (1) | 225.23 ± 8.16 |
| EC→EC→EC (2) | 242.27 ± 8.78 |
| EC→EC→EC (4) | 198.92 ± 7.21 |
| EC→EC→EC (4) | 249.36 ± 9.04 |
| Stilbenes | |
| trans-Resveratrol-3-O-glucoside | 51.92 ± 1.94 |
| Cyclohexanecarboxylic acid | |
| Quinic acid | 108.00 ± 4.02 |
| Hydroxycinnamic acids | |
| 3-O-Caffeoylquinic acid (chlorogenic acid) | 567.06 ± 20.55 |
| Caffeic acid-O-galactoside | 98.72 ± 3.58 |
| Caffeic acid-O-glucoside | 74.66 ± 2.71 |
| 5-O-Caffeoylquinic acid (neochlorogenic acid) | 906.08 ± 32.84 |
| p-Coumaric acid-O-glucoside | 236.37 ± 8.57 |
| 3-O-p-Coumaroylquinic acid | 399.47 ± 14.48 |
| Feruloylquinic acid | 248.93 ± 9.02 |
| 4-O-p-Coumaroylquinic acid | 219.83 ± 7.97 |
| Ferulic acid-O-galactoside | 131.66 ± 4.77 |
| Ferulic acid-O-glucoside | 122.26 ± 4.43 |
| Flavonol glycosides | |
| Quercetin-3-O-rhamnosyl-galactoside | 25.57 ± 0.93 |
| Quercetin-3-O-galactoside (hyperoside) | 29.17 ± 1.06 |
| Kaempferol-3-O-galactoside | 11.15 ± 0.40 |
| Quercetin-3-O-rhamnosyl-glucoside | 20.35 ± 0.74 |
| Quercetin-3-O-glucoside (isoquercetin) | 22.80 ± 0.83 |
| Kaempferol-3-O-glucoside (astragalin) | 9.94 ± 0.36 |
| Quercetin-3-O-arabinoside (guaiaverin) | 16.77 ± 0.61 |
| Quercetin-3-O-xyloside | 13.97 ± 0.51 |
| Kaempferol-3-O-rhamnosyl-galactoside | 12.52 ± 0.45 |
| Kaempferol-3-O-rhamnosyl-glucoside | 9.15 ± 0.33 |
| Kaempferol-3-O-arabinoside | 11.15 ± 0.40 |
| Kaempferol-3-O-xyloside | 12.80 ± 0.46 |
| Total analyzed polyphenols | 5840.50 |
| Gene Name | Forward Primer (5′–3′) | Reverse Primer (5′–3′) |
|---|---|---|
| Actb (β-actin) | ACGGCCAGGTCATCACTATTG | CAAGAAGGAAGGCTGGAAAAG |
| Ptgs2 (COX2) | TGAGCAACTATTCCAAACCAGC | GCACGTAGTCTTCGATCACTATC |
| iNOS | GGCAGCCTGTGAGACCTTTG | GCATTGGAAGTGAAGCGTTTC |
| TNFα | CCCTCACACTCAGATCATCTTCT | GCTACGACGTGGGCTACAG |
| IL-6 | GAGTTGTGCAATGGCAATTCTG | GCAAGTGCATCATCGTTGTTCAT |
| IL-1β | TTCAGGCAGGCAGTATCACTC | CCACGGGAAAGACACAGGTAG |
| Ccl2 (MCP-1) | AGGTGTCCCAAAGAAGCTGTA | ATGTCTGGACCCATTCCTTCT |
| Sirt-1 | TGATTGGCACCGATCCTCG | CCACAGCGTCATATCATCCAG |
| IL-1ra | GCTCATTGCTGGGTACTTACAA | CCAGACTTGGCACAAGACAGG |
| Icam1 | GACCCCAAGGAGATCACATTC | GAAGATCGAAAGTCCGGA |
| Noxo1 | AGAGGAGCCCTTATCCCAACC | TGTCCAGAATTTCTTGAGCCTTG |
| Fabp4 (aP2) | AGTGAAAACTTCGATGATTACATGAA | GCCTGCCACTTTCCTTGTG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tasinov, O.; Dincheva, I.; Badjakov, I.; Kiselova-Kaneva, Y.; Galunska, B.; Nogueiras, R.; Ivanova, D. Phytochemical Composition, Anti-Inflammatory and ER Stress-Reducing Potential of Sambucus ebulus L. Fruit Extract. Plants 2021, 10, 2446. https://doi.org/10.3390/plants10112446
Tasinov O, Dincheva I, Badjakov I, Kiselova-Kaneva Y, Galunska B, Nogueiras R, Ivanova D. Phytochemical Composition, Anti-Inflammatory and ER Stress-Reducing Potential of Sambucus ebulus L. Fruit Extract. Plants. 2021; 10(11):2446. https://doi.org/10.3390/plants10112446
Chicago/Turabian StyleTasinov, Oskan, Ivayla Dincheva, Ilian Badjakov, Yoana Kiselova-Kaneva, Bistra Galunska, Ruben Nogueiras, and Diana Ivanova. 2021. "Phytochemical Composition, Anti-Inflammatory and ER Stress-Reducing Potential of Sambucus ebulus L. Fruit Extract" Plants 10, no. 11: 2446. https://doi.org/10.3390/plants10112446









