LED Illumination for High-Quality High-Yield Crop Growth in Protected Cropping Environments
Abstract
:1. Introduction
2. LED Illumination
3. Photosynthesis
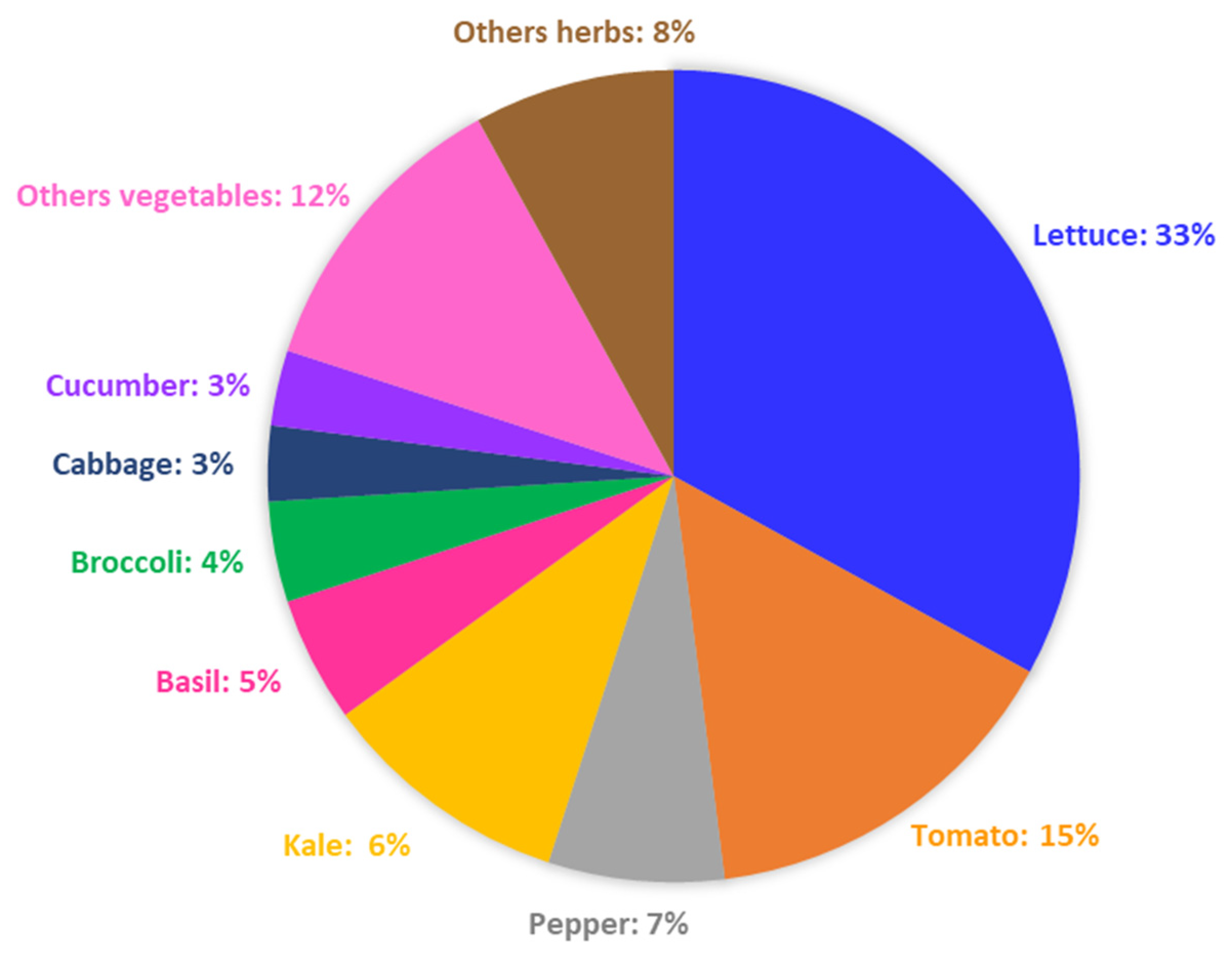
4. Methodology for Comparative Analysis of LED Effects on Vegetables and Herbs
5. Effect of LED on Nutrients
5.1. Antioxidant Properties
5.2. Phenolic Compound
5.3. Essential Vitamins
5.4. Carotenoids
6. Effect of LED on Physiology
7. Effect of LED on Postharvest Quality and Resource Use Efficiency
8. The Implication of LED on Plants Development and Its Future Policy
9. Summary and Suggestions
10. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Silva, C.S.; Seider, W.D.; Lior, N. Exergy efficiency of plant photosynthesis. Chem. Eng. Sci. 2015, 130, 151–171. [Google Scholar] [CrossRef] [Green Version]
- Kami, C.; Lorrain, S.; Hornitschek, P.; Fankhauser, C. Light-regulated plant growth and development. Curr. Top. Dev. Biol. 2010, 91, 29–66. [Google Scholar] [PubMed] [Green Version]
- Carruthers, T.J.; Longstaff, B.J.; Dennison, W.C.; Abal, E.G.; Aioi, K. Measurement of light penetration in relation to seagrass. In Global Seagrass Research Methods; Elsevier: Amsterdam, The Netherlands, 2001; pp. 370–392. [Google Scholar]
- Sæbø, A.; Krekling, T.; Appelgren, M. Light quality affects photosynthesis and leaf anatomy of birch plantlets in vitro. Plant Cell Tissue Organ Cult. 1995, 41, 177–185. [Google Scholar] [CrossRef]
- Zou, J.; Fanourakis, D.; Tsaniklidis, G.; Cheng, R.; Yang, Q.; Li, T. Lettuce growth, morphology and critical leaf trait responses to far-red light during cultivation are low fluence and obey the reciprocity law. Sci. Hortic. 2021, 289, 110455. [Google Scholar] [CrossRef]
- Li, X.; Lu, W.; Hu, G.; Wang, X.C.; Zhang, Y.; Sun, G.X.; Fang, Z. Effects of light-emitting diode supplementary lighting on the winter growth of greenhouse plants in the Yangtze River Delta of China. Bot. Stud. 2016, 57, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wali, A.F.; Majid, S.; Rasool, S.; Shehada, S.B.; Abdulkareem, S.K.; Firdous, A.; Beigh, S.; Shakeel, S.; Mushtaq, S.; Akbar, I. Natural products against cancer: Review on phytochemicals from marine sources in preventing cancer. Saudi Pharm. J. 2019, 27, 767–777. [Google Scholar] [CrossRef]
- Martineau, V.; Lefsrud, M.; Naznin, M.T.; Kopsell, D.A. Comparison of light-emitting diode and high-pressure sodium light treatments for hydroponics growth of Boston lettuce. HortScience 2012, 47, 477–482. [Google Scholar] [CrossRef] [Green Version]
- Jishi, T.; Fujiwara, K. Time-varying Photosynthetic Photon Flux Density and Relative Spectral Photon Flux Density Distribution to Improve Plant Growth and Morphology in Plant Factories with Artificial Lighting. Hortic. J. 2021, 90, 147–153. [Google Scholar] [CrossRef]
- Hopkins, W.G. Introduction to Plant Physiology; John Wiley and Sons: Hoboken, NJ, USA, 1999. [Google Scholar]
- Liu, J.; van Iersel, M.W. Photosynthetic Physiology of Blue, Green, and Red Light: Light Intensity Effects and Underlying Mechanisms. Front. Plant Sci. 2021, 12, 328. [Google Scholar] [CrossRef]
- Hogewoning, S.W.; Wientjes, E.; Douwstra, P.; Trouwborst, G.; Van Ieperen, W.; Croce, R.; Harbinson, J. Photosynthetic quantum yield dynamics: From photosystems to leaves. Plant Cell 2012, 24, 1921–1935. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Kaiser, E.; Zhang, Y.; Yang, Q.; Li, T. Red/blue light ratio strongly affects steady-state photosynthesis, but hardly affects photosynthetic induction in tomato (Solanum lycopersicum). Physiol. Plant. 2019, 167, 144–158. [Google Scholar] [CrossRef]
- Ouzounis, T.; Heuvelink, E.; Ji, Y.; Schouten, H.; Visser, R.; Marcelis, L. Blue and red LED lighting effects on plant biomass, stomatal conductance, and metabolite content in nine tomato genotypes. In Proceedings of the VIII International Symposium on Light in Horticulture 1134, East Lansing, MI, USA, 22 May 2016; pp. 251–258. [Google Scholar]
- Johkan, M.; Shoji, K.; Goto, F.; Hashida, S.-N.; Yoshihara, T. Blue light-emitting diode light irradiation of seedlings improves seedling quality and growth after transplanting in red leaf lettuce. HortScience 2010, 45, 1809–1814. [Google Scholar] [CrossRef] [Green Version]
- Terashima, I.; Fujita, T.; Inoue, T.; Chow, W.S.; Oguchi, R. Green light drives leaf photosynthesis more efficiently than red light in strong white light: Revisiting the enigmatic question of why leaves are green. Plant Cell Physiol. 2009, 50, 684–697. [Google Scholar] [CrossRef] [Green Version]
- Semenova, N.A.; Smirnov, A.A.; Grishin, A.A.; Pishchalnikov, R.Y.; Chesalin, D.D.; Gudkov, S.V.; Chilingaryan, N.O.; Skorokhodova, A.N.; Dorokhov, A.S.; Izmailov, A.Y. The Effect of Plant Growth Compensation by Adding Silicon-Containing Fertilizer under Light Stress Conditions. Plants 2021, 10, 1287. [Google Scholar] [CrossRef] [PubMed]
- Smith, H.L.; McAusland, L.; Murchie, E.H. Don’t ignore the green light: Exploring diverse roles in plant processes. J. Exp. Bot. 2017, 68, 2099–2110. [Google Scholar] [CrossRef]
- Zhen, S.; Haidekker, M.; van Iersel, M.W. Far-red light enhances photochemical efficiency in a wavelength-dependent manner. Physiol. Plant. 2019, 167, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Stutte, G.W.; Edney, S.; Skerritt, T. Photoregulation of bioprotectant content of red leaf lettuce with light-emitting diodes. HortScience 2009, 44, 79–82. [Google Scholar] [CrossRef] [Green Version]
- Kubota, C.; Chia, P.; Yang, Z.; Li, Q. Applications of far-red light emitting diodes in plant production under controlled environments. In Proceedings of the International Symposium on Advanced Technologies and Management Towards Sustainable Greenhouse Ecosystems: Greensys, Athens, Greece, 5–10 June 2011; Volume 952, pp. 59–66. [Google Scholar]
- Guidi, L.; Chaffron, S.; Bittner, L.; Eveillard, D.; Larhlimi, A.; Roux, S.; Darzi, Y.; Audic, S.; Berline, L.; Brum, J.R. Plankton networks driving carbon export in the oligotrophic ocean. Nature 2016, 532, 465–470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goins, G.D.; Yorio, N.C.; Sanwo, M.; Brown, C. Photomorphogenesis, photosynthesis, and seed yield of wheat plants grown under red light-emitting diodes (LEDs) with and without supplemental blue lighting. J. Exp. Bot. 1997, 48, 1407–1413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinho, P.; Jokinen, K.; Halonen, L. The influence of the LED light spectrum on the growth and nutrient uptake of hydroponically grown lettuce. Lighting Res. Technol. 2017, 49, 866–881. [Google Scholar] [CrossRef]
- Tan, C.; Wang, D.; Zhou, J.; Du, Y.; Luo, M.; Zhang, Y.; Guo, W. Remotely assessing fraction of photosynthetically active radiation (FPAR) for wheat canopies based on hyperspectral vegetation indexes. Front. Plant Sci. 2018, 9, 776. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.; Chory, J.; Fankhauser, C. Light signal transduction in higher plants. Annu. Rev. Genet. 2004, 38, 87–117. [Google Scholar] [CrossRef] [Green Version]
- Galvão, V.C.; Fankhauser, C. Sensing the light environment in plants: Photoreceptors and early signaling steps. Curr. Opin. Neurobiol. 2015, 34, 46–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tilbrook, K.; Arongaus, A.B.; Binkert, M.; Heijde, M.; Yin, R.; Ulm, R. The UVR8 UV-B photoreceptor: Perception, signaling and response. Arab. Book/Am. Soc. Plant Biol. 2013, 11, e0164. [Google Scholar] [CrossRef] [Green Version]
- Yadav, A.; Singh, D.; Lingwan, M.; Yadukrishnan, P.; Masakapalli, S.K.; Datta, S. Light signaling and UV-B-mediated plant growth regulation. J. Integr. Plant Biol. 2020, 62, 1270–1292. [Google Scholar] [CrossRef] [PubMed]
- Nelson, J.A.; Bugbee, B. Economic analysis of greenhouse lighting: Light emitting diodes vs. high intensity discharge fixtures. PLoS ONE 2014, 9, e99010. [Google Scholar] [CrossRef] [Green Version]
- Liao, Y.; Suzuki, K.; Yu, W.; Zhuang, D.; Takai, Y.; Ogasawara, R.; Shimazu, T.; Fukui, H. Night break effect of LED light with different wavelengths on floral bud differentiation of chrysanthemum morifolium Ramat ‘Jimba’and Iwa no hakusen. Environ. Control Biol. 2014, 52, 45–50. [Google Scholar] [CrossRef] [Green Version]
- Tokuno, A.; Ibaraki, Y.; Ito, S.-I.; Araki, H.; Yoshimura, K.; Osaki, K. Disease suppression in greenhouse tomato by supplementary lighting with 405 nm LED. Environ. Control Biol. 2012, 50, 19–29. [Google Scholar] [CrossRef] [Green Version]
- Yan, W.-H.; Wang, P.; Chen, H.-X.; Zhou, H.-J.; Li, Q.-P.; Wang, C.-R.; Ding, Z.-H.; Zhang, Y.-S.; Yu, S.-B.; Xing, Y.-Z. A major QTL, Ghd8, plays pleiotropic roles in regulating grain productivity, plant height, and heading date in rice. Mol. Plant 2011, 4, 319–330. [Google Scholar] [CrossRef]
- Ibaraki, Y.; Shigemoto, C. Estimation of supplemental lighting efficiency based on PPFD distribution on the canopy surface. J. Agric. Meteorol. 2013, 69, 47–54. [Google Scholar] [CrossRef] [Green Version]
- Van Ieperen, W.; Savvides, A.; Fanourakis, D. Red and blue light effects during growth on hydraulic and stomatal conductance in leaves of young cucumber plants. In Proceedings of the VII International Symposium on Light in Horticultural Systems 956, Wageningen, The Netherlands, 14 October 2012; pp. 223–230. [Google Scholar]
- Park, Y.; Runkle, E.S. Spectral effects of light-emitting diodes on plant growth, visual color quality, and photosynthetic photon efficacy: White versus blue plus red radiation. PLoS ONE 2018, 13, e0202386. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Kubota, C. Effects of supplemental light quality on growth and phytochemicals of baby leaf lettuce. Environ. Exp. Bot. 2009, 67, 59–64. [Google Scholar] [CrossRef]
- Massa, G.D.; Kim, H.-H.; Wheeler, R.M.; Mitchell, C.A. Plant productivity in response to LED lighting. HortScience 2008, 43, 1951–1956. [Google Scholar] [CrossRef]
- Gupta, S.D.; Jatothu, B. Fundamentals and applications of light-emitting diodes (LEDs) in in vitro plant growth and morphogenesis. Plant Biotechnol. Rep. 2013, 7, 211–220. [Google Scholar] [CrossRef]
- McCree, K.J. Test of current definitions of photosynthetically active radiation against leaf photosynthesis data. Agric. Meteorol. 1972, 10, 443–453. [Google Scholar] [CrossRef]
- Hoover, W.H. The dependence of carbon dioxide assimilation in a higher plant on wave length of radiation. Smithson. Misc. Collect. 1937, 95, 21. [Google Scholar]
- Samuolienė, G.; Brazaitytė, A.; Jankauskienė, J.; Viršilė, A.; Sirtautas, R.; Novičkovas, A.; Sakalauskienė, S.; Sakalauskaitė, J.; Duchovskis, P. LED irradiance level affects growth and nutritional quality of Brassica microgreens. Open Life Sci. 2013, 8, 1241–1249. [Google Scholar] [CrossRef]
- Azad, M.O.K.; Kim, W.W.; Park, C.H.; Cho, D.H. Effect of artificial LED light and far infrared irradiation on phenolic compound, isoflavones and antioxidant capacity in soybean (Glycine max L.) sprout. Foods 2018, 7, 174. [Google Scholar] [CrossRef] [Green Version]
- Naznin, M.T.; Lefsrud, M.; Gravel, V.; Azad, M.O.K. Blue light added with Red LEDs enhance growth characteristics, pigments content, and antioxidant capacity in lettuce, spinach, kale, basil, and sweet pepper in a controlled environment. Plants 2019, 8, 93. [Google Scholar] [CrossRef] [Green Version]
- Izzo, L.G.; Mele, B.H.; Vitale, L.; Vitale, E.; Arena, C. The role of monochromatic red and blue light in tomato early photomorphogenesis and photosynthetic traits. Environ. Exp. Bot. 2020, 179, 104195. [Google Scholar] [CrossRef]
- ECU-Worldsearch; Edith Cowan University: Joondalup, Australia, 2020.
- Rohart, F.; Gautier, B.; Singh, A.; Lê Cao, K.-A. mixOmics: An R package for ‘omics feature selection and multiple data integration. PLoS Comput. Biol. 2017, 13, e1005752. [Google Scholar] [CrossRef] [Green Version]
- Lepetit, B.; Dietzel, L. Light signaling in photosynthetic eukaryotes with ‘green’and ‘red’chloroplasts. Environ. Exp. Bot. 2015, 114, 30–47. [Google Scholar] [CrossRef]
- Bednarczyk, D.; Aviv-Sharon, E.; Savidor, A.; Levin, Y.; Charuvi, D. Influence of short-term exposure to high light on photosynthesis and proteins involved in photo-protective processes in tomato leaves. Environ. Exp. Bot. 2020, 179, 104198. [Google Scholar] [CrossRef]
- Hasan, M.; Bashir, T.; Ghosh, R.; Lee, S.K.; Bae, H. An overview of LEDs’ effects on the production of bioactive compounds and crop quality. Molecules 2017, 22, 1420. [Google Scholar] [CrossRef] [Green Version]
- Singh, D.; Basu, C.; Meinhardt-Wollweber, M.; Roth, B. LEDs for energy efficient greenhouse lighting. Renew. Sustain. Energy Rev. 2015, 49, 139–147. [Google Scholar] [CrossRef] [Green Version]
- Meiramkulova, K.; Tanybayeva, Z.; Kydyrbekova, A.; Turbekova, A.; Aytkhozhin, S.; Zhantasov, S.; Taukenov, A. The Efficiency of LED Irradiation for Cultivating High-Quality Tomato Seedlings. Sustainability 2021, 13, 9426. [Google Scholar] [CrossRef]
- Craine, J.M.; Dybzinski, R. Mechanisms of plant competition for nutrients, water and light. Funct. Ecol. 2013, 27, 833–840. [Google Scholar] [CrossRef]
- Masclaux-Daubresse, C.; Daniel-Vedele, F.; Dechorgnat, J.; Chardon, F.; Gaufichon, L.; Suzuki, A. Nitrogen uptake, assimilation and remobilization in plants: Challenges for sustainable and productive agriculture. Ann. Bot. 2010, 105, 1141–1157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wojciechowska, R.; Długosz-Grochowska, O.; Kołton, A.; Żupnik, M. Effects of LED supplemental lighting on yield and some quality parameters of lamb’s lettuce grown in two winter cycles. Sci. Hortic. 2015, 187, 80–86. [Google Scholar] [CrossRef]
- Piovene, C.; Orsini, F.; Bosi, S.; Sanoubar, R.; Bregola, V.; Dinelli, G.; Gianquinto, G. Optimal red: Blue ratio in led lighting for nutraceutical indoor horticulture. Sci. Hortic. 2015, 193, 202–208. [Google Scholar] [CrossRef]
- Li, Y.; Shi, R.; Jiang, H.; Wu, L.; Zhang, Y.; Song, S.; Su, W.; Liu, H. End-Of-Day LED Lightings Influence the Leaf Color, Growth and Phytochemicals in Two Cultivars of Lettuce. Agronomy 2020, 10, 1475. [Google Scholar] [CrossRef]
- Lee, M.K.; Arasu, M.V.; Park, S.; Byeon, D.H.; Chung, S.-O.; Park, S.U. LED lights enhance metabolites and antioxidants in Chinese cabbage and kale. Braz. Arch. Biol. Technol. 2016, 59. [Google Scholar] [CrossRef] [Green Version]
- Samuolienė, G.; Sirtautas, R.; Brazaitytė, A.; Duchovskis, P. LED lighting and seasonality effects antioxidant properties of baby leaf lettuce. Food Chem. 2012, 134, 1494–1499. [Google Scholar] [CrossRef] [PubMed]
- Ohtake, N.; Ishikura, M.; Suzuki, H.; Yamori, W.; Goto, E. Continuous irradiation with alternating red and blue light enhances plant growth while keeping nutritional quality in lettuce. HortScience 2018, 53, 1804–1809. [Google Scholar] [CrossRef] [Green Version]
- Mizuno, T.; Amaki, W.; Watanabe, H. Effects of monochromatic light irradiation by LED on the growth and anthocyanin contents in leaves of cabbage seedlings. In Proceedings of the VI International Symposium on Light in Horticulture 907, Tsukuba, Japan, 1 September 2011; pp. 179–184. [Google Scholar]
- Samuolienė, G.; Viršilė, A.; Brazaitytė, A.; Jankauskienė, J.; Duchovskis, P.; Novičkovas, A.; Bliznikas, Z.; Zukauskas, A. Effect of supplementary pre-harvest LED lighting on the antioxidant and nutritional properties of green vegetables. In Proceedings of the XXVIII International Horticultural Congress on Science and Horticulture for People (IHC2010): International Symposium on 939, Lisbon, Portugal, 30 November 2012; pp. 85–91. [Google Scholar]
- Bian, Z.H.; Yang, Q.C.; Liu, W.K. Effects of light quality on the accumulation of phytochemicals in vegetables produced in controlled environments: A review. J. Sci. Food Agric. 2015, 95, 869–877. [Google Scholar] [CrossRef]
- Novičkovas, A.; Brazaitytė, A.; Duchovskis, P.; Jankauskienė, J.; Samuolienė, G.; Virsilė, A.; Sirtautas, R.; Bliznikas, Z.; Zukauskas, A. Solid-state lamps (LEDs) for the short-wavelength supplementary lighting in greenhouses: Experimental results with cucumber. In Proceedings of the XXVIII International Horticultural Congress on Science and Horticulture for People (IHC2010): International Symposium on 927, Lisbon, Portugal, 28 February 2012; pp. 723–730. [Google Scholar]
- Vauzour, D. Dietary polyphenols as modulators of brain functions: Biological actions and molecular mechanisms underpinning their beneficial effects. Oxidative Med. Cell. Longev. 2012, 2012, 914273. [Google Scholar] [CrossRef] [Green Version]
- Samuolienė, G.; Urbonavičiūtė, A.; Brazaitytė, A.; Šabajevienė, G.; Sakalauskaitė, J.; Duchovskis, P. The impact of LED illumination on antioxidant properties of sprouted seeds. Open Life Sci. 2011, 6, 68–74. [Google Scholar] [CrossRef]
- Alrifai, O.; Hao, X.; Liu, R.; Lu, Z.; Marcone, M.F.; Tsao, R. Amber, red and blue LEDs modulate phenolic contents and antioxidant activities in eight Cruciferous microgreens. J. Food Bioact. 2020, 11. [Google Scholar] [CrossRef]
- Urbonavičiūtė, A.; Samuolienė, G.; Brazaitytė, A.; Duchovskis, P.; Ruzgas, V.; Žukauskas, A. The effect of variety and lighting qualityon wheatgrass antioxidant properties. Zemdirb.-Agric. 2009, 96, 119–128. [Google Scholar]
- Kim, D.-O.; Chun, O.K.; Kim, Y.J.; Moon, H.-Y.; Lee, C.Y. Quantification of polyphenolics and their antioxidant capacity in fresh plums. J. Agric. Food Chem. 2003, 51, 6509–6515. [Google Scholar] [CrossRef]
- Connor, A.M.; Stephens, M.J.; Hall, H.K.; Alspach, P.A. Variation and heritabilities of antioxidant activity and total phenolic content estimated from a red raspberry factorial experiment. J. Am. Soc. Hortic. Sci. 2005, 130, 403–411. [Google Scholar] [CrossRef] [Green Version]
- Huyut, Z.; Beydemir, Ş.; Gülçin, İ. Antioxidant and antiradical properties of selected flavonoids and phenolic compounds. Biochem. Res. Int. 2017, 2017, 7616791. [Google Scholar] [CrossRef]
- Karakaya, S.N.E.; Taş, A.A. Antioxidant activity of some foods containing phenolic compounds. Int. J. Food Sci. Nutr. 2001, 52, 501–508. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects—A review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- AL-Ishaq, R.K.; Abotaleb, M.; Kubatka, P.; Kajo, K.; Büsselberg, D. Flavonoids and their anti-diabetic effects: Cellular mechanisms and effects to improve blood sugar levels. Biomolecules 2019, 9, 430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warren, C.R.; Dreyer, E.; Adams, M.A. Photosynthesis-Rubisco relationships in foliage of Pinus sylvestris in response to nitrogen supply and the proposed role of Rubisco and amino acids as nitrogen stores. Trees 2003, 17, 359–366. [Google Scholar] [CrossRef]
- Lee, M.-J.; Son, K.-H.; Oh, M.-M. Increase in biomass and bioactive compounds in lettuce under various ratios of red to far-red LED light supplemented with blue LED light. Hortic. Environ. Biotechnol. 2016, 57, 139–147. [Google Scholar] [CrossRef]
- Manivannan, A.; Soundararajan, P.; Halimah, N.; Ko, C.H.; Jeong, B.R. Blue LED light enhances growth, phytochemical contents, and antioxidant enzyme activities of Rehmannia glutinosa cultured in vitro. Hortic. Environ. Biotechnol. 2015, 56, 105–113. [Google Scholar] [CrossRef]
- Shiga, T.; Shoji, K.; Shimada, H.; Hashida, S.-N.; Goto, F.; Yoshihara, T. Effect of light quality on rosmarinic acid content and antioxidant activity of sweet basil, Ocimum basilicum L. Plant Biotechnol. 2009, 26, 255–259. [Google Scholar] [CrossRef] [Green Version]
- Luthria, D.L.; Mukhopadhyay, S.; Krizek, D.T. Content of total phenolics and phenolic acids in tomato (Lycopersicon esculentum Mill.) fruits as influenced by cultivar and solar UV radiation. J. Food Compos. Anal. 2006, 19, 771–777. [Google Scholar] [CrossRef]
- Lee, H.; Oh, I.-N.; Kim, J.; Jung, D.; Cuong, N.P.; Kim, Y.; Lee, J.; Kwon, O.; Park, S.U.; Lim, Y. Phenolic compound profiles and their seasonal variations in new red-phenotype head-forming Chinese cabbages. LWT 2018, 90, 433–439. [Google Scholar] [CrossRef]
- OSU. Environmental Factors Affecting Plant Growth. Available online: https://extension.oregonstate.edu/gardening/techniques/environmental-factors-affecting-plant-growth (accessed on 11 October 2021).
- Chen, W.; Xu, Z.; Liu, X.; Yang, Y.; Wang, Z.; Song, F. Effect of LED light source on the growth and quality of different lettuce varieties. Acta Bot. Boreali-Occident. Sin. 2011, 31, 1434–1440. [Google Scholar]
- Samuolienė, G.; Brazaitytė, A.; Sirtautas, R.; Novičkovas, A.; Duchovskis, P. The effect of supplementary LED lighting on the antioxidant and nutritional properties of lettuce. In Proceedings of the International Symposium on Advanced Technologies and Management Towards Sustainable Greenhouse Ecosystems: Greensys, Athens, Greece, 5–10 June 2011; Volume 952, pp. 835–841. [Google Scholar]
- Bian, Z.-H.; Cheng, R.-F.; Yang, Q.-C.; Wang, J.; Lu, C. Continuous light from red, blue, and green light-emitting diodes reduces nitrate content and enhances phytochemical concentrations and antioxidant capacity in lettuce. J. Am. Soc. Hortic. Sci. 2016, 141, 186–195. [Google Scholar] [CrossRef] [Green Version]
- Duchovskis, P.; Novickovas, A. Supplementary red-LED lighting affects phytochemicals and nitrate of baby leaf lettuce. J. Food Agric. Environ. 2011, 9, 271–274. [Google Scholar]
- Li, H.; Tang, C.; Xu, Z.; Liu, X.; Han, X. Effects of different light sources on the growth of non-heading Chinese cabbage (Brassica campestris L.). J. Agric. Sci. 2012, 4, 262. [Google Scholar] [CrossRef] [Green Version]
- Sivakumar, D.; Chen, L.; Sultanbawa, Y. A comprehensive review on beneficial dietary phytochemicals in common traditional Southern African leafy vegetables. Food Sci. Nutr. 2018, 6, 714–727. [Google Scholar] [CrossRef] [Green Version]
- Lefsrud, M.; Kopsell, D.; Wenzel, A.; Sheehan, J. Changes in kale (Brassica oleracea L. var. acephala) carotenoid and chlorophyll pigment concentrations during leaf ontogeny. Sci. Hortic. 2007, 112, 136–141. [Google Scholar] [CrossRef]
- Botella-Pavía, P.; Rodríguez-Concepción, M. Carotenoid biotechnology in plants for nutritionally improved foods. Physiol. Plant. 2006, 126, 369–381. [Google Scholar] [CrossRef]
- Morrow, R.C. LED lighting in horticulture. HortScience 2008, 43, 1947–1950. [Google Scholar] [CrossRef] [Green Version]
- He, R.; Zhang, Y.; Song, S.; Su, W.; Hao, Y.; Liu, H. UV-A and FR irradiation improves growth and nutritional properties of lettuce grown in an artificial light plant factory. Food Chem. 2021, 345, 128727. [Google Scholar] [CrossRef]
- Lefsrud, M.G.; Kopsell, D.A.; Sams, C.E. Irradiance from distinct wavelength light-emitting diodes affect secondary metabolites in kale. HortScience 2008, 43, 2243–2244. [Google Scholar] [CrossRef] [Green Version]
- Krinsky, N.I.; Landrum, J.T.; Bone, R.A. Biologic mechanisms of the protective role of lutein and zeaxanthin in the eye. Annu. Rev. Nutr. 2003, 23, 171–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kopsell, D.A.; Sams, C.E. Increases in shoot tissue pigments, glucosinolates, and mineral elements in sprouting broccoli after exposure to short-duration blue light from light emitting diodes. J. Am. Soc. Hortic. Sci. 2013, 138, 31–37. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.-L.; Guo, W.-Z.; Xue, X.-Z.; Wang, L.-C.; Qiao, X.-J. Growth and quality responses of ‘Green Oak Leaf’lettuce as affected by monochromic or mixed radiation provided by fluorescent lamp (FL) and light-emitting diode (LED). Sci. Hortic. 2014, 172, 168–175. [Google Scholar] [CrossRef]
- Wu, M.-C.; Hou, C.-Y.; Jiang, C.-M.; Wang, Y.-T.; Wang, C.-Y.; Chen, H.-H.; Chang, H.-M. A novel approach of LED light radiation improves the antioxidant activity of pea seedlings. Food Chem. 2007, 101, 1753–1758. [Google Scholar] [CrossRef]
- Samuolienė, G.; Brazaitytė, A.; Viršilė, A.; Jankauskienė, J.; Sakalauskienė, S.; Duchovskis, P. Red light-dose or wavelength-dependent photoresponse of antioxidants in herb microgreens. PLoS ONE 2016, 11, e0163405. [Google Scholar] [CrossRef]
- Brazaitytė, A.; Sakalauskienė, S.; Samuolienė, G.; Jankauskienė, J.; Viršilė, A.; Novičkovas, A.; Sirtautas, R.; Miliauskienė, J.; Vaštakaitė, V.; Dabašinskas, L. The effects of LED illumination spectra and intensity on carotenoid content in Brassicaceae microgreens. Food Chem. 2015, 173, 600–606. [Google Scholar] [CrossRef]
- Sikora, E.; Bodziarczyk, I. Composition and antioxidant activity of kale (Brassica oleracea L. var. acephala) raw and cooked. Acta Sci. Pol. Technol. Aliment. 2012, 11, 239–248. [Google Scholar]
- Cui, J.; Ma, Z.; Xu, Z.; Zhang, H.; Chang, T.; Liu, H. Effects of supplemental lighting with different light qualities on growth and physiological characteristics of cucumber, pepper and tomato seedlings. Acta Hortic. Sin. 2009, 36, 663–670. [Google Scholar]
- Kalaitzoglou, P.; Van Ieperen, W.; Harbinson, J.; van der Meer, M.; Martinakos, S.; Weerheim, K.; Nicole, C.; Marcelis, L. Effects of continuous or end-of-day far-red light on tomato plant growth, morphology, light absorption and fruit production. Front. Plant Sci. 2019, 10, 322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaiser, E.; Weerheim, K.; Schipper, R.; Dieleman, J.A. Partial replacement of red and blue by green light increases biomass and yield in tomato. Sci. Hortic. 2019, 249, 271–279. [Google Scholar] [CrossRef]
- Degni, B.F.; Haba, C.T.; Dibi, W.G.; Soro, D.; Zoueu, J.T. Effect of light spectrum on growth, development, and mineral contents of okra (Abelmoschus esculentus L.). Open Agric. 2021, 6, 276–285. [Google Scholar] [CrossRef]
- Hogewoning, S.W.; Trouwborst, G.; Maljaars, H.; Poorter, H.; van Ieperen, W.; Harbinson, J. Blue light dose–responses of leaf photosynthesis, morphology, and chemical composition of Cucumis sativus grown under different combinations of red and blue light. J. Exp. Bot. 2010, 61, 3107–3117. [Google Scholar] [CrossRef] [PubMed]
- Samuolienė, G.; Brazaitytė, A.; Duchovskis, P.; Viršilė, A.; Jankauskienė, J.; Sirtautas, R.; Novičkovas, A.; Sakalauskienė, S.; Sakalauskaitė, J. Cultivation of vegetable transplants using solid-state lamps for the short-wavelength supplementary lighting in greenhouses. In Proceedings of the International Symposium on Advanced Technologies and Management towards Sustainable Greenhouse Ecosystems: Greensys, Athens, Greece, 5–10 June 2011; Volume 952, pp. 885–892. [Google Scholar]
- Fanwoua, J.; Vercambre, G.; Buck-Sorlin, G.; Dieleman, J.A.; de Visser, P.; Génard, M. Supplemental LED lighting affects the dynamics of tomato fruit growth and composition. Sci. Hortic. 2019, 256, 108571. [Google Scholar] [CrossRef]
- Ajdanian, L.; Babaei, M.; Aroiee, H. The growth and development of cress (Lepidium sativum) affected by blue and red light. Heliyon 2019, 5, e02109. [Google Scholar] [CrossRef] [Green Version]
- Hosseini, A.; Mehrjerdi, M.Z.; Aliniaeifard, S.; Seif, M. Photosynthetic and growth responses of green and purple basil plants under different spectral compositions. Physiol. Mol. Biol. Plants 2019, 25, 741–752. [Google Scholar] [CrossRef]
- Zha, L.; Liu, W. Effects of light quality, light intensity, and photoperiod on growth and yield of cherry radish grown under red plus blue LEDs. Hortic. Environ. Biotechnol. 2018, 59, 511–518. [Google Scholar] [CrossRef]
- Naznin, M.; Lefsrud, M.; Gravel, V.; Hao, X. Different ratios of red and blue LED light effects on coriander productivity and antioxidant properties. In Proceedings of the VIII International Symposium on Light in Horticulture 1134, East Lansing, MI, USA, 22 May 2016; pp. 223–230. [Google Scholar]
- Lu, N.; Maruo, T.; Johkan, M.; Hohjo, M.; Tsukagoshi, S.; Ito, Y.; Ichimura, T.; Shinohara, Y. Effects of supplemental lighting with light-emitting diodes (LEDs) on tomato yield and quality of single-truss tomato plants grown at high planting density. Environ. Control Biol. 2012, 50, 63–74. [Google Scholar] [CrossRef] [Green Version]
- Tarakanov, I.; Yakovleva, O.; Konovalova, I.; Paliutina, G.; Anisimov, A. Light-emitting diodes: On the way to combinatorial lighting technologies for basic research and crop production. In Proceedings of the VII International Symposium on Light in Horticultural Systems 956, Wageningen, The Netherlands, 14 October 2012; pp. 171–178. [Google Scholar] [CrossRef]
- Pacheco, F.V.; Alvarenga, I.C.A.; Junior, P.M.R.; Pereira Pinto, J.E.B.; de Paula Avelar, R.; Alvarenga, A.A. Growth and production of secondary compounds in monkey-pepper (Piper aduncum L.) leaves cultivated under altered ambient light. Aust. J. Crop Sci. 2014, 8, 1510. [Google Scholar]
- Stutte, G.W. Light-emitting diodes for manipulating the phytochrome apparatus. HortScience 2009, 44, 231–234. [Google Scholar] [CrossRef]
- Murchie, E.; Pinto, M.; Horton, P. Agriculture and the new challenges for photosynthesis research. New Phytol. 2009, 181, 532–552. [Google Scholar] [CrossRef]
- Son, K.-H.; Oh, M.-M. Growth, photosynthetic and antioxidant parameters of two lettuce cultivars as affected by red, green, and blue light-emitting diodes. Hortic. Environ. Biotechnol. 2015, 56, 639–653. [Google Scholar] [CrossRef]
- Keefe, T. The Nature of Light; Community College of Rhode Island: Warwick, RI, USA, 2007. [Google Scholar]
- Naznin, M.T.; Lefsrud, M. An Overview of LED Lighting and Spectral Quality on Plant Photosynthesis. In Light Emitting Diodes for Agriculture; Springer: Berlin/Heidelberg, Germany, 2017; pp. 101–111. [Google Scholar]
- Zhang, Y.; Ji, J.; Song, S.; Su, W.; Liu, H. Growth, Nutritional Quality and Health-Promoting Compounds in Chinese Kale Grown under Different Ratios of Red: Blue LED Lights. Agronomy 2020, 10, 1248. [Google Scholar] [CrossRef]
- Sergejeva, D.; Alsina, I.; Duma, M.; Dubova, L.; Augspole, I.; Erdberga, I.; Berzina, K. Evaluation of different lighting sources on the growth and chemical composition of lettuce. Agron. Res. 2018, 16, 3. [Google Scholar]
- Camejo, D.; Frutos, A.; Mestre, T.C.; del Carmen Piñero, M.; Rivero, R.M.; Martínez, V. Artificial light impacts the physical and nutritional quality of lettuce plants. Hortic. Environ. Biotechnol. 2020, 61, 69–82. [Google Scholar] [CrossRef]
- Gupta, S.D.; Agarwal, A. Light-Emitting Diodes in Postharvest Quality Preservation and Microbiological Food Safety. In Light Emitting Diodes for Agriculture; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Braidot, E.; Petrussa, E.; Peresson, C.; Patui, S.; Bertolini, A.; Tubaro, F.; Wählby, U.; Coan, M.; Vianello, A.; Zancani, M. Low-intensity light cycles improve the quality of lamb’s lettuce (Valerianella olitoria [L.] Pollich) during storage at low temperature. Postharvest Biol. Technol. 2014, 90, 15–23. [Google Scholar] [CrossRef]
- Kader, A.A.; Rolle, R.S. The Role of Post-Harvest Management in Assuring the Quality and Safety of Horticultural Produce; Food & Agriculture Org.: Rome, Italy, 2004; Volume 152. [Google Scholar]
- Zhan, L.; Li, Y.; Hu, J.; Pang, L.; Fan, H. Browning inhibition and quality preservation of fresh-cut romaine lettuce exposed to high intensity light. Innov. Food Sci. Emerg. Technol. 2012, 14, 70–76. [Google Scholar] [CrossRef]
- Jin, P.; Yao, D.; Xu, F.; Wang, H.; Zheng, Y. Effect of light on quality and bioactive compounds in postharvest broccoli florets. Food Chem. 2015, 172, 705–709. [Google Scholar] [CrossRef] [PubMed]
- Noodén, L.D.; Schneider, M.J. Light control of senescence. In Plant Cell Death Processes; Elsevier: Amsterdam, The Netherlands, 2004; pp. 375–383. [Google Scholar]
- Li, Y.; Dvořák, M.; Nesterenko, P.N.; Nuchtavorn, N.; Macka, M. High power deep UV-LEDs for analytical optical instrumentation. Sens. Actuators B Chem. 2018, 255, 1238–1243. [Google Scholar] [CrossRef]
- Glowacz, M.; Mogren, L.M.; Reade, J.P.; Cobb, A.H.; Monaghan, J.M. High-but not low-intensity light leads to oxidative stress and quality loss of cold-stored baby leaf spinach. J. Sci. Food Agric. 2015, 95, 1821–1829. [Google Scholar] [CrossRef] [Green Version]
- Hasperué, J.H.; Rodoni, L.M.; Guardianelli, L.M.; Chaves, A.R.; Martínez, G.A. Use of LED light for Brussels sprouts postharvest conservation. Sci. Hortic. 2016, 213, 281–286. [Google Scholar] [CrossRef]
- Muneer, S.; Kim, E.J.; Park, J.S.; Lee, J.H. Influence of green, red and blue light emitting diodes on multiprotein complex proteins and photosynthetic activity under different light intensities in lettuce leaves (Lactuca sativa L.). Int. J. Mol. Sci. 2014, 15, 4657–4670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kozuki, A.; Ishida, Y.; Kakibuchi, K.; Mishima, T.; Sakurai, N.; Murata, Y.; Nakano, R.; Ushijima, K.; Kubo, Y. Effect of postharvest short-term radiation of near infrared light on transpiration of lettuce leaf. Postharvest Biol. Technol. 2015, 108, 78–85. [Google Scholar] [CrossRef]
- Rahman, M.M.; Vasiliev, M.; Alameh, K. LED Illumination spectrum manipulation for increasing the yield of sweet basil (Ocimum basilicum L.). Plants 2021, 10, 344. [Google Scholar] [CrossRef] [PubMed]
- Bian, S.; Li, X.; Mainali, H.; Chen, L.; Dhaubhadel, S. Genome-wide analysis of DWD proteins in soybean (Glycine max): Significance of Gm08DWD and GmMYB176 interaction in isoflavonoid biosynthesis. PLoS ONE 2017, 12, e0178947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yano, Y.; Nakamura, T.; Maruyama, A. Consumer perception and understanding of vegetables produced at plant factories with artificial lighting. In LED Lighting for Urban Agriculture; Springer: Berlin/Heidelberg, Germany, 2016; pp. 347–363. [Google Scholar]
- Cary, M.; Stutte, G.W. Sole-Source Lighting for Controlled-Environment Agriculture. In Lighting up Profits Understanding Greenhouse Lighting, 2nd ed.; Runkle, E., Lopez, R., Eds.; Meister Media Worldwide: Cordova, TN, USA, 2015. [Google Scholar]
- Schubert, E.F. Light-Emitting Diodes; Mont, F.W., Kim, J.K., Eds.; Rensselaer Polytechnic Institute: Troy, NY, USA, 2018. [Google Scholar]
- Park, Y.; Runkle, E.S. Far-red radiation promotes growth of seedlings by increasing leaf expansion and whole-plant net assimilation. Environ. Exp. Bot. 2017, 136, 41–49. [Google Scholar] [CrossRef] [Green Version]
- Zhen, S.; van Iersel, M.W. Far-red light is needed for efficient photochemistry and photosynthesis. J. Plant Physiol. 2017, 209, 115–122. [Google Scholar] [CrossRef] [Green Version]
- Craver, J.K.; Miller, C.T.; Williams, K.A.; Bello, N.M. Ultraviolet radiation affects intumescence development in ornamental sweetpotato (Ipomoea batatas). HortScience 2014, 49, 1277–1283. [Google Scholar] [CrossRef]
- Snowden, M.C.; Cope, K.R.; Bugbee, B. Sensitivity of seven diverse species to blue and green light: Interactions with photon flux. PLoS ONE 2016, 11, e0163121. [Google Scholar]
- Ouzounis, T.; Rosenqvist, E.; Ottosen, C.-O. Spectral effects of artificial light on plant physiology and secondary metabolism: A review. HortScience 2015, 50, 1128–1135. [Google Scholar] [CrossRef] [Green Version]
- Hayashi, E. Current status of commercial plant factories with LED lighting market in Asia, Europe, and other regions. In LED Lighting for Urban Agriculture; Springer: Berlin/Heidelberg, Germany, 2016; pp. 295–308. [Google Scholar]
- Jishi, T.; Kimura, K.; Matsuda, R.; Fujiwara, K. Effects of temporally shifted irradiation of blue and red LED light on cos lettuce growth and morphology. Sci. Hortic. 2016, 198, 227–232. [Google Scholar] [CrossRef]
- Cope, K.R.; Bugbee, B. Spectral effects of three types of white light-emitting diodes on plant growth and development: Absolute versus relative amounts of blue light. HortScience 2013, 48, 504–509. [Google Scholar] [CrossRef]
- Gómez, C.; Mitchell, C.A. Growth responses of tomato seedlings to different spectra of supplemental lighting. HortScience 2015, 50, 112–118. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, C. Plant lighting in controlled environments for space and earth applications. In Proceedings of the VII International Symposium on Light in Horticultural Systems 956, Wageningen, The Netherlands, 14 October 2012; pp. 23–36. [Google Scholar]
- Gislerød, H.R.; Mortensen, L.; Torre, S.; Pettersen, H.; Dueck, T.; Sand, A. Light and energy saving in modern greenhouse production. In Proceedings of the VII International Symposium on Light in Horticultural Systems 956, Wageningen, The Netherlands, 14 October 2012; pp. 85–97. [Google Scholar]
- Voss, J. Market Special: Greenhouse Farming in Germany; The Ministry of Economics Affairs, Agriculture and Innovation, NL, EVD International: Den Haag, The Netherlands, 2011. [Google Scholar]





| Abbreviation | Definition | Units |
|---|---|---|
| PAR | Photosynthetic Active Radiation | μmol m−2 s−1 |
| PPF | Photosynthetic Photon Flux | μmol s−1 |
| PPFD | Photosynthetic Photon Flux Density | μmol m−2 s−1 |
| FPAR | Fraction of Photosynthetically Active Radiation | NA |
| IPPC | Illuminated Leaves Per Unit Power Consumption | µmol s−1 W−1 |
| Power Density | Power Transferred Per Unit Area | W/m2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahman, M.M.; Field, D.L.; Ahmed, S.M.; Hasan, M.T.; Basher, M.K.; Alameh, K. LED Illumination for High-Quality High-Yield Crop Growth in Protected Cropping Environments. Plants 2021, 10, 2470. https://doi.org/10.3390/plants10112470
Rahman MM, Field DL, Ahmed SM, Hasan MT, Basher MK, Alameh K. LED Illumination for High-Quality High-Yield Crop Growth in Protected Cropping Environments. Plants. 2021; 10(11):2470. https://doi.org/10.3390/plants10112470
Chicago/Turabian StyleRahman, Md Momtazur, David Luke Field, Soyed Mohiuddin Ahmed, Md Tanvir Hasan, Mohammad Khairul Basher, and Kamal Alameh. 2021. "LED Illumination for High-Quality High-Yield Crop Growth in Protected Cropping Environments" Plants 10, no. 11: 2470. https://doi.org/10.3390/plants10112470
APA StyleRahman, M. M., Field, D. L., Ahmed, S. M., Hasan, M. T., Basher, M. K., & Alameh, K. (2021). LED Illumination for High-Quality High-Yield Crop Growth in Protected Cropping Environments. Plants, 10(11), 2470. https://doi.org/10.3390/plants10112470






