Chemotypes and Their Stability in Mentha longifolia (L.) L.—A Comprehensive Study of Five Accessions
Abstract
:1. Introduction
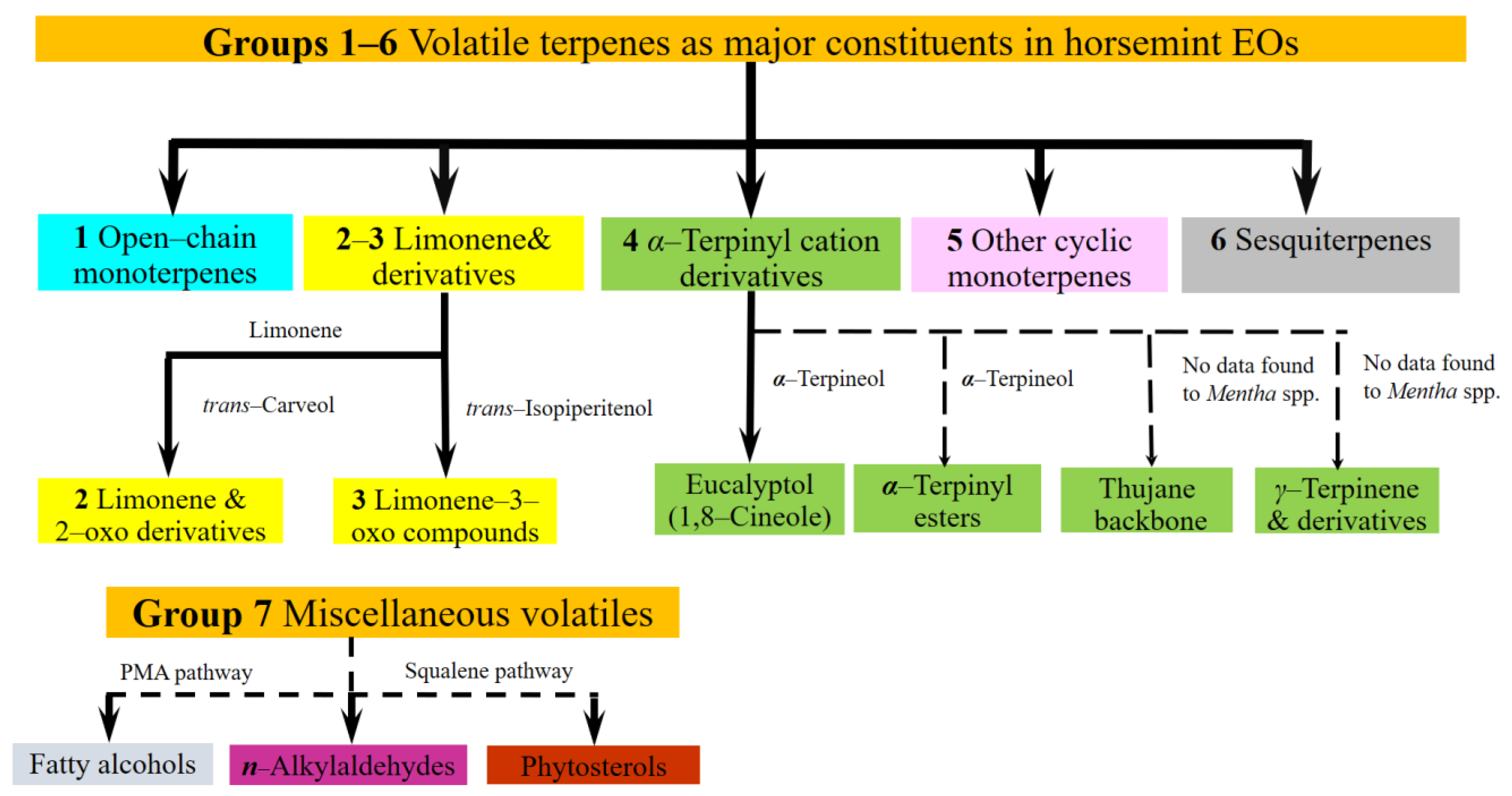
- −
- −
- −
- −
- Group 4 Derivatives of α-terpinyl ion 1,8-cineole [3] accompanies both open-chains and limonene 3-oxo derivatives in concentration 7–44%. Other terpinyl daughter compounds are seldom: α-terpinyl acetate [14,20,21], terpineoles [20], derivatives of γ-terpinene [22,23,24,25,26], and thujane [2,20,21,22,23,24,25,26]. In a single case, borneol [3] was found in high concentration, 29%
- −
- −
- −
2. Results and Discussion
2.1. Essential Oil Yield Depending on Accession and Year
2.2. Essential Oil Composition and Its Variability in the Five Accessions
2.3. Chemotaxonomic Evaluation of the Studied Accessions
3. Conclusions
4. Materials and Methods
4.1. Plant Material
4.2. Experimental Design, Propagation and Maintenance of Plots
4.3. Sampling and Preparation to Chemical Analyses
4.4. Method of EO Extraction (Distillation), Measurement of Essential Oil Yield
4.5. Assessment of Qualitative and Quantitative Composition of Essential Oils
4.6. Statistical Evaluation of Essential Oil Composition and Yield
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tucker, A.O.; Naczi, R.F.C. Mentha: An overview of its classification and relationships. In Mint: The Genus Mentha—Medicinal and Aromatic Plants—Industrial Profiles; Lawrence, B.M., Ed.; CRC Press: Boca Raton, FL, USA, 2007; pp. 21–26. [Google Scholar]
- Vining, K.J.; Zhang, Q.; Tucker, A.O.; Smith, C.; Davis, T.M. Mentha longifolia (L.) L.: A model species for mint genetic research. HortScience 2005, 40, 1225–1229. [Google Scholar] [CrossRef]
- Moshrefi Araghi, A.; Nemati, H.; Azizi, M.; Moshtaghi, N.; Shoora, M.; Hadian, J. Assessment of phytochemical and agro-morphological variability among different wild accessions of Mentha longifolia L. cultivated in field condition. Ind. Crops Prod. 2019, 140, 111698. [Google Scholar] [CrossRef]
- Başer, K.H.C.; Kürkçüoğlu, M.; Tarimcilar, G.; Kaynak, G. Essential oils of Mentha species from Northern Turkey. J. Essent. Oil Res. 1999, 11, 579–588. [Google Scholar] [CrossRef]
- Petrulaitis, L.; Gudžinskas, Z. What are we conserving? A case study of Mentha longifolia and allied species from Lithuania. Botanica 2018, 24, 3–14. [Google Scholar] [CrossRef] [Green Version]
- Kew Royal Botanical Garden The Plants of the World/Mentha longifolia L. Available online: http://www.plantsoftheworldonline.org/taxon/urn:lsid:ipni.org:names:450735-1 (accessed on 22 September 2021).
- Gobert, V.; Moja, S.; Colson, M.; Taberlet, P. Hybridization in the section Mentha (Lamiaceae) inferred from AFLP markers. Am. J. Bot. 2002, 89, 2017–2023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patonay, K.; Németh-Zámboriné, É. Horsemint as a potential raw material for the food industry: Survey on the chemistry of a less studied mint species. Phytochem. Rev. 2020, 20, 631–652. [Google Scholar] [CrossRef]
- Lawrence, B.M. Oil composition of other Mentha species and hybrids. In Mint: The Genus Mentha—Medicinal and Aromatic Plants—Industrial Profiles; Lawrence, B.M., Ed.; CRC Press: Boca Raton, FL, USA, 2007; pp. 327–347. [Google Scholar]
- Németh-Zámboriné, É. Natural variability of essential oil components. In Handbook of Essential Oils, 2nd ed.; Başer, K.H.C., Buchbauer, G., Eds.; CRC Press-Taylor and Francis Group LLC: Boca Raton, FL, USA, 2016; p. 95. [Google Scholar]
- Llorens-Molina, J.A.; Vacas, S.; Castell, V.; Verdeguer, M. Seasonal variations of essential oils from five accessions of Mentha longifolia (L.) L. with selected chemical profiles. J. Essent. Oil Res. 2020, 32, 419–428. [Google Scholar] [CrossRef]
- Kowalczyk, A.; Przychodna, M.; Sopata, S.; Bodalska, A.; Fecka, I. Thymol and thyme essential oil—New insights into selected therapeutic applications. Molecules 2020, 25, 4125. [Google Scholar] [CrossRef]
- Németh, É.Z.; Thi Nguyen, H. Thujone, a widely debated volatile compound: What do we know about it? Phytochem. Rev. 2020, 19, 405–423. [Google Scholar] [CrossRef]
- Başer, K.H.C.; Kürkçüoğlu, M.; Demirci, B.; Özek, T.; Tarimcilar, G. Essential oils of Mentha species from Marmara region of Turkey. J. Essent. Oil. Res. 2012, 24, 265–272. [Google Scholar] [CrossRef]
- Mimica-Dukić, N.; Bozin, B. Mentha L. species (Lamiaceae) as promising sources of bioactive secondary metabolites. Curr. Pharm. Des. 2008, 14, 3141–3150. [Google Scholar] [CrossRef]
- Al-Okbi, Y.S.; Fadel, H.H.M.; Mohamed, D.A. Phytochemical constituents, antioxidant and anticancer activity of Mentha citrata and Mentha longifolia. Res. J. Pharm. Biol. Chem. Sci. 2015, 6, 739–751. [Google Scholar]
- Venskutonis, P.R. A chemotype of Mentha longifolia L. from Lithuania rich in piperitenone oxide. J. Essent. Oil Res. 1996, 8, 91–95. [Google Scholar] [CrossRef]
- Younis, M.H.Y.; Beshir, S.M. Carvone-rich essential oils from Mentha longifolia (L.) Huds. ssp. schimperi Briq. and Mentha spicata L. grown in Sudan. J. Essent. Oil Res. 2011, 16, 539–541. [Google Scholar] [CrossRef]
- Sharopov, F.S.; Sulaimanova, V.A.; Setzer, W.N. Essential oil composition of Mentha longifolia from wild populations growing in Tajikistan. J. Med. Act. Plants 2012, 1, 76–84. [Google Scholar] [CrossRef]
- Llorens-Molina, J.A.; García-Rellán, D.; Vacas, S.; Bonet, A. Individual sampling approach to study the chemodiversity of volatile and semivolatile compounds of Mentha longifolia L. growing wild in Jiloca basin, Spain. Int. J. Biosci. 2015, 7, 166–177. [Google Scholar] [CrossRef]
- Kapp, K. Polyphenolic and Essential Oil Composition of Mentha and Their Antimicrobial Effect. Ph.D. Thesis, University of Helsinki, Helsinki, Finland, 2015. Available online: https://helda.helsinki.fi/bitstream/handle/10138/158806/polyphen.pdf?sequence=1 (accessed on 22 September 2021).
- Ćavar Zeljković, S.; Šišková, J.; Komzáková, K.; De Diego, N.; Kaffková, K.; Tarkowski, P. Phenolic compounds and biological activity of selected Mentha species. Plants 2021, 10, 550. [Google Scholar] [CrossRef]
- Mimica-Dukić, N.; Kite, G.; Gasic, O.; Stajner, D.; Pavkov, R.; Jancic, R.; Fellows, L. Comparative study of volatile constituents and an timicrobial activity of Mentha species. Acta Hortic. 1993, 344, 110–115. [Google Scholar] [CrossRef]
- Akşit, H.; Demirtas, I.; Telci, I.; Tarımcılar, G. Chemical diversity in essential oil composition of Mentha longifolia (L.) Hudson subsp. typhoides (Briq.) Harley var. typhoides from Turkey. J. Essent. Oil Res. 2013, 25, 430–437. [Google Scholar] [CrossRef]
- Hassanzadeh, M.K.; Emami, S.A.; Asili, J. Review of the essential oil composition of Iranian Lamiaceae. J. Essent. Oil Res. 2011, 23, 35–74. [Google Scholar] [CrossRef]
- Golparvar, R.; Hadipanah, A.; Gheisari, M.M.; Salehi, S.; Khaliliazar, R.; Ghasemi, O. Comparative analysis of chemical composition of Mentha longifolia (L.) Huds. J. Herbal. Drugs 2017, 7, 235–241. [Google Scholar]
- Karasawa, D.; Erdenechimeg, A.; Okamoto, Y.; Tateba, H.; Shimizu, S. A study on Mongolian Mints. A new chemotype from Mentha asiatica Borriss and constituents of M. arvensis L. and M. piperita L. J. Essent. Oil Res. 1995, 7, 255–260. [Google Scholar] [CrossRef]
- Ali, H.M.; Elgat, W.A.A.A.; El-Hefny, M.; Salem, M.Z.M.; Taha, A.S.; Al Farraj, D.A.; Elshikh, M.S.; Hatamleh, A.A.; Abdel-Salam, A. New approach for using of Mentha longifolia L. and Citrus reticulata L. essential oils as wood-biofungicides: GC-MS, SEM, and MNDO quantum chemical studies. Materials 2021, 14, 1361. [Google Scholar] [CrossRef] [PubMed]
- Güllüce, M.; Sahin, F.; Sokmen, M.; Ozer, H.; Daferera, D.; Sokmen, A.; Polissiou, M.; Adiguzel, A.; Ozkan, H. Antimicrobial and antioxidant properties of essential oils and methanolic extract from Mentha longifolia ssp. longifolia. Food Chem. 2007, 103, 1449–1456. [Google Scholar] [CrossRef]
- Hajlaoui, H.; Trabelsi, N.; Noumi, E.; Snoussi, M.; Fallah, H.; Ksouri, R.; Bakhrouf, A. Biological activities of the essential oils and methanol extract of two cultivated mint species (Mentha longifolia and Mentha pulegium) used in the Tunisian folkloric medicine. World J. Microbiol. Biotechnol. 2009, 25, 2227–2238. [Google Scholar] [CrossRef]
- Ghoulami, S.; Il-Idrissi, A.; Fkih-Tetouani, S. Phytochemical study of Mentha longifolia of Morocco. Fitoterapia 2001, 72, 596–598. [Google Scholar] [CrossRef]
- Murad, H.A.S.; Abdallah, H.M.; Ali, S.S. Mentha longifolia protects against acetic acid induced colitis in rats. J. Ethnopharm. 2016, 190, 354–361. [Google Scholar] [CrossRef]
- Tunçtürk, M.; Tunçtürk, R.; Sekeroglu, N.; Ertus, M.M.; Ozgokce, F. Lead concentrations of herbs used in Van Herby cheese. Nat. Prod. Commun. 2011, 6, 1473–1474. [Google Scholar]
- Patonay, K.; Korózs, M.; Murányi, Z.; Pénzesné Kónya, E. Polyphenols in northern Hungarian Mentha longifolia (L.) L. treated with ultrasonic extraction for potential oenological uses. Turk. J. Agric. For. 2017, 41, 208–217. [Google Scholar] [CrossRef]
- Patonay, K.; Szalontai, H.; Csugány, J.; Szabó-Hudák, O.; Pénzesné Kónya, E.; Zámboriné Németh, É. Comparison of extraction methods for the assessment of total polyphenol content and in vitro antioxidant capacity of horsemint (Mentha longifolia (L.) L.). Appl. Res. Med. Aromat. Plants 2019, 15, 100220. [Google Scholar] [CrossRef]
- Patonay, K.; Szabó-Hudák, O.; Szalontai, H.; Jánószky, M.; Kónya, E.; Németh, É. Extraction and identification of major polyphenol constituents of Northern Hungarian horsemint (Mentha longifolia L. (L.)). Acta Biol. Plant. Agriensis 2020, 8, 53–68. [Google Scholar] [CrossRef]
- De Sousa Barros, A.; de Morais, S.M.; Travassos, F.P.A.; Gusmão Pinto Vieira, Í.; Aragão Craveiro, A.; Oliveira dos Santos Fontenelle, R.; Silva Alencar de Menezes, J.E.; Walber Ferreira da Silva, F.; Araújo de Sousa, H. Chemical composition and functional properties of essential oils from Mentha species. Ind. Crops Prod. 2015, 76, 557–564. [Google Scholar] [CrossRef]
- Fleisher, A.; Fleisher, Z. The essential oils from Mentha longifolia growing in Sinai and Israel. J. Essent. Oil. Res. 1991, 3, 57–58. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corp.: Carol Stream, IL, USA, 2017. [Google Scholar]
- Stein, S.; Mirokhin, Y.; Tchekhovskoi, D.; Maillard, G.; Mikaia, A.; Neta, P.; Sparkman, D.; White, E.; Yang, X.; Zaikin, V.; et al. Agilent Techologies NIST Mass Spectral Library Revision 2005. (The NIST Mass Spectrometry Data Center (2011) Standard Reference Database NIST 2.0). The NIST Mass spectral search program for the library was distributed by the The Standard Reference Data Program of The National Institute of Standards and Technology of the United States. 19 May 2011. [Google Scholar]
- Gosztola, B. Morphological and Chemical Diversity of Different Chamomile (Matricaria recutita L.) Populations of the Great Hungarian Plain. (Alföldi Vadon Termő Orvosi Kamilla (Matricaria recutita L.) Populációk Diverzitásának Értékelése Morfológiai és Beltartalmi Szempontból). Ph.D. Thesis, Corvinus University, Budapest, Hungary, 2012; p. 16. Available online: http://phd.lib.uni-corvinus.hu/600/2/Gosztola_Beata_ten.pdf (accessed on 22 September 2021).
- Lawrence, B.M. The composition of commercially important mints. In Mint: The Genus Mentha—Medicinal and Aromatic Plants—Industrial Profiles; Lawrence, B.M., Ed.; CRC Press: Boca Raton, FL, USA, 2007; pp. 217–325. [Google Scholar]
- Salehi, B.; Stojanović-Radić, Z.; Matejić, J.; Sharopov, F.; Antolak, H.; Kręgiel, D.; Sen, S.; Sharifi-Rad, M.; Acharya, K.; Sharifi-Rad, R.; et al. Plants of Genus Mentha: From farm to food factory. Plants 2018, 7, 70. [Google Scholar] [CrossRef] [Green Version]
- Zeinali, H.; Arzani, A.; Razmjoo, K.; Rezaee, M.B. Evaluation of oil compositions of Iranian mints (Mentha spp.). J. Essent. Oil Res. 2005, 17, 156–159. [Google Scholar] [CrossRef]
- Regulation (EC) No 1334/2008 of the European Parliament and of the Council of 16 December 2008 on flavourings and certain food ingredients with flavouring properties for use in and on foods and amending Council Regulation (EEC) No 1601/91, Regulations (EC) No 2232/96 and (EC) No 110/2008 and Directive 2000/13/EC. Off. J. Eur. Union L 2008, 354, 34–50.
- Verghese, J. Dihydrocarvone. Perfum. Flavorist 1980, 5, 23–26. Available online: https://www.perfumerflavorist.com/fragrance/application/multiuse/Dihydrocarvone-376780731.html (accessed on 22 September 2021).
- Bertoli, A.; Leonardi, M.; Krzyzanowska, J.; Ołeszek, W.; Pistelli, L. Mentha longifolia in vitro cultures as safe source of flavouring ingredients. Acta Biochim. Pol. 2011, 58, 581–587. [Google Scholar] [CrossRef]
- Nieto, G. A review on applications and uses of Thymus in the food industry. Plants 2020, 9, 961. [Google Scholar] [CrossRef]
- Szűcs, P.; Táborská, J.; Baranyi, G.; Pénzes-Kónya, E. Short-term changes in the bryophyte flora in the botanical garden of Eszterházy Károly University (Eger, NE Hungary). Acta Biol. Plant. Agriensis 2017, 5, 52–60. [Google Scholar] [CrossRef]
- Van den Dool, H.; Kratz, P. A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J. Chrom. A 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Bicchi, C.; Chaintreau, A.; Joulain, D. Technical editorial: Identification of flavour and fragrance constituents. Flavour. Fragr. J. 2018, 33, 201–202. [Google Scholar] [CrossRef] [Green Version]



| Accession | Year | Habitat EGR | Habitat SOR | ||
|---|---|---|---|---|---|
| YIELD Mean (N = 3) | S.D. 1 | YIELD Mean (N = 2) | S.D. | ||
| KBÁ | 2019 | 1.84 | 0.10 | 2.06 | 0.08 |
| HV1 | 1.09 | 0.05 | 1.33 | 0.08 | |
| HV2 | 1.99 | 0.06 | 2.00 | 0.09 | |
| EGR3 | 1.25 | 0.05 | 1.21 | 0.08 | |
| SZD | 1.32 | 0.06 | 1.17 | 0.00 | |
| KBÁ | 2020 | 1.78 | 0.03 | 2.08 | 0.13 |
| HV1 | 1.10 | 0.17 | 0.94 | 0.06 | |
| HV2 | 1.75 | 0.09 | 1.12 | 0.05 | |
| EGR3 | 1.08 | 0.05 | 0.87 | 0.00 | |
| SZD | 1.25 | 0.03 | 1.04 | 0.05 | |
| Name | tR, min | LRI | ID | Habitat: EGR | Habitat: SOR | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Year: 2019 | Year: 2020 | Year: 2019 | Year: 2020 | ||||||||||||||||||||
| KBÁ | HV1 | HV2 | EGR3 | SZD | KBÁ | HV1 | HV2 | EGR3 | SZD | KBÁ | HV1 | HV2 | EGR3 | SZD | KBÁ | HV1 | HV2 | EGR3 | SZD | ||||
| α-Thujene | 5.31 | 928 | 1, 2 | 0 | 0 | 0.05 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | No data available No data available | 0.1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| α-Pinene | 5.56 | 938 | 1, 2, 3 | 0.26 | 0.16 | 0.34 | 0.16 | 0.20 | 0.3 | 0.57 | 0.46 | 0.40 | 0.36 | 0.23 | 0.59 | 0.49 | 0.29 | 0.34 | 0.66 | 0.76 | 0.31 | 0.26 | |
| Camphene | 5.95 | 952 | 1, 2, 3 | 0 | 0.02 | 0.10 | 0 | 0 | 0 | 0.05 | 0.12 | 0 | 0.01 | 0 | 0.11 | 0.01 | 0.02 | 0 | 0.04 | 0.06 | 0 | 0 | |
| Sabinene | 6.52 | 976 | 1, 2, 3 | 0.30 | 0.99 | 0.35 | 0.30 | 0.20 | 0.30 | 1.3 | 0.58 | 0.60 | 0.30 | 0.28 | 0.57 | 0.61 | 0.26 | 0.33 | 1.32 | 1.44 | 0.52 | 0.27 | |
| β-Pinene | 6.64 | 980 | 1, 2, 3 | 0.47 | 1.07 | 0.55 | 0.40 | 0.40 | 0.50 | 1.36 | 0.74 | 0.70 | 0.50 | 0.44 | 0.9 | 0.86 | 0.49 | 0.55 | 1.49 | 1.49 | 0.63 | 0.43 | |
| β-Myrcene | 6.99 | 994 | 1, 2, 3 | 0.31 | 1.15 | 0.40 | 0.35 | 0.30 | 0.30 | 1.48 | 0.62 | 0.60 | 0.32 | 0.29 | 0.55 | 0.59 | 0.27 | 0.32 | 1.45 | 1.44 | 0.52 | 0.27 | |
| 3-Octanol | 7.14 | 1000 | 1, 2, 3 | 0.23 | 0.09 | 0.34 | 0.06 | 0.10 | 0.30 | 0.17 | 0.29 | 0.10 | 0.10 | 0.20 | 0.25 | 0.07 | 0 | 0.21 | 0.11 | 0.06 | 0 | 0.06 | |
| α-Phellandrene | 7.43 | 1008 | 1, 2 | 0 | 0.10 | 0 | 0 | 0 | 0 | 0.15 | 0.06 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.13 | 0.13 | 0 | 0 | |
| α-Terpinene | 7.79 | 1017 | 1, 2, 3 | 0 | 0.88 | 0.09 | 0 | 0 | 0 | 1.21 | 0.39 | 0 | 0 | 0 | 0.1 | 0 | 0 | 0 | 0.85 | 2.34 | 0 | 0 | |
| para-Cymene | 8.09 | 1025 | 1, 2, 3 | 0 | 7.24 | 0.38 | 0 | 0 | 0.10 | 8.01 | 1.66 | 0 | 0 | 0 | 0.62 | 0 | 0 | 0 | 7.67 | 9.36 | 0 | 0 | |
| Limonene | 8.19 | 1028 | 1, 2, 3 | 0.41 | 0.5 | 2.73 | 0.62 | 0.40 | 0.60 | 0.59 | 2.66 | 1.20 | 0.64 | 0.48 | 3.3 | 1.32 | 0.35 | 0.43 | 0.5 | 0.91 | 1.76 | 0.36 | |
| 1,8-cineole | 8.38 | 1033 | 1, 2, 3 | 2.81 | 17.50 | 3.06 | 3.70 | 1.50 | 2.60 | 14.87 | 4.38 | 5.00 | 1.54 | 2.68 | 4.64 | 5.16 | 1.57 | 2.60 | 17.10 | 14.90 | 4.94 | 2.17 | |
| (Z)-Ocimene | 8.5 | 1036 | 1, 2 | 0.50 | 0.25 | 0.38 | 0.64 | 0.30 | 0.40 | 0.22 | 0.32 | 1.00 | 0.40 | 0.32 | 0.25 | 0.63 | 0.17 | 0.46 | 0.24 | 0.37 | 0.87 | 0.43 | |
| (E)-Ocimene | 8.85 | 1046 | 1, 2 | 0.06 | 0.03 | 0.03 | 0.06 | 0 | 0.10 | 0.04 | 0.03 | 0.10 | 0.04 | 0.04 | 0 | 0.06 | 0.02 | 0.05 | 0.04 | 0.05 | 0.06 | 0.04 | |
| γ-Terpinene | 9.2 | 1055 | 1, 2, 3 | 0 | 4.15 | 0.49 | 0 | 0 | 0 | 4.74 | 1.75 | 0.40 | 0 | 0 | 0.44 | 0 | 0 | 0 | 3.57 | 7.22 | 0 | 0 | |
| Terpinene-4-acetate | 9.59 | 1065 | 1, 2 | 0.01 | 0.69 | 0 | 0.01 | 0 | 0 | 0.83 | 0.20 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.77 | 0.59 | 0.03 | 0 | |
| Linalool | 10.76 | 1097 | 1, 2, 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0.19 | 0.39 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.17 | 0.71 | 0 | 0 | |
| 3-Octyl acetate | 11.58 | 1120 | 1, 2 | 0 | 0.3 | 0 | 0 | 0 | 0 | 0.24 | 0.06 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.39 | 0.18 | 0 | 0 | |
| Menthone | 12.84 | 1147 | 1, 2, 3 | 53.90 | 0.42 | 0.32 | 0.68 | 64.00 | 49.00 | 0.90 | 0 | 6.10 | 55.6 | 62.7 | 0.08 | 0.09 | 66.3 | 55.78 | 0.41 | 0 | 0 | 46.67 | |
| Isomenthone | 13.26 | 1157 | 1, 2, 3 | 6.34 | 0 | 0 | 0.41 | 13.00 | 5.70 | 0.20 | 0 | 1.00 | 15.14 | 6.16 | 0 | 0 | 11.70 | 6.74 | 0.04 | 0 | 0 | 9.57 | |
| Borneol | 13.43 | 1162 | 1, 2, 3 | 0 | 0 | 0.64 | 0 | 0.10 | 0.20 | 0 | 0.73 | 0 | 0.22 | 0 | 0.42 | 0 | 0.22 | 0 | 0 | 0 | 0 | 0 | |
| cis-dehydro-α-terpineole | 13.49 | 1164 | 1, 2 | 0 | 0.60 | 0 | 0 | 0 | 0 | 0.66 | 0 | 0.20 | 0 | 0 | 0 | 0 | 0.12 | 0 | 0.63 | 0 | 0 | 0 | |
| Menthol | 13.68 | 1168 | 1, 2, 3 | 4.41 | 0 | 0 | 0 | 0.20 | 3.70 | 0 | 0 | 0 | 0.30 | 1.83 | 0 | 0 | 0.09 | 3.35 | 0 | 0 | 0 | 0.20 | |
| trans-Isopulegone | 13.81 | 1171 | 1, 2 | 3.23 | 0 | 0 | 0 | 0.50 | 4.50 | 0 | 0 | 0 | 0.52 | 3.90 | 0 | 0 | 0.56 | 2.88 | 0 | 0 | 0 | 0.31 | |
| Terpinene-4-ol | 13.96 | 1174 | 1, 2, 3 | 0 | 0.32 | 0 | 0 | 0 | 0 | 0.41 | 0.40 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.27 | 1.11 | 0.21 | 0 | |
| Isomenthol | 14.13 | 1178 | 1, 2, 3 | 0.08 | 1.70 | 0 | 0 | 0 | 0.10 | 0 | 0 | 0 | 0.06 | 0 | 0 | 0 | 0.06 | 0.07 | 0 | 0 | 0 | 0.10 | |
| α-Terpineol | 14.55 | 1189 | 1, 2, 3 | 0.55 | 0 | 0.53 | 0.79 | 0 | 0.50 | 1.74 | 0.08 | 0.90 | 0.39 | 0.58 | 0.58 | 0.83 | 0.33 | 0.48 | 1.64 | 1.13 | 0.74 | 0.41 | |
| cis-Dihydrocarvone | 14.74 | 1194 | 1, 2 | 0.02 | 0.22 | 57.06 | 0.18 | 0 | 0.40 | 1.99 | 47.57 | 0 | 0.07 | 0.04 | 54.5 | 0 | 0.47 | 0 | 0.31 | 8.45 | 0.05 | 0.18 | |
| trans-Dihydrocarvone | 15.05 | 1201 | 1, 2, 3 | 0 | 0.07 | 11.66 | 0 | 0 | 0.10 | 0.37 | 9.93 | 0 | 0 | 0 | 12.3 | 0 | 0.08 | 0 | 0.04 | 1.43 | 0 | 0 | |
| Octanol acetate | 15.22 | 1205 | 1, 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.08 | 0 | 0 | |
| 1,6-Dihydrocarveol | 15.49 | 1211 | 1, 2 | 0 | 0 | 0.17 | 0 | 0 | 0 | 0 | 0.16 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| trans-Carveol | 15.67 | 1215 | 1, 2, 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.05 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Carvenone | 15.99 | 1223 | 1, 2 | 0 | 0 | 0 | 0.53 | 0 | 0 | 0 | 0 | 0.50 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.44 | 0 | |
| cis-Dihydrocarveol | 16.03 | 1224 | 1, 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.28 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Citronellol | 16.12 | 1225 | 1, 2, 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0.17 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.12 | 0.08 | 0 | 0 | |
| cis-3-Hexenyl isovalerate | 16.24 | 1229 | 1, 2 | 0 | 0 | 0 | 0 | 0 | 0.10 | 0.20 | 0.16 | 0.1 | 0.15 | 0 | 0 | 0 | 0 | 0.12 | 0.25 | 0.28 | 0 | 0.15 | |
| Pulegone | 16.38 | 1232 | 1, 2, 3 | 8.45 | 0.17 | 0 | 0 | 0.10 | 15 | 0.76 | 0 | 0 | 0.53 | 6.3 | 0 | 0 | 0.04 | 6.59 | 0 | 0 | 0 | 0.13 | |
| Carvone | 16.71 | 1241 | 1, 2, 3 | 0 | 0 | 0.33 | 0 | 0 | 0 | 0 | 0.36 | 0 | 0 | 0 | 0.22 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Piperitone | 17.06 | 1248 | 1, 2, 3 | 0.08 | 0 | 0.25 | 0 | 0.70 | 0.10 | 0.05 | 0.28 | 0 | 0 | 0 | 0.23 | 0 | 0.46 | 0.13 | 0 | 0.42 | 0 | 0 | |
| cis-Piperitone epoxide | 17.08 | 1249 | 1, 2 | 0 | 0 | 0 | 53.50 | 0 | 0 | 0.13 | 1.80 | 44.00 | 2.82 | 0 | 0 | 55.34 | 0 | 0 | 0.14 | 0.09 | 52.10 | 12.20 | |
| Citronellyl formate | 17.77 | 1265 | 1, 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Geranial; Citral A | 17.83 | 1268 | 1, 2, 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0.08 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Neomenthyl acetate | 17.84 | 1267 | 1, 2 | 0.24 | 0 | 0 | 0.09 | 0.10 | 0.20 | 0 | 0 | 0.10 | 0.08 | 0.14 | 0 | 0 | 0.11 | 0.21 | 0 | 0 | 0.11 | 0.14 | |
| Dihydroedulan I. | 18.37 | 1279 | 1, 2 | 0.44 | 0.44 | 0.44 | 0.63 | 0.40 | 0.20 | 0.31 | 0.51 | 0.50 | 0.42 | 0.19 | 0.36 | 0.30 | 0.23 | 0.22 | 0.36 | 0.59 | 0.38 | 0.47 | |
| Menthyl acetate | 18.71 | 1281 | 1, 2 | 1.18 | 0 | 0 | 0 | 0 | 0.90 | 0 | 0 | 0 | 0 | 0.5 | 0 | 0 | 0 | 0.59 | 0 | 0 | 0 | 0 | |
| Thymol | 18.81 | 1290 | 1, 2, 3 | 0 | 13.9 | 1.34 | 0.60 | 0 | 0.30 | 13.75 | 4.30 | 1.10 | 0.09 | 0 | 1.39 | 0.65 | 0 | 0.09 | 13.4 | 19.8 | 0.69 | 0.33 | |
| Carvacrol | 19.2 | 1299 | 1, 2, 3 | 0 | 20.6 | 0.46 | 0.44 | 0.10 | 0.30 | 20.23 | 1.44 | 0.90 | 0.14 | 0 | 0.11 | 0.41 | 0 | 0.10 | 19.3 | 1.28 | 0.61 | 0.18 | |
| Dihydrocarvyl acetate | 20.02 | 1321 | 1, 2 | 0 | 0 | 0.3 | 0 | 0 | 0 | 0 | 0.23 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Citronellyl acetate | 20.02 | 1322 | 1, 2, 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0.07 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.1 | 0.05 | 0 | 0 | |
| Thymyl acetate | 21.13 | 1352 | 1, 2 | 0 | 0 | 0 | 0 | 0 | 0.2 | 0.19 | 0.06 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.64 | 0 | 0 | |
| Eugenol | 21.36 | 1358 | 1, 2, 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.12 | 0.18 | 0 | 0 | |
| Carvacryl acetate | 21.95 | 1374 | 1, 2 | 0 | 10.4 | 0 | 0 | 0 | 0 | 8.14 | 0.43 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 8.81 | 0.46 | 0 | 0 | |
| β-Bourbonene | 22.26 | 1383 | 1, 2 | 0.18 | 0.03 | 0.12 | 0.33 | 0.2 | 0.1 | 0.04 | 0.14 | 0.30 | 0.22 | 0 | 0.14 | 0 | 0.32 | 0.13 | 0.04 | 0.13 | 0.21 | 0.24 | |
| β-Elemene | 22.55 | 1391 | 1, 2, 3 | 0 | 0 | 0 | 0 | 0 | 0.1 | 0.04 | 0.14 | 0.30 | 0.11 | 0 | 0 | 0 | 0 | 0.1 | 0.05 | 0.09 | 0.18 | 0.17 | |
| cis-Jasmone | 23.09 | 1405 | 1, 2 | 0 | 0 | 0 | 0 | 0 | 0.1 | 0.1 | 0.08 | 0.10 | 0.03 | 0 | 0 | 0 | 0 | 0.08 | 0.08 | 0.34 | 0 | 0.02 | |
| β-Caryophyllene | 23.68 | 1419 | 1, 2, 3 | 6.45 | 9.95 | 5.98 | 16.5 | 6 | 4.7 | 8.27 | 6.83 | 13 | 6.54 | 5.64 | 7.98 | 15.2 | 5.8 | 6.57 | 10.7 | 9.95 | 16.5 | 9.22 | |
| α-Humulene | 25.07 | 1454 | 1, 2, 3 | 0.62 | 0.82 | 0.23 | 1.70 | 0.50 | 0.50 | 0.73 | 0.39 | 1.60 | 0.63 | 0.52 | 0.51 | 1.60 | 0.56 | 0.68 | 0.95 | 0.36 | 1.67 | 0.93 | |
| β-Farnesene | 25.27 | 1459 | 1, 2 | 0.27 | 0.07 | 0 | 1.14 | 0.20 | 0.30 | 0.09 | 0.11 | 0 | 0.24 | 0.19 | 0 | 1.07 | 0 | 0.31 | 0 | 0.14 | 0 | 0.35 | |
| Germacrene D | 26.18 | 1481 | 1, 2 | 4.92 | 3.31 | 5.88 | 10.9 | 6.70 | 3.60 | 3.36 | 6.41 | 11.00 | 7.86 | 3.87 | 5.28 | 8.79 | 6.51 | 5.82 | 3.9 | 7.29 | 11.1 | 9.32 | |
| 1-Acetoxy-p-menth-3-on | 26.2 | 1482 | 1, 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.80 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.52 | 0 | |
| Bicyclogermacrene | 26.81 | 1497 | 1, 2 | 0.75 | 0 | 1.50 | 1.79 | 1.40 | 0.50 | 0.14 | 1.51 | 1.80 | 2.03 | 0.42 | 0.8 | 0.97 | 1 | 0.93 | 0.16 | 0.69 | 1.44 | 1.91 | |
| β-Cadinene | 29.87 | 1580 | 1, 2 | 0 | 0 | 0 | 0.62 | 0 | 0.10 | 0.13 | 0.44 | 0.80 | 0.34 | 0 | 0 | 0 | 0 | 0.15 | 0 | 0.36 | 0.70 | 0.37 | |
| Spathulenol | 29.98 | 1584 | 1, 2 | 0.09 | 0 | 0.18 | 0.35 | 0.10 | 0.20 | 0 | 0.16 | 0.50 | 0.2 | 0 | 0 | 0.30 | 0 | 0.13 | 0 | 0 | 0.48 | 0.34 | |
| Caryophyllene oxide | 30.2 | 1590 | 1, 2, 3 | 0.18 | 0.06 | 0 | 0.49 | 0 | 0.60 | 0.08 | 0.12 | 0.60 | 0.17 | 0 | 0.12 | 0 | 0 | 0.23 | 0.10 | 0.12 | 0.68 | 0.39 | |
| Viridiflorol | 30.49 | 1598 | 1, 2 | 1.11 | 0 | 0 | 0 | 0 | 1.20 | 0 | 0 | 0 | 0 | 0.86 | 0 | 0 | 0 | 1.38 | 0 | 0 | 0 | 0 | |
| Monoterpenes | 84.10 | 73.40 | 82.45 | 64.10 | 83.00 | 87.00 | 78.06 | 82.74 | 66.00 | 80.65 | 87.10 | 82.40 | 67.35 | 84.20 | 82.43 | 73.5 | 77.6 | 64.9 | 75.27 | ||||
| Hydrocarbons | 1.46 | 13.40 | 4.93 | 1.54 | 1.10 | 1.60 | 15.75 | 7.74 | 3.20 | 1.55 | 1.27 | 5.76 | 2.58 | 0.85 | 1.49 | 13.8 | 21.3 | 3.00 | 1.15 | ||||
| Open-chain | 0.87 | 1.43 | 0.81 | 1.05 | 0.60 | 0.80 | 1.75 | 0.97 | 1.70 | 0.76 | 0.65 | 0.80 | 1.28 | 0.46 | 0.83 | 1.73 | 1.86 | 1.45 | 0.74 | ||||
| Limonene & byproducts 1 | 1.44 | 2.72 | 3.97 | 1.48 | 1.30 | 1.60 | 3.81 | 4.44 | 2.80 | 1.81 | 1.43 | 5.36 | 3.28 | 1.39 | 1.65 | 3.97 | 4.60 | 3.22 | 1.32 | ||||
| α-Terpinyl pw | 0 | 0.98 | 0.09 | 0 | 0 | 0 | 1.35 | 0.45 | 0 | 0 | 0 | 0.10 | 0 | 0 | 0 | 0.98 | 2.47 | 0 | 0 | ||||
| γ-Terpinene pw | 0 | 11.4 | 0.87 | 0 | 0 | 0.1 | 12.75 | 3.41 | 0.40 | 0 | 0 | 1.06 | 0 | 0 | 0 | 11.20 | 16.60 | 0 | 0 | ||||
| Oxygenated | 82.00 | 67.00 | 76.90 | 61.60 | 81.00 | 85.00 | 66.41 | 74.00 | 61.00 | 78.17 | 85.20 | 75.10 | 62.85 | 82.30 | 80.16 | 64.00 | 52.40 | 60.30 | 73.27 | ||||
| Open-chain, alcohol | 0 | 0 | 0 | 0 | 0 | 0 | 0.35 | 0.39 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.29 | 0.79 | 0 | 0 | ||||
| Open-chain, aldehyde | 0 | 0 | 0 | 0 | 0 | 0 | 0.08 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||
| Open-chain, ester | 0 | 0 | 0 | 0 | 0 | 0 | 0.07 | 0 | 0.10 | 0 | 0 | 0 | 0 | 0 | 0 | 0.10 | 0.05 | 0 | 0 | ||||
| Limonene-2-oxo, alcohol | 0 | 0 | 0.17 | 0 | 0 | 0 | 0 | 0.48 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||
| Limonene-2-oxo, ketone | 0.02 | 0.29 | 69.05 | 0.71 | 0 | 0.50 | 2.36 | 57.86 | 0.50 | 0.07 | 0.04 | 67.00 | 0 | 0.55 | 0 | 0.35 | 9.88 | 0.49 | 0.18 | ||||
| Limonene-3-oxo, alcohol | 4.41 | 0 | 0 | 0 | 0.20 | 3.70 | 0 | 0 | 0 | 0.30 | 1.83 | 0 | 0 | 0.09 | 3.35 | 0 | 0 | 0 | 0.20 | ||||
| Limonene-3-oxo, ketone | 72.00 | 0.59 | 0.57 | 1.09 | 79.00 | 75.00 | 1.91 | 0.28 | 7.90 | 71.79 | 79 | 0.31 | 0.09 | 79 | 72.12 | 0.45 | 0.42 | 0.52 | 56.68 | ||||
| Limonene-3-oxo, epoxide | 0 | 0 | 0 | 53.50 | 0 | 0 | 0.13 | 1.80 | 44.00 | 2.82 | 0 | 0 | 55.34 | 0 | 0 | 0.14 | 0.09 | 52.10 | 12.20 | ||||
| Limonene-3-oxo, ester | 1.42 | 0 | 0 | 0.09 | 0.10 | 1.10 | 0 | 0 | 0.10 | 0.08 | 0.64 | 0 | 0 | 0.11 | 0.80 | 0 | 0 | 0.11 | 0.14 | ||||
| α-Terpinyl pw., alcohol | 0.55 | 0.32 | 1.17 | 0.79 | 0.10 | 0.70 | 2.15 | 1.21 | 0.90 | 0.61 | 0.58 | 1 | 0.83 | 0.55 | 0.48 | 1.91 | 2.24 | 0.95 | 0.41 | ||||
| α-Terpinyl pw., ether | 3.25 | 17.50 | 3.06 | 3.70 | 1.50 | 2.60 | 14.87 | 4.38 | 5.00 | 1.54 | 2.68 | 4.64 | 5.16 | 1.57 | 2.60 | 17.1 | 14.9 | 4.94 | 2.17 | ||||
| α-Terpinyl pw., ester | 0.01 | 0.69 | 0 | 0.01 | 0 | 0 | 0.83 | 0.2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.77 | 0.59 | 0.03 | 0 | ||||
| γ-Terpinene pw, phenol | 0 | 34.50 | 1.8 | 1.04 | 0.10 | 0.60 | 33.99 | 5.74 | 2.00 | 0.22 | 0 | 1.50 | 1.060 | 0 | 0.19 | 32.6 | 21.10 | 1.3.00 | 0.51 | ||||
| γ-Terpinene pw, phenol ester | 0 | 10.40 | 0 | 0 | 0 | 0.20 | 8.34 | 0.49 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 8.81 | 1.10 | 0 | 0 | ||||
| Sesquiterpenes | 14.60 | 14.20 | 13.89 | 33.80 | 15.00 | 12.00 | 12.98 | 16.34 | 31.00 | 18.39 | 11.50 | 14.80 | 27.93 | 14.20 | 16.51 | 15.90 | 19.50 | 33.40 | 23.26 | ||||
| Hydrocarbons | 13.20 | 14.20 | 13.71 | 33.00 | 15.00 | 9.90 | 12.80 | 15.97 | 29.00 | 17.99 | 10.60 | 14.70 | 27.63 | 14.20 | 14.69 | 15.80 | 19.00 | 31.70 | 22.51 | ||||
| Oxygenated | 1.38 | 0.06 | 0.18 | 0.84 | 0.10 | 2.00 | 0.08 | 0.28 | 1.20 | 0.37 | 0.86 | 0.12 | 0.30 | 0 | 1.74 | 0.10 | 0.12 | 1.16 | 0.73 | ||||
| Volatile shikimates | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.12 | 0.18 | 0 | 0 | ||||
| Volatile PMAs (FA & esters) | 0.23 | 0.39 | 0.34 | 0.06 | 0.10 | 0.40 | 0.61 | 0.50 | 0.20 | 0.25 | 0.20 | 0.25 | 0.07 | 0 | 0.33 | 0.75 | 0.52 | 0 | 0.21 | ||||
| Compound Type | Compound Name | Loadings for Major Compounds Calculated to PC1-PC3. | ||
|---|---|---|---|---|
| Loading/PC1 | Loading/PC2 | Loading/PC3 | ||
| Limonene-2-oxo | cis-Dihydrocarvone | −0.055332 | 0.727988 | −0.627070 |
| Limonene-2-oxo | trans-Dihydrocarvone | −0.044463 | 0.727524 | −0.617217 |
| Limonene-3-oxo | Menthone | 0.546769 | 0.169262 | 0.647878 |
| Limonene-3-oxo | Isomenthone | 0.492990 | 0.108175 | 0.453511 |
| Limonene-3-oxo | Pulegone | 0.312915 | 0.149585 | 0.637364 |
| Limonene-3-oxo | cis-Piperitone epoxide | 0.307598 | −0.714431 | −0.431420 |
| α-Terpinyl derivative | 1,8-Cineole | −0.934298 | −0.193698 | 0.077340 |
| γ-Terpinene derivative | γ-Terpinene | −0.958503 | −0.059386 | −0.002698 |
| γ-Terpinene derivative | para-Cymene | −0.980025 | −0.080735 | 0.105628 |
| γ-Terpinene derivative | Thymol | −0.972715 | −0.094191 | 0.034730 |
| γ-Terpinene derivative | Carvacrol | −0.757757 | −0.101103 | 0.243685 |
| γ-Terpinene derivative | Carvacryl acetate | −0.738520 | −0.082496 | 0.265629 |
| Sesquiterpene | β-Caryophyllene | −0.061425 | −0.762830 | −0.432633 |
| Sesquiterpene | Germacrene D | 0.412524 | −0.605841 | −0.595122 |
| Compound | Concentrations (Area%) in Samples | CV, % | Homogeneity | |||
|---|---|---|---|---|---|---|
| EGR_2019 | EGR_2020 | SOR_2019 | SOR_2020 | |||
| Accession: KBÁ | ||||||
| Menthone | 53.91 | 49.09 | 62.65 | 55.78 | 10.15 | homogeneous |
| Isomenthone | 6.34 | 5.74 | 6.16 | 6.74 | 6.67 | homogeneous |
| Pulegone | 8.45 | 15.48 | 6.30 | 6.59 | 46.62 | heterogeneous |
| β-Caryophyllene | 6.45 | 4.72 | 5.64 | 6.57 | 14.68 | homogeneous |
| Germacrene D | 4.92 | 3.56 | 3.87 | 5.82 | 22.74 | heterogeneous |
| Accession: SZD | ||||||
| Menthone | 64.00 | 55.60 | 66.30 | 46.67 | 15.35 | homogeneous |
| Isomenthone | 13.43 | 15.14 | 11.68 | 9.57 | 19.17 | borderline case |
| β-Caryophyllene | 6.00 | 6.54 | 5.80 | 9.22 | 22.99 | heterogeneous |
| Germacrene D | 6.69 | 7.86 | 6.51 | 9.32 | 17.07 | homogeneous |
| cis-Piperitone epoxide | 0.00 | 2.82 | 0.00 | 12.20 | 154.02 | extrem. heterogeneous |
| Accession: EGR3 | ||||||
| cis-Piperitone epoxide | 53.47 | 44.42 | 55.34 | 19176 | 9.34 | extrem. homogeneous |
| β-Caryophyllene | 16.49 | 13.42 | 15.20 | 16.46 | 9.38 | extrem. homogeneous |
| Germacrene D | 10.89 | 10.67 | 8.79 | 11.07 | 10.20 | homogeneous |
| 1,8-cineole | 3.70 | 4.97 | 5.16 | 4.94 | 14.25 | homogeneous |
| Menthone | 0.68 | 6.13 | 0.09 | 0.00 | 171.14 | extrem. heterogeneous |
| Accession: HV2 | ||||||
| cis-Dihydrocarvone | 57.06 | 47.57 | 54.51 | 8.45 | 54.08 | extrem. heterogeneous |
| trans-Dihydrocarvone | 11.66 | 9.93 | 12.28 | 1.43 | 56.99 | extrem. heterogeneous |
| β-Caryophyllene | 5.98 | 6.83 | 7.98 | 9.95 | 22.35 | heterogeneous |
| Germacrene D | 5.88 | 6.41 | 5.28 | 7.29 | 13.71 | homogeneous |
| Thymol | 1.34 | 4.30 | 1.39 | 19.79 | 131.75 | extrem. heterogeneous |
| 1,8-cineole | 3.06 | 4.38 | 4.64 | 14.93 | 81.38 | extrem. heterogeneous |
| γ-Terpinene | 0.49 | 1.75 | 0.44 | 7.22 | 130.14 | extrem. heterogeneous |
| para-Cymene | 0.38 | 1.66 | 0.62 | 9.36 | 142.14 | extrem. heterogeneous |
| Accession: HV1 | ||||||
| Carvacrol | 20.56 | 20.23 | No data available | 19.28 | 3.32 | extrem. homogeneous |
| 1,8-cineole | 17.45 | 14.87 | 17.11 | 8.50 | extrem. homogeneous | |
| Thymol | 13.9 | 13.75 | 13.36 | 2.04 | extrem. homogeneous | |
| Carvacryl acetate | 10.40 | 8.14 | 8.81 | 12.72 | homogenous | |
| para-Cymene | 7.24 | 8.01 | 7.67 | 5.05 | extrem. homogeneous | |
| β-Caryophyllene | 9.95 | 8.27 | 10.66 | 12.73 | homogenous | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patonay, K.; Szalontai, H.; Radácsi, P.; Zámboriné-Németh, É. Chemotypes and Their Stability in Mentha longifolia (L.) L.—A Comprehensive Study of Five Accessions. Plants 2021, 10, 2478. https://doi.org/10.3390/plants10112478
Patonay K, Szalontai H, Radácsi P, Zámboriné-Németh É. Chemotypes and Their Stability in Mentha longifolia (L.) L.—A Comprehensive Study of Five Accessions. Plants. 2021; 10(11):2478. https://doi.org/10.3390/plants10112478
Chicago/Turabian StylePatonay, Katalin, Helga Szalontai, Péter Radácsi, and Éva Zámboriné-Németh. 2021. "Chemotypes and Their Stability in Mentha longifolia (L.) L.—A Comprehensive Study of Five Accessions" Plants 10, no. 11: 2478. https://doi.org/10.3390/plants10112478
APA StylePatonay, K., Szalontai, H., Radácsi, P., & Zámboriné-Németh, É. (2021). Chemotypes and Their Stability in Mentha longifolia (L.) L.—A Comprehensive Study of Five Accessions. Plants, 10(11), 2478. https://doi.org/10.3390/plants10112478






