Effects of Exogenous Application of Indole-3-Butyric Acid on Maize Plants Cultivated in the Presence or Absence of Cadmium
Abstract
:1. Introduction
2. Results
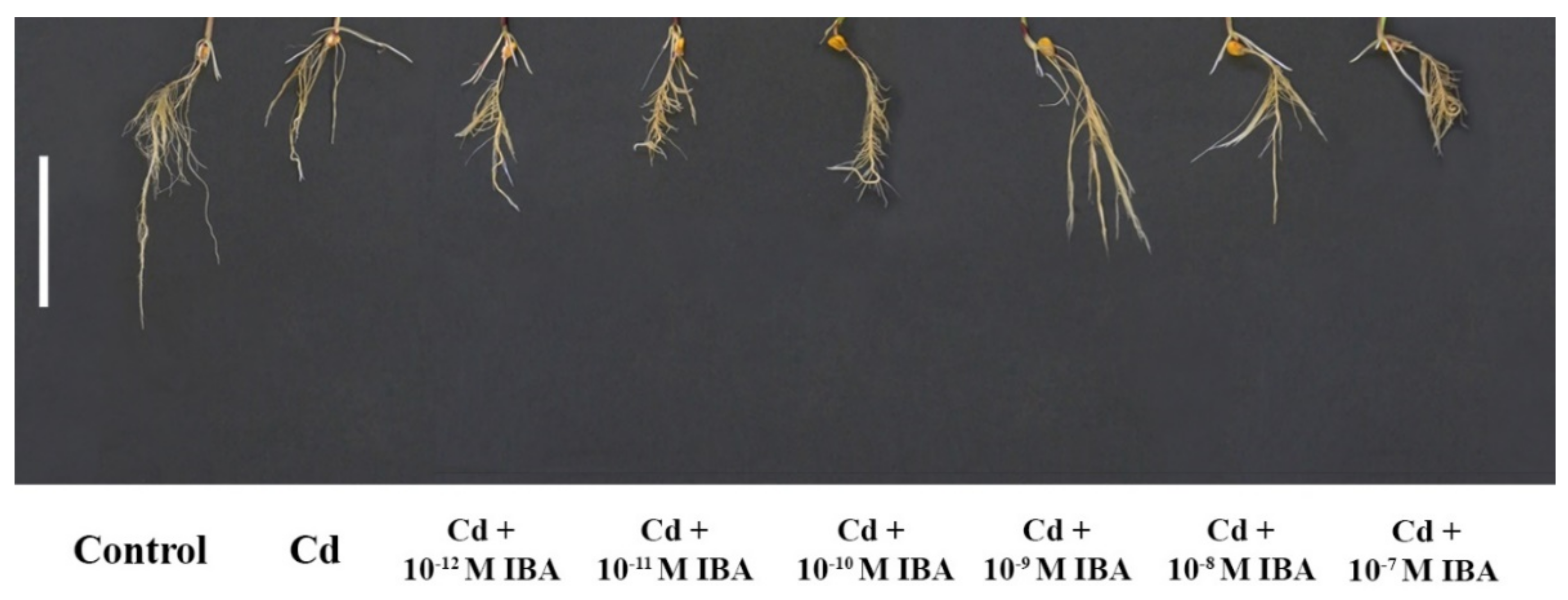
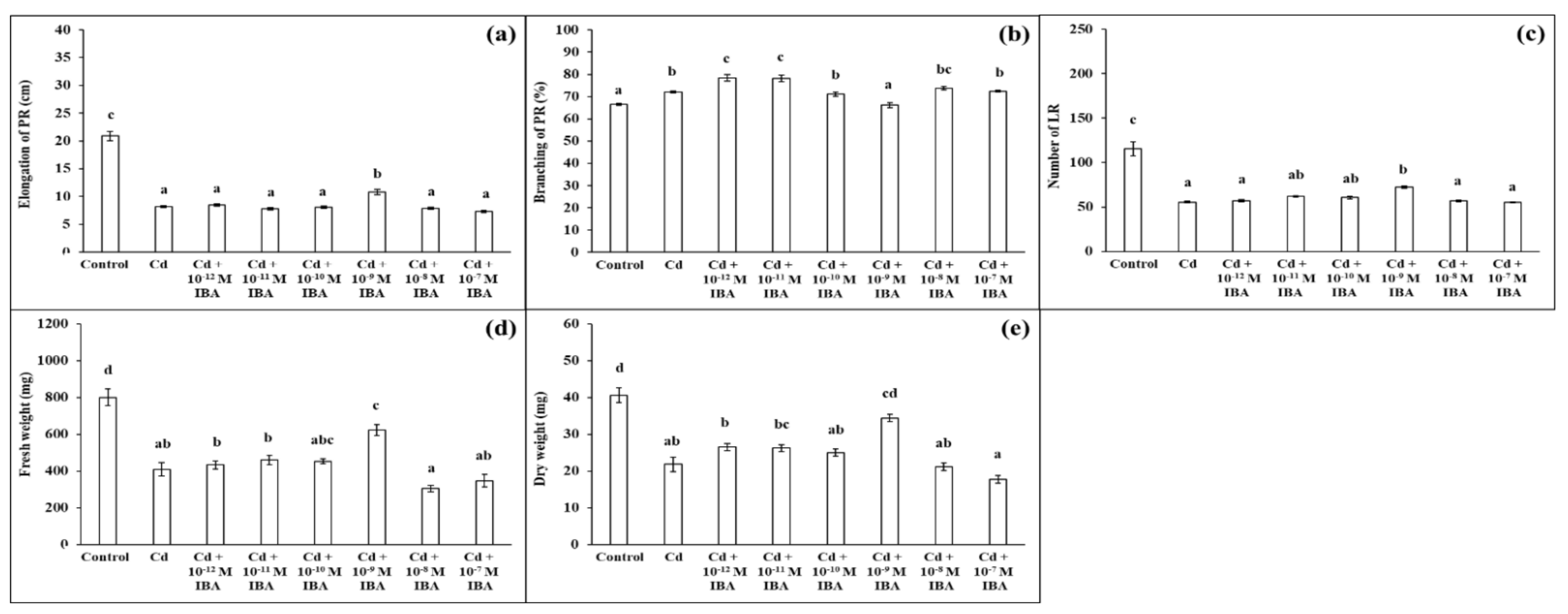
2.1. Effects of IBA on the Growth Parameters in the Absence and Presence of Cd
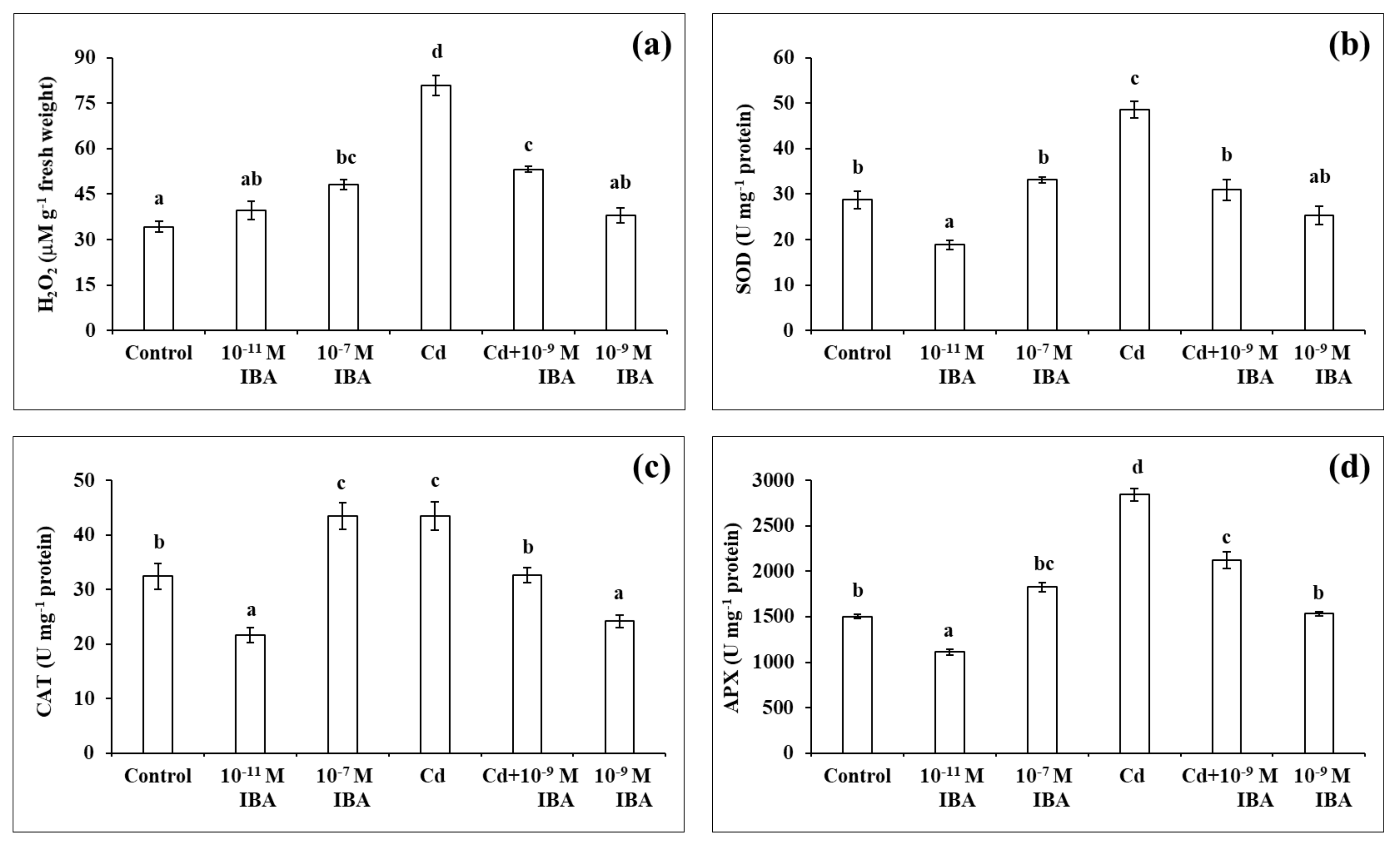
2.2. Effects of IBA and Cd on the Concentrations of H2O2 and on the Activity of Antioxidant Enzymes
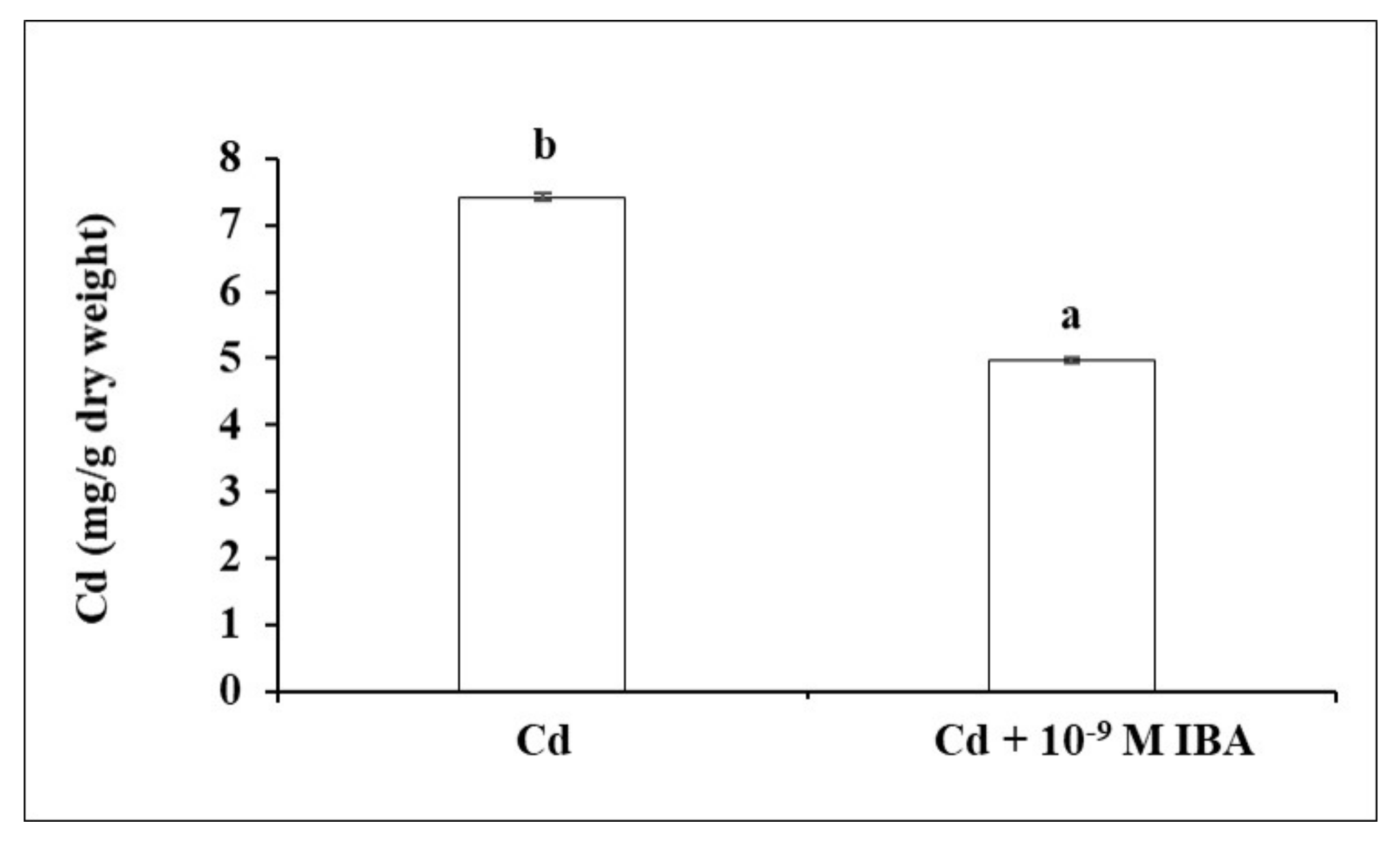
2.3. Effect of IBA on the Concentration of Cd
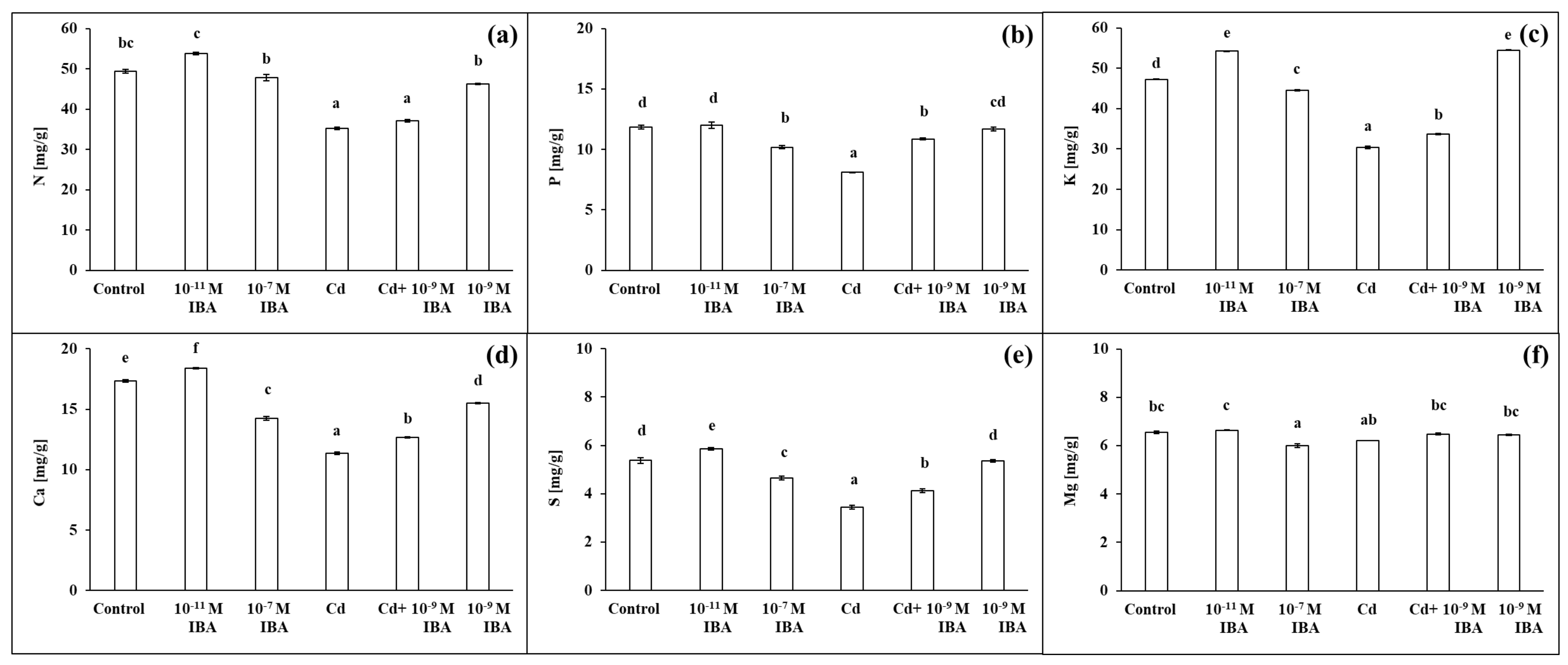
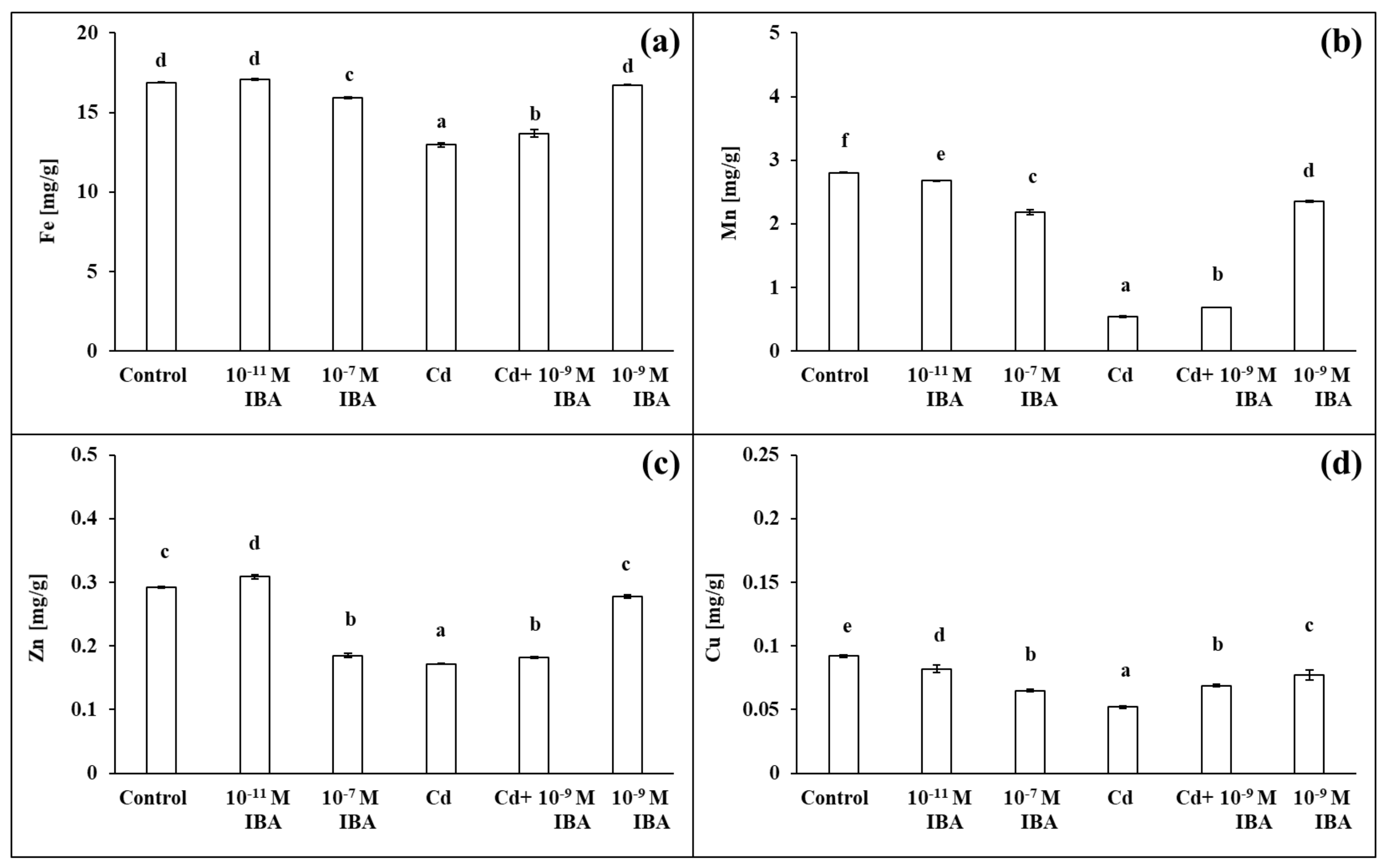
2.4. Effects of IBA and Cd on the Concentration of Mineral Nutrients
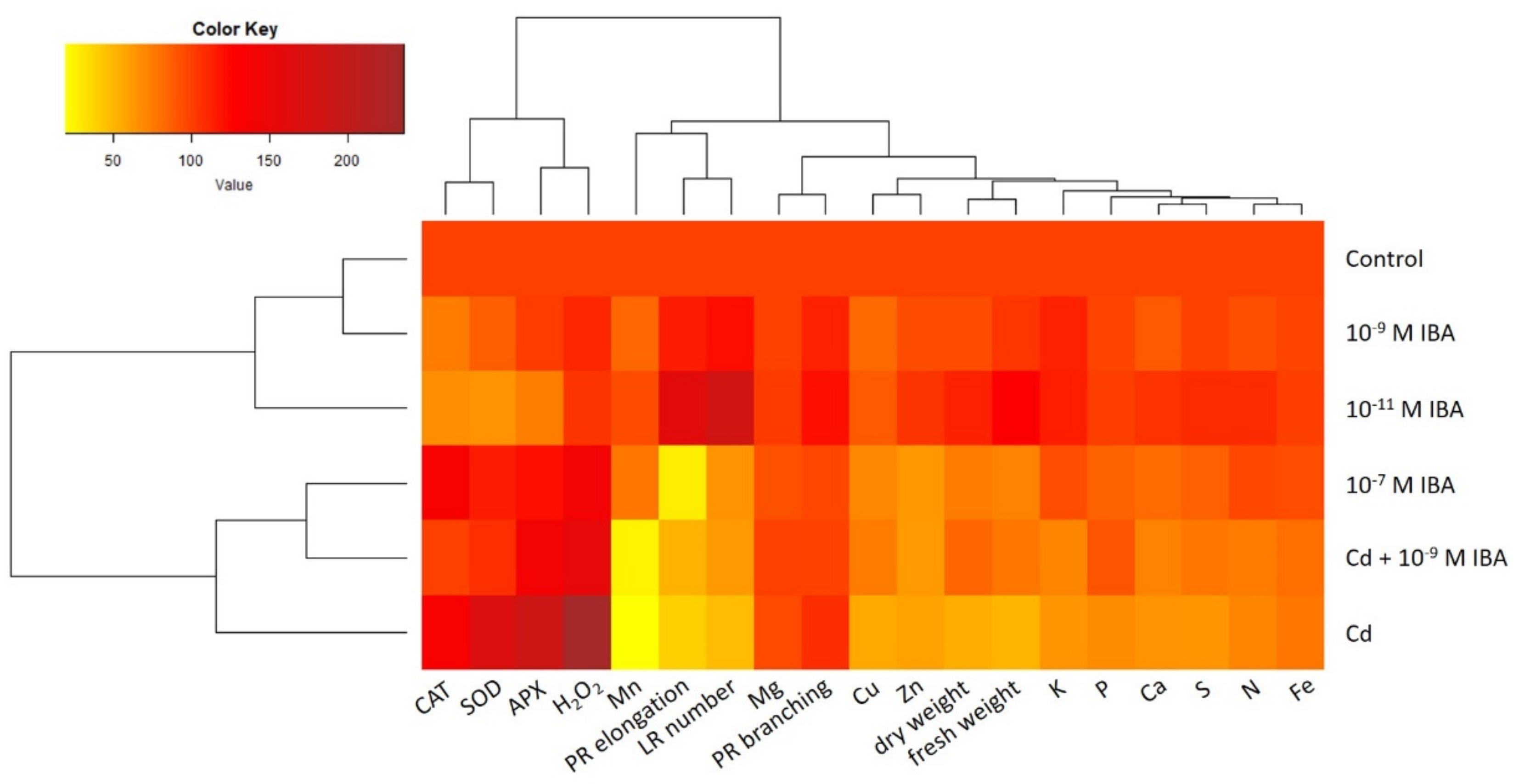
2.5. Data Visualization and Analysis—Relationship among Different Treatments and Observed Parameters
3. Discussion
4. Materials and Methods
4.1. Plant Material and Cultivation
4.2. Measurement of the Growth Parameters
4.3. Determination of H2O2
4.4. Determination of Antioxidant Enzymes Activity
4.5. Determination of Selected Mineral Nutrients
4.6. Data Visualization and Analysis—Heatmap and Correlation Matrix Heatmap
4.7. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tognetti, V.B.; Mühlenbock, P.; van Breusegem, F. Stress homeostasis—The redox and auxin perspective. Plant Cell Environ. 2012, 35, 321–333. [Google Scholar] [CrossRef] [PubMed]
- Depuydt, S.; Hardtke, C.S. Hormone signalling crosstalk in plant growth. Curr. Biol. 2011, 21, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Garg, N.; Manchanda, G. ROS generation in plants: Boon or bane? Plant Biosyst. 2009, 143, 81–96. [Google Scholar] [CrossRef]
- Ahmad, P.; Tripathi, D.K.; Deshmukh, R.; Singh, V.P.; Corpas, F.J. Revisiting the role of ROS and RNS in plants under changing environment. Environ. Exp. Bot. 2019, 161, 1–3. [Google Scholar] [CrossRef]
- Kohli, S.K.; Khanna, K.; Bhardwaj, R.; Abd Allah, E.F.; Ahmad, P.; Corpas, F.J. Assessment of Subcellular ROS and NO Metabolism in Higher Plants: Multifunctional Signaling Molecules. Antioxidants 2019, 8, 641. [Google Scholar] [CrossRef] [Green Version]
- Asgher, M.; Khan, M.I.R.; Anjum, N.A.; Khan, N.A. Minimising toxicity of cadmium in plants—Role of plant growth regulators. Protoplasma 2015, 252, 399–413. [Google Scholar] [CrossRef] [PubMed]
- Jan, S.; Alyemeni, M.N.; Wijaya, L.; Alam, P.; Siddique, K.H.; Ahmad, P. Interactive effect of 24-epibrassinolide and silicon alleviates cadmium stress via the modulation of antioxidant defense and glyoxalase systems and macronutrient content in Pisum sativum L. seedlings. BMC Plant Biol. 2018, 18, 146. [Google Scholar] [CrossRef]
- Hernandez-Baranda, Y.; Rodriguez-Hernandez, P.; Pena-Icart, M.; Merino-Hernandez, Y.; Cartya-Rubio, O. Toxicity of Cadmium in plants and strategies to reduce its effects. Case study: The tomato. Cultiv. Trop. 2019, 40, e10. [Google Scholar] [CrossRef] [Green Version]
- Wagner, G.J. Accumulation of cadmium in crop plants and its consequences to human health. In Advances in Agronomy; Wagner, G.J., Ed.; Elsevier Academic Press: San Diego, CA, USA, 1993; Volume 51, pp. 173–212. [Google Scholar] [CrossRef]
- Clemens, A.; Aarts, M.G.; Thomine, S.; Verbuggen, N. Plant science: The key to prevent slow cadmium poisoning. Trend Plant Sci. 2013, 18, 92–99. [Google Scholar] [CrossRef]
- Järup, L.; Åkesson, A. Current status of cadmium as an environmental health problem. Toxicol. Appl. Pharmacol. 2009, 238, 201–208. [Google Scholar] [CrossRef]
- Bashri, G.; Prasad, S.M. Exogenous IAA differentially affects growth, oxidative stress and antioxidants system in Cd stressed Trigonella foenum-graecum L. seedlings: Toxicity alleviation by up-regulation of ascorbate-glutathione cycle. Ecotoxicol. Environ. Saf. 2016, 132, 329–338. [Google Scholar] [CrossRef]
- Khan, M.Y.; Prakash, V.; Yadav, V.; Chauhan, D.K.; Prasad, S.M.; Ramawat, N.; Singh, V.P.; Tripathi, D.K.; Sharma, S. Regulation of cadmium toxicity in roots of tomato by indole acetic acid with special emphasis on reactive oxygen species production and their scavenging. Plant Physiol. Biochem. 2019, 142, 193–201. [Google Scholar] [CrossRef]
- Demecsová, L.; Zelinová, V.; Liptáková, Ľ.; Valentovičová, K.; Tamás, L. Indole-3-butyric acid priming reduced cadmium toxicity in barley root tip via NO genereation and enhanced glutathione peroxidase activity. Planta 2020, 252, 46–62. [Google Scholar] [CrossRef]
- Piacentini, D.; Rovere, F.D.; Sofo, A.; Fattorini, L.; Falasca, G.; Altamura, M.M. Nitric oxide cooperates with auxin to mitigate the alterations in the root system caused by cadmium and arsenic. Front. Plant Sci. 2020, 11, 1182–1198. [Google Scholar] [CrossRef]
- López, M.L.; Peralta-Videa, J.R.; Benitez, T.; Duarte-Gardea, M.; Gardea-Torresdey, J.L. Effects of lead, EDTA, and IAA on nutrient uptake by alfalfa plants. J. Plant Nutr. 2007, 30, 1247–1261. [Google Scholar] [CrossRef]
- Wang, H.H.; Shan, X.Q.; Wen, B.; Owens, G.; Fang, J.; Zhang, S.Z. Effect of indole-3-acetic acid on lead accumulation in maize (Zea mays L.) seedlings and the relevant antioxidant response. Environ. Exp. Bot. 2007, 61, 246–253. [Google Scholar] [CrossRef]
- Nordström, A.C.; Jacobs, F.A.; Efiasson, L. Effect of exogenous indole-3-acetic acid and indole-3-butyric acid on internal levels of the respective auxins and their conjugation with aspartic acid during adventitious root formation in pea cuttings. Plant Physiol. 1991, 96, 856–861. [Google Scholar] [CrossRef] [Green Version]
- Kaczmarek, D.K.; Parus, A.; Lozynsko, M.; Pernak, J. Use of ammonium salts or binary mixtures derived from amino acids, glycine betaine, choline and indole-3-butyric ciad as plant regulators. RSC Adv. 2020, 10, 43058. [Google Scholar] [CrossRef]
- Kaczmarek, D.K.; Kleiber, T.; Wenping, L.; Niemczak, M.; Chrzanowski, L.; Pernak, J. Transformation of indole-3-butyric acid into ionic liquids as a sustainable strategy leading to highy efficient plant growth stimulators. ACS Sustain. Chem. Eng. 2020, 8, 1591–1598. [Google Scholar] [CrossRef]
- Jones, J.B. Complet Guide for Growing Plants Hydroponically, 1st ed.; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Redjala, T.; Zelko, I.; Sterckeman, T.; Legue, V.; Lux, A. Relationship between root structure and root cadmium uptake in maize. Envir. Exper. Bot. 2011, 71, 241–247. [Google Scholar] [CrossRef]
- Štefančič, M.; Štampar, F.; Veberič, R.; Osterc, G. The levels of IAA, IAAsp and some phenolics in cherry rootstock ‘GiSelA 5’ leafy cuttings pretreated with IAA and IBA. Sci. Hortic. 2007, 112, 399–405. [Google Scholar] [CrossRef]
- Çakmakçı, R.; Mosber, G.; Milton, A.H.; Alatürk, F.; Ali, B. The Effect of Auxin and Auxin-Producing Bacteria on the Growth, Essential Oil Yield, and Composition in Medicinal and Aromatic Plants. Curr. Microbiol. 2020, 77, 564–577. [Google Scholar] [CrossRef]
- Majda, M.; Robert, S. The role of auxin in cell wall expansion. Int. J. Mol. Sci. 2018, 19, 951. [Google Scholar] [CrossRef] [Green Version]
- Marquez, G.; Alarcon, M.V.; Salguero, J. Differential responses of primary and lateral roots to indole-3-acetic acid, indole-3-butyric acid, and 1-naphthaleneacetic acid in maize seedlings. Biol. Plant 2016, 60, 367–375. [Google Scholar] [CrossRef]
- True, J.H.; Shaw, S.L. Exogenous auxin induces transverse microtubule arrays throught transporter Transport inhibitor response1/auxin signaling f-box receptors. Plant Physiol. 2020, 182, 892–907. [Google Scholar] [CrossRef]
- Wasteneys, G.O. Progress in understanding the role of microtubules in plan cells. Curr. Opin. Plant Biol. 2004, 7, 651–660. [Google Scholar] [CrossRef]
- Šípošová, K.; Kollárová, K.; Lišková, D.; Vivodová, Z. The effects of IBA on the composition of maize root cell walls. J. Plant Physiol. 2019, 239, 10–17. [Google Scholar] [CrossRef]
- Demecsová, L.; Tamás, L. Reactive oxygen species, auxin and nitric oxide in metal-stressed roots: Toxicity or defence. BioMetals 2019, 32, 717–744. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Abbas, T.; Zia-ur-Rehman, M.; Hannan, F.; Keller, C.; Al-Wabel, M.I.; Ok, Y.S. Cadmium minimization in wheat: A critical review. Ecotoxicol. Environ. Saf. 2016, 130, 43–53. [Google Scholar] [CrossRef]
- Zhu, X.F.; Wang, Z.W.; Dong, F.; Lei, G.J.; Shi, Y.Z.; Li, G.X.; Zheng, S.J. Exogenous auxin alleviates cadmium toxicity in Arabidopsis thaliana by stimulating synthesis of hemicellulose 1 and increasing the cadmium fixation capacity of root cell walls. J. Hazard. Mater. 2013, 263, 398–403. [Google Scholar] [CrossRef]
- Choudhury, S.; Panda, S.K. Role of salicylic acid in regulating cadmium induced oxidative stress in Oryza sativa L. roots. Bulg. J. Plant Physiol. 2004, 30, 95–110. [Google Scholar]
- Hasanuzzaman, M.; Hossain, M.A.; Fujita, M. Exogenous selenium pretreatment protects rapeseed seedlings from cadmium-induced oxidative stress by upregulating antioxidant defense and methylglyoxal detoxification systems. Biol. Trace Elem. Res. 2012, 149, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Černý, M.; Habánová, H.; Berka, M.; Luklová, M.; Brzobohatý, B. Hydrogen peroxide: Its role in plant biology and crosstalk with signalling networks. Int. J. Mol. Sci. 2018, 19, 2812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mishra, S.; Srivastava, V.; Mehrotra, S.; Quadri, S.N. The regulation of plant development: Cross-talk of reactive oxygen species and plant hormones. In Reactive Oxygen Species in Plants: Boon or Bane—Revisiting the Role of ROS; Singh, V.P., Sing, S., Tripathi, D.K., Prasad, S.M., Eds.; Wiley: Hoboken, NJ, USA, 2017; pp. 243–260. [Google Scholar]
- El-Gaied, L.F.; Abu El-Heba, G.A.; El-Sherif, N.A. Effect of growth hormones on some antioxidant parameters and gene expression in tomato. GM Crops Food 2013, 4, 67–73. [Google Scholar] [CrossRef]
- Tyburski, J.; Dunajska, K.; Mazurek, P.; Piotrowska, B.; Tretyn, A. Exogenous auxin regulates H2O2 metabolism in roots of tomato (Lycopersicon esculentum Mill.) seedlings affecting the expression and activity of CuZn-superoxide dismutase, catalase, and peroxidase. Acta Physiol. Plant. 2009, 31, 249–260. [Google Scholar] [CrossRef]
- Saini, S.; Kaur, N.; Pati, P.K. Phytohormones: Key players in the modulation of heavy metal stress tolernace in plants. Ecotoxicol. Environ. Saf. 2021, 223, 112578. [Google Scholar] [CrossRef]
- Singh, S.; Singh, S.; Ramachandran, V.; Eapen, S. Copper tolerance and response of antioxidative enzymes in axenically grown Brassica juncea (L.) plants. Ecotoxicol. Environ. Saf. 2010, 73, 1975–1981. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Singh, H.; Bhat, J.A.; Singh, V.P.; Corpas, F.J.; Yadav, S.R. Auxin metabolic network regulates the plant response to metalloids stress. J. Hazard. Mater. 2021, 405, 124250. [Google Scholar] [CrossRef]
- Lin, L.; Ma, Q.; Wang, J.; Lv, X.; Liao, M.; Xia, H.; Chen, S.; Lai, Y.; Chen, C.; Wang, X.; et al. Effects of indole-3-butyric acid (IBA) on growth and cadmium accumulation in the accumulator plant Stellaria Media Environ. Prog. Sustain. Energy 2018, 37, 733–737. [Google Scholar] [CrossRef]
- Ran, J.; Zheng, W.; Wang, H.; Wang, H.; Li, Q. Indole-3-acetic acid promotes cadmium (Cd) accumulation in a Cd hyperaccumulator and a non-hyperaccumulator by different physiological responses. Ecotoxicol. Environ. Saf. 2020, 191, 110213. [Google Scholar] [CrossRef]
- Agami, R.A.; Mohamed, G.F. Exogenous treatment with indole-3-acetic acid and salicylic acid alleviates cadmium toxicity in wheat seedlings. Ecotoxicol. Environ. Saf. 2013, 94, 164–171. [Google Scholar] [CrossRef]
- Li, Z.; Zhu, J.; Wang, Y.; Lin, L. Effects of exogenous indole acetic acid on growth and cadmium accumulation of Cyphomandra betacea seedlings. Int. J. Environ. Anal. Chem. 2020. [CrossRef]
- San-Francisco, S.; Houdusse, F.; Zamarreno, A.M.; Garnica, M.; Casanova, E.; Garcia-Mina, J.M. Effects of IAA and IAA precursors on the development, mineral nutrition, IAA content and free polyamine content of pepper plants cultivated in hydroponic conditions. Sci. Hortic. 2005, 106, 38–52. [Google Scholar] [CrossRef]
- Huang, X.; Duan, S.; Wu, Q.; Yu, M.; Shabala, S. Reducing cadmium accumulation in plants: Structure–function relations and tissue-specific operation of transporters in the spotlight. Plants 2020, 9, 223. [Google Scholar] [CrossRef] [Green Version]
- Huang, D.; Wang, R.; Liu, Y.; Zeng, G.; Lai, C.; Xu, P.; Lu, B.; Xu, J.; Wang, C.; Huang, C. Application of molecularly imprinted polymers in wastewater treatment: A review. Environ. Sci. Pollut. Res. 2015, 22, 936–977. [Google Scholar] [CrossRef]
- Keller, C.; Ludwig, C.; Davoli, C.; Wochele, J. Thermal treatment of metal-enriched biomass produced from heavy metal phytoextraction. Environ. Sci. Technol. 2005, 39, 3359–3367. [Google Scholar] [CrossRef]
- Danelli, T.; Sepulcri, A.; Masetti, G.; Colombo, F.; Sangiorgio, S.; Cassani, E.; Anelli, S.; Adani, F.; Pilu, R. Arundo donax L. biomass production in a polluted area: Effects of two harvest timings on heavy metals uptake. Appl. Sci. 2021, 11, 1147. [Google Scholar] [CrossRef]
- Edgar, V.-N.; Fabián, F.-L.; Mario, P.-C.J.; Ileana, V.-R. Coupling plant biomass derived from phytoremediation of potential toxic-metal-polluted soils to bioenergy production and high-value by-products—A review. Appl. Sci. 2021, 11, 2982. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnin, D.I. The water-culture method growing plants without soil. Circ. Calif. Agric. Exp. Stn. 1950, 347, 1–39. [Google Scholar]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Madamanchi, N.R.; Donahue, J.L.; Cramer, C.L.; Alscher, R.G.; Pedersen, K. Differential response of Cu, Zn superoxide dismutases in two pea cultivars during a short-term exposure to sulfur dioxide. Plant Mol. Biol. 1994, 26, 95–103. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Hodges, D.M.; Andrews, C.J.; Johnson, D.A.; Hamilton, R.I. Antioxidant enzyme responses to chilling stress in differentially sensitive inbred maize lines. J. Exp. Bot. 1997, 48, 1105–1113. [Google Scholar] [CrossRef]
- Claiborne, A. Catalase activity. In Handbook of Methods for Oxygen Radical Research, 1st ed.; Greenwald, R.A., Ed.; CRC Press: Boca Raton, FL, USA, 1985; pp. 283–284. [Google Scholar]
- Warnes, G.R.; Bolker, B.; Bonebakker, L.; Gentleman, R.; Huber, W.; Liaw, A.; Lumley, T.; Maechler, M.; Magnusson, A.; Moeller, S.; et al. gplots: Various R Programming Tools for Plotting Data. R Package Version 3.0.4. Available online: https://CRAN.R-project.org/package=gplots (accessed on 7 September 2020).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria; Available online: https://www.r-project.org/ (accessed on 22 June 2020).
- Schober, P.; Boer, C.; Schwarte, L.A. Correlation coefficients: Appropriate use and interpretation. Anesth. Analg. 2018, 126, 1763–1768. [Google Scholar] [CrossRef]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šípošová, K.; Labancová, E.; Kučerová, D.; Kollárová, K.; Vivodová, Z. Effects of Exogenous Application of Indole-3-Butyric Acid on Maize Plants Cultivated in the Presence or Absence of Cadmium. Plants 2021, 10, 2503. https://doi.org/10.3390/plants10112503
Šípošová K, Labancová E, Kučerová D, Kollárová K, Vivodová Z. Effects of Exogenous Application of Indole-3-Butyric Acid on Maize Plants Cultivated in the Presence or Absence of Cadmium. Plants. 2021; 10(11):2503. https://doi.org/10.3390/plants10112503
Chicago/Turabian StyleŠípošová, Kristína, Eva Labancová, Danica Kučerová, Karin Kollárová, and Zuzana Vivodová. 2021. "Effects of Exogenous Application of Indole-3-Butyric Acid on Maize Plants Cultivated in the Presence or Absence of Cadmium" Plants 10, no. 11: 2503. https://doi.org/10.3390/plants10112503
APA StyleŠípošová, K., Labancová, E., Kučerová, D., Kollárová, K., & Vivodová, Z. (2021). Effects of Exogenous Application of Indole-3-Butyric Acid on Maize Plants Cultivated in the Presence or Absence of Cadmium. Plants, 10(11), 2503. https://doi.org/10.3390/plants10112503






