Sorption–Desorption Behavior of Doxycycline in Soil–Manure Systems Amended with Mesquite Wood Waste Biochar
Abstract
:1. Introduction
2. Results and Discussion
2.1. Physiochemical Characteristics of Soil and Manure
2.2. Biochar Characterization
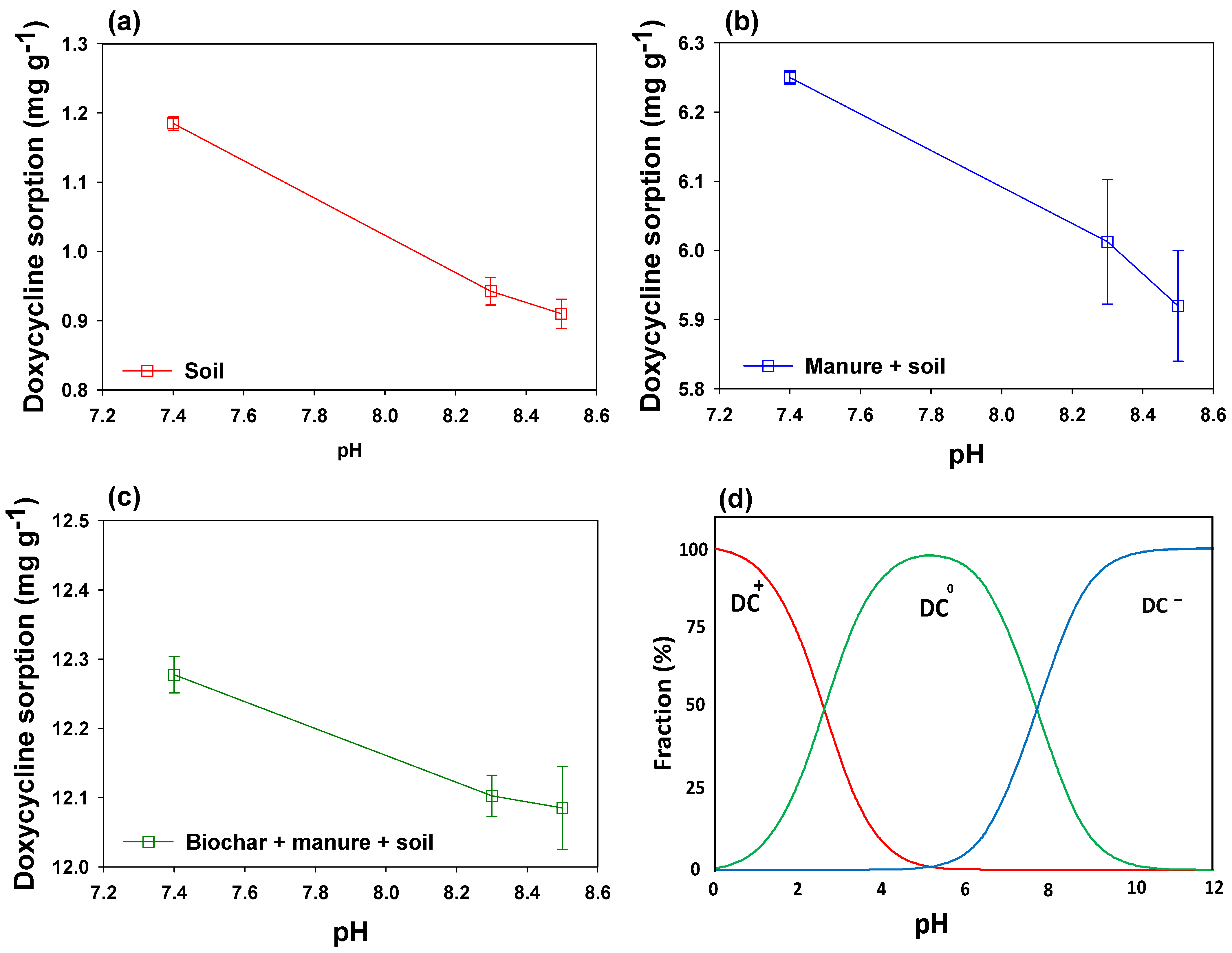
2.3. Effects of Soil Type on Doxycycline Sorption
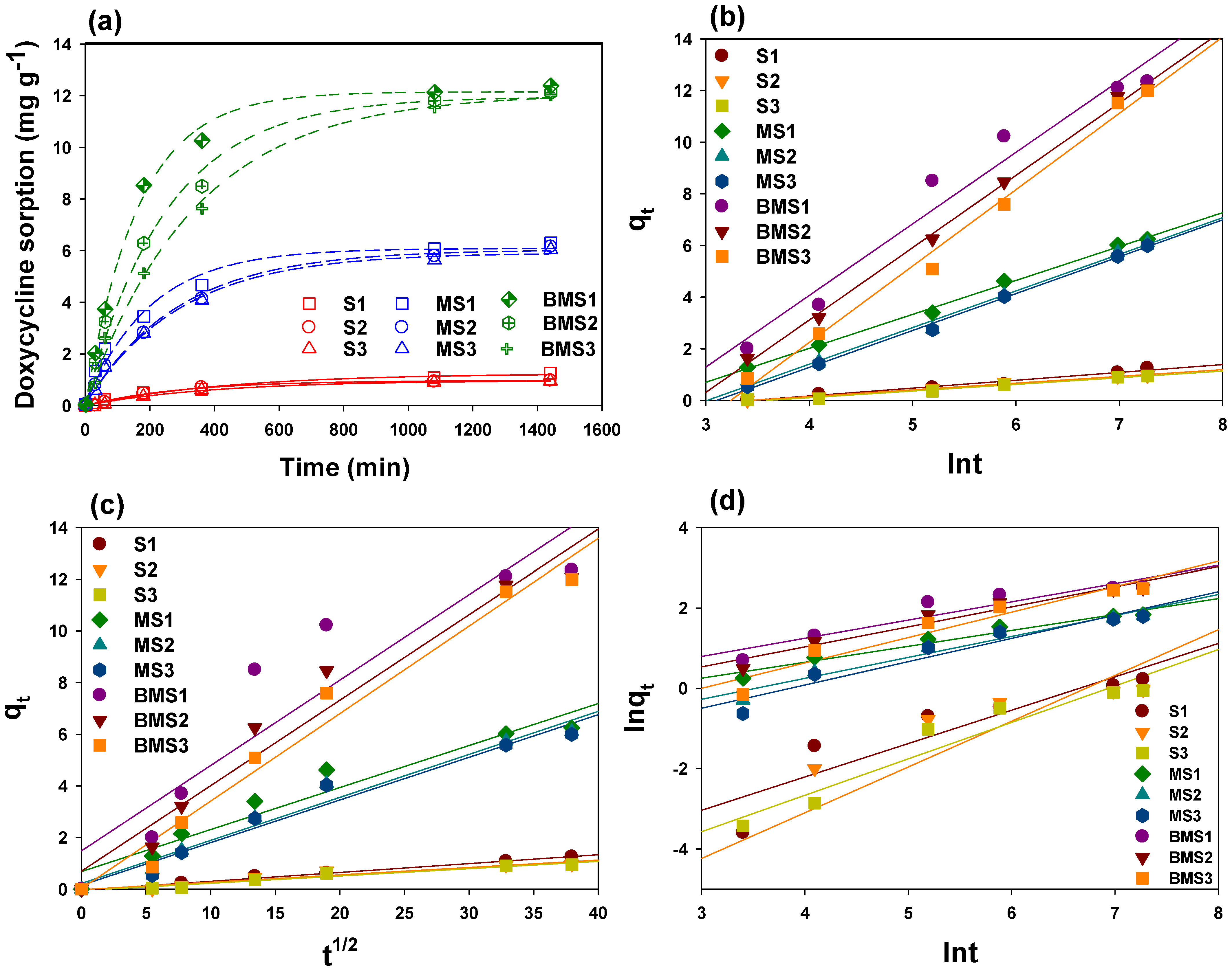
2.4. Sorption Kinetics
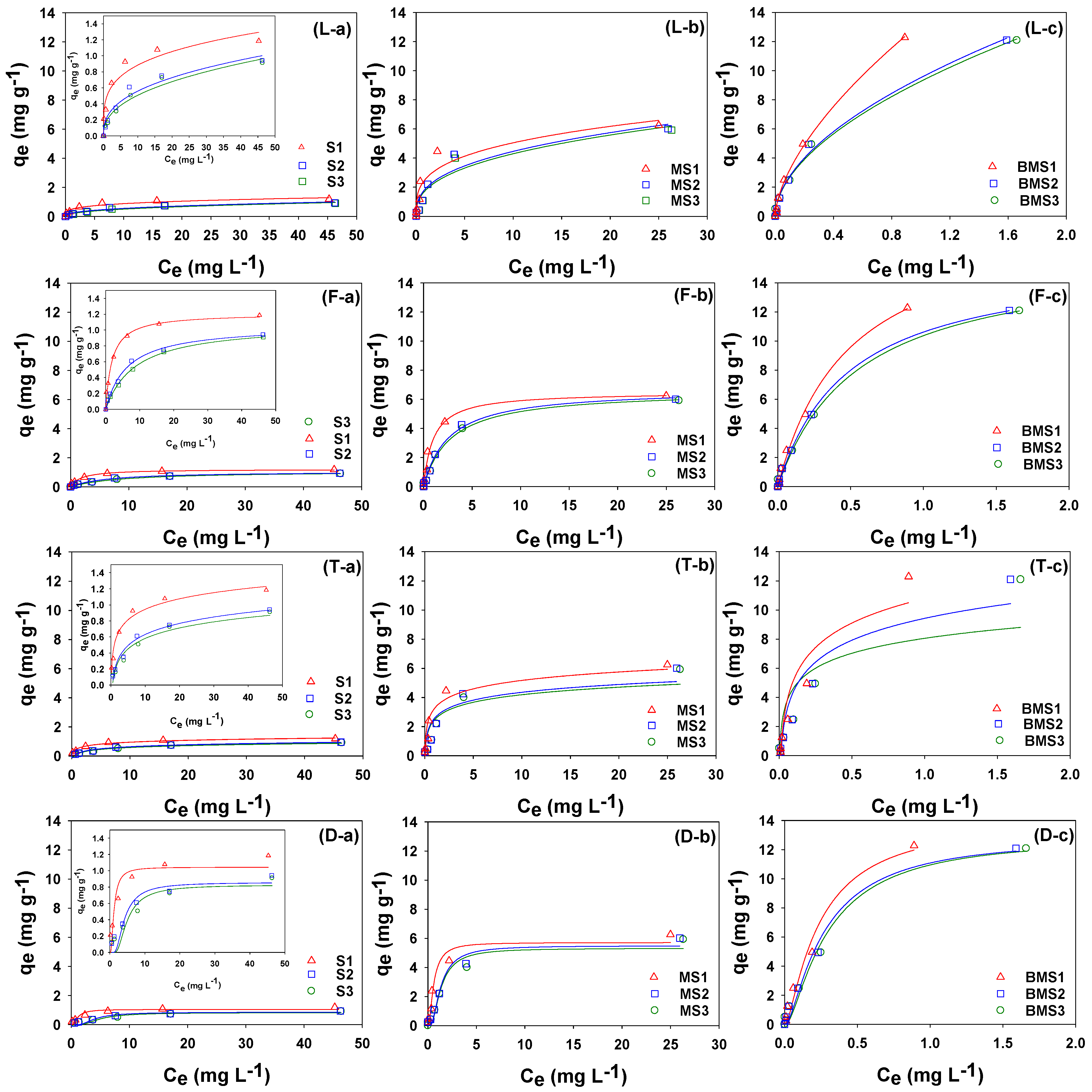
2.5. Sorption Equilibrium
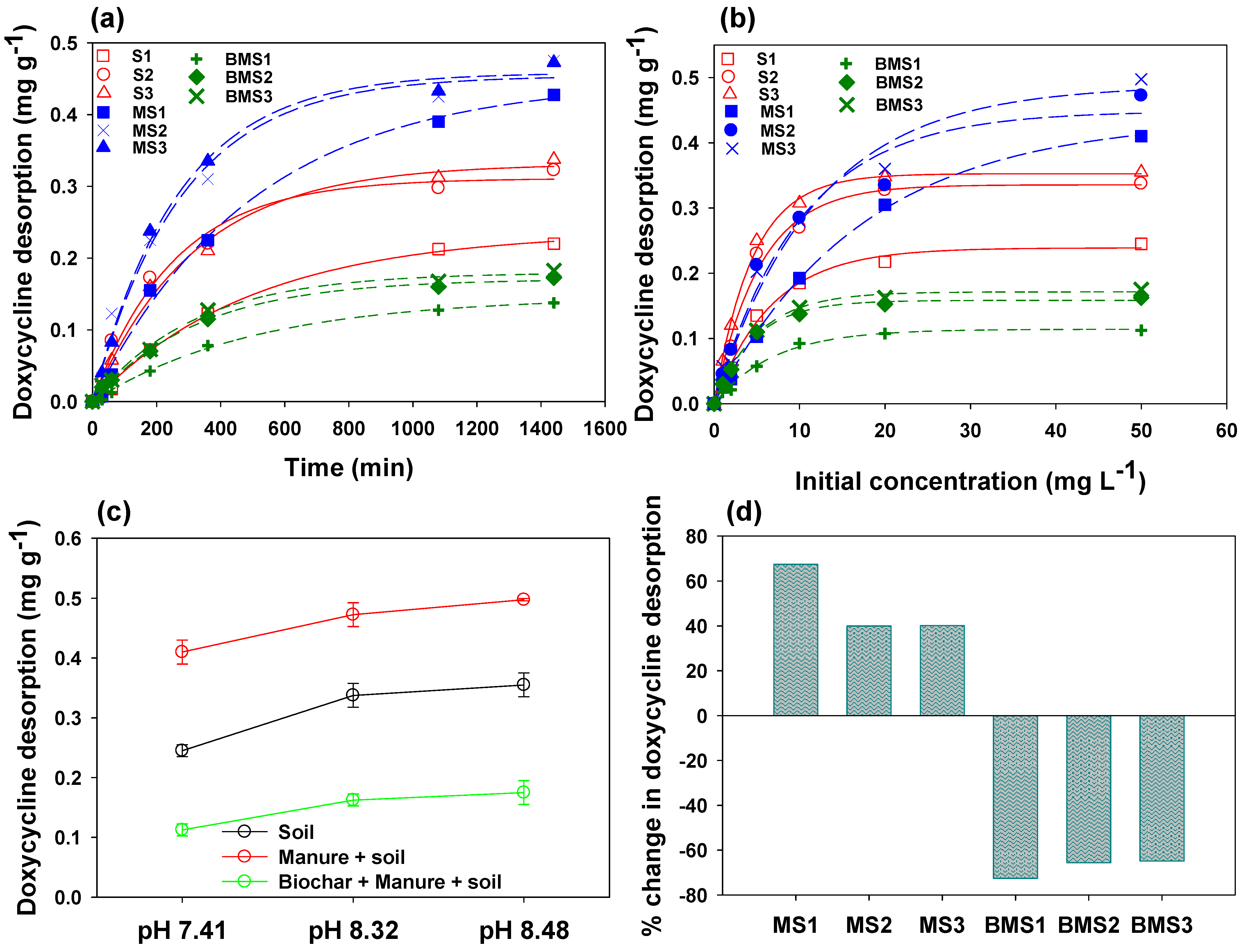
2.6. Desorption of Doxycycline
2.7. Environmental Implications
3. Materials and Methods
3.1. Materials
3.2. Soil and Manure Sample Collection and Characterization
3.3. Biochar Production and Characterization
3.4. Sorption–Desorption Experiments
3.5. Solid Phase Extraction
3.6. Quantification of Doxycycline
3.7. Kinetics Modeling
3.8. Isotherm Modeling
3.9. Statistical Analyses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, K.; Gao, L.; Zhang, Q.; Shang, J. Accumulation of sulfamethazine and ciprofloxacin on grain surface decreases the transport of biochar colloids in saturated porous media. J. Hazard. Mater. 2021, 417, 125908. [Google Scholar] [CrossRef] [PubMed]
- McManus, P.S.; Stockwell, V.O.; Sundin, G.W.; Jones, A.L. Antibiotic Use in Plant Agriculture. Annu. Rev. Phytopathol. 2002, 40, 443–465. [Google Scholar] [CrossRef] [PubMed]
- Carr, C.; Hersom, M.J.; Jeong, K.C.; Di Lorenzo, N.; Scheffler, J.M.; Faniola, G.; Miller, S.; Denney, H.; Roberts, V.; Williams, N.; et al. Antibiotic Use and Resistance for Beef Cattle Producers. EDIS 2019, 2019, 1–5. [Google Scholar] [CrossRef]
- Sarmah, A.K.; Meyer, M.T.; Boxall, A.B.A. A Global Perspective on the Use, Sales, Exposure Pathways, Occurrence, Fate and Effects of Veterinary Antibiotics (VAs) in the Environment. Chemosphere 2006, 65, 725–759. [Google Scholar] [CrossRef]
- Boeckel, T.P.V.; Brower, C.; Gilbert, M.; Grenfell, B.T.; Levin, S.A.; Robinson, T.P.; Teillant, A.; Laxminarayan, R. Global Trends in Antimicrobial Use in Food Animals. Proc. Natl. Acad. Sci. USA 2015, 112, 5649–5654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuppusamy, S.; Kakarla, D.; Venkateswarlu, K.; Megharaj, M.; Yoon, Y.-E.; Lee, Y.B. Veterinary Antibiotics (VAs) Contamination as a Global Agro-Ecological Issue: A Critical View. Agric. Ecosyst. Environ. 2018, 257, 47–59. [Google Scholar] [CrossRef]
- Zhu, Y.G.; Gillings, M.; Simonet, P.; Stekel, D.; Banwart, S.; Penuelas, J. Microbial Mass Movements. Science 2017, 357, 1099–1100. [Google Scholar] [CrossRef] [Green Version]
- Biel-Maeso, M.; Corada-Fernández, C.; Lara-Martín, P.A. Monitoring the Occurrence of Pharmaceuticals in Soils Irrigated with Reclaimed Wastewater. Environ. Pollut. 2018, 235, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Muurinen, J.; Stedtfeld, R.; Karkman, A.; Pärnänen, K.; Tiedje, J.; Virta, M. Influence of Manure Application on the Environmental Resistome under Finnish Agricultural Practice with Restricted Antibiotic Use. Environ. Sci. Technol. 2017, 51, 5989–5999. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Liu, W. Occurrence, Fate, and Ecotoxicity of Antibiotics in Agro-Ecosystems. A Review. Agron. Sustain. Dev. 2012, 32, 309–327. [Google Scholar] [CrossRef] [Green Version]
- Akkaya Sayğılı, G.; Sayğılı, H.; Koyuncu, F.; Güzel, F. Development and Physicochemical Characterization of a New Magnetic Nanocomposite as an Economic Antibiotic Remover. Process Saf. Environ. Prot. 2015, 94, 441–451. [Google Scholar] [CrossRef]
- Hamscher, G.; Sczesny, S.; Höper, H.; Nau, H. Determination of Persistent Tetracycline Residues in Soil Fertilized with Liquid Manure by High-Performance Liquid Chromatography with Electrospray Ionization Tandem Mass Spectrometry. Anal. Chem. 2002, 74, 1509–1518. [Google Scholar] [CrossRef]
- Jannat Abadi, M.H.; Nouri, S.M.M.; Zhiani, R.; Heydarzadeh, H.D.; Motavalizadehkakhky, A. Removal of Tetracycline from Aqueous Solution Using Fe-Doped Zeolite. Int. J. Ind. Chem. 2019, 10, 291–300. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, V.-T.; Hung, C.-M.; Nguyen, T.-B.; Chang, J.-H.; Wang, T.-H.; Wu, C.-H.; Lin, Y.-L.; Chen, C.-W.; Dong, C.-D. Efficient Heterogeneous Activation of Persulfate by Iron-Modified Biochar for Removal of Antibiotic from Aqueous Solution: A Case Study of Tetracycline Removal. Catalysts 2019, 9, 49. [Google Scholar] [CrossRef] [Green Version]
- Ye, Z.; Weinberg, H.S.; Meyer, M.T. Trace Analysis of Trimethoprim and Sulfonamide, Macrolide, Quinolone, and Tetracycline Antibiotics in Chlorinated Drinking Water Using Liquid Chromatography Electrospray Tandem Mass Spectrometry. Anal. Chem. 2007, 79, 1135–1144. [Google Scholar] [CrossRef]
- Widyasari-Mehta, A.; Hartung, S.; Kreuzig, R. From the Application of Antibiotics to Antibiotic Residues in Liquid Manures and Digestates: A Screening Study in One European Center of Conventional Pig Husbandry. J. Environ. Manage. 2016, 177, 129–137. [Google Scholar] [CrossRef]
- Szatmári, I.; Laczay, P.; Borbély, Z. Degradation of Doxycycline in Aged Pig Manure. Acta Vet. Hung. 2011, 59, 1–10. [Google Scholar] [CrossRef]
- Xiong, W.; Zeng, Z.; Li, X.; Zeng, G.; Xiao, R.; Yang, Z.; Xu, H.; Chen, H.; Cao, J.; Zhou, C.; et al. Ni-doped MIL-53 (Fe) nanoparticles for optimized doxycycline removal by using response surface methodology from aqueous solution. Chemosphere 2019, 232, 186–194. [Google Scholar] [CrossRef]
- Ahmad, J.; Naeem, S.; Ahmad, M.; Usman, A.R.; Al-Wabel, M.I. A critical review on organic micropollutants contamination in wastewater and removal through carbon nanotubes. J. Environ. Manage. 2019, 246, 214–228. [Google Scholar] [CrossRef]
- Igwegbe, C.; Aniagor, C.O.; Ighalo, J.; Oba, S. Adsorption of Doxycycline from Aqueous Media: A Review. J. Mol. Liq. 2021, 334, 116124. [Google Scholar] [CrossRef]
- Damian, G.E.; Micle, V.; Sur, I.M. Removal of heavy metals from contaminated soil using chitosan as washing agent a-preliminary study. J. Environ. Protec. Ecol. 2020, 21, 823–829. [Google Scholar]
- Seleiman, M.F.; Alotaibi, M.A.; Alhammad, B.A.; Alharbi, B.M.; Refay, Y.; Badawy, S.A. Effects of ZnO nanoparticles and biochar of rice straw and cow manure on characteristics of contaminated soil and sunflower productivity, oil quality, and heavy metals uptake. Agronomy 2020, 10, 790. [Google Scholar] [CrossRef]
- Chao, Y.; Zhu, W.; Wu, X.; Hou, F.; Xun, S.; Wu, P.; Ji, H.; Xu, H.; Li, H. Application of Graphene-like Layered Molybdenum Disulfide and Its Excellent Adsorption Behavior for Doxycycline Antibiotic. Chem. Eng. J. 2014, 243, 60–67. [Google Scholar] [CrossRef]
- Song, Y.; Wang, F.; Kengara, F.O.; Yang, X.; Gu, C.; Jiang, X. Immobilization of Chlorobenzenes in Soil Using Wheat Straw Biochar. J. Agric. Food Chem. 2013, 61, 4210–4217. [Google Scholar] [CrossRef]
- Guo, H.; Jiao, T.; Zhang, Q.; Guo, W.; Peng, Q.; Yan, X. Preparation of Graphene Oxide-Based Hydrogels as Efficient Dye Adsorbents for Wastewater Treatment. Nanoscale Res. Lett. 2015, 10, 272. [Google Scholar] [CrossRef] [Green Version]
- Rajapaksha, A.U.; Alam, M.d.S.; Chen, N.; Alessi, D.S.; Igalavithana, A.D.; Tsang, D.C.W.; Ok, Y.S. Removal of Hexavalent Chromium in Aqueous Solutions Using Biochar: Chemical and Spectroscopic Investigations. Sci. Total Environ. 2018, 625, 1567–1573. [Google Scholar] [CrossRef]
- Liu, N.; Zhu, M.; Wang, H.; Ma, H. Adsorption Characteristics of Direct Red 23 from Aqueous Solution by Biochar. J. Mol. Liq. 2016, 223, 335–342. [Google Scholar] [CrossRef]
- Liu, Z.; Tian, D.; Hu, J.; Shen, F.; Long, L.; Zhang, Y.; Yang, G.; Zeng, Y.; Zhang, J.; He, J.; et al. Functionalizing Bottom Ash from Biomass Power Plant for Removing Methylene Blue from Aqueous Solution. Sci. Total Environ. 2018, 634, 760–768. [Google Scholar] [CrossRef]
- Mandal, A.; Singh, N.; Purakayastha, T.J. Characterization of Pesticide Sorption Behaviour of Slow Pyrolysis Biochars as Low Cost Adsorbent for Atrazine and Imidacloprid Removal. Sci. Total Environ. 2017, 577, 376–385. [Google Scholar] [CrossRef]
- Chen, D.; Liu, X.; Bian, R.; Cheng, K.; Zhang, X.; Zheng, J.; Joseph, S.; Crowley, D.; Pan, G.; Li, L. Effects of biochar on availability and plant uptake of heavy metals—Ameta-analysis. J. Environ. Manag. 2018, 222, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Abdin, Y.; Usman, A.; Ok, Y.S.; Tsang, Y.F.; Al-Wabel, M. Competitive sorption and availability of coexisting heavy metals in mining-contaminated soil: Contrasting effects of mesquite and fishbone biochars. Environ. Res. 2020, 181, 108846. [Google Scholar] [CrossRef]
- Kazmi, S.J.; Shaikh, S.; Zamir, U.B.; Zafar, H.; Rasool, A.; Tariq, F.; Afzal, A.; Arif, T. Ecological and Socio-Economic Evaluation of the Use of Prosopis Juliflora for Biochar Production in Pakistan. Drynet Pak. 2009, 1–54. [Google Scholar]
- Wahba, M.M.; Fawkia, L.; Zaghloul, A. Management of Calcareous Soils in Arid Region. Int. J. Environ. Pollut. 2019, 2, 248–258. [Google Scholar]
- Sun, X.; Yang, L.; Li, Q.; Zhao, J.; Li, X.; Wang, X.; Liu, H. Amino-Functionalized Magnetic Cellulose Nanocomposite as Adsorbent for Removal of Cr(VI): Synthesis and Adsorption Studies. Chem. Eng. J. 2014, 241, 175–183. [Google Scholar] [CrossRef]
- Al-Wabel, M.I.; Al-Omran, A.; El-Naggar, A.H.; Nadeem, M.; Usman, A.R.A. Pyrolysis Temperature Induced Changes in Characteristics and Chemical Composition of Biochar Produced from Conocarpus Wastes. Bioresour. Technol. 2013, 131, 374–379. [Google Scholar] [CrossRef]
- Zhao, Y.; Geng, J.; Wang, X.; Gu, X.; Gao, S. Tetracycline Adsorption on Kaolinite: pH, Metal Cations and Humic Acid Effects. Ecotoxicology 2011, 20, 1141–1147. [Google Scholar] [CrossRef]
- Park, J.-H.; Wang, J.J.; Kim, S.-H.; Kang, S.-W.; Jeong, C.Y.; Jeon, J.-R.; Park, K.H.; Cho, J.-S.; Delaune, R.D.; Seo, D.-C. Cadmium Adsorption Characteristics of Biochars Derived Using Various Pine Tree Residues and Pyrolysis Temperatures. J. Colloid Interface Sci. 2019, 553, 298–307. [Google Scholar] [CrossRef]
- Usman, A.R.A.; Abduljabbar, A.; Vithanage, M.; Ok, Y.S.; Ahmad, M.; Ahmad, M.; Elfaki, J.; Abdulazeem, S.S.; Al-Wabel, M.I. Biochar Production from Date Palm Waste: Charring Temperature Induced Changes in Composition and Surface Chemistry. J. Anal. Appl. Pyrolysis 2015, 115, 392–400. [Google Scholar] [CrossRef]
- Zhao, Y.; Huang, L.; Chen, Y. Biochars Derived from Giant Reed (Arundo Donax L.) with Different Treatment: Characterization and Ammonium Adsorption Potential. Environ. Sci. Pollut. Res. 2017, 24, 25889–25898. [Google Scholar] [CrossRef]
- Rafique, M.I.; Usman, A.R.A.; Ahmad, M.; Al-Wabel, M.I. Immobilization and Mitigation of Chromium Toxicity in Aqueous Solutions and Tannery Waste-Contaminated Soil Using Biochar and Polymer-Modified Biochar. Chemosphere 2021, 266, 129198. [Google Scholar] [CrossRef]
- Bavariani, M.Z.; Ronaghi, A.; Ghasemi, R. Influence of Pyrolysis Temperatures on FTIR Analysis, Nutrient Bioavailability, and Agricultural Use of Poultry Manure Biochars. Commun. Soil Sci. Plant Anal. 2019, 50, 402–411. [Google Scholar] [CrossRef]
- Jones, A.D.; Bruland, G.L.; Agrawal, S.G.; Vasudevan, D. Factors Influencing the Sorption of Oxytetracycline to Soils. Environ. Toxicol. Chem. 2005, 24, 761–770. [Google Scholar] [CrossRef]
- Wang, Y.-J.; Sun, R.-J.; Xiao, A.-Y.; Wang, S.-Q.; Zhou, D.-M. Phosphate Affects the Adsorption of Tetracycline on Two Soils with Different Characteristics. Geoderma 2010, 156, 237–242. [Google Scholar] [CrossRef]
- Aristilde, L.; Marichal, C.; Miéhé-Brendlé, J.; Lanson, B.; Charlet, L. Interactions of oxytetracycline with a smectite clay: A spectroscopic study with molecular simulations. Environ. Sci. Technol. 2010, 4, 7839–7845. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Lim, S.; Han, M.; Cho, J. Sorption characteristics of oxytetracycline, amoxicillin, and sulfathiazole in two different soil types. Geoderma 2012, 185, 97–101. [Google Scholar] [CrossRef]
- Kahle, M.; Stamm, C. Sorption of the Veterinary Antimicrobial Sulfathiazole to Organic Materials of Different Origin. Environ. Sci. Technol. 2007, 41, 132–138. [Google Scholar] [CrossRef]
- Zhang, X.; Bai, B.; Li Puma, G.; Wang, H.; Suo, Y. Novel Sea Buckthorn Biocarbon SBC@β-FeOOH Composites: Efficient Removal of Doxycycline in Aqueous Solution in a Fixed-Bed through Synergistic Adsorption and Heterogeneous Fenton-like Reaction. Chem. Eng. J. 2016, 284, 698–707. [Google Scholar] [CrossRef] [Green Version]
- Lagaly, G.; Ogawa, M.; Dékány, I. Chapter 10.3—Clay mineral-organic interactions. In Developments in Clay Science; Handbook of Clay, Science; Bergaya, F., Lagaly, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 5, pp. 435–505. [Google Scholar]
- Wei, J.; Liu, Y.; Li, J.; Zhu, Y.; Yu, H.; Peng, Y. Adsorption and Co-Adsorption of Tetracycline and Doxycycline by One-Step Synthesized Iron Loaded Sludge Biochar. Chemosphere 2019, 236, 124254. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Ge, C.; Feng, D.; Yu, H.; Luo, J.; Li, J.; Strong, P.J.; Sarmah, A.K.; Bolan, N.S.; Wang, H. Effects of Metal Ions and PH on Ofloxacin Sorption to Cassava Residue-Derived Biochar. Sci. Total Environ. 2018, 616–617, 1384–1391. [Google Scholar] [CrossRef]
- Ahmad, M.; Ahmad, M.; Usman, A.R.A.; Al-Faraj, A.S.; Ok, Y.S.; Hussain, Q.; Abduljabbar, A.S.; Al-Wabel, M.I. An Efficient Phosphorus Scavenging from Aqueous Solution Using Magnesiothermally Modified Bio-Calcite. Environ. Technol. 2018, 39, 1638–1649. [Google Scholar] [CrossRef]
- Tan, X.; Liu, Y.; Gu, Y.; Xu, Y.; Zeng, G.; Hu, X.; Liu, S.; Wang, X.; Liu, S.; Li, J. Biochar-Based Nano-Composites for the Decontamination of Wastewater: A Review. Bioresour. Technol. 2016, 212, 318–333. [Google Scholar] [CrossRef] [PubMed]
- Conde-Cid, M.; Álvarez-Esmorís, C.; Paradelo-Núñez, R.; Nóvoa-Muñoz, J.C.; Arias-Estévez, M.; Álvarez-Rodríguez, E.; Fernández-Sanjurjo, M.J.; Núñez-Delgado, A. Occurrence of Tetracyclines and Sulfonamides in Manures, Agricultural Soils and Crops from Different Areas in Galicia (NW Spain). J. Clean. Prod. 2018, 197, 491–500. [Google Scholar] [CrossRef]
- Park, J.Y.; Huwe, B. Effect of PH and Soil Structure on Transport of Sulfonamide Antibiotics in Agricultural Soils. Environ. Pollut. 2016, 213, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Al-Wabel, M.I.; Ahmad, M.; Rafique, M.I.; Akanji, M.A.; Usman, A.R.; Al-Farraj, A.S. Sulfamethoxazole Leaching from Manure-Amended Sandy Loam Soil as Affected by the Application of Jujube Wood Waste-Derived Biochar. Molecules 2021, 26, 4674. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhang, Y.; Gao, Y.; Boyd, S.A.; Zhu, D.; Li, H. Influence of dissolved organic matter on tetracycline bioavailability to an antibiotic-resistant bacterium. Environ. Sci. Technol. 2015, 49, 10903–10910. [Google Scholar] [CrossRef]
- Chen, Y.; Hu, C.; Deng, D.; Li, Y.; Luo, L. Factors affecting sorption behaviors of tetracycline to soils: Importance of soil organic carbon, pH and Cd contamination. Ecotox. Environ. Saf. 2020, 197, 110572. [Google Scholar] [CrossRef]
- Pan, M.; Chu, L.M. Adsorption and degradation of five selected antibiotics in agricultural soil. Sci. Total Environ. 2016, 545, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Tan, X.; Liu, Y.; Tian, S.; Zeng, G.; Jiang, L.; Liu, S.; Li, J.; Liu, N.; Yin, Z. Comprehensive Adsorption Studies of Doxycycline and Ciprofloxacin Antibiotics by Biochars Prepared at Different Temperatures. Front. Chem. 2018, 93, 1075–1084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmad, M.; Usman, A.R.A.; Rafique, M.I.; Al-Wabel, M.I. Engineered Biochar Composites with Zeolite, Silica, and Nano-Zerovalent Iron for the Efficient Scavenging of Chlortetracycline from Aqueous Solutions. Environ. Sci. Pollut. Res. 2019, 26, 15136–15152. [Google Scholar] [CrossRef] [PubMed]
- Sassman, S.A.; Lee, L.S. Sorption of Three Tetracyclines by Several Soils: Assessing the Role of pH and Cation Exchange. Environ. Sci. Technol. 2005, 39, 7452–7459. [Google Scholar] [CrossRef] [PubMed]
- Boxall, A.; Tiede, K.; Bryning, G.; Bevan, R.; Levy, L. Desk-based study of current knowledge on veterinary medicines in drinking water and estimation of potential levels. Report Ref. DWI 2011, 70, 235. [Google Scholar]
- Peiris, C.; Gunatilake, S.R.; Mlsna, T.E.; Mohan, D.; Vithanage, M. Biochar Based Removal of Antibiotic Sulfonamides and Tetracyclines in Aquatic Environments: A Critical Review. Bioresour. Technol. 2017, 246, 150–159. [Google Scholar] [CrossRef]
- Xie, M.; Chen, W.; Xu, Z.; Zheng, S.; Zhu, D. Adsorption of Sulfonamides to Demineralized Pine Wood Biochars Prepared under Different Thermochemical Conditions. Environ. Pollut. 2014, 186, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Abel, S.; Nybom, I.; Mäenpää, K.; Hale, S.E.; Cornelissen, G.; Akkanen, J. Mixing and Capping Techniques for Activated Carbon Based Sediment Remediation—Efficiency and Adverse Effects for Lumbriculus Variegatus. Water Res. 2017, 114, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Fernández, C.; Alonso, C.; Babín, M.M.; Pro, J.; Carbonell, G.; Tarazona, J.V. Ecotoxicological Assessment of Doxycycline in Aged Pig Manure Using Multispecies Soil Systems. Sci. Total Environ. 2004, 323, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, S.E.; Halling-Sørensen, B. Drugs in the Environment. Chemosphere 2000, 40, 691–699. [Google Scholar] [CrossRef]
- Hesse, P.R. A Textbook of Soil Chemical Analysis; (No. 631.41 H4); Chemical Publishing Company: Revere, MA, USA, 1971. [Google Scholar]
- ASTM, Designation. 84 Standard Test Method for Chemical Analysis of Wood Charcoal; ASTM International: West Conshohocken, PA, USA, 1762; Volume 84, pp. 1–2. [Google Scholar]
- Al-Wabel, M.I.; Ahmad, M.; Ahmad, J.; Lubis, N.M.; Usman, A.R.; Al-Farraj, A.S. Assessing the prevalence of veterinary antibiotics and associated potential ecological risk in dryland soil, manure, and compost: A case study from Saudi Arabia. J. King Saud Uni. -Sci. 2021, 33, 101558. [Google Scholar] [CrossRef]
- Pils, J.R.V.; Laird, D.A. Sorption of Tetracycline and Chlortetracycline on K- and Ca-Saturated Soil Clays, Humic Substances, and Clay-Humic Complexes. Environ. Sci. Technol. 2007, 41, 1928–1933. [Google Scholar] [CrossRef]
- Sparks, D.L. Kinetics and Mechanisms of Chemical Reactions at the Soil Mineral/Water Interface. In Soil Physical Chemistry; CRC Press: Boca Raton, FL, USA, 1998; ISBN 978-0-203-73928-0. [Google Scholar]
- Ho, Y.S.; McKay, G. Pseudo-Second Order Model for Sorption Processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Insights into the Modeling of Adsorption Isotherm Systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
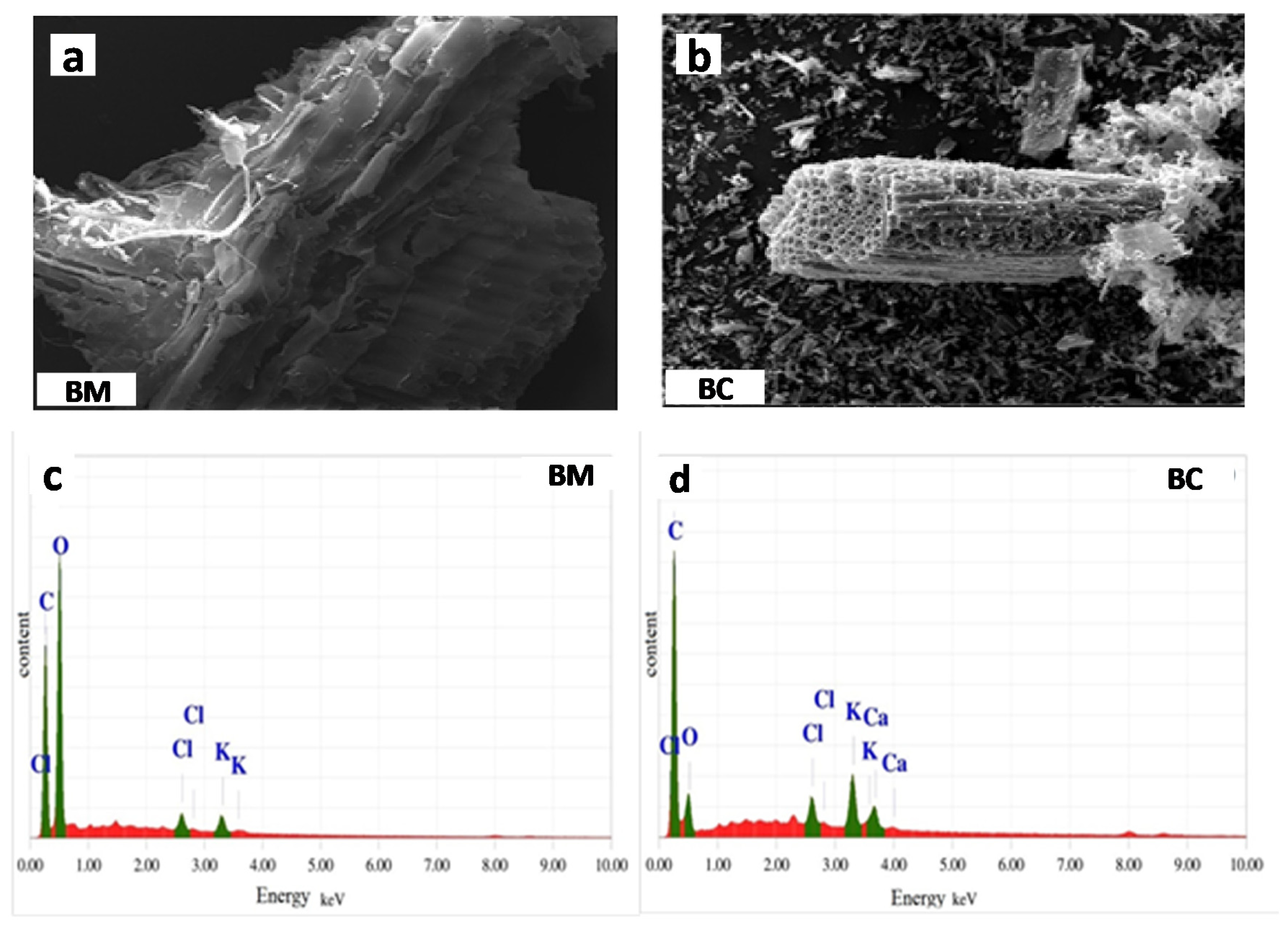


| Property | Unit | S1 | S2 | S3 | Manure |
|---|---|---|---|---|---|
| Sand | % | 56.40 ± 4.31 | 73.90 ± 5.15 | 81.40 ± 5.20 | - |
| Silt | % | 17.50 ± 1.45 | 10.00 ± 0.91 | 2.50 ± 0.44 | - |
| Clay | % | 26.10 ± 3.98 | 16.10 ± 2.98 | 16.10 ± 1.12 | - |
| Texture | - | Sandy clay loam | Sandy loam | Sandy loam | - |
| pH | - | 7.41 ± 0.24 | 8.32 ± 0.21 | 8.48 ± 0.30 | 7.72 ± 0.22 |
| EC | dS m−1 | 4.82 ± 0.12 | 1.42 ± 0.11 | 2.30 ± 0.02 | 6.61 ± 0.65 |
| Organic matter | % | 0.86 ± 0.09 | 0.71 ± 0.08 | 0.67 ± 0.02 | 10.34 ± 0.84 |
| CEC | cmol kg−1 | 26.39 ± 2.64 | 18.28 ± 1.98 | 19.89 ± 0.87 | 57.29 ± 5.61 |
| CaCO3 | % | 8.55 ± 1.01 | 9.86 ± 1.80 | 9.33 ± 0.21 | - |
| Available P | mg kg−1 | 11.89 ± 1.85 | 8.68 ± 1.35 | 7.81 ± 0.33 | 1970.82 ± 18.71 |
| Available K | mg kg−1 | 107 ± 8.15 | 96 ± 5.41 | 68 ± 3.64 | 40.16 ± 5.56 |
| Available Na | mg kg−1 | 34.75 ± 2.87 | 22.87 ± 1.55 | 26.43 ± 1.21 | 46.09 ± 3.67 |
| Doxycycline | μg kg−1 | N.D. | N.D. | N.D. | N.D. |
| Moisture | % | 1.58 ± 0.01 | 1.51 ± 0.01 | 1.43 ± 0.02 | 13.03 ± 0.28 |
| Volatiles | % | - | - | - | 38.72 ± 3.59 |
| Fixed carbon | % | - | - | - | 19.29 ± 1.76 |
| Ash | % | - | - | - | 28.96 ± 2.87 |
| Property | Unit | BM | BC |
|---|---|---|---|
| Yield | % | – | 30.2 ± 4.32 |
| Moisture | % | 8.1 ± 0.95 | 2.10 ± 0.82 |
| Ash | % | 1.0 ± 0.16 | 14.5 ± 1.32 |
| Volatiles | % | 72.4 ± 6.71 | 7.6 ± 0.81 |
| Fixed carbon | % | 22.6 ± 1.23 | 75.8 ± 6.13 |
| Surface area | m2 g−1 | - | 196 ± 8.18 |
| Doxycycline | μg kg−1 | - | N.D. |
| C | % | - | 80.08 ± 6.20 |
| O | % | - | 2.49 ± 0.11 |
| H | % | - | 1.64 ± 0.05 |
| N | % | - | 1.30 ± 0.08 |
| O/C molar ratio | - | - | 0.02 ± 0.00 |
| H/C molar ratio | - | - | 0.24 ± 0.00 |
| (O + N)/C molar ratio | - | - | 0.04 ± 0.00 |
| Soil Alone | Manure + Soil | Biochar + Manure + Soil | ||
|---|---|---|---|---|
| pH | r | −0.999 | −0.996 | −0.998 |
| p-value | 0.017 | 0.047 | 0.036 | |
| Phosphorus | r | 0.995 | 0.997 | 0.992 |
| p-value | 0.060 | 0.045 | 0.077 | |
| CEC | r | 0.956 | 0.894 | 0.963 |
| p-value | 0.189 | 0.295 | 0.172 | |
| CaCO3 | r | −0.866 | −0.771 | −0.879 |
| p-value | 0.332 | 0.438 | 0.315 | |
| Clay | r | 0.996 | 0.967 | 0.996 |
| p-value | 0.049 | 0.045 | 0.050 | |
| EC | r | 0.935 | 0.864 | 0.944 |
| p-value | 0.229 | 0.335 | 0.212 | |
| Moisture | r | 0.898 | 0.959 | 0.887 |
| p-value | 0.288 | 0.182 | 0.305 | |
| Organic matter | r | 0.996 | 0.997 | 0.997 |
| p-value | 0.050 | 0.047 | 0.045 | |
| Sand | r | −0.982 | −0.998 | −0.977 |
| p-value | 0.119 | 0.013 | 0.136 | |
| Silt | r | 0.915 | 0.969 | 0.904 |
| p-value | 0.264 | 0.158 | 0.280 |
| Sorbent | Parameters | S1 | S2 | S3 | MS1 | MS2 | MS3 | BMS1 | BMS2 | BMS3 |
|---|---|---|---|---|---|---|---|---|---|---|
| First order | k1 | 0.001 | 0.002 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.002 |
| R2 | 0.308 | 0.225 | 0.314 | 0.665 | 0.706 | 0.659 | 0.520 | 0.618 | 0.654 | |
| Second order | k2 | −0.008 | −0.007 | −0.009 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| R2 | 0.126 | 0.154 | 0.215 | 0.153 | 0.181 | 0.166 | 0.147 | 0.172 | 0.166 | |
| Pseudo-first order | −0.001 | −0.001 | −0.001 | −0.002 | −0.002 | −0.002 | −0.002 | −0.002 | −0.002 | |
| qe | −0.027 | −0.414 | −0.348 | 1.447 | 1.554 | 1.545 | 2.067 | 2.242 | 2.334 | |
| R2 | 0.245 | 0.159 | 0.171 | 0.684 | 0.774 | 0.790 | 0.746 | 0.818 | 0.879 | |
| Pseudo-second order | 7.2 × 10−4 | 3.9 × 10−2 | 1.262 | 0.983 | 1.319 | 2.474 | 1.237 | 0.756 | 0.880 | |
| qe | 1.870 | 0.022 | 7.0 × 10−4 | 9.2 × 10−4 | 6.7 × 10−4 | 3.5 × 10−4 | 7.1 × 10−4 | 1.1 × 10−3 | 1.0 × 10−3 | |
| h | 2.5 × 10−3 | 1.9 × 10−5 | 6.2 × 10−7 | 8.1 × 10−7 | 5.9 × 10−7 | 3.1 × 10−7 | 6.3 × 10−7 | 1.0 × 10−7 | 8.9 × 10−7 | |
| R2 | 0.397 | 0.103 | 0.105 | 0.104 | 0.104 | 0.104 | 0.104 | 0.104 | 0.104 | |
| Elovich | a | 0.173 | 0.150 | 0.142 | 0.910 | 0.886 | 0.875 | 1.897 | 1.793 | 1.763 |
| β | 0.282 | 0.243 | 0.251 | 0.880 | 1.164 | 1.208 | 1.914 | 2.207 | 2.611 | |
| R2 | 0.977 | 0.992 | 0.981 | 0.997 | 0.993 | 0.997 | 0.965 | 0.997 | 0.992 | |
| Intraparticle diffusion | kid | 0.034 | 0.029 | 0.028 | 0.163 | 0.167 | 0.165 | 0.330 | 0.331 | 0.339 |
| c | −0.039 | −0.017 | −0.054 | 0.683 | 0.222 | 0.152 | 1.485 | 0.698 | 0.023 | |
| R2 | 0.984 | 0.930 | 0.958 | 0.942 | 0.971 | 0.966 | 0.871 | 0.956 | 0.967 | |
| Power function | kf | 0.142 | 0.185 | 0.123 | 0.280 | 0.293 | 0.302 | 0.383 | 0.378 | 0.396 |
| b | −1.507 | −2.087 | −1.715 | −0.255 | −0.501 | −0.610 | −0.155 | −0.263 | −0.516 | |
| R2 | 0.830 | 0.751 | 0.925 | 0.962 | 0.958 | 0.926 | 0.888 | 0.947 | 0.926 |
| Isotherms | Parameters | S1 | S2 | S3 | MS1 | MS2 | MS3 | BMS1 | BMS2 | BMS3 |
|---|---|---|---|---|---|---|---|---|---|---|
| Langmuir | QL (mg g−1) | 1.210 | 1.059 | 1.083 | 6.497 | 6.708 | 6.604 | 18.930 | 15.792 | 16.073 |
| KL (L g−1) | 0.548 | 0.159 | 0.114 | 1.006 | 0.388 | 0.357 | 2.049 | 2.050 | 1.818 | |
| R2 | 0.988 | 0.995 | 0.994 | 0.984 | 0.992 | 0.992 | 0.995 | 0.999 | 0.997 | |
| Freundlich | KF (L g−1) | 0.494 | 0.232 | 0.194 | 2.582 | 1.919 | 1.803 | 13.263 | 9.518 | 9.266 |
| 1/n | 0.253 | 0.382 | 0.417 | 0.291 | 0.366 | 0.377 | 0.618 | 0.540 | 0.539 | |
| R2 | 0.949 | 0.961 | 0.973 | 0.916 | 0.928 | 0.937 | 0.996 | 0.992 | 0.993 | |
| Temkin | b (J mol−1) | 1.23 × 104 | 1.14 × 104 | 1.20 × 104 | 2.71 × 103 | 2.93 × 103 | 3.03 × 103 | 9.35 × 102 | 1.04 × 103 | 1.53 × 103 |
| A (L g−1) | 15.078 | 2.430 | 2.213 | 48.203 | 27.544 | 26.895 | 84.938 | 76.535 | 228.050 | |
| R2 | 0.968 | 0.980 | 0.952 | 0.904 | 0.787 | 0.777 | 0.907 | 0.906 | 0.725 | |
| Dubinin–Radushkevich | qD (mg g−1) | 1.044 | 0.856 | 0.825 | 5.707 | 5.487 | 5.313 | 13.833 | 12.765 | 12.777 |
| E (kJ g−1) | 8.0 × 10−4 | 6.6 × 10−3 | 9.1 × 10−3 | 3.0 × 10−4 | 9.0 × 10−4 | 1.0 × 10−3 | 1.0 × 10−3 | 1.0 × 10−4 | 1.0 × 10−3 | |
| R2 | 0.863 | 0.881 | 0.873 | 0.951 | 0.962 | 0.960 | 0.974 | 0.984 | 0.984 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Wabel, M.I.; Ahmad, M.; Al-Swadi, H.A.; Ahmad, J.; Abdin, Y.; Usman, A.R.A.; Al-Farraj, A.S.F. Sorption–Desorption Behavior of Doxycycline in Soil–Manure Systems Amended with Mesquite Wood Waste Biochar. Plants 2021, 10, 2566. https://doi.org/10.3390/plants10122566
Al-Wabel MI, Ahmad M, Al-Swadi HA, Ahmad J, Abdin Y, Usman ARA, Al-Farraj ASF. Sorption–Desorption Behavior of Doxycycline in Soil–Manure Systems Amended with Mesquite Wood Waste Biochar. Plants. 2021; 10(12):2566. https://doi.org/10.3390/plants10122566
Chicago/Turabian StyleAl-Wabel, Mohammad I., Munir Ahmad, Hamed A. Al-Swadi, Jahangir Ahmad, Yassir Abdin, Adel R. A. Usman, and Abdullah S. F. Al-Farraj. 2021. "Sorption–Desorption Behavior of Doxycycline in Soil–Manure Systems Amended with Mesquite Wood Waste Biochar" Plants 10, no. 12: 2566. https://doi.org/10.3390/plants10122566
APA StyleAl-Wabel, M. I., Ahmad, M., Al-Swadi, H. A., Ahmad, J., Abdin, Y., Usman, A. R. A., & Al-Farraj, A. S. F. (2021). Sorption–Desorption Behavior of Doxycycline in Soil–Manure Systems Amended with Mesquite Wood Waste Biochar. Plants, 10(12), 2566. https://doi.org/10.3390/plants10122566










