Physiological Responses of Two Contrasting Kiwifruit (Actinidia spp.) Rootstocks against Waterlogging Stress
Abstract
:1. Introduction
2. Results
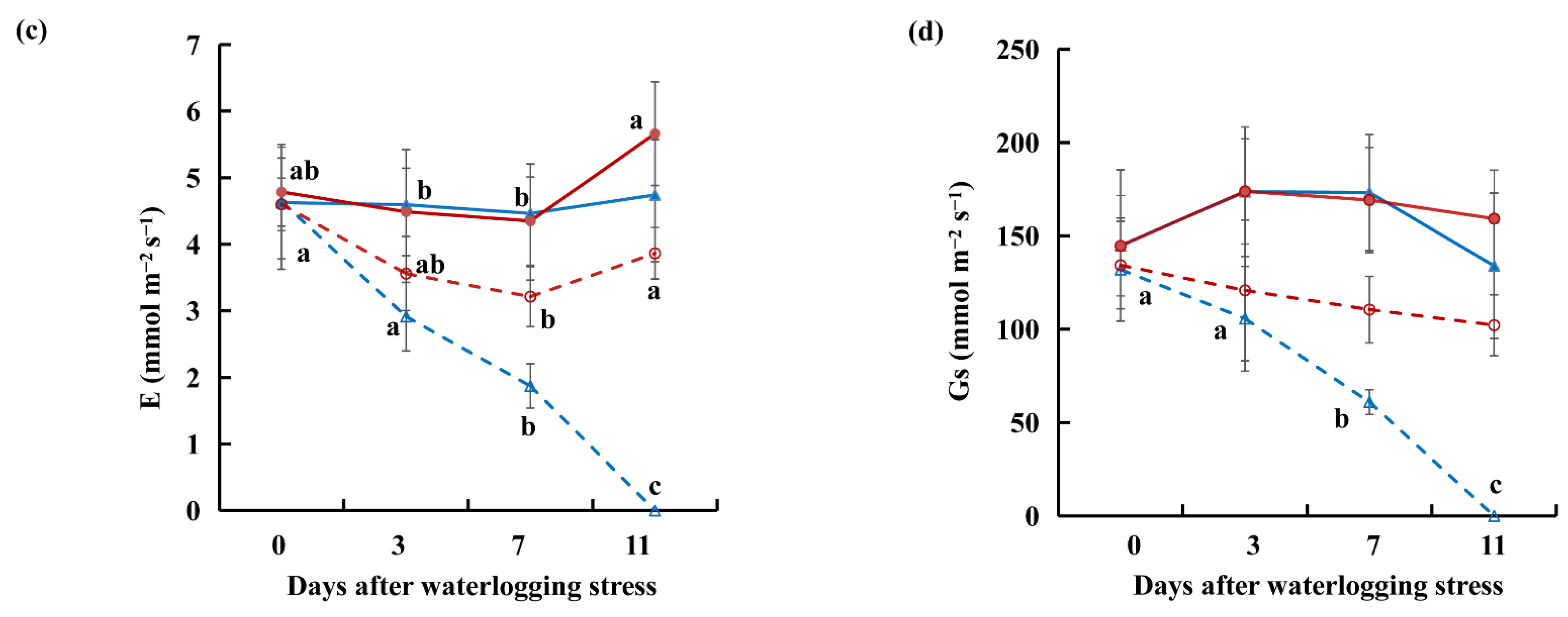
2.1. Relative Water Content, Malonaldehyde, and Gas Exchange Data under Waterlogging Stress
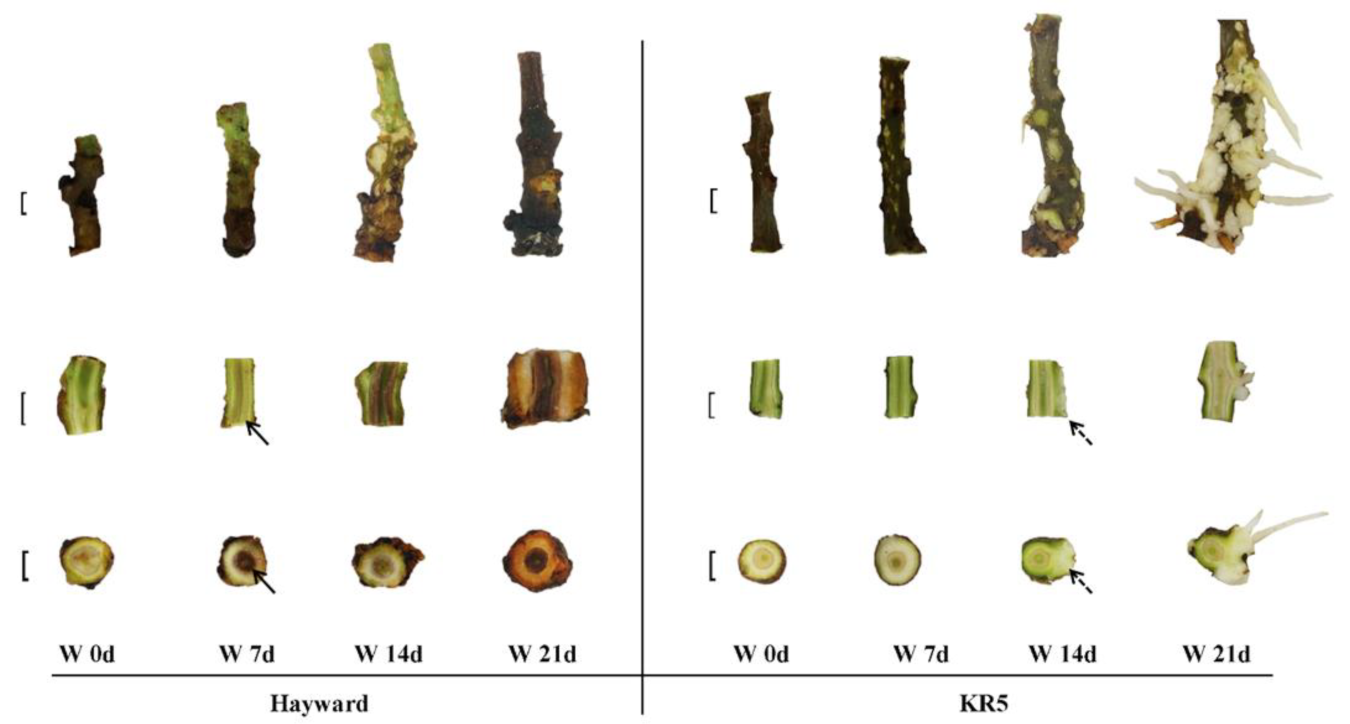
2.2. Morphological Changes of Stem Base and Root under Waterlogging Stress
2.3. Changes of Sucrose Content under Waterlogging Stress
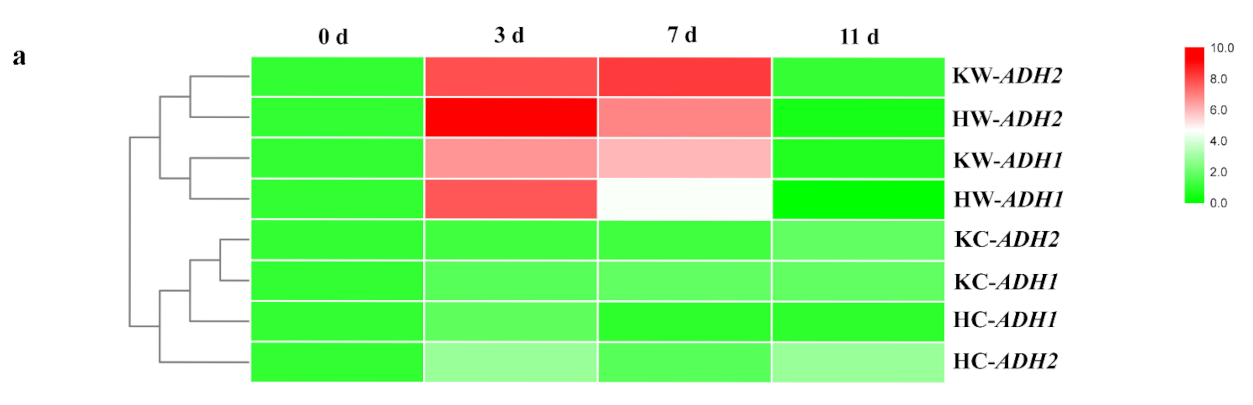
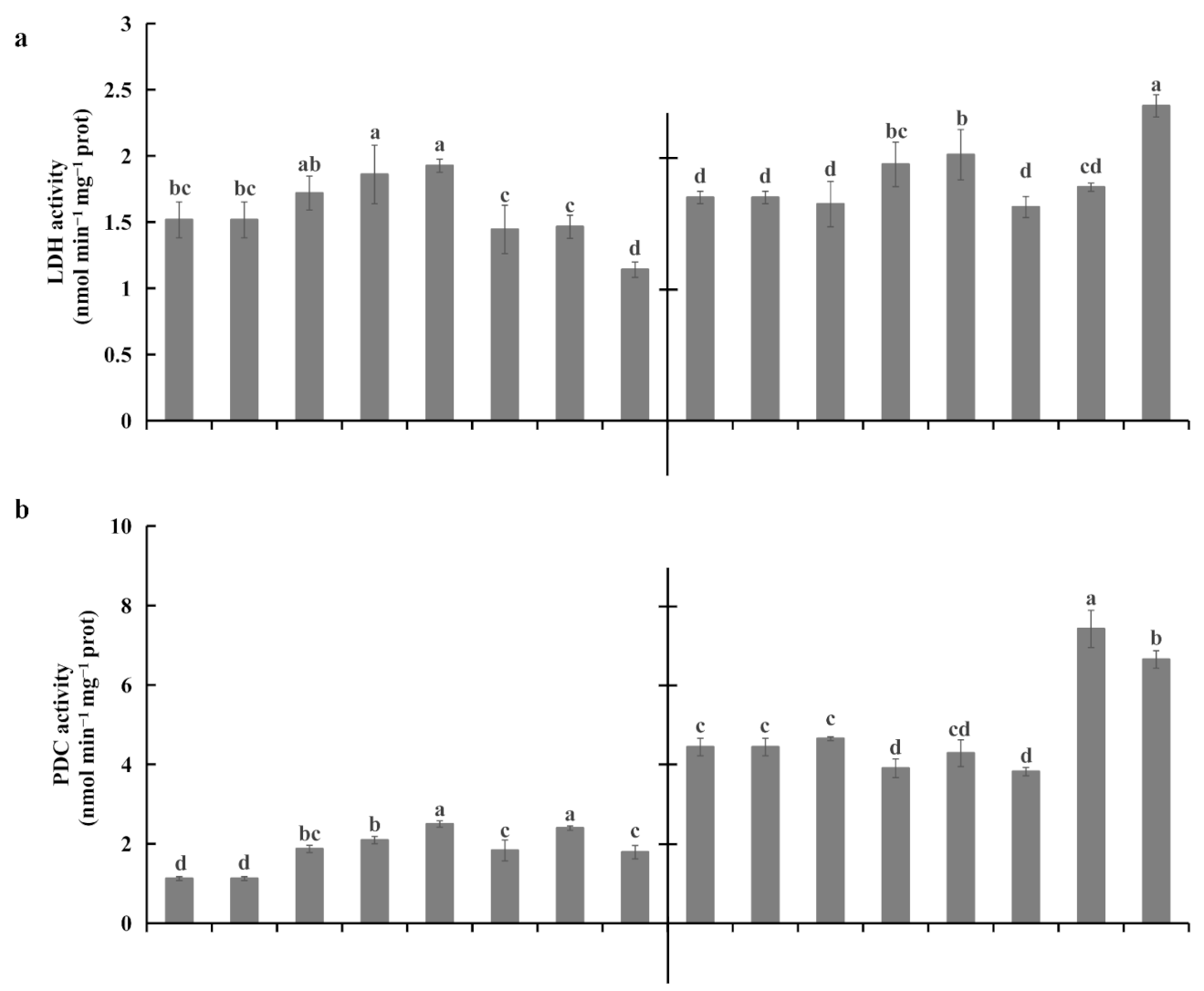
2.4. Root Fermentations under Waterlogging Stress
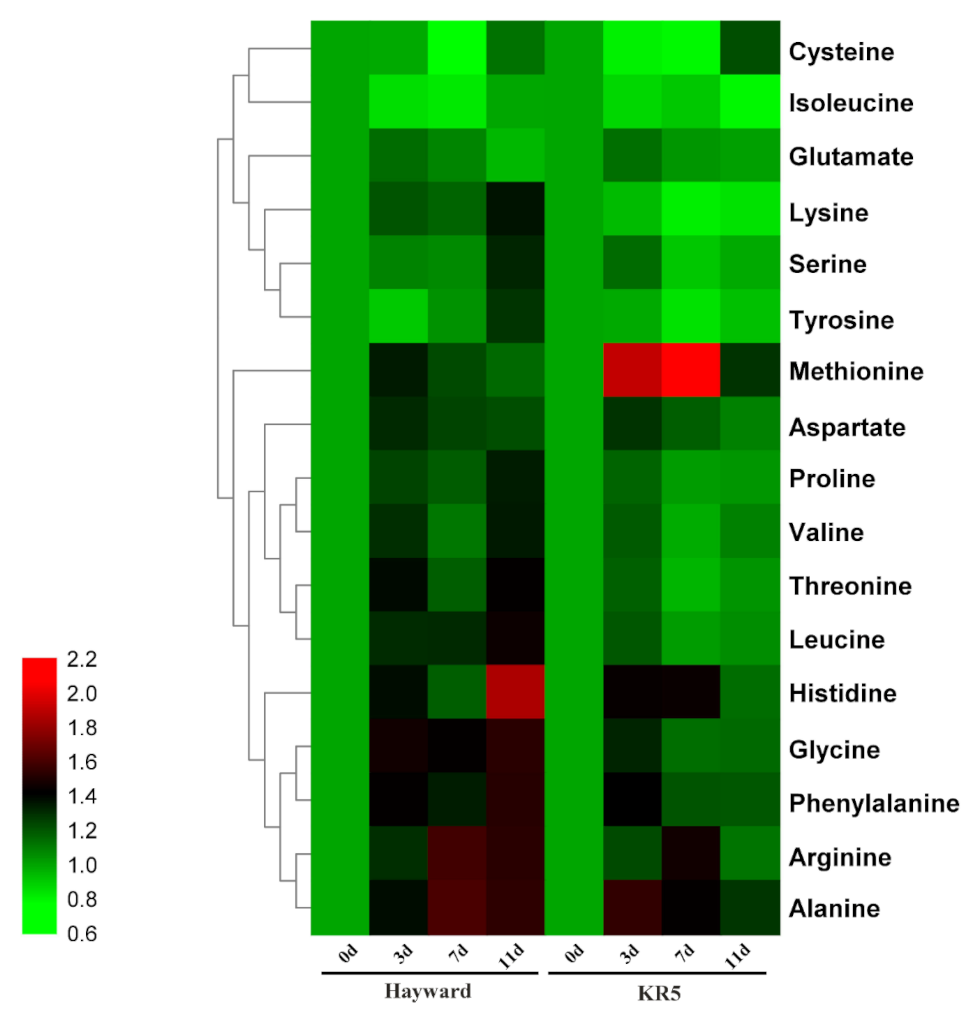
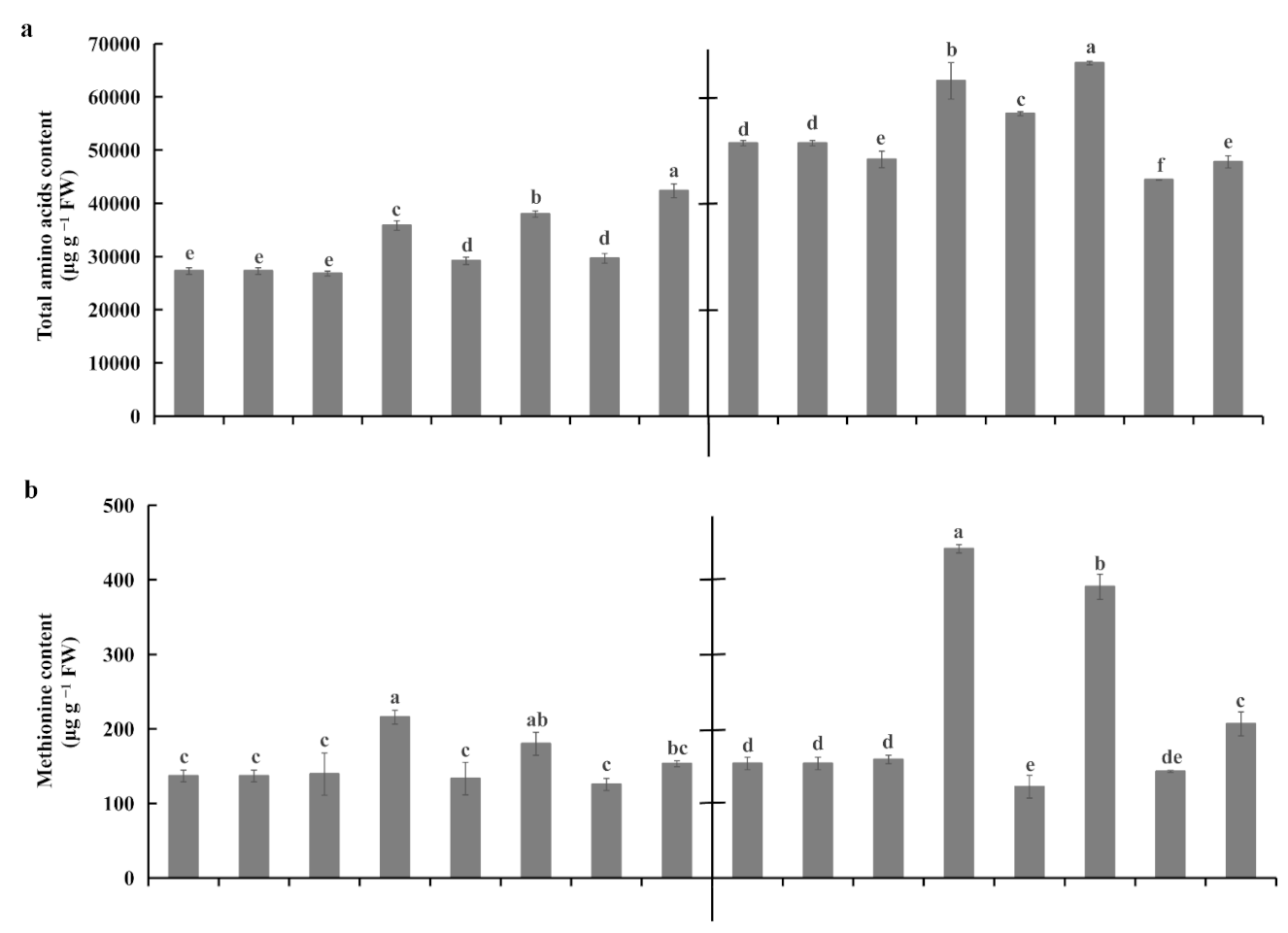
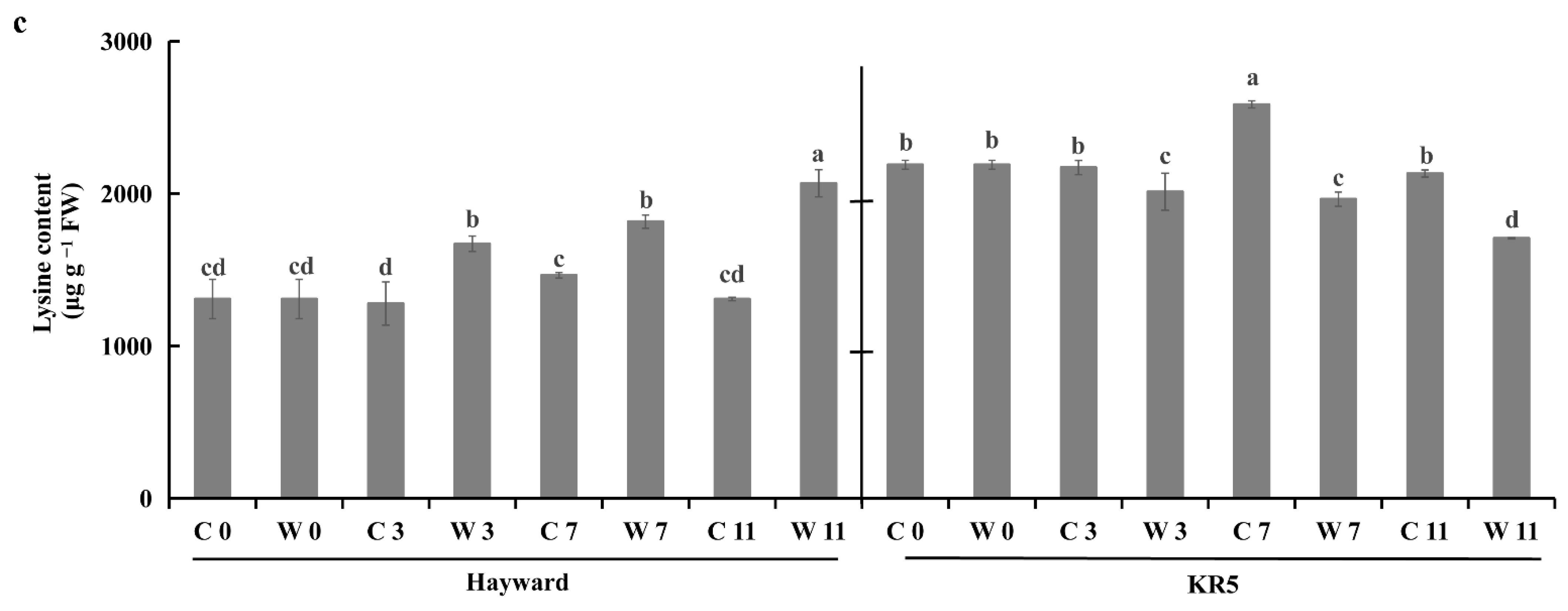
2.5. Root Amino Acid Contents Under Waterlogging Stress
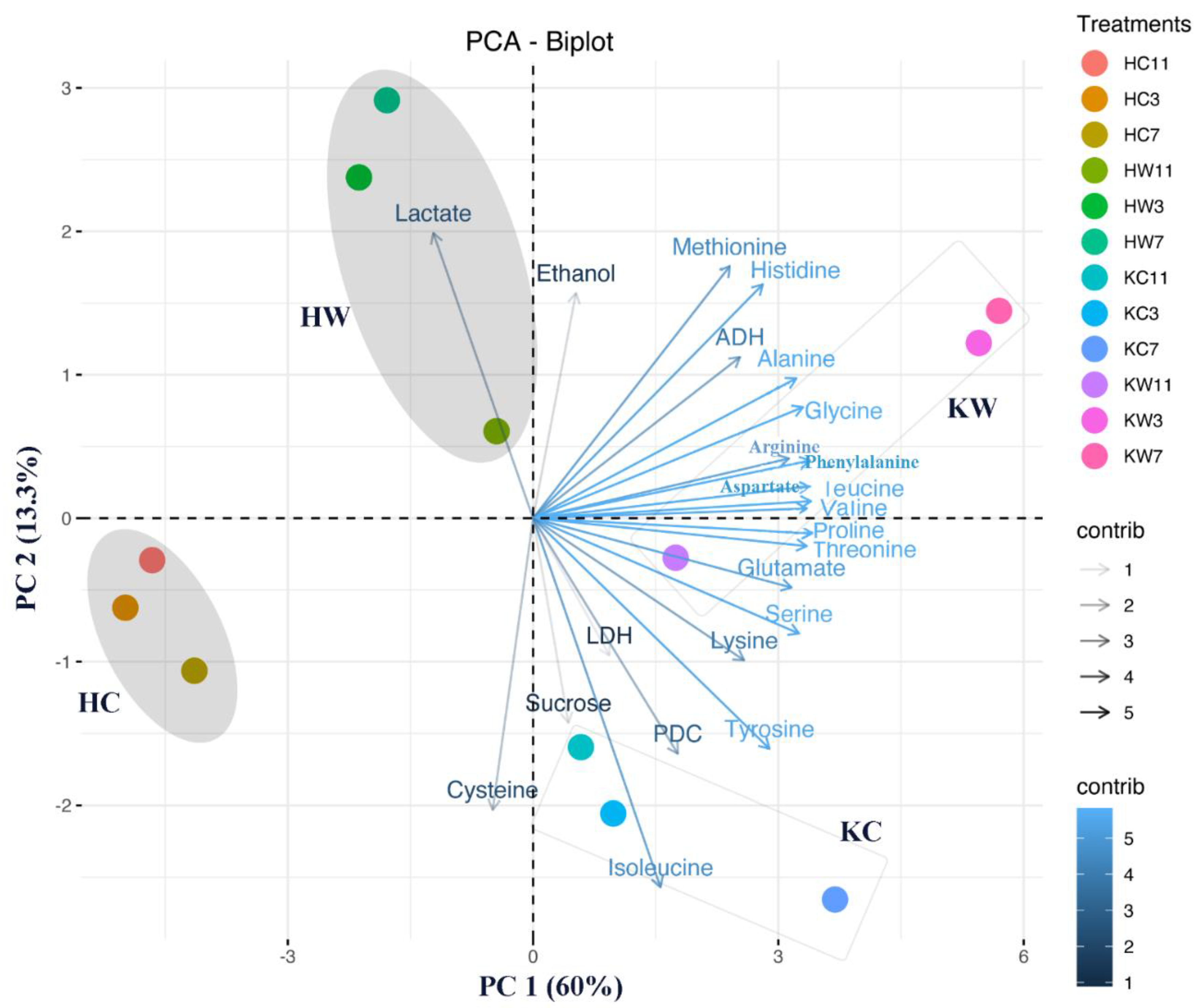
2.6. Multivariate Data Analysis
3. Discussion
3.1. Kiwifruit Plant Growth under Waterlogging Stress
3.2. Root Sugar and Fermentative Metabolisms under Waterlogging Stress
3.3. Root Amino Acid Metabolisms under Waterlogging Stress
4. Materials and Methods
4.1. Plant Materials and Waterlogging Treatments
4.2. Measurements of Relative Water Content
4.3. Gas Exchange Data and Measurement of Malondialdehyde Contents
4.4. Measurements of Sucrose Contents, Enzymatic Activities, and Metabolite Contents
4.5. Measurements of Amino Acid Contents
4.6. Gene Expression Analysis
4.7. Statistics Analysis and Heatmap Illustration
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Konnerup, D.; Toro, G.; Pedersen, O.; Colmer, T.D. Waterlogging tolerance, tissue nitrogen and oxygen transport in the forage legume Melilotus siculus: A comparison of nodulated and nitrate-fed plants. Ann. Bot. 2018, 121, 699–709. [Google Scholar] [CrossRef]
- Ahmed, S.; Nawata, E.; Hosokawa, M.; Domae, Y.; Sakuratani, T. Alterations in photosynthesis and some antioxidant enzymatic activities of mungbean subjected to waterlogging. Plant Sci. 2002, 163, 117–123. [Google Scholar] [CrossRef]
- Yetisir, H.; Caliskan, M.E.; Soylu, S.; Sakar, M. Some physiological and growth responses of watermelon [Citrullus lanatus (Thunb.) Matsum. and Nakai] grafted onto Lagenaria siceraria to flooding. Environ. Exp Bot. 2006, 58, 1–8. [Google Scholar] [CrossRef]
- Ren, B.; Zhang, J.; Dong, S.; Liu, P.; Zhao, B. Effects of waterlogging on leaf mesophyll cell ultrastructure and photosynthetic characteristics of summer maize. PLoS ONE 2016, 11, e0161424. [Google Scholar] [CrossRef] [Green Version]
- Tian, L.X.; Bi, W.S.; Liu, X.; Sun, L.; Li, J. Effects of waterlogging stress on the physiological response and grain-filling characteristics of spring maize (Zea mays L.) under field conditions. Acta Physiol. Plant 2019, 41, 1–14. [Google Scholar] [CrossRef]
- Pedersen, O.; Sauter, M.; Colmer, T.D.; Nakazono, M. Regulation of root adaptive anatomical and morphological traits during low soil oxygen. New Phytol. 2020, 229, 42–49. [Google Scholar] [CrossRef] [Green Version]
- Qi, X.H.; Li, Q.Q.; Ma, X.T.; Qian, C.L.; Wang, H.H.; Ren, N.N.; Shen, C.X.; Huang, S.M.; Xu, X.W.; Xu, Q.; et al. Waterlogging-induced adventitious root formation in cucumber is regulated by ethylene and auxin through reactive oxygen species signalling. Plant Cell Environ. 2019, 42, 1458–1470. [Google Scholar] [CrossRef]
- Vidoz, M.L.; Loreti, E.; Mensuali, A.; Alpi, A.; Perata, P. Hormonal interplay during adventitious root formation in flooded tomato plants. Plant J. 2010, 63, 551–562. [Google Scholar] [CrossRef]
- Fukao, T.; Bailey-Serres, J. Plant responses to hypoxia—is survival a balancing act? Trends Plant Sci. 2004, 9, 449–456. [Google Scholar] [CrossRef]
- Mignolli, F.; Barone, J.O.; Vidoz, M.L. Root submergence enhances respiration and sugar accumulation in the stem of flooded tomato plants. Plant Cell Environ. 2021, 1–12. [Google Scholar] [CrossRef]
- Cho, H.Y.; Loreti, E.; Shih, M.C.; Perata, P. Energy and sugar signaling during hypoxia. New Phytol. 2021, 229, 57–63. [Google Scholar] [CrossRef] [Green Version]
- Germain, V.; Ricard, B.; Raymond, P.; Saglio, P.H. The role of sugars, hexokinase, and sucrose synthase in the determination of hypoxically induced tolerance to anoxia in tomato roots. Plant Physiol. 1997, 114, 167–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, J.W.; Sharif, R.; Xu, X.W.; Chen, X.H. Mechanisms of waterlogging tolerance in plants: Research progress and prospects. Front Plant Sci. 2021, 11, 627331. [Google Scholar] [CrossRef] [PubMed]
- Dat, J.F.; Capelli, N.; Folzer, H.; Bourgeade, P.; Badot, P.M. Sensing and signalling during plant flooding. Plant Physiol. Biochem. 2004, 42, 273–282. [Google Scholar] [CrossRef]
- Machado, M.; Machado, S.; Pimentel, F.B.; Freitas, V.; Alves, R.C.; Oliveira, M.B.P.P. Amino acid profile and protein quality assessment of macroalgae produced in an integrated multi-trophic aquaculture system. Foods 2020, 9, 1382. [Google Scholar] [CrossRef] [PubMed]
- Bailey-Serres, J.; Fukao, T.; Gibbs, D.J.; Holdsworth, M.J.; Lee, S.C.; Licausi, F.; Perata, P.; Voesenek, L.A.C.J.; van Dongen, J.T. Making sense of low oxygen sensing. Trends Plant Sci. 2012, 17, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Sousa, C.A.F.d.; Sodek, L. The metabolic response of plants to oxygen deficiency. Braz. J. Plant Physiol. 2002, 14, 83–94. [Google Scholar] [CrossRef] [Green Version]
- Limami, A.M.; Glevarec, G.; Ricoult, C.; Cliquet, J.B.; Planchet, E. Concerted modulation of alanine and glutamate metabolism in young Medicago truncatula seedlings under hypoxic stress. J. Exp. Bot. 2008, 59, 2325–2335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, M.O.; Kim, K.P.; Kim, B.G.; Hahn, J.S.; Hong, C.B. Flooding stress-induced glycine-rich RNA-binding protein from Nicotiana tabacum. Mol. Cells 2009, 27, 47–54. [Google Scholar] [CrossRef]
- Bai, D.F.; Li, Z.; Qi, X.J.; Chen, J.Y.; Gu, H.; Huang, W.Q.; Ren, J.J.; Zhong, Y.P.; Fang, J.B. Physiological responses and tolerance evaluation of four species of Actinidia to waterlogging stress. J. Fruit Sci. 2019, 36, 163–173. [Google Scholar]
- Chen, Y.M.; Shi, M.Q.; Zhang, C.; Xu, K. Physiological effects of inundated rootstock on the growth and flooding stress of kiwifruit. Hubei Agric. Sci. 2018, 57, 77–80. [Google Scholar]
- Ni, M.; Liu, F.; Hu, Q.; Hu, J.; Gao, Y.; Liu, X.; Xu, K. Effects of waterlogging stress on the leaf chlorophyll florescence characteristics of kiwifruit grafted on different rootstocks. J. South China Fruits 2016, 45, 29–33. [Google Scholar]
- Zhang, J.Y.; Huang, S.N.; Chen, Y.H.; Wang, G.; Guo, Z.R. Identification and characterization of two waterlogging responsive alcohol dehydrogenase genes (AdADH1 and AdADH2) in Actinidia deliciosa. Mol. Breed. 2017, 37, 52. [Google Scholar] [CrossRef]
- Irfan, M.; Hayat, S.; Hayat, Q.; Afroz, S.; Ahmad, A. Physiological and biochemical changes in plants under waterlogging. Protoplasma 2010, 241, 3–17. [Google Scholar] [CrossRef]
- Ploschuk, R.A.; Miralles, D.J.; Colmer, T.D.; Ploschuk, E.L.; Striker, G.G. Waterlogging of winter crops at early and late stages: Impacts on leaf physiology, growth and yield. Front. Plant Sci. 2018, 9, 1863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angeles, G.; Evert, R.F.; Kozlowski, T.T. Development of lenticels and adventitious roots in flooded Ulmusamericana seedlings. Can. J. For. Res. 1986, 16, 585–590. [Google Scholar] [CrossRef]
- Herrera, A. Responses to flooding of plant water relations and leaf gas exchange in tropical tolerant trees of a black-water wetland. Front. Plant Sci. 2013, 4, 106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Provost, G.; Lesur, I.; Lalanne, C.; Da Silva, C.; Labadie, K.; Aury, J.M.; Leple, J.C.; Plomion, C. Implication of the suberin pathway in adaptation to waterlogging and hypertrophied lenticels formation in pedunculate oak (Quercus robur L.). Tree Physiol. 2016, 36, 1330–1342. [Google Scholar] [PubMed] [Green Version]
- Gunawardena, A.H.; Pearce, D.M.; Jackson, M.B.; Hawes, C.R.; Evans, D.E. Characterisation of programmed cell death during aerenchyma formation induced by ethylene or hypoxia in roots of maize (Zea mays L.). Planta 2001, 212, 205–214. [Google Scholar] [CrossRef]
- Kreuzwieser, J.; Rennenberg, H. Molecular and physiological responses of trees to waterlogging stress. Plant Cell Environ. 2014, 37, 2245–2259. [Google Scholar] [CrossRef]
- Kreuzwieser, J.; Hauberg, J.; Howell, K.A.; Carroll, A.; Rennenberg, H.; Millar, A.H.; Whelan, J. Differential response of gray poplar leaves and roots underpins stress adaptation during hypoxia. Plant Physiol. 2009, 149, 461–473. [Google Scholar] [CrossRef] [Green Version]
- Sala, A.; Woodruff, D.R.; Meinzer, F.C. Carbon dynamics in trees: Feast or famine? Tree Physiol. 2012, 32, 764–775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loreti, E.; Poggi, A.; Novi, G.; Alpi, A.; Perata, P. A genome-wide analysis of the effects of sucrose on gene expression in Arabidopsis seedlings under anoxia. Plant Physiol. 2005, 137, 1130–1138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, J.; Cho, L.H.; Tun, W.; Jeon, J.S.; An, G. Sucrose signaling in higher plants. Plant Sci. 2021, 302, 110703. [Google Scholar] [CrossRef]
- Dolferus, R.; Wolansky, M.; Carroll, R.; Miyashita, Y.; Ismond, K.; Good, A. Functional analysis of lactate dehydrogenase during hypoxic stress in Arabidopsis. Funct Plant Biol. 2008, 35, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Ferner, E.; Rennenberg, H.; Kreuzwieser, J. Effect of flooding on C metabolism of flood-tolerant (Quercus robur) and non-tolerant (Fagus sylvatica) tree species. Tree Physiol. 2012, 32, 135–145. [Google Scholar] [CrossRef] [Green Version]
- Vitor, S.C.; Sodek, L. Products of anaerobic metabolism in waterlogged roots of soybean are exported in the xylem. Plant Sci. 2019, 284, 82–90. [Google Scholar] [CrossRef]
- Licausi, F.; Perata, P. Low oxygen signaling and tolerance in plants. Adv. Bot. Res. 2009, 50, 139–198. [Google Scholar]
- Veena, J.; Singla, N.K.; Sunita, J.; Kaushalya, G. Activities of enzymes of fermentation pathways in the leaves and roots of contrasting cultivars of sorghum (Sorghum bicolor L.) during flooding. Physiol. Mol. Biol. Plants 2010, 16, 241–247. [Google Scholar]
- Xu, X.; Wang, H.; Qi, X.; Xu, Q.; Chen, X. Waterlogging-induced increase in fermentation and related gene expression in the root of cucumber (Cucumis sativus L.). Sci. Hortic. 2014, 179, 388–395. [Google Scholar]
- Ohnson, J.R.; Cobb, B.G.; Drew, M.C. Hypoxic induction of anoxia tolerance in roots of Adh1 null Zea mays L. Plant Physiol. 1994, 105, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, H.; Takano, T.; Takeda, G.; Uchimiya, H. Adh1 is transcriptionally active but its translational product is reduced in a rad mutant of rice (Oryza sativa L.), which is vulnerable to submergence stress. Theor. Appl. Genet. 1998, 97, 1197–1203. [Google Scholar] [CrossRef]
- Zabalza, A.; Van Dongen, J.T.; Froehlich, A.; Oliver, S.N.; Faix, B.; Gupta, K.J.; Schmalzlin, E.; Igal, M.; Orcaray, L.; Royuela, M.; et al. Regulation of respiration and fermentation to control the plant internal oxygen concentration. Plant Physiol. 2009, 149, 1087–1098. [Google Scholar] [CrossRef] [Green Version]
- Campalans, A.; Messeguer, R.; Goday, A.; Pages, M. Plant responses to drought, from ABA signal transduction events to the action of the induced proteins. Plant Physiol. Biochem. 1999, 37, 327–340. [Google Scholar] [CrossRef]
- Stadtman, E.R. Oxidation of free amino acids and amino acid residues in proteins by radiolysis and by metal-catalyzed reactions. Annu. Rev. Biochem. 1993, 62, 797–821. [Google Scholar] [CrossRef] [PubMed]
- Du, H.Y.; Liu, D.X.; Liu, G.T.; Liu, H.P.; Kurtenbach, R. Relationship between polyamines and anaerobic respiration of wheat seedling root under water-logging stress. Russ. J. Plant Physiol. 2018, 65, 874–881. [Google Scholar] [CrossRef]
- Yiu, J.C.; Juang, L.D.; Fang, D.Y.T.; Liu, C.W.; Wu, S.J. Exogenous putrescine reduces flooding-induced oxidative damage by increasing the antioxidant properties of Welsh onion. Sci. Hortic. 2009, 120, 306–314. [Google Scholar] [CrossRef]
- Chandana, T.; Venkatesh, Y.P. Occurrence, functions and biological significance of arginine-rich proteins. Curr. Protein Peptide Sci. 2016, 17, 507–516. [Google Scholar] [CrossRef]
- Rocha, M.; Licausi, F.; Araújo, W.L.; Nunes-Nesi, A.; Sodek, L.; Fernie, A.R.; van Dongen, J.T. Glycolysis and the tricarboxylic acid cycle are linked by alanine aminotransferase during hypoxia induced by waterlogging of Lotus japonicus. Plant Physiol. 2010, 152, 1501–1513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, M.; Wu, H.; Clay, K.L.; Jung, R.; Larkins, B.A.; Gibbon, B.C. Identification and characterization of lysine-rich proteins and starch biosynthesis genes in the opaque2 mutant by transcriptional and proteomic analysis. BMC Plant Biol. 2013, 13, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Zhong, Y.P.; Bai, D.F.; Lin, M.M.; Qi, X.J.; Fang, J.B. Comparative analysis of physiological traits of three Actinidia valvata Dunn genotypes during waterlogging and post-waterlogging recovery. Hortic. Environ. Biotechnol. 2020, 61, 825–836. [Google Scholar] [CrossRef]
- Buwalda, J.G. The impact of canopy growth and temporal changes in radiation on the dynamics of canopy carbon assimilation for kiwifruit (Actinidia deliciosa) vines during spring. Environ. Exp. Bot. 1994, 34, 141–151. [Google Scholar] [CrossRef]
- Salah, A.; Li, J.; Ge, J.; Cao, C.; Li, H.; Wang, Y.; Liu, Z.; Zhan, M.; Zhao, M. Morphological and physiological responses of maize seedlings under drought and waterlogging. J. Agric. Sci. Technol. 2019, 21, 1199–1214. [Google Scholar]
- Lin, Y.X.; Zhang, H.; Chen, R.K. HPLC-RID determination of sucrose, glucose and fructose in sugarcane internodes. J. Fujian Agric. For. Univ. 2015, 44, 232–235. [Google Scholar]
- Zhao, Y.M.; Long, L.Y.; Ding, X.Y.; Bu, Y.H.; Cao, J.K. Studies on simultaneous determination of ethanol and acetaldehyde in plum fruit by static head-space gas chromatography. Food Res. Dev. 2013, 34, 80–83. [Google Scholar]
- Xu, Y.; Li, K.; Wang, H.; Chen, L.; Gong, X. Determination of eight organic acids in blueberries by HPLC. Food Sci. 2015, 36, 127–131. [Google Scholar]
- Yu, H.C.; Ma, R.S.; Guo, Q.M.; Zhou, F.Q.; Guo, J.G. Determination of amino acids in the fruits of Sambucus williamsii by PITC pre-column derivation and RP-HPLC method. Guizhou Agric. Sci. 2015, 43, 213–216. [Google Scholar]
- Yin, X.R.; Allan, A.C.; Xu, Q.; Burdon, J.; Dejnoprat, S.; Chen, K.S.; Ferguson, I.B. Differential expression of kiwifruit ERF genes in response to postharvest abiotic stress. Postharvest Biol. Technol. 2012, 66, 1–7. [Google Scholar] [CrossRef]
- Le, S.; Josse, J.; Husson, F. FactoMineR: An R package for multivariate analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Bai, D.; Zhong, Y.; Abid, M.; Qi, X.; Hu, C.; Fang, J. Physiological Responses of Two Contrasting Kiwifruit (Actinidia spp.) Rootstocks against Waterlogging Stress. Plants 2021, 10, 2586. https://doi.org/10.3390/plants10122586
Li Z, Bai D, Zhong Y, Abid M, Qi X, Hu C, Fang J. Physiological Responses of Two Contrasting Kiwifruit (Actinidia spp.) Rootstocks against Waterlogging Stress. Plants. 2021; 10(12):2586. https://doi.org/10.3390/plants10122586
Chicago/Turabian StyleLi, Zhi, Danfeng Bai, Yunpeng Zhong, Muhammad Abid, Xiujuan Qi, Chungen Hu, and Jinbao Fang. 2021. "Physiological Responses of Two Contrasting Kiwifruit (Actinidia spp.) Rootstocks against Waterlogging Stress" Plants 10, no. 12: 2586. https://doi.org/10.3390/plants10122586





