Sunscreen Effect Exerted by Secondary Carotenoids and Mycosporine-like Amino Acids in the Aeroterrestrial Chlorophyte Coelastrella rubescens under High Light and UV-A Irradiation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Identification and Characterization of a New Strain Coelastrella Rubescens NAMSU R1
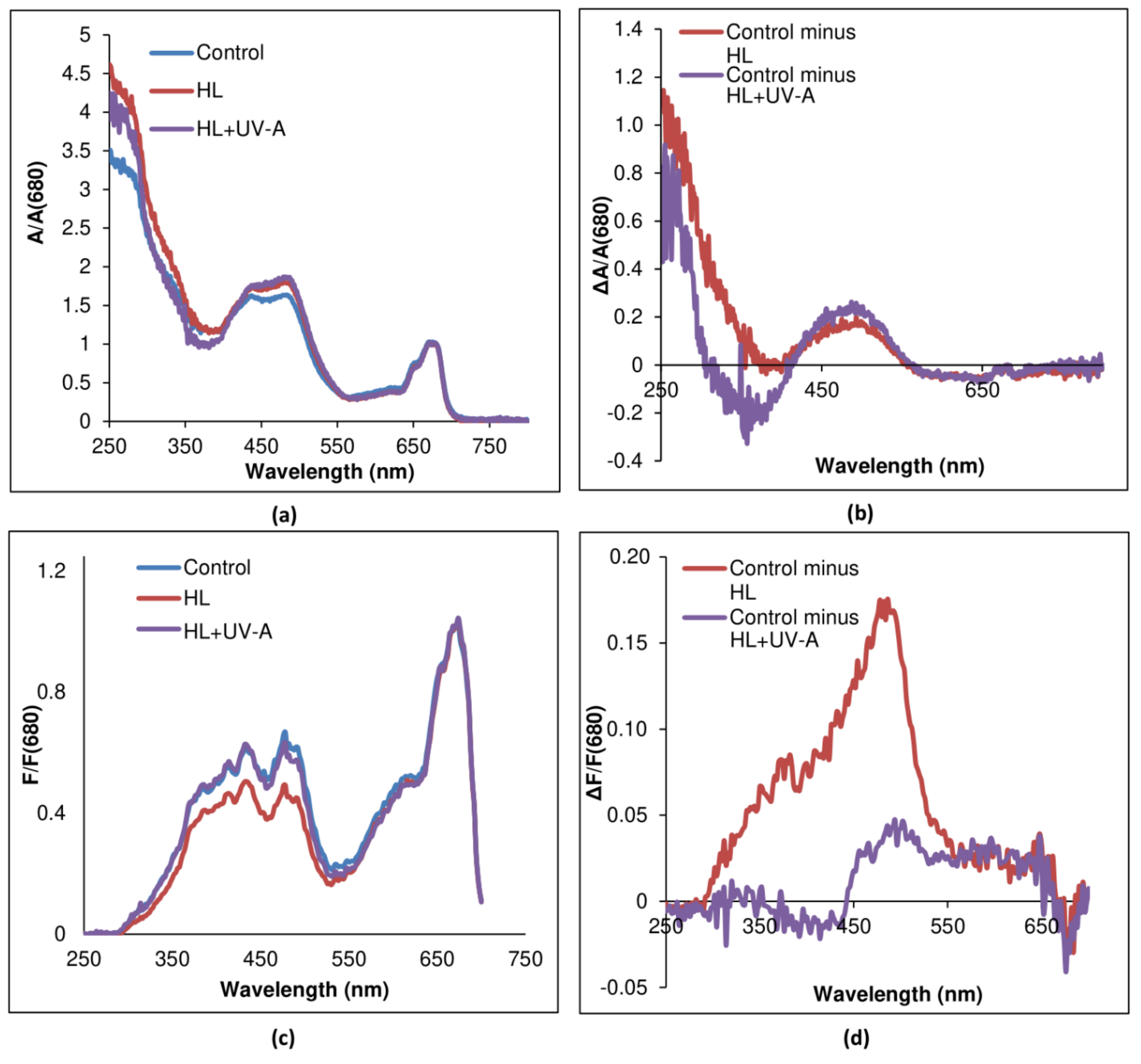
2.2. Sunscreen Effect in the Visible Region of the Spectrum
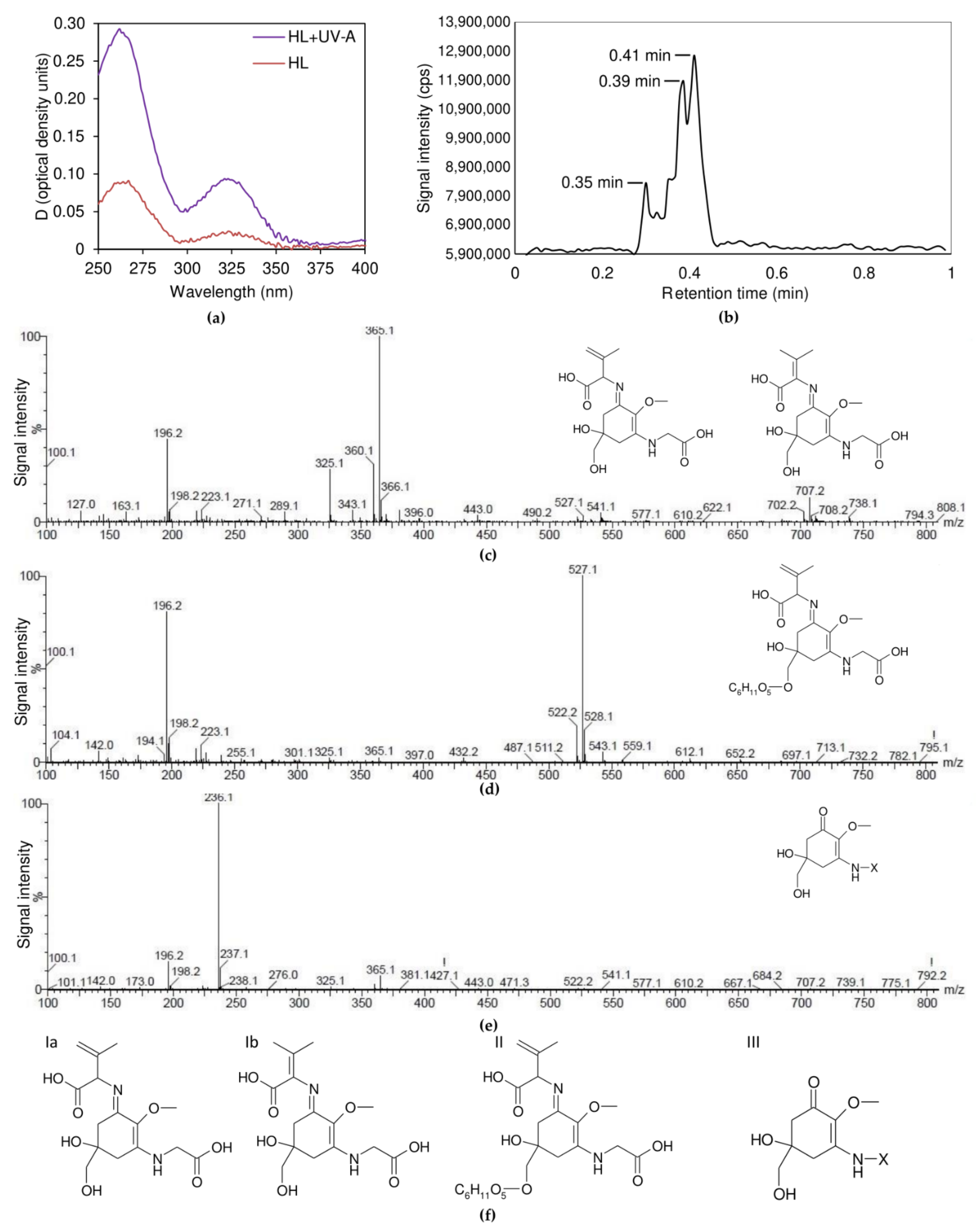
2.3. Sunscreen Effect in the UV Range
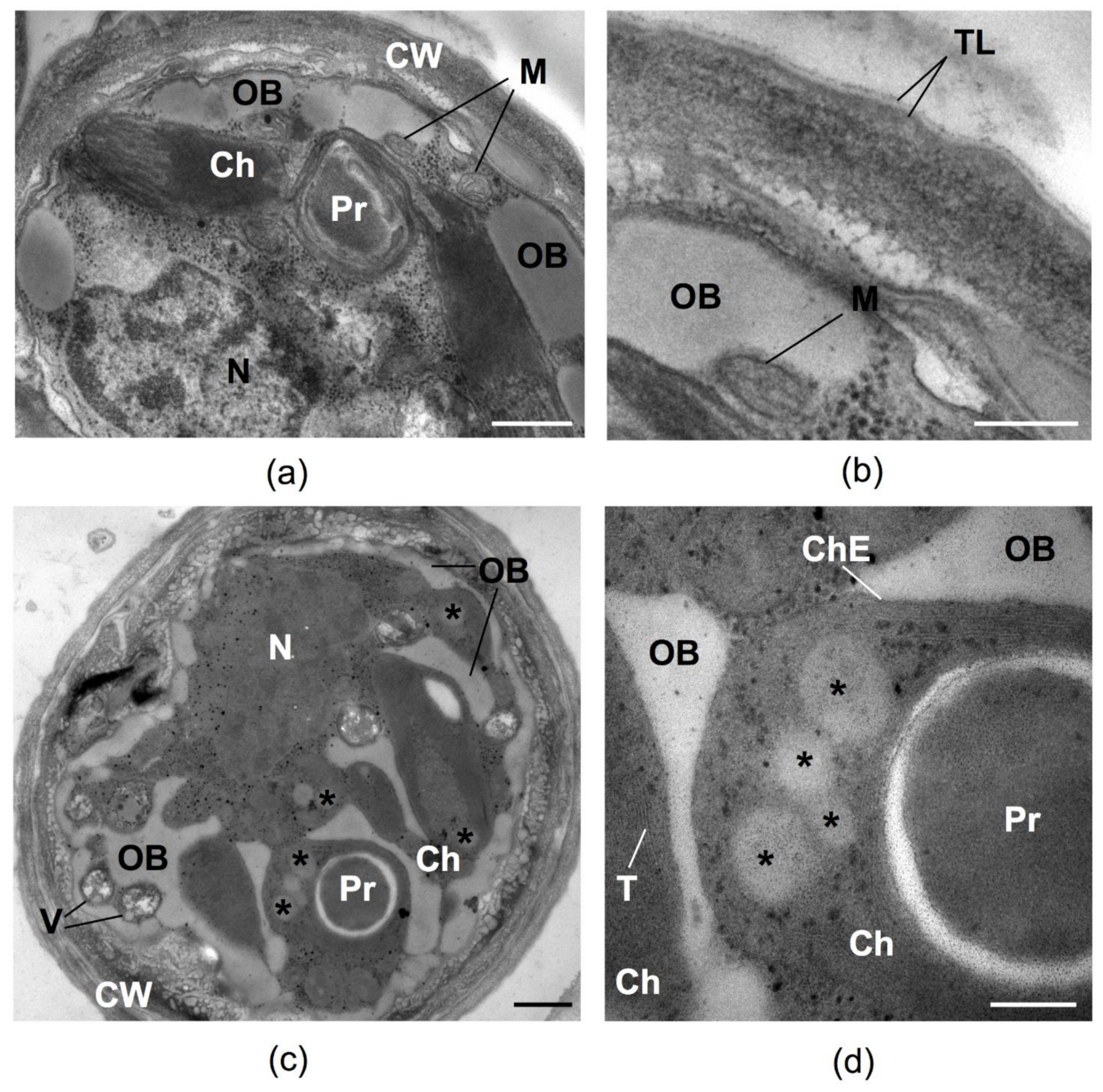
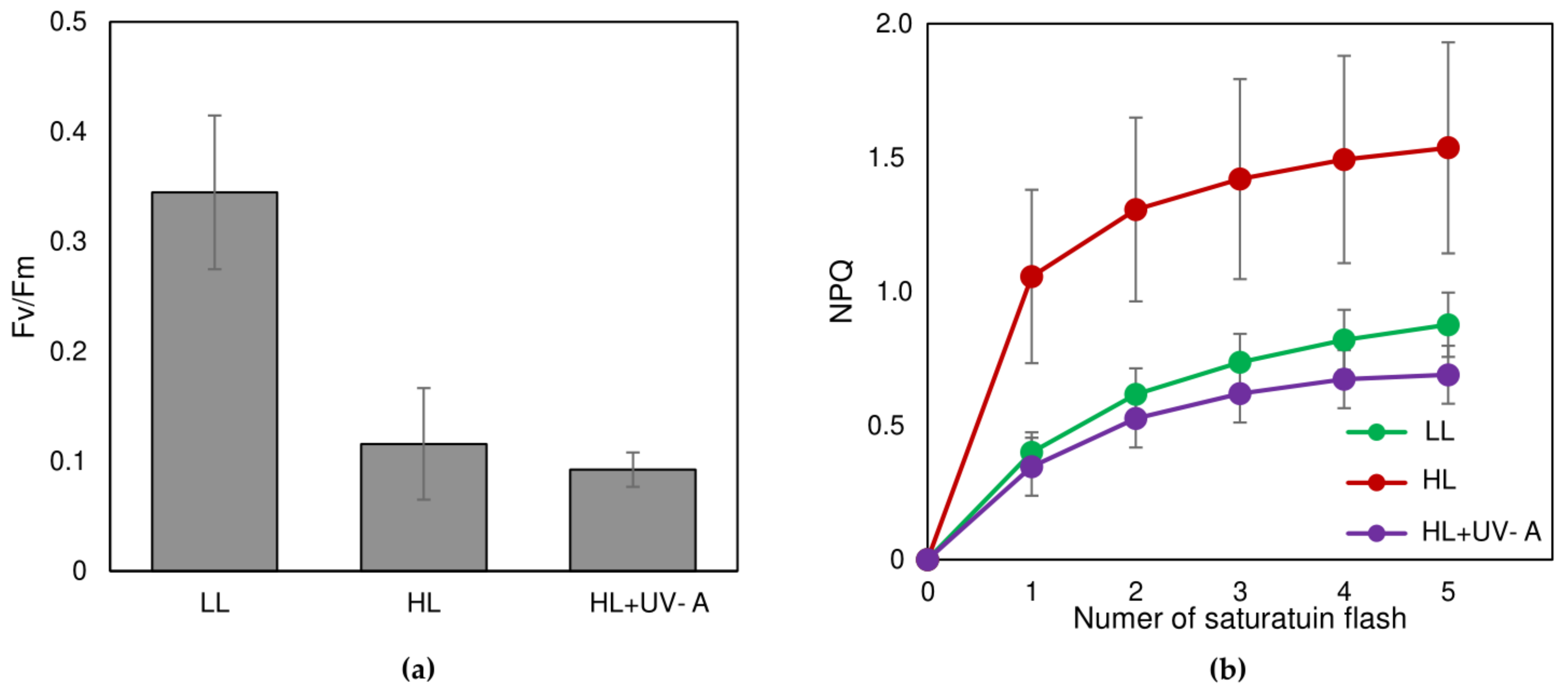
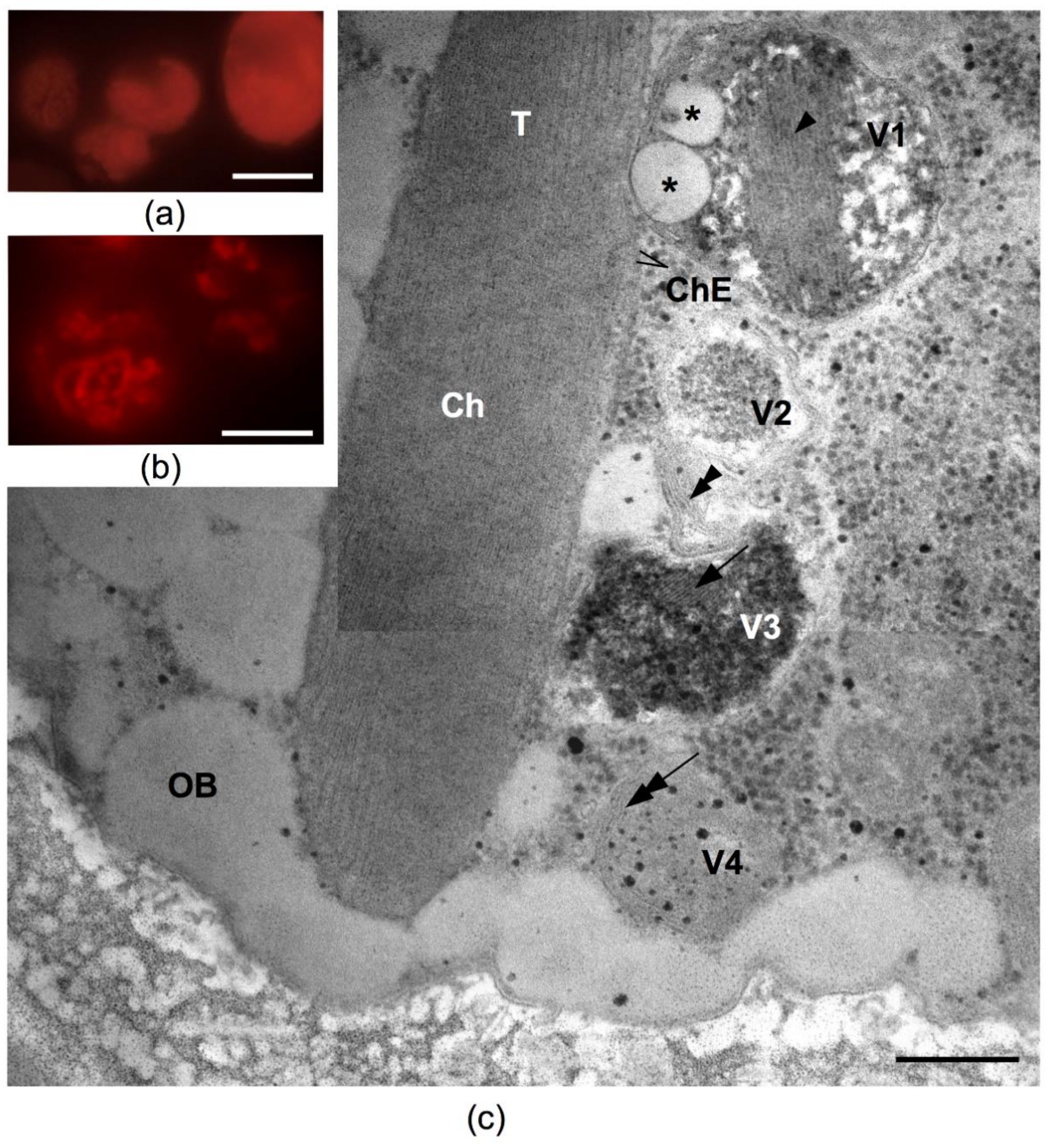
2.4. Possible Photoprotective Mechanisms Additional to Sunscreen
3. Materials and Methods
3.1. Strain Isolation and Identification
3.2. Induction of Photoprotectants’ Synthesis
3.3. Microscopy
3.3.1. Light Microscopy
3.3.2. Electron Microscopy
3.4. Spectroscopy
3.4.1. Absorbance Spectra of Cell Suspensions
3.4.2. UV-VIS-Absorbance Spectra of Cell Extracts
3.4.3. Excitation Spectra
3.5. Chromatography
3.5.1. Thin Layer Chromatography
3.5.2. Ultra-Performance Liquid Chromatography—Mass Spectrometry
3.6. The Analysis of Chlorophyll Fluorescence Induction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Karsten, U.; Rindi, F. Ecophysiological performance of an urban strain of the aeroterrestrial green alga Klebsormidium sp. (Klebsormidiales, Klebsormidiophyceae). Eur. J. Phycol. 2010, 45, 426–435. [Google Scholar] [CrossRef] [Green Version]
- Karsten, U.; Lembcke, S.; Schumann, R. The effects of ultraviolet radiation on photosynthetic performance, growth and sunscreen compounds in aeroterrestrial biofilm algae isolated from building facades. Planta 2007, 225, 991–1000. [Google Scholar] [CrossRef]
- Holzinger, A.; Karsten, U. Desiccation stress and tolerance in green algae: Consequences for ultrastructure, physiological and molecular mechanisms. Front. Plant Sci. 2013, 4, 327. [Google Scholar] [CrossRef] [Green Version]
- Sen, S.; Mallick, N. Mycosporine-like amino acids: Algal metabolites shaping the safety and sustainability profiles of commercial sunscreens. Algal Res. 2021, 58, 102425. [Google Scholar] [CrossRef]
- Xiong, F.; Komenda, J.; Kopecký, J.; Nedbal, L. Strategies of ultraviolet-B protection in microscopic algae. Physiol. Plant. 1997, 100, 378–388. [Google Scholar] [CrossRef]
- Goldberg, B.; Klein, W.H. Variations in the spectral distribution of daylight at various geographical locations on the earth’s surface. Sol. Energy 1977, 19, 3–13. [Google Scholar] [CrossRef]
- Blumthaler, M.; Ambach, W.; Ellinger, R. Increase in solar UV radiation with altitude. J. Photochem. Photobiol. B Biol. 1997, 39, 130–134. [Google Scholar] [CrossRef]
- Kotilainen, T.; Aphalo, P.J.; Brelsford, C.C.; Böök, H.; Devraj, S.; Heikkilä, A.; Hernández, A.; Kylling, A.V.; Lindfors, T.M.; Robson, T.M. Patterns in the spectral composition of sunlight and biologically meaningful spectral photon ratios as affected by atmospheric factors. Agric. For. Meteorol. 2020, 291, 108041. [Google Scholar] [CrossRef]
- Karsten, U.; Holzinger, A. Green algae in alpine biological soil crust communities: Acclimation strategies against ultraviolet radiation and dehydration. Biodivers. Conserv. 2014, 23, 1845–1858. [Google Scholar] [CrossRef] [Green Version]
- Verdaguer, D.; Jansen, M.A.; Llorens, L.; Morales, L.O.; Neugart, S. UV-A radiation effects on higher plants: Exploring the known unknown. Plant. Sci. 2017, 255, 72–81. [Google Scholar] [CrossRef]
- Vanhaelewyn, L.; Van Der Straeten, D.; De Coninck, B.; Vandenbussche, F. Ultraviolet radiation from a plant perspective: The plant-microorganism context. Front. Plant Sci. 2020, 11, 1984. [Google Scholar] [CrossRef]
- Cockell, C.S.; Knowland, J. Ultraviolet radiation screening compounds. Biol. Rev. 1999, 74, 311–345. [Google Scholar] [CrossRef]
- Milito, A.; Castellano, I.; Damiani, E. From Sea to Skin: Is There a Future for Natural Photoprotectants? Mar. Drugs 2021, 19, 379. [Google Scholar] [CrossRef]
- Vega, J.; Schneider, G.; Moreira, B.R.; Herrera, C.; Bonomi-Barufi, J.; Figueroa, F.L. Mycosporine-Like Amino Acids from Red Macroalgae: UV-Photoprotectors with Potential Cosmeceutical Applications. Appl. Sci. 2021, 11, 5112. [Google Scholar] [CrossRef]
- Oren, A.; Gunde-Cimerman, N. Mycosporines and mycosporine-like amino acids: UV protectants or multipurpose secondary metabolites? FEMS Microbiol. Lett. 2007, 269, 1–10. [Google Scholar] [CrossRef]
- Singh, A.; Čížková, M.; Bišová, K.; Vítová, M. Exploring Mycosporine-Like Amino Acids (MAAs) as Safe and Natural Protective Agents against UV-Induced Skin Damage. Antioxidants 2021, 10, 683. [Google Scholar] [CrossRef]
- Geraldes, V.; Pinto, E. Mycosporine-like Amino Acids (MAAs): Biology. Chemistry and Identification Features. Pharmaceuticals 2021, 14, 63. [Google Scholar] [CrossRef]
- Wada, N.; Sakamoto, T.; Matsugo, S. Mycosporine-like amino acids and their derivatives as natural antioxidants. Antioxidants 2015, 4, 603–646. [Google Scholar] [CrossRef]
- Karsten, U.; Friedl, T.; Schumann, R.; Hoyer, K.; Lembcke, S. Mycosporine-like amino acids and phylogenies in green algae: Prasiola and its relatives from the Trebouxiophyceae (Chlorophyta). J. Phycol. 2005, 41, 557–566. [Google Scholar] [CrossRef]
- Karentz, D.; McEuen, F.S.; Land, M.C.; Dunlap, W.C. Survey of mycosporine-like amino acid compounds in Antarctic marine organisms: Potential protection from ultraviolet exposure. Mar. Biol. 1991, 108, 157–166. [Google Scholar] [CrossRef]
- Hartmann, A.; Glaser, K.; Holzinger, A.; Ganzera, M.; Karsten, U. Klebsormidin A and B, two new UV-sunscreen compounds in green microalgal Interfilum and Klebsormidium species (Streptophyta) from terrestrial habitats. Front. Microbiol. 2020, 11, 499. [Google Scholar] [CrossRef]
- Singh, S.P.; Kumari, S.; Rastogi, R.P.; Singh, K.L.; Sinha, R.P. Mycosporine-like amino acids (MAAs): Chemical structure, biosynthesis and significance as UV-absorbing/screening compounds. J. Exp. Biol. 2008, 46, 7–17. [Google Scholar]
- Nazifi, E.; Wada, N.; Asano, T.; Nishiuchi, T.; Iwamuro, Y.; Chinaka, S.; Matsugo, S.; Sakamoto, T. Characterization of the chemical diversity of glycosylated mycosporine-like amino acids in the terrestrial cyanobacterium Nostoc commune. J. Photochem. Photobiol. B Biol. 2015, 142, 154–168. [Google Scholar] [CrossRef] [Green Version]
- Rosic, N.N. Mycosporine-like amino acids: Making the foundation for organic personalized sunscreens. Mar. Drugs 2019, 17, 638. [Google Scholar] [CrossRef] [Green Version]
- Holzinger, A.; Pichrtová, M. Abiotic stress tolerance of charophyte green algae: New challenges for omics techniques. Front. Plant Sci. 2016, 7, 678. [Google Scholar] [CrossRef] [Green Version]
- Procházková, L.; Remias, D.; Bilger, W.; Křížková, H.; Řezanka, T.; Nedbalová, L. Cysts of the snow alga Chloromonas krienitzii (Chlorophyceae) show increased tolerance to ultraviolet radiation and elevated visible light. Front. Plant Sci. 2020, 11, 2068. [Google Scholar] [CrossRef]
- Carletti, G.; Nervo, G.; Cattivelli, L. Flavonoids and melanins: A common strategy across two kingdoms. Int. J. Biol. Sci. 2014, 10, 1159. [Google Scholar] [CrossRef] [Green Version]
- Solhaug, K.A.; Gauslaa, Y.; Nybakken, L.; Bilger, W. UV-induction of sun-screening pigments in lichens. New Phytol. 2003, 158, 91–100. [Google Scholar] [CrossRef]
- Rao, D.N.; LeBlanc, F. A possible role of atranorin in the lichen thallus. Bryologist 1965, 68, 284–289. [Google Scholar] [CrossRef]
- McEvoy, M.; Solhaug, K.A.; Gauslaa, Y. Solar radiation screening in usnic acid-containing cortices of the lichen Nephroma arcticum. Symbiosis 2007, 43, 143–150. [Google Scholar]
- Gauslaa, Y.; Ustvedt, E.M. Is parietin a UV-B or a blue-light screening pigment in the lichen Xanthoria parietina? Photochem. Photobiol. Sci. 2003, 2, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Asada, K. Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant. Physiol. 2006, 141, 391–396. [Google Scholar] [CrossRef] [Green Version]
- Merzlyak, M.N.; Chivkunova, O.B. Light-stress-induced pigment changes and evidence for anthocyanin photoprotection in apples. J. Photochem. Photobiol. B Biol. 2000, 55, 155–163. [Google Scholar] [CrossRef]
- Solovchenko, A. Screening pigments: General questions. In Photoprotection in Plants; Springer: Berlin/Heidelberg, Germany, 2010; Volume 14, pp. 9–31. [Google Scholar]
- Fernandes, Â.; Figueiredo, S.; Finimundy, T.C.; Pinela, J.; Tzortzakis, N.; Ivanov, M.; Soković, M.; Ferreira, I.; Petropoulos, S.A.; Barros, L. Chemical composition and bioactive properties of purple French bean (Phaseolus vulgaris L.) as affected by water deficit irrigation and biostimulants application. Sustainability 2021, 13, 6869. [Google Scholar] [CrossRef]
- Merzlyak, M.; Solovchenko, A.; Pogosyan, S. Optical properties of rhodoxanthin accumulated in Aloe arborescens Mill. leaves under high-light stress with special reference to its photoprotective function. Photochem. Photobiol. Sci. 2005, 4, 333–340. [Google Scholar] [CrossRef]
- Solovchenko, A.; Neverov, K. Carotenogenic response in photosynthetic organisms: A colorful story. Photosynth. Res. 2017, 133, 31–47. [Google Scholar] [CrossRef]
- Fan, L.; Vonshak, A.; Zarka, A.; Boussiba, S. Does astaxanthin protect Haematococcus against light damage? Z. Nat. C 1998, 53, 93–100. [Google Scholar] [CrossRef]
- Peled, E.; Pick, U.; Zarka, A.; Shimoni, E.; Leu, S.; Boussiba, S. Light-induced oil globule migration in Haematococcus pluvialis (Chlorophyceae). J. Phycol. 2012, 48, 1209–1219. [Google Scholar] [CrossRef]
- Chekanov, K.; Schastnaya, E.; Neverov, K.; Leu, S.; Boussiba, S.; Zarka, A.; Solovchenko, A. Non-photochemical quenching in the cells of the carotenogenic chlorophyte Haematococcus lacustris under favorable conditions and under stress. Biochim. Biophys. Acta Gen. Subj. 2019, 1863, 1429–1442. [Google Scholar] [CrossRef]
- Chekanov, K.; Lobakova, E.; Selyakh, I.; Semenova, L.; Sidorov, R.; Solovchenko, A. Accumulation of astaxanthin by a new Haematococcus pluvialis strain BM1 from the White Sea coastal rocks (Russia). Mar. Drugs 2014, 12, 4504–4520. [Google Scholar] [CrossRef] [Green Version]
- Takaichi, S. Carotenoids in algae: Distributions, biosyntheses and functions. Mar. Drugs 2011, 9, 1101–1118. [Google Scholar] [CrossRef] [PubMed]
- Boussiba, S.; Vonshak, A. Astaxanthin accumulation in the green alga Haematococcus pluvialis. Plant Cell Physiol. 1991, 32, 1077–1082. [Google Scholar] [CrossRef] [Green Version]
- Lemoine, Y.; Schoefs, B. Secondary ketocarotenoid astaxanthin biosynthesis in algae: A multifunctional response to stress. Photosynth. Res. 2010, 106, 155–177. [Google Scholar] [CrossRef]
- Boussiba, S. Carotenogenesis in the green alga Haematococcus pluvialis: Cellular physiology and stress response. Physiol. Plant. 2000, 108, 111–117. [Google Scholar] [CrossRef]
- Ben-Amotz, A.; Shaish, A.; Avron, M. Mode of action of the massively accumulated β-carotene of Dunaliella bardawil in protecting the alga against damage by excess irradiation. Plant. Physiol. 1989, 91, 1040–1043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ben-Amotz, A.; Avron, M. The biotechnology of mass culturing Dunaliella for products of commercial interest. In Algal and Cyanobacterial Biotechnology; Cresswell, R.C., Rees, T.A.V., Shah, N., Eds.; Longman Scientific & Technical: Harlow, UK, 1989; pp. 91–114. [Google Scholar]
- Czygan, F.C. Sekundär-Carotinoide in Grünalgen. Arch. Mikrobiol. 1968, 62, 209–236. [Google Scholar] [CrossRef]
- Minyuk, G.S.; Chelebieva, E.S.; Chubchikova, I.N. Secondary carotenogenesis of the green microalga Bracteacoccus minor (Chodat) Petrova (Chlorophyta) in a two-stage culture. Int. J. Algae 2014, 16, 354–368. [Google Scholar] [CrossRef]
- Chekanov, K.; Litvinov, D.; Fedorenko, T.; Chivkunova, O.; Lobakova, E. Combined Production of Astaxanthin and β-Carotene in a New Strain of the Microalga Bracteacoccus aggregatus BM5/15 (IPPAS C-2045) Cultivated in Photobioreactor. Biology 2021, 10, 643. [Google Scholar] [CrossRef] [PubMed]
- Chekanov, K.; Fedorenko, T.; Kublanovskaya, A.; Litvinov, D.; Lobakova, E. Diversity of carotenogenic microalgae in the White Sea polar region. FEMS Microbiol. Ecol. 2020, 96, fiz183. [Google Scholar] [CrossRef]
- Procházková, L.; Leya, T.; Křížková, H.; Nedbalová, L. Sanguina nivaloides and Sanguina aurantia gen. et spp. nov. (Chlorophyta): The taxonomy, phylogeny, biogeography and ecology of two newly recognised algae causing red and orange snow. FEMS Microbiol. Ecol. 2019, 95, fiz064. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Sun, D.; Cheng, K.W.; Chen, F. Inhibition of autophagy modulates astaxanthin and total fatty acid biosynthesis in Chlorella zofingiensis under nitrogen starvation. Biores. Technol. 2018, 247, 610–615. [Google Scholar] [CrossRef]
- Kawasaki, S.; Yoshida, R.; Ohkoshi, K.; Toyoshima, H. Coelastrella astaxanthina sp. nov. (Sphaeropleales, Chlorophyceae), a novel microalga isolated from an asphalt surface in midsummer in Japan. Phycol. Res. 2020, 68, 107–114. [Google Scholar] [CrossRef]
- Tschaikner, A.; Ingolić, E.; Stoyneva, M.P.; Gärtner, G. Autosporulation in the soil alga Coelastrella terrestris (Chlorophyta, Scenedesmaceae, Scenedesmoideae). Phytol. Balc. 2007, 13, 29–34. [Google Scholar]
- Minyuk, G.; Chelebieva, E.; Chubchikova, I.; Dantsyuk, N.; Drobetskaya, I.; Sakhon, E.; Chekanov, K.; Solovchenko, A. Stress-induced secondary carotenogenesis in Coelastrella rubescens (Scenedesmaceae, Chlorophyta), a producer of value-added keto-carotenoids. Algae 2017, 32, 245–259. [Google Scholar] [CrossRef]
- Kaufnerová, V.; Eliáš, M. The demise of the genus Scotiellopsis Vinatzer (Chlorophyta). Nova Hedwig. 2013, 97, 415–428. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Song, H.; Liu, X.; Liu, B.; Hu, Z.; Liu, G. Morphology and molecular phylogeny of coccoid green algae Coelastrella sensu lato (Scenedesmaceae, Sphaeropeales), including the description of three new species and two new varieties. J. Phycol. 2019, 55, 1290–1305. [Google Scholar] [CrossRef]
- Goecke, F.; Noda, J.; Paliocha, M.; Gislerød, H.R. Revision of Coelastrella (Scenedesmaceae, Chlorophyta) and first register of this green coccoid microalga for continental Norway. World J. Microbiol. Biotechnol. 2020, 36, 1–17. [Google Scholar] [CrossRef]
- Orosa, M.; Torres, E.; Fidalgo, P.; Abalde, J. Production and analysis of secondary carotenoids in green algae. J. Appl. Phycol. 2000, 12, 553–556. [Google Scholar] [CrossRef] [Green Version]
- Melis, A. Photosystem-II damage and repair cycle in chloroplasts: What modulates the rate of photodamage in vivo? Trends Plant. Sci. 1999, 4, 130–135. [Google Scholar] [CrossRef]
- Mehler, A.H. Studies on reactions of illuminated chloroplasts: I. Mechanism of the reduction of oxygen and other hill reagents. Arch. Biochem. Biophys. 1951, 33, 65–77. [Google Scholar] [CrossRef]
- Chekanov, K.; Vasilieva, S.; Solovchenko, A.; Lobakova, E. Reduction of photosynthetic apparatus plays a key role in survival of the microalga Haematococcus pluvialis (Chlorophyceae) at freezing temperatures. Photosynthetica 2018, 56, 1268–1277. [Google Scholar] [CrossRef]
- Kim, J.E.; Cheng, K.M.; Craft, N.E.; Hamberger, B.; Douglas, C.J. Over-expression of Arabidopsis thaliana carotenoid hydroxylases individually and in combination with a β-carotene ketolase provides insight into in vivo functions. Phytochemistry 2010, 71, 168–178. [Google Scholar] [CrossRef]
- Damiani, M.C.; Leonardi, P.I.; Pieroni, O.I.; Cáceres, E.J. Ultrastructure of the cyst wall of Haematococcus pluvialis (Chlorophyceae): Wall development and behaviour during cyst germination. Phycologia 2006, 45, 616–623. [Google Scholar] [CrossRef]
- Polle, J.E.; Roth, R.; Ben-Amotz, A.; Goodenough, U. Ultrastructure of the green alga Dunaliella salina strain CCAP19/18 (Chlorophyta) as investigated by quick-freeze deep-etch electron microscopy. Algal Res. 2020, 49, 101953. [Google Scholar] [CrossRef]
- Sun, Z.; Cunningham, F.X.; Gantt, E. Differential expression of two isopentenyl pyrophosphate isomerases and enhanced carotenoid accumulation in a unicellular chlorophyte. Proc. Natl. Acad. Sci. USA 1998, 95, 11482–11488. [Google Scholar] [CrossRef] [Green Version]
- Cunningham, F.X., Jr.; Gantt, E. Genes and enzymes of carotenoid biosynthesis in plants. Annu. Rev. Plant Biol. 1998, 49, 557–583. [Google Scholar] [CrossRef]
- Parailloux, M.; Godin, S.; Fernandes, S.; Lobinski, R. Untargeted Analysis for Mycosporines and Mycosporine-Like Amino Acids by Hydrophilic Interaction Liquid Chromatography (HILIC)—Electrospray Orbitrap MS2/MS3. Antioxidants 2020, 9, 1185. [Google Scholar] [CrossRef]
- Kochkin, D.V.; Galishev, B.A.; Glagoleva, E.S.; Titova, M.V.; Nosov, A.M. Rare triterpene glycoside of ginseng (ginsenoside malonyl-Rg 1) detected in plant cell suspension culture of Panax japonicus var. repens. Russ. J. Plant Physiol. 2017, 64, 649–656. [Google Scholar] [CrossRef]
- Kochkin, D.V.; Galishev, B.A.; Titova, M.V.; Popova, E.V.; Nosov, A.M. Chromato-Mass-Spectrometric Identification of Glycosides of Phenylethylamides of Hydroxycinnamic Acids in a Suspension Cell Culture of Mandragora turcomanica. Russ. J. Plant Physiol. 2020, 68, 973–980. [Google Scholar] [CrossRef]
- Geraldes, V.; de Medeiros, L.S.; Lima, S.T.; Alvarenga, D.O.; Gacesa, R.; Long, P.F.; Fiore, M.F.; Pinto, E. Genetic and biochemical evidence for redundant pathways leading to mycosporine-like amino acid biosynthesis in the cyanobacterium Sphaerospermopsis torques-reginae ITEP-024. Algae 2020, 35, 177–187. [Google Scholar] [CrossRef]
- Cardozo, K.H.M.; Vessecchi, R.; Carvalho, V.M.; Pinto, E.; Gates, P.J.; Colepicolo, P.; Galembeck, S.E.; Lopes, N.P. A theoretical and mass spectrometry study of the fragmentation of mycosporine-like amino acids. Int. J. Mass Spectrom. 2008, 273, 11–19. [Google Scholar] [CrossRef]
- Cardozo, K.H.; Carvalho, V.M.; Pinto, E.; Colepicolo, P. Fragmentation of mycosporine-like amino acids by hydrogen/deuterium exchange and electrospray ionisation tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2006, 20, 253–258. [Google Scholar] [CrossRef]
- D’Agostino, P.M.; Javalkote, V.S.; Mazmouz, R.; Pickford, R.; Puranik, P.R.; Neilan, B.A. Comparative profiling and discovery of novel glycosylated mycosporine-like amino acids in two strains of the cyanobacterium Scytonema cf. crispum. Appl. Environ. Microbiol. 2016, 82, 5951–5959. [Google Scholar] [CrossRef] [Green Version]
- Matsuyama, K.; Matsumoto, J.; Yamamoto, S.; Nagasaki, K.; Inoue, Y.; Nishijima, M.; Mori, T. pH-independent charge resonance mechanism for UV protective functions of shinorine and related mycosporine-like amino acids. J. Phys. Chem. A 2015, 119, 12722–12729. [Google Scholar] [CrossRef]
- Burczyk, J.; Zych, M.; Ioannidis, N.E.; Kotzabasis, K. Polyamines in cell walls of chlorococcalean microalgae. Zeitschrift für Naturforschung C 2014, 69, 75–80. [Google Scholar] [CrossRef] [Green Version]
- Atkinson, J.A.; Gunning, B.E.S.; John, P.C.L. Sporopollenin in the cell wall of Chlorella and other algae: Ultrastructure, chemistry, and incorporation of 14C-acetate, studied in synchronous cultures. Planta 1972, 107, 1–32. [Google Scholar] [CrossRef]
- Montsant, A.; Zarka, A.; Boussiba, S. Presence of a nonhydrolyzable biopolymer in the cell wall of vegetative cells and astaxanthin-rich cysts of Haematococcus pluvialis (Chlorophyceae). Mar. Biotechnol. 2021, 3, 515–521. [Google Scholar] [CrossRef]
- Goiris, K.; Muylaert, K.; Voorspoels, S.; Noten, B.; De Paepe, D.; Baart, G.J.E.; De Cooman, L. Detection of flavonoids in microalgae from different evolutionary lineages. J. Phycol. 2014, 50, 483–492. [Google Scholar] [CrossRef]
- Chekanov, K.; Lukyanov, A.; Boussiba, S.; Aflalo, C.; Solovchenko, A. Modulation of photosynthetic activity and photoprotection in Haematococcus pluvialis cells during their conversion into haematocysts and back. Photosynth. Res. 2016, 128, 313–323. [Google Scholar] [CrossRef]
- Torzillo, G.; Goksan, T.; Faraloni, C.; Kopecky, J.; Masojídek, J. Interplay between photochemical activities and pigment composition in an outdoor culture of Haematococcus pluvialis during the shift from the green to red stage. J. Appl. Phycol. 2003, 15, 127–136. [Google Scholar] [CrossRef]
- Gu, W.; Li, H.; Zhao, P.; Yu, R.; Pan, G.; Gao, S.; Xie, X.; Huang, A.; He, L.; Wang, G. Quantitative proteomic analysis of thylakoid from two microalgae (Haematococcus pluvialis and Dunaliella salina) reveals two different high light-responsive strategies. Sci. Rep. 2014, 4, 1–12. [Google Scholar] [CrossRef]
- Gorelova, O.; Baulina, O.; Ismagulova, T.; Kokabi, K.; Lobakova, E.; Selyakh, I.; Semenova, L.; Chivkunova, O.; Karpova, O.; Scherbakov, P.; et al. Stress-induced changes in the ultrastructure of the photosynthetic apparatus of green microalgae. Protoplasma 2019, 256, 261–277. [Google Scholar] [CrossRef]
- Solovchenko, A.; Baulina, O.; Ptushenko, O.; Gorelova, O. Ultrastructural patterns of photoacclimation and photodamage to photosynthetic algae cell under environmental stress. Physiol. Plant. 2019, 166, 251–263. [Google Scholar] [CrossRef]
- Lazár, D. Parameters of photosynthetic energy partitioning. J. Plant Physiol. 2015, 175, 131–147. [Google Scholar] [CrossRef]
- Fratamico, A.; Tocquin, P.; Franck, F. The chlorophyll a fluorescence induction curve in the green microalga Haematococcus pluvialis: Further insight into the nature of the P–S–M fluctuation and its relationship with the “low-wave” phenomenon at steady-state. Photosynth. Res. 2016, 128, 271–285. [Google Scholar] [CrossRef]
- Izumi, M.; Ishida, H.; Nakamura, S.; Hidema, J. Entire photodamaged chloroplasts are transported to the central vacuole by autophagy. Plant Cell 2017, 29, 377–394. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Pérez, M.E.; Crespo, J.L. Autophagy in the model alga Chlamydomonas reinhardtii. Autophagy 2010, 6, 562–563. [Google Scholar] [CrossRef] [Green Version]
- Shebanova, A.; Ismagulova, T.; Solovchenko, A.; Baulina, O.; Lobakova, E.; Ivanova, A.; Moiseenko, A.; Shaitan, K.; Polshakov, V.; Nedbal, L.; et al. Versatility of the green microalga cell vacuole function as revealed by analytical transmission electron microscopy. Protoplasma 2017, 254, 1323–1340. [Google Scholar] [CrossRef]
- Baulina, O.; Gorelova, O.; Solovchenko, A.; Chivkunova, O.; Semenova, L.; Selyakh, I.; Scherbakov, P.; Burakova, O.; Lobakova, E. Diversity of the nitrogen starvation responses in subarctic Desmodesmus sp. (Chlorophyceae) strains isolated from symbioses with invertebrates. FEMS Microbiol. Ecol. 2016, 92, fiw031. [Google Scholar] [CrossRef] [Green Version]
- Scherbakov, P.; Ismagulova, T.; Chernov, T.; Gorelova, O.; Selyakh, I.; Semenova, L.; Baulina, O.; Chivkunova, O.; Solovchenko, A. A new subarctic strain of Tetradesmus obliquus. Part II: Comparative studies of CO2-stress tolerance. J. Appl. Phycol. 2018, 30, 2751–2761. [Google Scholar] [CrossRef]
- Goodson, C.; Roth, R.; Wang, Z.T.; Goodenough, U. Structural correlates of cytoplasmic and chloroplast lipid body synthesis in Chlamydomonas reinhardtii and stimulation of lipid body production with acetate boost. Eukaryot. Cell 2011, 10, 1592–1606. [Google Scholar] [CrossRef] [Green Version]
- Goncalves, E.C.; Johnson, J.V.; Rathinasabapathi, B. Conversion of membrane lipid acyl groups to triacylglycerol and formation of lipid bodies upon nitrogen starvation in biofuel green algae Chlorella UTEX29. Planta 2013, 238, 895–906. [Google Scholar] [CrossRef]
- Kong, D.X.; Li, Y.Q.; Wang, M.L.; Bai, M.; Zou, R.; Tang, H.; Wu, H. Effects of light intensity on leaf photosynthetic characteristics, chloroplast structure, and alkaloid content of Mahonia bodinieri (Gagnep.) Laferr. Acta Physiol. Plant. 2016, 38, 120. [Google Scholar] [CrossRef]
- Wang, X.; Song, Y.; Liu, B.; Hang, W.; Li, R.; Cui, H.; Li, R.; Jia, X. Enhancement of astaxanthin biosynthesis in Haematococcus pluvialis via inhibition of autophagy by 3-methyladenine under high light. Algal Res. 2020, 50, 101991. [Google Scholar] [CrossRef]
- Stanier, R.Y.; Kunisawa, R.; Mandel, M.C.B.G.; Cohen-Bazire, G. Purification and properties of unicellular blue-green algae (order Chroococcales). Bacteriol. Rev. 1971, 35, 171–205. [Google Scholar] [CrossRef]
- Ismagulova, T.; Chekanov, K.; Gorelova, O.; Baulina, O.; Semenova, L.; Selyakh, I.; Chivkunova, O.; Karpova, O.; Lobakova, E.; Solovchenko, A. A new subarctic strain of Tetradesmus obliquus—Part I: Identification and fatty acid profiling. J. Appl. Phycol. 2018, 30, 2737–2750. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: A multiple sequence alignment method with reduced time and space complexity. BMC Bioinform. 2004, 5, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Aldrich, J. RA Fisher and the making of maximum likelihood 1912–1922. Stat. Sci. 1997, 12, 162–176. [Google Scholar] [CrossRef]
- Kimura, M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef]
- Gorelova, O.A.; Baulina, O.I.; Solovchenko, A.E.; Chekanov, K.A.; Chivkunova, O.B.; Fedorenko, T.A.; Lobakova, E.S. Similarity and diversity of the Desmodesmus spp. microalgae isolated from associations with White Sea invertebrates. Protoplasma 2015, 252, 489–503. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, E.S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J. Cell Biol. 1963, 17, 208–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merzlyak, M.N.; Naqvi, K.R. On recording the true absorption spectrum and the scattering spectrum of a turbid sample: Application to cell suspensions of the cyanobacterium Anabaena variabilis. J. Photochem. Photobiol. B Biol. 2000, 58, 123–129. [Google Scholar] [CrossRef]
- Merzlyak, M.N.; Chivkunova, O.B.; Maslova, I.P.; Naqvi, K.R.; Solovchenko, A.E.; Klyachko-Gurvich, G.L. Light absorption and scattering by cell suspensions of some cyanobacteria and microalgae. Russ. J. Plant. Physiol. 2008, 55, 420–425. [Google Scholar]
- Folch, J.; Lees, M.; Stanley, G.S. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Chekanov, K.; Lobakova, E. Photosynthesis measurements on the upper and lower side of the thallus of the foliose lichen Nephroma arcticum (L.) Torss. Photosynth. Res. 2021, 149, 289–301. [Google Scholar]

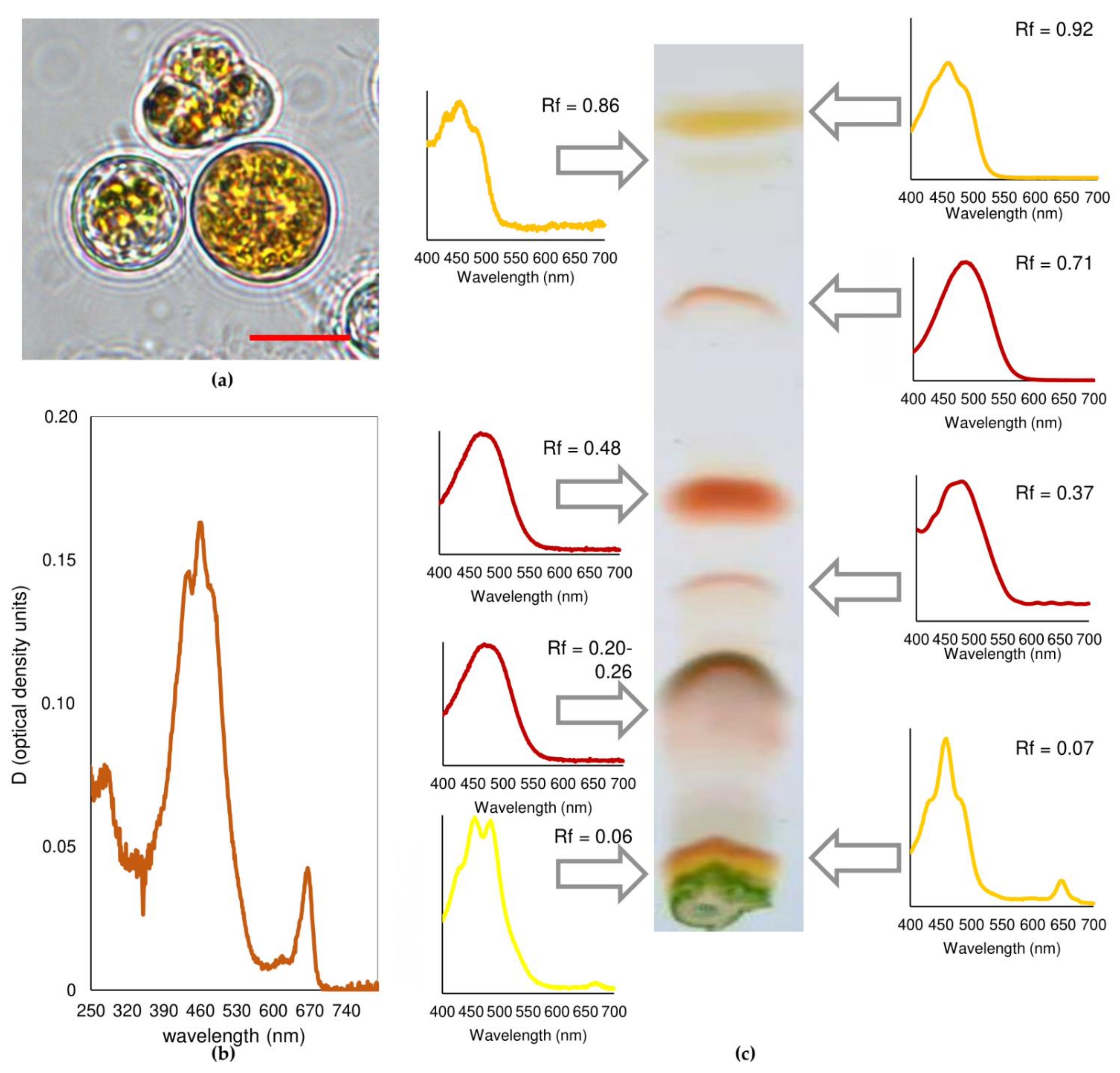
| Rf | Pigment | Content (%-of Total Carotenoid) |
|---|---|---|
| 0.92 | β-carotene | 7.65 |
| 0.86 | α-carotene | 0.62 |
| 0.71 | Echinenone | 1.96 |
| 0.48 | astaxanthin diesters | 27.99 |
| 0.37 | Canthaxanthin | 0.97 |
| 0.20–0.26 | astaxanthin monoesters | 35.32 |
| 0.07 | free ketocarotenoids + photosynthetic xanthophylls 1 | 4.94 |
| 0.06 | photosynthetic xanthophylls | 20.55 |
| Retention Time, Min. | m/z | |||||
|---|---|---|---|---|---|---|
| [M+H]+ | [M+NH4]+ | [M+Na]+ | [M+K]+ | Cluster Ion | Fragment Ions | |
| 0.35 | 505.1 | 522.1 | 527.1 | 543.1 | [2M+Na]+ 1031.3 [2M+NH4]+ 1026.3 | 487 365 325 432 |
| 0.39 | 343.1 | 360.1 | 365.1 | 381.1 | [2M+Na]+ 707.2 [2M+NH4]+ 702.2 [2M+H]+ 685.2 [3M+Na]+ 1049.3 [3M+NH4]+ 1044.3 [3M+H]+ 1027.3 [4M+Na]+ 1391.4 [4M+NH4]+ 1386.4 [4M+H]+ 1369.4 [5M+Na]+ 1733.6 [5M+NH4]+ 1728.6 [5M+H]+ 1711.6 | 325 307 289 271 297 279 281 275 328 |
| 0.41 | 236.1 | - | 258.1 | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaytseva, A.; Chekanov, K.; Zaytsev, P.; Bakhareva, D.; Gorelova, O.; Kochkin, D.; Lobakova, E. Sunscreen Effect Exerted by Secondary Carotenoids and Mycosporine-like Amino Acids in the Aeroterrestrial Chlorophyte Coelastrella rubescens under High Light and UV-A Irradiation. Plants 2021, 10, 2601. https://doi.org/10.3390/plants10122601
Zaytseva A, Chekanov K, Zaytsev P, Bakhareva D, Gorelova O, Kochkin D, Lobakova E. Sunscreen Effect Exerted by Secondary Carotenoids and Mycosporine-like Amino Acids in the Aeroterrestrial Chlorophyte Coelastrella rubescens under High Light and UV-A Irradiation. Plants. 2021; 10(12):2601. https://doi.org/10.3390/plants10122601
Chicago/Turabian StyleZaytseva, Anna, Konstantin Chekanov, Petr Zaytsev, Daria Bakhareva, Olga Gorelova, Dmitry Kochkin, and Elena Lobakova. 2021. "Sunscreen Effect Exerted by Secondary Carotenoids and Mycosporine-like Amino Acids in the Aeroterrestrial Chlorophyte Coelastrella rubescens under High Light and UV-A Irradiation" Plants 10, no. 12: 2601. https://doi.org/10.3390/plants10122601
APA StyleZaytseva, A., Chekanov, K., Zaytsev, P., Bakhareva, D., Gorelova, O., Kochkin, D., & Lobakova, E. (2021). Sunscreen Effect Exerted by Secondary Carotenoids and Mycosporine-like Amino Acids in the Aeroterrestrial Chlorophyte Coelastrella rubescens under High Light and UV-A Irradiation. Plants, 10(12), 2601. https://doi.org/10.3390/plants10122601







