Research and Progress on the Mechanism of Iron Transfer and Accumulation in Rice Grains
Abstract
:1. Introduction
2. Physiological Function of Fe and Its Nutritional Requirements in Organisms
2.1. Physiological Function of Fe in Organisms
2.2. Range Variation of Fe Concentration in Rice Grains and Demand of Fe Concentration for Human
3. Fe Absorption and Transport Mechanism in Rice Plants
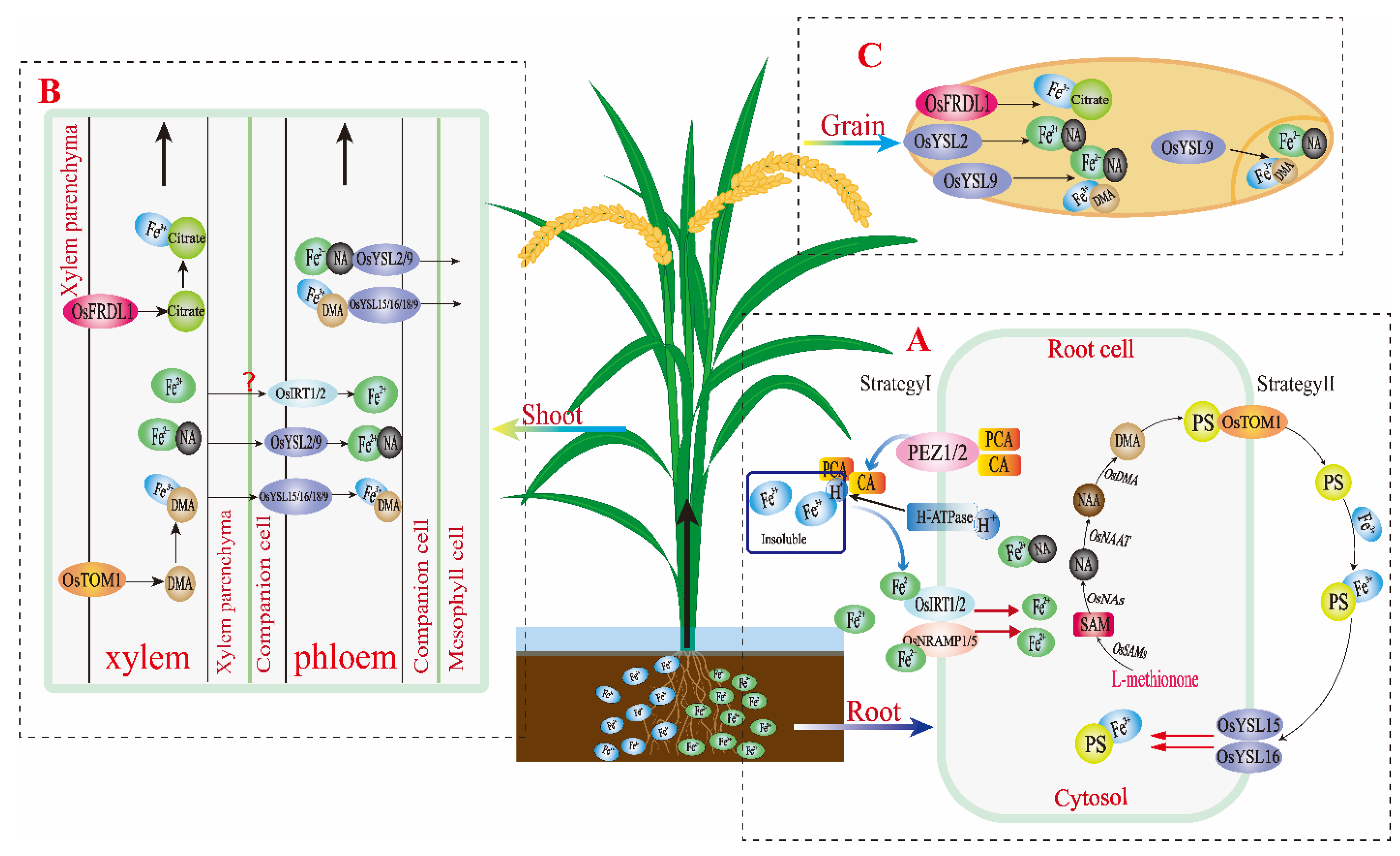
3.1. Absorption Mechanism of Fe into Rice Roots
3.2. Fe Transport Mechanism in Rice Plants
3.3. Accumulation Form and Distribution Characteristics of Fe in Rice Grain
4. Interrelationship and Balance between Fe and Other Elements in Rice
4.1. Interrelationship and Balance between Fe and Other Metal Elements in Rice
4.2. Interaction between Fe and S and P Elements
5. Strengthening Route of Micronutrient Fe Nutrition in Rice Grains
5.1. Strengthening Grain Fe Nutrition through Conventional Breeding Methods
5.2. Agronomic Optimization to Improve Rice Grain Fe Concentration and Bioavailability
5.3. Genetic Engineering Technology Improves Fe Capacity in Rice Grain
6. Suggestions for Further Research
7. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. WHO Global Anaemia Estimates, 2021 Edition. Available online: https://www.who.int/data/gho/data/themes/topics/anaemia_in_women_and_children (accessed on 10 November 2021).
- Kulkarni, A.; Khade, M.; Arun, S.; Badami, P.; Kumar, G.R.K.; Dattaroy, T.; Soni, B.; Dasgupta, S. An overview on mechanism, cause, prevention and multi-nation policy level interventions of dietary iron deficiency. Crit. Rev. Food Sci. Nutr. 2021, 2, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, Y.; Bhullar, N.K. Potential implications of interactions between Fe and S on cereal Fe biofortification. J. Integr. Plant Biol. 2020, 21, 2827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maganti, S.; Swaminathan, R.; Parida, A. Variation in Iron and Zinc Content in Traditional Rice Genotypes. Agric. Res. 2020, 9, 316–328. [Google Scholar] [CrossRef]
- Broadley, M.; Brown, P.; Cakmak, I.; Rengel, Z.; Zhao, F. Function of Nutrients: Micronutrients. In Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Marschner, P., Ed.; Academic Press: San Diego, CA, USA, 2012; pp. 191–248. [Google Scholar] [CrossRef]
- Stevens, G.A.; Finucane, M.M.; De-Regil, L.M.; Paciorek, C.J.; Flaxman, S.R.; Branca, F.; Peña-Rosas, J.P.; Bhutta, Z.A.; Ezzati, M. Global, regional, and national trends in haemoglobin concentration and prevalence of total and severe anaemia in children and pregnant and non-pregnant women for 1995–2011: A systematic analysis of population-representative data. Lancet Glob. Health 2013, 1, e16–e25. [Google Scholar] [CrossRef] [Green Version]
- Briat, J.-F.; Dubos, C.; Gaymard, F. Iron nutrition, biomass production, and plant product quality. Trends Plant Sci. 2015, 20, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Muthayya, S.; Sugimoto, J.D.; Montgomery, S.; Maberly, G.F. An overview of global rice production, supply, trade, and consumption. Ann. N. Y. Acad. Sci. 2014, 1324, 7–14. [Google Scholar] [CrossRef]
- Kumar, A.; Lal, M.K.; Kar, S.S.; Nayak, L.; Ngangkham, U.; Samantaray, S.; Sharma, S.G. Bioavailability of iron and zinc as affected by phytic acid content in rice grain. J. Food Biochem. 2017, 41, e12413. [Google Scholar] [CrossRef]
- Wei, Y.Y.; Wang, S.; Gu, M.H.; He, L.X.; Shen, F.K.; Cai, Z.Q. Genotypic Variations in Concentrations of Selenium, Iron and Zinc in Rice Grain and Its Dietary Exposure Assessment. Food Ind. 2020, 41, 217–220. [Google Scholar]
- Shi, Y.M.; Liu, B.L.; Wang, W.H.; Zheng, T.Q.; Xu, J.L.; Wei, S.F.; Wu, H.; Liao, Z.B. Preliminary Report on the Screening of Functional Components of Fe, Zn and Se in Some Rice Germplasms and Rice Breeding Material. Seed 2019, 38, 75–77. [Google Scholar]
- Lu, L.; Tian, S.; Liao, H.; Zhang, J.; Yang, X.; Labavitch, J.M.; Chen, W. Analysis of Metal Element Distributions in Rice (Oryza sativa L.) Seeds and Relocation during Germination Based on X-Ray Fluorescence Imaging of Zn, Fe, K, Ca, and Mn. PLoS ONE 2013, 8, e57360. [Google Scholar] [CrossRef]
- Trumbo, P.; Yates, A.A.; Schlicker, S.; Poos, M. Dietary Reference Intakes: Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. J. Am. Diet. Assoc. 2001, 101, 294–301. [Google Scholar] [CrossRef]
- Sohn, E. Contamination: The toxic side of rice. Nature 2014, 514, S62–S63. [Google Scholar] [CrossRef] [PubMed]
- Bouis, H.E.; Hotz, C.; McClafferty, B.; Meenakshi, J.V.; Pfeiffer, W.H. Biofortification: A New Tool to Reduce Micronutrient Malnutrition. Food Nutr. Bull. 2011, 32, S31–S40. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Nakanishi, H.; Nishizawa, N.K. Recent insights into iron homeostasis and their application in graminaceous crops. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2010, 86, 900–913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, T.; Nishizawa, N.K. Iron Uptake, Translocation, and Regulation in Higher Plants. Annu. Rev. Plant Biol. 2012, 63, 131–152. [Google Scholar] [CrossRef] [Green Version]
- Ishimaru, Y.; Suzuki, M.; Tsukamoto, T.; Suzuki, K.; Nakazono, M.; Kobayashi, T.; Wada, Y.; Watanabe, S.; Matsuhashi, S.; Takahashi, M.; et al. Rice plants take up iron as an Fe3+-phytosiderophore and as Fe2+. Plant J. 2006, 45, 335–346. [Google Scholar] [CrossRef]
- Vert, G.; Grotz, N.; Dédaldéchamp, F.; Gaymard, F.; Guerinot, M.L.; Briat, J.F.; Curie, C. Correction: IRT1, an arabidopsis transporter essential for iron uptake from the soil and for plant growth. Plant Cell 2021, 33, 439–440. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, R.; Ishimaru, Y.; Senoura, T.; Shimo, H.; Ishikawa, S.; Arao, T.; Nakanishi, H.; Nishizawa, N.K. The OsNRAMP1 iron transporter is involved in Cd accumulation in rice. J. Exp. Bot. 2011, 62, 4843–4850. [Google Scholar] [CrossRef] [Green Version]
- Ishimaru, Y.; Bashir, K.; Nakanishi, H.; Nishizawa, N.K. OsNRAMP5, a major player for constitutive iron and manganese uptake in rice. Plant Signal Behav. 2012, 7, 763–766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cesco, S.; Neumann, G.; Tomasi, N.; Pinton, R.; Weisskopf, L. Release of plant-borne flavonoids into the rhizosphere and their role in plant nutrition. Plant Soil 2010, 329, 1–25. [Google Scholar] [CrossRef]
- Ishimaru, Y.; Kakei, Y.; Shimo, H.; Bashir, K.; Sato, Y.; Sato, Y.; Uozumi, N.; Nakanishi, H.; Nishizawa, N.K. A Rice Phenolic Efflux Transporter Is Essential for Solubilizing Precipitated Apoplasmic Iron in the Plant Stele. J. Biol. Chem. 2011, 286, 24649–24655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inoue, H.; Higuchi, K.; Takahashi, M.; Nakanishi, H.; Mori, S.; Nishizawa, N.K. Three rice nicotianamine synthase genes, OsNAS1, OsNAS2, and OsNAS3 are expressed in cells involved in long-distance transport of iron and differentially regulated by iron. Plant J. 2003, 36, 366–381. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.A.T.; Kyriacou, B.; Callahan, D.L.; Carruthers, L.; Stangoulis, J.; Lombi, E.; Tester, M. Constitutive Overexpression of the OsNAS Gene Family Reveals Single-Gene Strategies for Effective Iron- and Zinc-Biofortification of Rice Endosperm. PLoS ONE 2011, 6, e24476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.; Kim, Y.-S.; Jeon, U.S.; Kim, Y.-K.; Schjoerring, J.K.; An, G. Activation of Rice nicotianamine synthase 2 (OsNAS2) enhances iron availability for biofortification. Mol. Cells 2012, 33, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Chiecko, J.C.; Kim, S.A.; Walker, E.L.; Lee, Y.; Guerinot, M.L.; An, G. Disruption of OsYSL15 Leads to Iron Inefficiency in Rice Plants. Plant Physiol. 2009, 150, 786–800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kakei, Y.; Ishimaru, Y.; Kobayashi, T.; Yamakawa, T.; Nakanishi, H.; Nishizawa, N.K. OsYSL16 plays a role in the allocation of iron. Plant Mol. Biol. 2012, 79, 583–594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roschzttardtz, H.; Conéjéro, G.; Divol, F.; Alcon, C.; Verdeil, J.-L.; Curie, C.; Mari, S. New insights into Fe localization in plant tissues. Front. Plant Sci. 2013, 4, 350. [Google Scholar] [CrossRef] [Green Version]
- Ishimaru, Y.; Masuda, H.; Bashir, K.; Inoue, H.; Tsukamoto, T.; Takahashi, M.; Nakanishi, H.; Aoki, N.; Hirose, T.; Ohsugi, R.; et al. Rice metal-nicotianamine transporter, OsYSL2, is required for the long-distance transport of iron and manganese. Plant J. 2010, 62, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, R.; Kato, M.; Nagata, S.; Yanagisawa, S.; Yoneyama, T. Identification of Zn–Nicotianamine and Fe–2′-Deoxymugineic Acid in the Phloem Sap from Rice Plants (Oryza sativa L.). Plant Cell Physiol. 2012, 53, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Yokosho, K.; Yamaji, N.; Ueno, D.; Mitani, N.; Ma, J.F. OsFRDL1 Is a Citrate Transporter Required for Efficient Translocation of Iron in Rice. Plant Physiol. 2008, 149, 297–305. [Google Scholar] [CrossRef] [Green Version]
- Bonneau, J.; Baumann, U.; Beasley, J.; Li, Y.; Johnson, A.A.T. Identification and molecular characterization of the nicotianamine synthase gene family in bread wheat. Plant Biotechnol. J. 2016, 14, 2228–2239. [Google Scholar] [CrossRef] [Green Version]
- Inoue, H.; Kobayashi, T.; Nozoye, T.; Takahashi, M.; Kakei, Y.; Suzuki, K.; Nakazono, M.; Nakanishi, H.; Mori, S.; Nishizawa, N.K. Rice OsYSL15 Is an Iron-regulated Iron(III)-Deoxymugineic Acid Transporter Expressed in the Roots and Is Essential for Iron Uptake in Early Growth of the Seedlings. J. Biol. Chem. 2009, 284, 3470–3479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aoyama, T.; Kobayashi, T.; Takahashi, M.; Nagasaka, S.; Usuda, K.; Kakei, Y.; Ishimaru, Y.; Nakanishi, H.; Mori, S.; Nishizawa, N.K. OsYSL18 is a rice iron (III)–deoxymugineic acid transporter specifically expressed in reproductive organs and phloem of lamina joints. Plant Mol. Biol. 2009, 70, 681–692. [Google Scholar] [CrossRef] [Green Version]
- Koike, S.; Inoue, H.; Mizuno, D.; Takahashi, M.; Nakanishi, H.; Mori, S.; Nishizawa, N.K. OsYSL2 is a rice metal-nicotianamine transporter that is regulated by iron and expressed in the phloem. Plant J. 2004, 39, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Senoura, T.; Sakashita, E.; Kobayashi, T.; Takahashi, M.; Aung, M.S.; Masuda, H.; Nakanishi, H.; Nishizawa, N.K. The iron-chelate transporter OsYSL9 plays a role in iron distribution in developing rice grains. Plant Mol. Biol. 2017, 95, 375–387. [Google Scholar] [CrossRef] [PubMed]
- Prom-u-Thai, C.; Dell, B.; Thomson, G.; Rerkasem, B. Easy and rapid detection of iron in rice grain. ScienceAsia 2003, 29, 203–207. [Google Scholar] [CrossRef]
- Wang, K.M.; Wu, J.G.; Li, G.; Zhang, D.P.; Yang, Z.W.; Shi, C.H. Distribution of phytic acid and mineral elements in three indica rice (Oryza sativa L.) cultivars. J. Cereal Sci. 2011, 54, 116–121. [Google Scholar] [CrossRef]
- Radchuk, V.; Weier, D.; Radchuk, R.; Weschke, W.; Weber, H. Development of maternal seed tissue in barley is mediated by regulated cell expansion and cell disintegration and coordinated with endosperm growth. J. Exp. Bot. 2010, 62, 1217–1227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weber, G.; von Wirén, N.; Hayen, H. Investigation of ascorbate-mediated iron release from ferric phytosiderophores in the presence of nicotianamine. Biometals 2008, 21, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Ariga, T.; Hazama, K.; Yanagisawa, S.; Yoneyama, T. Chemical forms of iron in xylem sap from graminaceous and non-graminaceous plants. Soil Sci. Plant Nutr. 2014, 60, 460–469. [Google Scholar] [CrossRef] [Green Version]
- Moore, K.L.; Zhao, F.-J.; Gritsch, C.S.; Tosi, P.; Hawkesford, M.J.; McGrath, S.P.; Shewry, P.R.; Grovenor, C.R.M. Localisation of iron in wheat grain using high resolution secondary ion mass spectrometry. J. Cereal Sci. 2012, 55, 183–187. [Google Scholar] [CrossRef]
- Theil, E.C. Iron, Ferritin, and Nutrition. Annu. Rev. Nutr. 2004, 24, 327–343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stein, R.J.; Ricachenevsky, F.K.; Fett, J.P. Differential regulation of the two rice ferritin genes (OsFER1 and OsFER2). Plant Sci. 2009, 177, 563–569. [Google Scholar] [CrossRef]
- Oliva, N.; Chadha-Mohanty, P.; Poletti, S.; Abrigo, E.; Atienza, G.; Torrizo, L.; Garcia, R.; Dueñas, C.; Poncio, M.A.; Balindong, J.; et al. Large-scale production and evaluation of marker-free indica rice IR64 expressing phytoferritin genes. Mol. Breed. 2014, 33, 23–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kyriacou, B.; Moore, K.L.; Paterson, D.; de Jonge, M.D.; Howard, D.L.; Stangoulis, J.; Tester, M.; Lombi, E.; Johnson, A.A.T. Localization of iron in rice grain using synchrotron X-ray fluorescence microscopy and high resolution secondary ion mass spectrometry. J. Cereal Sci. 2014, 59, 173–180. [Google Scholar] [CrossRef]
- Foster, A.W.; Osman, D.; Robinson, N.J. Metal Preferences and Metallation. J. Biol. Chem. 2014, 289, 28095–28103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Briat, J.-F.; Rouached, H.; Tissot, N.; Gaymard, F.; Dubos, C. Integration of P, S, Fe, and Zn nutrition signals in Arabidopsis thaliana: Potential involvement of Phosphate Starvation Response 1 (PHR1). Front. Plant Sci. 2015, 6, 290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, T.; Nozoye, T.; Nishizawa, N.K. Iron transport and its regulation in plants. Free. Radic. Biol. Med. 2019, 133, 11–20. [Google Scholar] [CrossRef]
- Shanmugam, V.; Lo, J.-C.; Yeh, K.-C. Control of Zn uptake in Arabidopsis halleri: A balance between Zn and Fe. Front. Plant Sci. 2013, 4, 281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.; An, G. Over-expression of OsIRT1 leads to increased iron and zinc accumulations in rice. Plant Cell Environ. 2009, 32, 408–416. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, Y.; Zhang, L.; Hu, J.; Zhang, X.; Lu, K.; Dong, H.; Wang, D.; Zhao, F.-J.; Huang, C.-F.; et al. OsNRAMP5 contributes to manganese translocation and distribution in rice shoots. J. Exp. Bot. 2014, 65, 4849–4861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mani, A.; Sankaranarayanan, K. In Silico Analysis of Natural Resistance-Associated Macrophage Protein (NRAMP) Family of Transporters in Rice. Protein J. 2018, 37, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Haydon, M.J.; Cobbett, C.S. Transporters of ligands for essential metal ions in plants. New Phytol. 2007, 174, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, M.; Datta, K.; Oliva, N.; Khalekuzzaman, M.; Torrizo, L.; Krishnan, S.; Oliveira, M.; Goto, F.; Datta, S.K. Enhanced iron and zinc accumulation in transgenic rice with the ferritin gene. Plant Sci. 2003, 164, 371–378. [Google Scholar] [CrossRef]
- Suzuki, M.; Tsukamoto, T.; Inoue, H.; Watanabe, S.; Matsuhashi, S.; Takahashi, M.; Nakanishi, H.; Mori, S.; Nishizawa, N.K. Deoxymugineic acid increases Zn translocation in Zn-deficient rice plants. Plant Mol. Biol. 2008, 66, 609–617. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Jeon, U.S.; Lee, S.J.; Kim, Y.-K.; Persson, D.P.; Husted, S.; Schjørring, J.K.; Kakei, Y.; Masuda, H.; Nishizawa, N.K.; et al. Iron fortification of rice seeds through activation of the nicotianamine synthase gene. Proc. Natl. Acad. Sci. USA 2009, 106, 22014–22019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masuda, H.; Usuda, K.; Kobayashi, T.; Ishimaru, Y.; Kakei, Y.; Takahashi, M.; Higuchi, K.; Nakanishi, H.; Mori, S.; Nishizawa, N.K. Overexpression of the Barley Nicotianamine Synthase Gene HvNAS1 Increases Iron and Zinc Concentrations in Rice Grains. Rice 2009, 2, 155–166. [Google Scholar] [CrossRef] [Green Version]
- Masuda, H.; Ishimaru, Y.; Aung, M.S.; Kobayashi, T.; Kakei, Y.; Takahashi, M.; Higuchi, K.; Nakanishi, H.; Nishizawa, N.K. Iron biofortification in rice by the introduction of multiple genes involved in iron nutrition. Sci. Rep. 2012, 2, 543. [Google Scholar] [CrossRef] [Green Version]
- Trijatmiko, K.R.; Dueñas, C.; Tsakirpaloglou, N.; Torrizo, L.; Arines, F.M.; Adeva, C.; Balindong, J.; Oliva, N.; Sapasap, M.V.; Borrero, J.; et al. Biofortified indica rice attains iron and zinc nutrition dietary targets in the field. Sci. Rep. 2016, 6, 19792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, L.; Chang, J.; Chen, R.; Li, H.; Lu, H.; Tao, L.; Xiong, J. Comparison on cellular mechanisms of iron and cadmium accumulation in rice: Prospects for cultivating Fe-rich but Cd-free rice. Rice 2016, 9, 39. [Google Scholar] [CrossRef] [Green Version]
- Bashir, A.; Rizwan, M.; Ali, S.; Zia ur Rehman, M.; Ishaque, W.; Atif Riaz, M.; Maqbool, A. Effect of foliar-applied iron complexed with lysine on growth and cadmium (Cd) uptake in rice under Cd stress. Environ. Sci. Pollut. Res. 2018, 25, 20691–20699. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Ling, Q.; Dong, F.; de Dios, V.R.; Li, Z.; Zhang, W.; Huo, T.; Chen, Y.; Hu, X.; Wang, X.; et al. Iron and copper micronutrients influences cadmium accumulation in rice grains by altering its transport and allocation. Sci. Total Environ. 2021, 777, 146118. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Abbas, T.; Zia-ur-Rehman, M.; Hannan, F.; Keller, C.; Al-Wabel, M.I.; Ok, Y.S. Cadmium minimization in wheat: A critical review. Ecotoxicol. Environ. Saf. 2016, 130, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, B.; Wang, Y.; Qin, Y.; Zhou, Y.; Qian, H. Influence and interaction of iron and lead on seed germination in upland rice. Plant Soil 2020, 455, 187–202. [Google Scholar] [CrossRef]
- Astolfi, S.; Pii, Y.; Terzano, R.; Mimmo, T.; Celletti, S.; Allegretta, I.; Lafiandra, D.; Cesco, S. Does Fe accumulation in durum wheat seeds benefit from improved whole-plant sulfur nutrition? J. Cereal Sci. 2018, 83, 74–82. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, C.; Dai, C.; Ge, Y. Sufficient sulfur supply promotes seedling growth, alleviates oxidation stress, and regulates iron uptake and translocation in rice. Biol. Plant 2015, 59, 788–792. [Google Scholar] [CrossRef]
- Wu, Z.; Naveed, S.; Zhang, C.; Ge, Y. Adequate supply of sulfur simultaneously enhances iron uptake and reduces cadmium accumulation in rice grown in hydroponic culture. Environ. Pollut. 2020, 262, 114327. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Huang, F.; Narsai, R.; Wu, J.; Giraud, E.; He, F.; Cheng, L.; Wang, F.; Wu, P.; Whelan, J.; et al. Physiological and Transcriptome Analysis of Iron and Phosphorus Interaction in Rice Seedlings. Plant Physiol. 2009, 151, 262–274. [Google Scholar] [CrossRef] [Green Version]
- Rai, V.; Sanagala, R.; Sinilal, B.; Yadav, S.; Sarkar, A.K.; Dantu, P.K.; Jain, A. Iron Availability Affects Phosphate Deficiency-Mediated Responses, and Evidence of Cross-Talk with Auxin and Zinc in Arabidopsis. Plant Cell Physiol. 2015, 56, 1107–1123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valentinuzzi, F.; Venuti, S.; Pii, Y.; Marroni, F.; Cesco, S.; Hartmann, F.; Mimmo, T.; Morgante, M.; Pinton, R.; Tomasi, N.; et al. Common and specific responses to iron and phosphorus deficiencies in roots of apple tree (Malus × domestica). Plant Mol. Biol. 2019, 101, 129–148. [Google Scholar] [CrossRef]
- Bournier, M.; Tissot, N.; Mari, S.; Boucherez, J.; Lacombe, E.; Briat, J.-F.; Gaymard, F. Arabidopsis Ferritin 1 (AtFer1) Gene Regulation by the Phosphate Starvation Response 1 (AtPHR1) Transcription Factor Reveals a Direct Molecular Link between Iron and Phosphate Homeostasis. J. Biol. Chem. 2013, 288, 22670–22680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller, J.; Gödde, V.; Niehaus, K.; Zörb, C. Metabolic Adaptations of White Lupin Roots and Shoots under Phosphorus Deficiency. Front. Plant Sci. 2015, 6, 1014. [Google Scholar] [CrossRef] [Green Version]
- Dong, J.; Piñeros, M.A.; Li, X.; Yang, H.; Liu, Y.; Murphy, A.S.; Kochian, L.V.; Liu, D. An Arabidopsis ABC Transporter Mediates Phosphate Deficiency-Induced Remodeling of Root Architecture by Modulating Iron Homeostasis in Roots. Mol. Plant 2017, 10, 244–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Godon, C.; Mercier, C.; Wang, X.; David, P.; Richaud, P.; Nussaume, L.; Liu, D.; Desnos, T. Under phosphate starvation conditions, Fe and Al trigger accumulation of the transcription factor STOP1 in the nucleus of Arabidopsis root cells. Plant J. 2019, 99, 937–949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mora-Macías, J.; Ojeda-Rivera, J.O.; Gutiérrez-Alanís, D.; Yong-Villalobos, L.; Oropeza-Aburto, A.; Raya-González, J.; Jiménez-Domínguez, G.; Chávez-Calvillo, G.; Rellán-Álvarez, R.; Herrera-Estrella, L. Malate-dependent Fe accumulation is a critical checkpoint in the root developmental response to low phosphate. Proc. Natl. Acad. Sci. USA 2017, 114, E3563–E3572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanikenne, M.; Esteves, S.M.; Fanara, S.; Rouached, H. Coordinated homeostasis of essential mineral nutrients: A focus on iron. J. Exp. Bot. 2021, 72, 2136–2153. [Google Scholar] [CrossRef] [PubMed]
- López-Bucio, J.S.; Salmerón-Barrera, G.J.; Ravelo-Ortega, G.; Raya-González, J.; León, P.; de la Cruz, H.R.; Campos-García, J.; López-Bucio, J.; Guevara-García, Á.A. Mitogen-activated protein kinase 6 integrates phosphate and iron responses for indeterminate root growth in Arabidopsis thaliana. Planta 2019, 250, 1177–1189. [Google Scholar] [CrossRef] [PubMed]
- Ai, H.; Cao, Y.; Jain, A.; Wang, X.; Hu, Z.; Zhao, G.; Hu, S.; Shen, X.; Yan, Y.; Liu, X.; et al. The ferroxidase LPR5 functions in the maintenance of phosphate homeostasis and is required for normal growth and development of rice. J. Exp. Bot. 2020, 71, 4828–4842. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Ruan, W.; Zhang, Y.; Zhang, Y.; Wang, X.; Guo, Z.; Wang, L.; Zhou, T.; Paz-Ares, J.; Yi, K. A reciprocal inhibitory module for Pi and iron signaling. Mol. Plant 2021. [Google Scholar] [CrossRef] [PubMed]
- Saenchai, C.; Bouain, N.; Kisko, M.; Prom-u-thai, C.; Doumas, P.; Rouached, H. The Involvement of OsPHO1;1 in the Regulation of Iron Transport Through Integration of Phosphate and Zinc Deficiency Signaling. Front. Plant Sci. 2016, 7, 396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garg, M.; Sharma, N.; Sharma, S.; Kapoor, P.; Kumar, A.; Chunduri, V.; Arora, P. Biofortified Crops Generated by Breeding, Agronomy, and Transgenic Approaches Are Improving Lives of Millions of People around the World. Front. Nutr. 2018, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- Connorton, J.M.; Balk, J.; Rodríguez-Celma, J. Iron homeostasis in plants–a brief overview. Metallomics 2017, 9, 813–823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anandan, A.; Rajiv, G.; Eswaran, R.; Prakash, M. Genotypic Variation and Relationships between Quality Traits and Trace Elements in Traditional and Improved Rice (Oryza sativa L.) Genotypes. J. Food Sci. 2011, 76, H122–H130. [Google Scholar] [CrossRef] [PubMed]
- Palmgren, M.G.; Edenbrandt, A.K.; Vedel, S.E.; Andersen, M.M.; Landes, X.; Østerberg, J.T.; Falhof, J.; Olsen, L.I.; Christensen, S.B.; Sandøe, P.; et al. Are we ready for back-to-nature crop breeding? Trends Plant Sci. 2015, 20, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Descalsota, G.I.L.; Swamy, B.P.M.; Zaw, H.; Inabangan-Asilo, M.A.; Amparado, A.; Mauleon, R.; Chadha-Mohanty, P.; Arocena, E.C.; Raghavan, C.; Leung, H.; et al. Genome-Wide Association Mapping in a Rice MAGIC Plus Population Detects QTLs and Genes Useful for Biofortification. Front. Plant Sci. 2018, 9, 1347. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Li, L.; Zheng, X.; Zhang, Z.; Mou, T.; Hu, Z. Quantitative trait loci controlling Cu, Ca, Zn, Mn and Fe content in rice grains. J. Genet. 2008, 87, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Oliveira, A.L.; Tan, L.; Fu, Y.; Sun, C. Genetic Identification of Quantitative Trait Loci for Contents of Mineral Nutrients in Rice Grain. J. Integr. Plant Biol. 2009, 51, 84–92. [Google Scholar] [CrossRef]
- Norton, G.J.; Deacon, C.M.; Xiong, L.; Huang, S.; Meharg, A.A.; Price, A.H. Genetic mapping of the rice ionome in leaves and grain: Identification of QTLs for 17 elements including arsenic, cadmium, iron and selenium. Plant Soil 2010, 329, 139–153. [Google Scholar] [CrossRef]
- Anuradha, K.; Agarwal, S.; Rao, Y.V.; Rao, K.V.; Viraktamath, B.C.; Sarla, N. Mapping QTLs and candidate genes for iron and zinc concentrations in unpolished rice of Madhukar × Swarna RILs. Gene 2012, 508, 233–240. [Google Scholar] [CrossRef]
- Wu, X.-L.; Hu, Z.-L. Meta-analysis of QTL Mapping Experiments. In Quantitative Trait Loci (QTL): Methods and Protocols; Rifkin, S.A., Ed.; Humana Press: Totowa, NJ, USA, 2012; pp. 145–171. [Google Scholar] [CrossRef]
- Swamy, B.P.M.; Kaladhar, K.; Anuradha, K.; Batchu, A.K.; Longvah, T.; Sarla, N. QTL Analysis for Grain Iron and Zinc Concentrations in Two O. nivara Derived Backcross Populations. Rice Sci. 2018, 25, 197–207. [Google Scholar] [CrossRef]
- Fageria, N.K.; Baligar, V.C.; Clark, R.B. Micronutrients in Crop Production. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: Cambridge, MA, USA, 2002; Volume 77, pp. 185–268. [Google Scholar]
- El-Ramady, H.R.; Abdalla, N.; Fári, M.; Domokos-Szabolcsy, É. Selenium enriched vegetables as biofortification alternative for alleviating micronutrient malnutrition. Int. J. Hortic. Sci. 2014, 20, 75–81. [Google Scholar] [CrossRef]
- Rehman, H.; Farooq, M.; Basra, S. High grain Zn content results from increased Zn supply and remobilization during grain filling in water saving rice cultivation. In Proceedings of the 14th Congress of Soil Science, Lahore, Pakistan, 12–15 March 2012; pp. 12–15. [Google Scholar]
- Prasad, R.; Shivay, Y.S.; Kumar, D. Current Status, Challenges, and Opportunities in Rice Production. In Rice Production Worldwide; Chauhan, B.S., Jabran, K., Mahajan, G., Eds.; Springer International Publishing: Cham, Germany, 2017; pp. 1–32. [Google Scholar] [CrossRef]
- Phattarakul, N.; Mongon, J.; Rerkasem, B. Variation in rice grain zinc and their response to zinc fertilizer. In Proceedings of the 3rd international zinc symposium, Hyderabad, India, 10–14 October 2011; pp. 10–14. [Google Scholar]
- He, W.; Shohag, M.J.I.; Wei, Y.; Feng, Y.; Yang, X. Iron concentration, bioavailability, and nutritional quality of polished rice affected by different forms of foliar iron fertilizer. Food Chem. 2013, 141, 4122–4126. [Google Scholar] [CrossRef]
- Yuan, L.; Wu, L.; Yang, C.; Lv, Q. Effects of iron and zinc foliar applications on rice plants and their grain accumulation and grain nutritional quality. J. Sci. Food Agric. 2013, 93, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Qiao, X.; He, Y.; Wang, Z.; Li, X.; Zhang, K.; Zeng, H. Effect of foliar spray of zinc on chloroplast β-carbonic anhydrase expression and enzyme activity in rice (Oryza sativa L.) leaves. Acta Physiol. Plant 2014, 36, 263–272. [Google Scholar] [CrossRef]
- He, Y.; Luo, Y.; Wang, Q.; Sun, Y.; Duan, N.; Chen, Z.; Zeng, H. Spray treatment of leaves with Fe2+ promotes procyanidin biosynthesis by upregulating the expression of the F3H and ANS genes in red rice grains (Oryza sativa L.). J. Cereal Sci. 2021, 100, 103231. [Google Scholar] [CrossRef]
- Das, P.; Barua, S.; Sarkar, S.; Karak, N.; Bhattacharyya, P.; Raza, N.; Kim, K.-H.; Bhattacharya, S.S. Plant extract–mediated green silver nanoparticles: Efficacy as soil conditioner and plant growth promoter. J. Hazard. Mater. 2018, 346, 62–72. [Google Scholar] [CrossRef]
- Adisa, I.O.; Pullagurala, V.L.R.; Peralta-Videa, J.R.; Dimkpa, C.O.; Elmer, W.H.; Gardea-Torresdey, J.L.; White, J.C. Recent advances in nano-enabled fertilizers and pesticides: A critical review of mechanisms of action. Environ. Sci. Nano 2019, 6, 2002–2030. [Google Scholar] [CrossRef]
- Li, M.; Zhang, P.; Adeel, M.; Guo, Z.; Chetwynd, A.J.; Ma, C.; Bai, T.; Hao, Y.; Rui, Y. Physiological impacts of zero valent iron, Fe3O4 and Fe2O3 nanoparticles in rice plants and their potential as Fe fertilizers. Environ. Pollut. 2021, 269, 116134. [Google Scholar] [CrossRef]
- Qiao, J.-t.; Liu, T.-x.; Wang, X.-q.; Li, F.-b.; Lv, Y.-h.; Cui, J.-h.; Zeng, X.-d.; Yuan, Y.-z.; Liu, C.-p. Simultaneous alleviation of cadmium and arsenic accumulation in rice by applying zero-valent iron and biochar to contaminated paddy soils. Chemosphere 2018, 195, 260–271. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Fang, Q.; Yan, S.; Pan, L.; Tang, X.; Ye, W. Effects of zinc oxide nanoparticles on arsenic stress in rice (Oryza sativa L.): Germination, early growth, and arsenic uptake. Environ. Sci. Pollut. Res. 2020, 27, 26974–26981. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, I.; Mukherjee, A.; Mukherjee, A. In planta genotoxicity of nZVI: Influence of colloidal stability on uptake, DNA damage, oxidative stress and cell death. Mutagenesis 2017, 32, 371–387. [Google Scholar] [CrossRef] [PubMed]
- Bashir, K.; Takahashi, R.; Nakanishi, H.; Nishizawa, N. The road to micronutrient biofortification of rice: Progress and prospects. Front. Plant Sci. 2013, 4, 15. [Google Scholar] [CrossRef] [Green Version]
- Vasconcelos, M.W.; Gruissem, W.; Bhullar, N.K. Iron biofortification in the 21st century: Setting realistic targets, overcoming obstacles, and new strategies for healthy nutrition. Curr. Opin. Biotechnol. 2017, 44, 8–15. [Google Scholar] [CrossRef]
- Kawakami, Y.; Bhullar, N.K. Molecular processes in iron and zinc homeostasis and their modulation for biofortification in rice. J. Integr. Plant Biol. 2018, 60, 1181–1198. [Google Scholar] [CrossRef] [PubMed]
- Mulualem, T. Application of bio-fortification through plant breeding to improve the value of staple crops. Biomed. Biotechnol. 2015, 3, 11–19. [Google Scholar] [CrossRef]
- Lucca, P.; Hurrell, R.; Potrykus, I. Genetic engineering approaches to improve the bioavailability and the level of iron in rice grains. Theor. Appl. Genet. 2001, 102, 392–397. [Google Scholar] [CrossRef]
- Paul, S.; Ali, N.; Gayen, D.; Datta, S.K.; Datta, K. Molecular breeding of Osfer2 gene to increase iron nutrition in rice grain. GM Crop. Food 2012, 3, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Qu, L.Q.; Yoshihara, T.; Ooyama, A.; Goto, F.; Takaiwa, F. Iron accumulation does not parallel the high expression level of ferritin in transgenic rice seeds. Planta 2005, 222, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Banakar, R.; Alvarez Fernández, Á.; Abadía, J.; Capell, T.; Christou, P. The expression of heterologous Fe (III) phytosiderophore transporter HvYS1 in rice increases Fe uptake, translocation and seed loading and excludes heavy metals by selective Fe transport. Plant Biotechnol. J. 2017, 15, 423–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, M.; Morikawa, K.C.; Nakanishi, H.; Takahashi, M.; Saigusa, M.; Mori, S.; Nishizawa, N.K. Transgenic rice lines that include barley genes have increased tolerance to low iron availability in a calcareous paddy soil. Soil Sci. Plant Nutr. 2008, 54, 77–85. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, Y.-H.; Yi, H.-Y.; Gong, J.-M. Vacuolar membrane transporters OsVIT1 and OsVIT2 modulate iron translocation between flag leaves and seeds in rice. Plant J. 2012, 72, 400–410. [Google Scholar] [CrossRef]
- Bashir, K.; Takahashi, R.; Akhtar, S.; Ishimaru, Y.; Nakanishi, H.; Nishizawa, N.K. The knockdown of OsVIT2 and MIT affects iron localization in rice seed. Rice 2013, 6, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Che, J.; Yamaji, N.; Ma, J.F. Role of a vacuolar iron transporter OsVIT2 in the distribution of iron to rice grains. New Phytol. 2021, 230, 1049–1062. [Google Scholar] [CrossRef] [PubMed]
- Che, J.; Yokosho, K.; Yamaji, N.; Ma, J.F. A Vacuolar Phytosiderophore Transporter Alters Iron and Zinc Accumulation in Polished Rice Grains1. Plant Physiol. 2019, 181, 276–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogo, Y.; Itai, R.N.; Kobayashi, T.; Aung, M.S.; Nakanishi, H.; Nishizawa, N.K. OsIRO2 is responsible for iron utilization in rice and improves growth and yield in calcareous soil. Plant Mol. Biol. 2011, 75, 593–605. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Nagasaka, S.; Senoura, T.; Itai, R.N.; Nakanishi, H.; Nishizawa, N.K. Iron-binding haemerythrin RING ubiquitin ligases regulate plant iron responses and accumulation. Nat. Commun. 2013, 4, 2792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boonyaves, K.; Wu, T.-Y.; Gruissem, W.; Bhullar, N.K. Enhanced Grain Iron Levels in Rice Expressing an Iron-Regulated Metal Transporter, Nicotianamine Synthase, and Ferritin Gene Cassette. Front. Plant Sci. 2017, 8, 130. [Google Scholar] [CrossRef] [Green Version]
- Díaz-Benito, P.; Banakar, R.; Rodríguez-Menéndez, S.; Capell, T.; Pereiro, R.; Christou, P.; Abadía, J.; Fernández, B.; Álvarez-Fernández, A. Iron and Zinc in the Embryo and Endosperm of Rice (Oryza sativa L.) Seeds in Contrasting 2′-Deoxymugineic Acid/Nicotianamine Scenarios. Front. Plant Sci. 2018, 9, 1190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aung, M.; Masuda, H.; Kobayashi, T.; Nakanishi, H.; Yamakawa, T.; Nishizawa, N. Iron Biofortification of Myanmar Rice. Front. Plant Sci. 2013, 4, 158. [Google Scholar] [CrossRef] [Green Version]
- Ali, N.; Paul, S.; Gayen, D.; Sarkar, S.N.; Datta, K.; Datta, S.K. Development of Low Phytate Rice by RNAi Mediated Seed-Specific Silencing of Inositol 1,3,4,5,6-Pentakisphosphate 2-Kinase Gene (IPK1). PLoS ONE 2013, 8, e68161. [Google Scholar] [CrossRef] [Green Version]
- Narayanan, N.N.; Vasconcelos, M.W.; Grusak, M.A. Expression profiling of Oryza sativa metal homeostasis genes in different rice cultivars using a cDNA macroarray. Plant Physiol. Biochem. 2007, 45, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.-Y.; Zhao, F.-J. QTL pyramiding for producing nutritious and safe rice grains. J. Integr. Plant Biol. 2020, 62, 264–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.-L.; Gao, Z.-Y.; Shang, L.-G.; Yang, C.-H.; Ruan, B.-P.; Zeng, D.-L.; Guo, L.-B.; Zhao, F.-J.; Huang, C.-F.; Qian, Q. Natural variation in the promoter of OsHMA3 contributes to differential grain cadmium accumulation between Indica and Japonica rice. J. Integr. Plant Biol. 2020, 62, 314–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, H.; Wang, M.; Wong, M.H.; Ye, Z. Does radial oxygen loss and iron plaque formation on roots alter Cd and Pb uptake and distribution in rice plant tissues? Plant Soil 2014, 375, 137–148. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, J.; Zhang, B.; Liu, Y.; Xu, X.; Li, Y.-F.; Li, B.; Gao, Y.; Chai, Z. The influence of iron plaque on the absorption, translocation and transformation of mercury in rice (Oryza sativa L.) seedlings exposed to different mercury species. Plant Soil 2016, 398, 87–97. [Google Scholar] [CrossRef]
| Gene | Method | Tissue | Strengthen Fe Level | Reference |
|---|---|---|---|---|
| Absorption and transport | ||||
| OsIRT1 | overexpression | brown rice | 1.1-fold | [52] |
| OsYSL15 | overexpression | brown rice | 1.2-fold | [27] |
| Long-distance transport | ||||
| HvIDS3 | transgenic plant | polished rice | 1.4-fold | [117] |
| OsYSL2 | overexpression | polished rice | 4.0-fold | [30] |
| ubi-1 + HvYS1 | constitutive expression | endosperm | 1.9-fold | [116] |
| OsYSL9 | RNAi | embryo | ~3.0-fold | [37] |
| Storage protein | ||||
| PyFER | transgene | brown rice | 2.0-fold | [113] |
| GmFER | transgene | brown/polished rice | 3.0~3.7-fold | [56] |
| SoyFER H1 | transgene | brown rice | 1.3-fold | [115] |
| OsFER2 | overexpression | brown rice | 2.1-fold | [114] |
| GmFER H1 | transgene | polished rice | 3.4-fold | [46] |
| Vacuole-related transport/storage | ||||
| OsVIT1/OsVIT2 | mutation | brown rice | ~1.5-fold | [118] |
| OsVIT2 | T-DNA insert | brown/polished rice | >1.5-fold | [109,120] |
| OsVMT | knockout | polished rice | 1.8~2.1-fold | [121] |
| Chelate synthase and combination strategy | ||||
| OsNAS1/OsNAS2/OsNAS3 | overexpression | brown rice | 2.0~4.0-fold | [25,26] |
| HvNAS1, OsYSL2, SoyFER | overexpression | polished rice | 3.4-fold | [126] |
| AtIRT1, AtNAS1, PvFER | overexpression | endosperm | 4.2-fold | [124] |
| OsNAS1, HvNAAT | overexpression | embryo/endosperm | 1.3~2.9-fold | [125] |
| Transcription factor | ||||
| OsIRO2 | overexpression | brown rice | 3.0-fold | [122] |
| OsHRZ1/OsHRZ2 | knockout | brown/polished rice | 2.9~3.8-fold | [123] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Chen, M.; Hao, Q.; Zeng, H.; He, Y. Research and Progress on the Mechanism of Iron Transfer and Accumulation in Rice Grains. Plants 2021, 10, 2610. https://doi.org/10.3390/plants10122610
Wang Q, Chen M, Hao Q, Zeng H, He Y. Research and Progress on the Mechanism of Iron Transfer and Accumulation in Rice Grains. Plants. 2021; 10(12):2610. https://doi.org/10.3390/plants10122610
Chicago/Turabian StyleWang, Qian, Mengjie Chen, Qianyi Hao, Hanlai Zeng, and Ying He. 2021. "Research and Progress on the Mechanism of Iron Transfer and Accumulation in Rice Grains" Plants 10, no. 12: 2610. https://doi.org/10.3390/plants10122610






