A Novel Pear Scab (Venturia nashicola) Resistance Gene, Rvn3, from Interspecific Hybrid Pear (Pyrus pyrifolia × P. communis)
Abstract
:1. Introduction
2. Results
2.1. Evaluation of Pear Scab Resistance
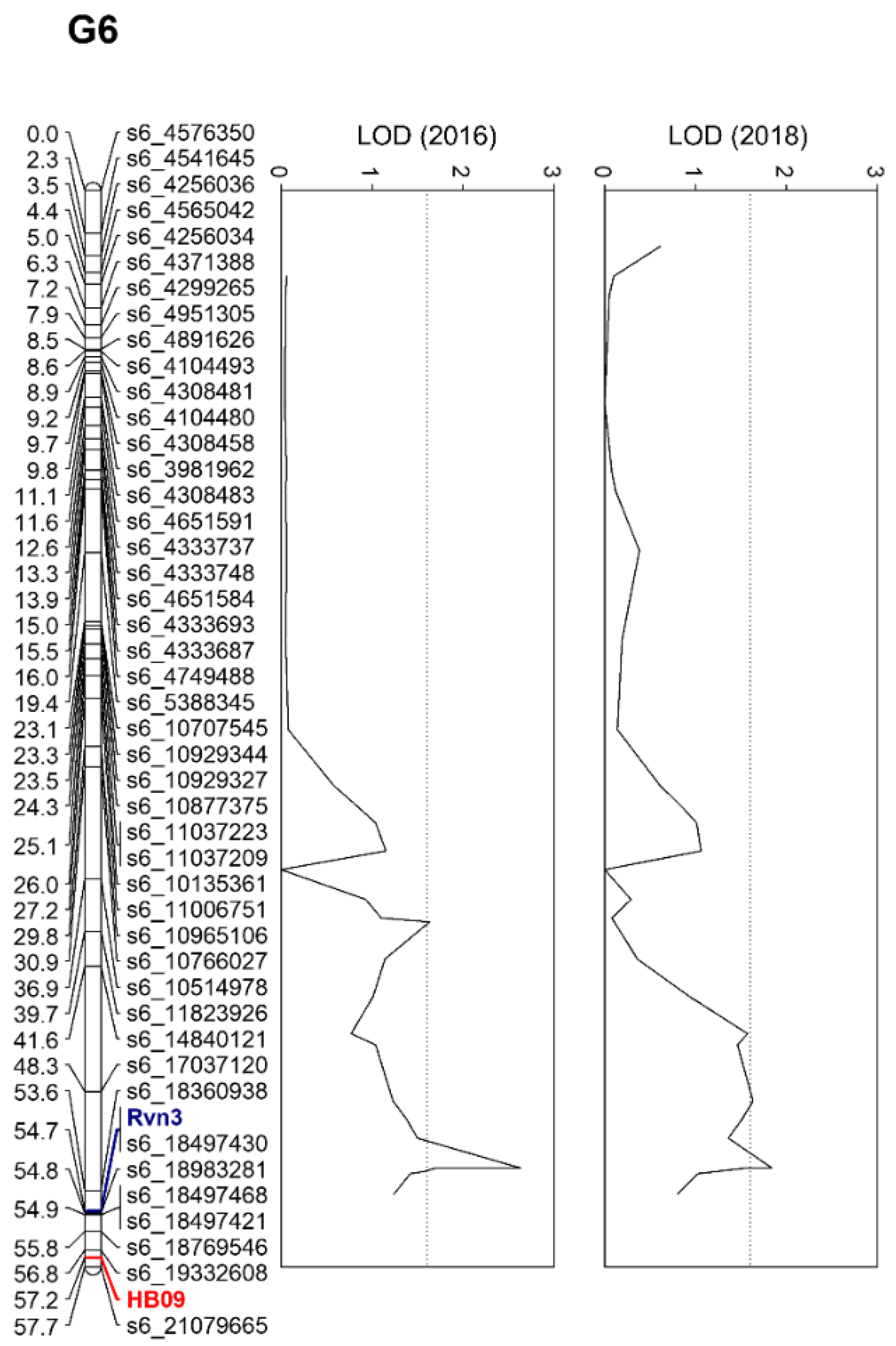
2.2. Construction of Genetic Linkage Maps
2.3. Identification of a Novel Scab Resistance Gene
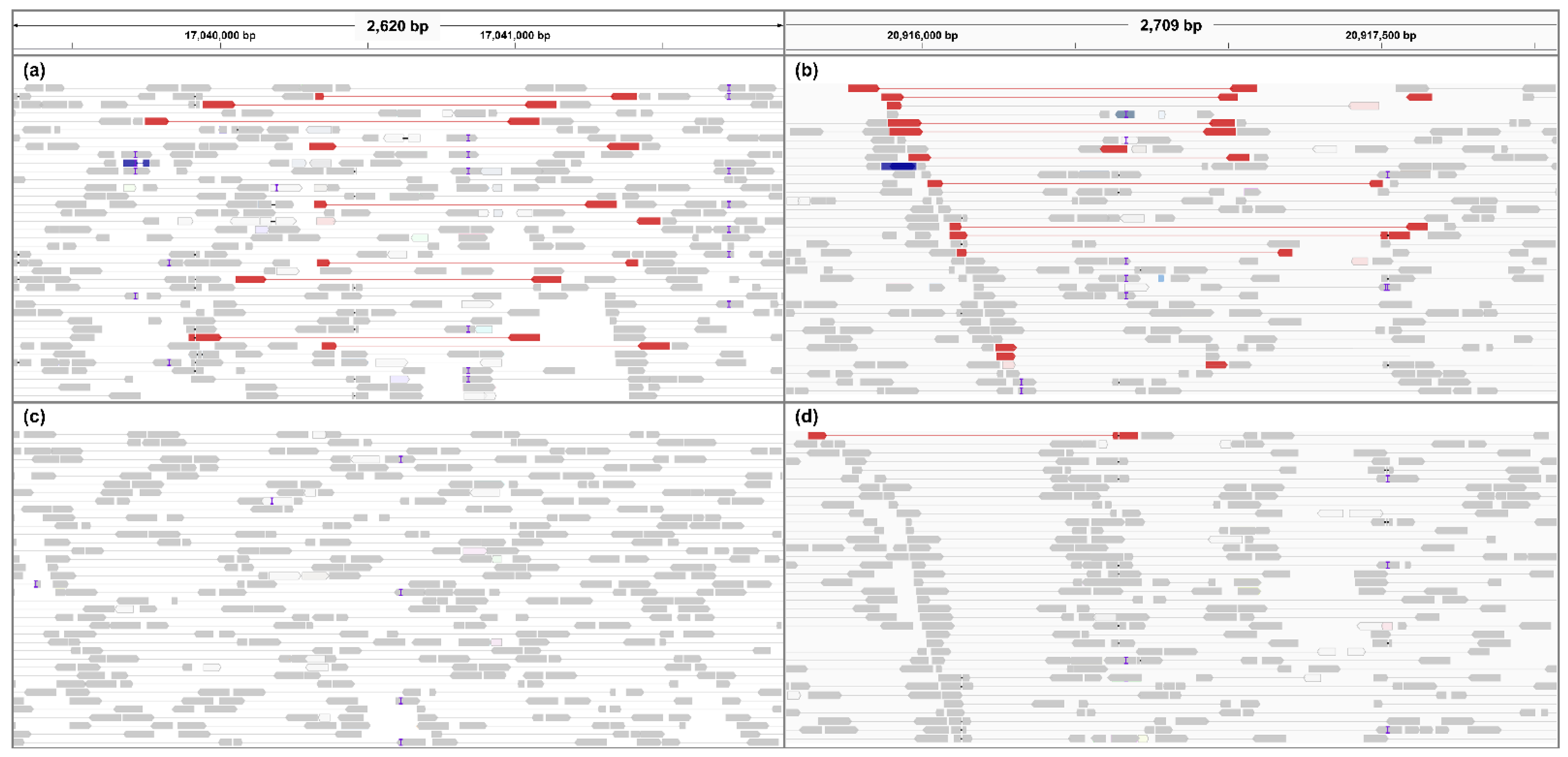
2.4. Insertion and Deletion Related with Scab Resistance
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Inoculation with V. nashicola and Scoring of Symptoms
4.3. Statistical Analysis
4.4. Linkage and QTL Analysis
4.5. Resequencing and InDel Detection
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Langford, M.H.; Keitt, E.N. Heterothallism and variability in Venturia pirina. Phytopathology 1942, 32, 357–369. [Google Scholar]
- Tanaka, S.; Yamamoto, S. Studies in pear scab. II. Taxonomy of the causal fungus of Japanese pear scab. Ann. Phytopathol. Soc. Jpn. 1964, 29, 128–136. [Google Scholar] [CrossRef]
- Abe, K.; Kotobuki, K.; Saito, T.; Terai, O. Inheritance of resistance to pear scab from European pears to Asian pears. Hortic. J. 2000, 69, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Terakami, S.; Shoda, M.; Adachi, Y.; Gonai, T.; Kasumi, M.; Sawamura, Y.; Iketani, H.; Kotobuki, K.; Patocchi, A.; Gessler, C.; et al. Genetic mapping of the pear scab resistance gene Vnk of Japanese pear cultivar Kinchaku. Theor. Appl. Genet. 2006, 113, 743–752. [Google Scholar] [CrossRef]
- Cho, K.H.; Shin, I.S.; Kim, K.T.; Suh, E.J.; Hong, S.S.; Lee, H.J. Development of AFLP and CAPS markers linked to the scab resistance gene, Rvn2, in an inter-specific hybrid pear (Pyrus spp.). J. Hortic. Sci. Biotechnol. 2009, 84, 619–624. [Google Scholar] [CrossRef]
- Pierantoi, L.; Dondini, L.; Cho, K.H.; Shin, I.S.; Gennari, F.; Chiodini, R.; Tartarini, S.; Kang, S.J.; Sansavini, S. Pear scab resistance QTLs via a European pear (Pyrus communis) linkage group. Tree Genet. Genomes 2007, 3, 311–317. [Google Scholar] [CrossRef]
- Bouvier, L.; Bourcy, M.; Boulay, M.; Tellier, M.; Guérif, P.; Denancé, C.; Durel, C.E.; Lespinasse, Y. A new pear scab resistance gene Rvp1 from the European pear cultivar ‘Navara’ maps in a genomic region syntenic to an apple scab resistance gene cluster on linkage group 2. Tree Genet. Genomes 2012, 8, 53–60. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, T.; Kimura, T.; Saito, T.; Kotobuki, M.; Matsuta, N.; Liebhard, R.; Gessler, C.; van de Weg, W.E.; Hayashi, T. Genetic linkage maps of Japanese and European pears aligned to the apple consensus map. Acta Hortic. 2004, 6636, 51–56. [Google Scholar] [CrossRef] [Green Version]
- Velasco, R.; Zharkikh, A.; Affourtit, J.; Dhingra, A.; Cestaro, A.; Kalyanaraman, A.; Fontana, P.; Bhatnagar, S.K.; Troggio, M.; Pruss, D.; et al. The genome of the domesticated apple (Malus × domestica). Nat. Genet. 2010, 42, 833–839. [Google Scholar] [CrossRef]
- Wu, J.; Wang, Z.; Shi, Z.; Zhang, S.; Ming, R.; Zhu, S.; Awais Khan, M.; Tao, S.; Korban, S.S.; Wang, H.; et al. The genome of the pear (Pyrus bretschneideri Rehd.). Genome Res. 2013, 23, 396–408. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.K.; Kang, S.S.; Won, K.H.; Shin, I.S.; Cho, K.S.; Ma, K.B.; Kim, M.S.; Choi, J.J.; Choi, J.H. Breeding of the scab-resistant pear cultivar ‘Greensis’. Korean J. Hortic. Sci. Technol. 2016, 34, 655–661. [Google Scholar]
- Iketani, H.; Abe, K.; Yamamoto, T.; Kotobuki, K.; Sato, Y.; Saito, T.; Terai, O.; Matsuta, N.; Hayashi, T. Mapping of disease-related genes in Japanese pear using a molecular linkage map with RAPD markers. Breed. Sci. 2001, 51, 179–184. [Google Scholar] [CrossRef] [Green Version]
- Shin, I.S.; Hyeon, I.H.; Hwang, H.S.; Hong, S.S.; Cho, K.H.; Cho, H.M. Screening of scab (Venturia nashicola) resistance germplasms in Pyrus species. Korean J. Hortic. Sci. Technol. 2004, 22, 63–68. [Google Scholar]
- Shay, J.R.; Hough, L.F. Evaluation of apple scab resistance in selections of Malus. Am. J. Bot. 1952, 39, 288–297. [Google Scholar] [CrossRef]
- Chevalier, M.; Lespinasse, Y.; Renaudin, S. A microscopic study of the different classes of symptoms coded by the Vf gene in apple for resistant to scab (Venturia inaequalis). Plant Pathol. 1991, 40, 249–256. [Google Scholar] [CrossRef]
- Bus, V.G.M.; Rikkerink, E.H.A.; Caffier, V.; Durel, C.E.; Plummer, K.M. Revision of the nomenclature of the differential host-pathogen interactions of Venturia inaequalis and Malus. Annu. Rev. Phytopathol. 2011, 49, 391–413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Broggini, G.A.L.; Galli, P.; Parravicini, G.; Gianfranceschi, L.; Gessler, C.; Patocchi, A. HcrVf paralogs are present on linkage groups 1 and 6 of Malus. Genome 2009, 52, 129–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Celton, J.M.; Chagné, D.; Tustin, S.D.; Terakami, S.; Nishitani, C.; Yamamoto, T.; Gardiner, S.E. Update on comparative genome mapping between Malus and Pyrus. BMC Res. Notes 2009, 2, 182. [Google Scholar] [CrossRef] [Green Version]
- Terakami, S.; Nishitani, C.; Kunihisa, M.; Shirasawa, K.; Sato, S.; Tabata, S.; Kurita, K.; Kanamori, H.; Katayose, Y.; Takada, N.; et al. Transcriptome-based single nucleotide polymorphism markers for genome mapping in Japanese pear (Pyrus pyrifolia Nakai). Tree Genet. Genomes 2014, 10, 853–863. [Google Scholar] [CrossRef]
- Chen, H.; Song, Y.; Li, L.T.; Khan, M.A.; Li, X.G.; Korban, S.S.; Wu, J.; Zhang, S.L. Construction of a high-density simple sequence repeat consensus genetic map for pear (Pyrus spp.). Plant Mol. Biol. Rep. 2015, 33, 316–325. [Google Scholar] [CrossRef]
- Soufflet-Freslon, V.; Gianfranceschi, L.; Patocchi, A.; Dure, C.E. Inheritance studies of apple scab resistance and identification of Rvi14, a new major gene that acts together with other broad-spectrum QTL. Genome 2008, 51, 657–667. [Google Scholar] [CrossRef] [Green Version]
- Vinatzer, B.A.; Patocchi, A.; Gianfranceschi, L.; Tartarini, S.; Zhang, H.B.; Gessler, C.; Sansavini, S. Apple contains receptor-like genes homologous to the Cladosporium fulvum resistance gene family of tomato with a cluster of genes cosegregating with Vf apple scab resistance. Am. Phytopathol. Soc. 2001, 14, 508–515. [Google Scholar] [CrossRef] [Green Version]
- Zhao, G.; Guo, D.; Wang, L.; Li, H.; Wang, C.; Guo, X. Functions of RPM1-interacting protein 4 in plant immunity. Planta 2021, 253, 11. [Google Scholar] [CrossRef] [PubMed]
- Park, P.; Ishii, H.; Adachi, Y.; Kanematsu, S.; Ieki, H.; Umemoto, S. Infection behavior of Venturia nashicola, the cause of scab on Asian pear. Biochem. Cell Biol. 2000, 90, 1209–1216. [Google Scholar] [CrossRef]
- Jiang, S.; Park, P.; Ishii, H. Ultrastructural study on scab resistance expressed in epidermal pectin layers of pear leaves. J. Gen. Plant Pathol. 2007, 73, 314–323. [Google Scholar] [CrossRef]
- Takemoto, D.; Hayashi, M.; Doke, N.; Nishimura, M.; Kawakita, K. Isolation of the gene for EILP, an elicitor-inducible LRR receptor-like protein, from tobacco by differential display. Plant Cell Physiol. 2000, 41, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Wu, D.; Chory, J. Plant receptor kinases: Systemin receptor identified. Proc. Natl. Acad. Sci. USA 2002, 99, 9090–9092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grattapagli, D.; Sederoff, R. Genetic linkage maps of Eucalyptus grandis and Eucalyptus urophylla using a pseudo-testcross: Mapping strategy and RAPD markers. Genetics 1994, 137, 1121–1137. [Google Scholar] [CrossRef]
- Oh, S.; Oh, Y.; Kim, K.; Han, H.; Kim, Y.; Won, K.; Kim, D. Construction of high-resolution genetic linkage map in pear pseudo-BC1 ((Pyrus pyrifolia × P. communis) × P. pyrifolia) using GBS-SNPs and SSRs. Hortic. Environ. Biotechnol. 2020, 61, 745–753. [Google Scholar] [CrossRef]
- Churchill, G.A.; Doerge, R.W. Empirical threshold values for quantitative trait mapping. Genetics 1994, 138, 963–971. [Google Scholar] [CrossRef]
- Cox, M.P.; Peterson, D.A.; Biggs, P.J. SolexaQA: At-a-glance quality assessment of Illumina second-generation sequencing data. BMC Bioinform. 2010, 11, 485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Xue, H.; Wang, S.; Yao, J.L.; Deng, C.H.; Wang, L.; Su, Y.; Zhang, H.; Zhou, H.; Sun, M.; Li, X.; et al. Chromosome level high-density integrated genetic maps improve the Pyrus bretschneideri ‘DangshanSuli’ v1.0 genome. BMC Genom. 2018, 19, 833. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Durbin, R. Fast and accurate short read alignment with burrows-wheeler transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R.; 1000 Genome Project Data Processing Subgroup. The sequence alignment/map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef] [Green Version]
- Picard. Available online: http://broadinstitute.github.io/picard (accessed on 16 October 2017).
- Robinson, J.T.; Thorvaldsdóttir, H.; Winckler, W.; Guttman, M.; Lander, E.S.; Getz, G.; Mesirov, J.P. Integrative genomics viewer. Nat. Biotechnol. 2011, 29, 24–26. [Google Scholar] [CrossRef] [Green Version]
| Year | No. of Individuals | Expected Segregation Ratio | χ2-Value | p | |
|---|---|---|---|---|---|
| Resistant | Susceptible | ||||
| 2016 | 39 | 54 | 1:1 | 2.42 | 0.12 |
| 2018 | 38 | 55 | 1:1 | 3.11 | 0.08 |
| Gene Description | Accession No. | Query Cover (%) | E-Value | Similarity (%) |
|---|---|---|---|---|
| Malus domestica probable serine/threonine protein kinase IRE | XM_029106090.1 | 16 | 3.00 × 10−72 | 84.42 |
| Malus domestica rust resistance kinase Lr10-like | XM_008356659.3 | 12 | 1.00 × 10−70 | 89.73 |
| Malus floribunda clone M18-6Bs Vf apple scab resistance protein HcrVf2-like gene, complete sequence | EU794447.1 | 8 | 2.00 × 10−53 | 92.21 |
| Pyrus × bretschneideri disease resistance protein RPM1-like | XM_018651218.1 | 40 | 6.00 × 10−138 | 97.91 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, S.; Han, H.; Kim, D. A Novel Pear Scab (Venturia nashicola) Resistance Gene, Rvn3, from Interspecific Hybrid Pear (Pyrus pyrifolia × P. communis). Plants 2021, 10, 2632. https://doi.org/10.3390/plants10122632
Oh S, Han H, Kim D. A Novel Pear Scab (Venturia nashicola) Resistance Gene, Rvn3, from Interspecific Hybrid Pear (Pyrus pyrifolia × P. communis). Plants. 2021; 10(12):2632. https://doi.org/10.3390/plants10122632
Chicago/Turabian StyleOh, Sewon, Hyeondae Han, and Daeil Kim. 2021. "A Novel Pear Scab (Venturia nashicola) Resistance Gene, Rvn3, from Interspecific Hybrid Pear (Pyrus pyrifolia × P. communis)" Plants 10, no. 12: 2632. https://doi.org/10.3390/plants10122632






