Plant Response to Mechanically-Induced Stress: A Case Study on Specialized Metabolites of Leafy Vegetables
Abstract
:1. Introduction
2. Results
2.1. Specialized Metabolites Content of Lettuce and Chicory
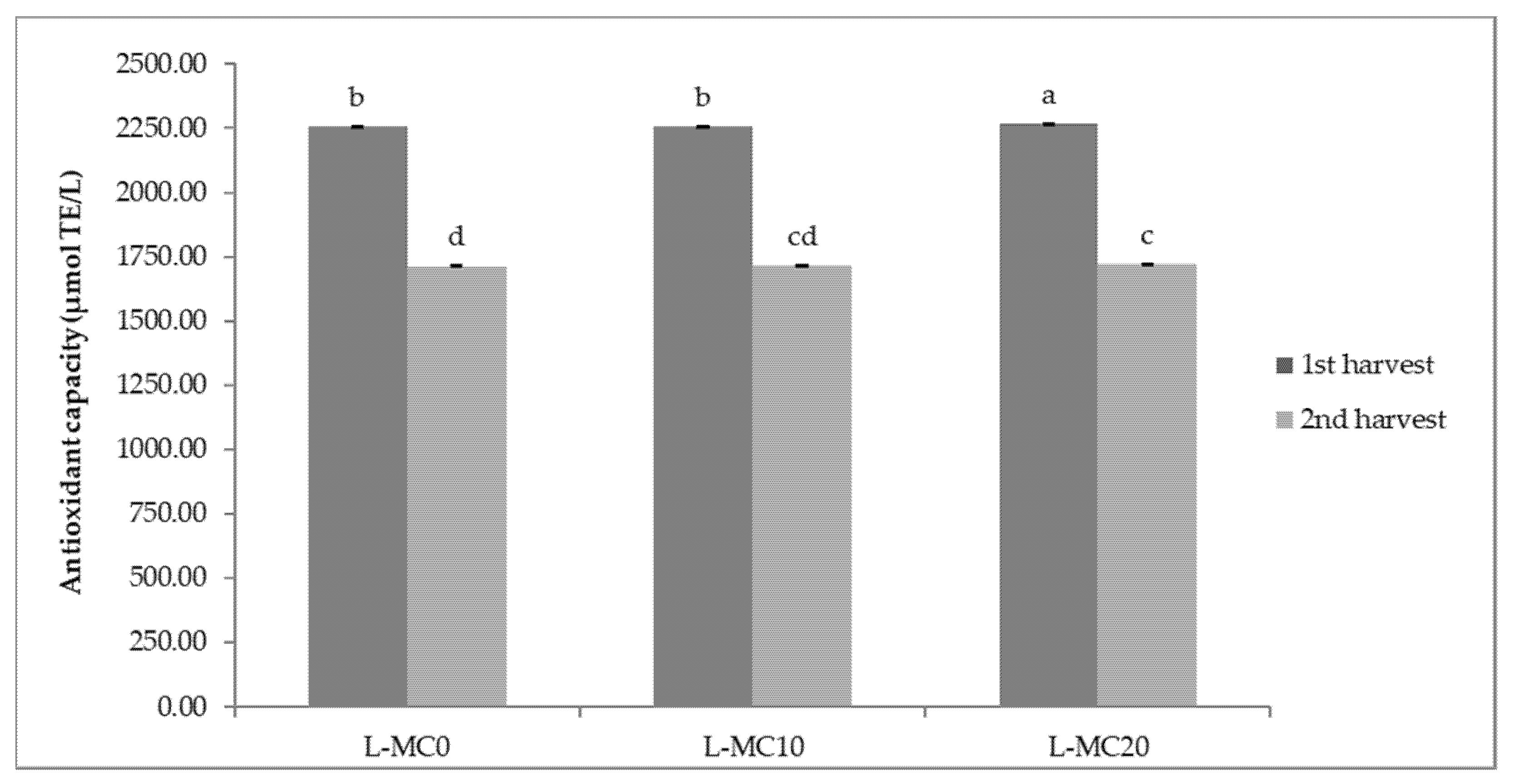
2.2. Antioxidant Capacity of Lettuce and Chicory
3. Discussion
3.1. Specialized Metabolites Content
3.2. Antioxidant Capacity
4. Materials and Methods
4.1. Plant Material
4.2. Floating Hydroponics
4.3. Mechanical Conditioning
4.4. Abiotic Parameters of Air and Nutrient Solution
4.5. Harvest Period
4.6. Determination of Specialized Metabolites Content
Chl_b = 21.426 × A644 − 4.65 × A622 [mg/L]
TCh = 5.134 × A662 + 20.436 × A644 [mg/L]
TCA = 4.695 × A440 − 0.268 × TCh [mg/L]
4.7. Determination of Antioxidant Capacity
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Radman, S.; Ćurko, J.; Toth, N.; Fabek, S.; Čoga, L.; Žutić, I.; Benko, B. Lamb’s lettuce mineral content in floating system. Acta Hortic. 2014, 1142, 343–348. [Google Scholar] [CrossRef]
- Toth, N.; Fabek, S.; Benko, B.; Žutić, I.; Stubljar, S.; Zeher, S. The effect of abiotic factors, sowing density and multiple harvest to arugula yield in floating hydroponic. Glas. Zašt. Bilja 2012, 35, 24–34. [Google Scholar]
- Gonnella, M.; Serio, F.; Conversa, G.; Santamaria, P. Yield and quality of lettuce grown in floating system using different sowing density and plant spatial arrangements. Acta Hortic. 2002, 614, 687–692. [Google Scholar] [CrossRef]
- Miceli, A.; Moncada, A.; Vetrano, F.; D’Anna, F. First results on yield and quality response of basil (Ocimum basilicum L.) grown in a floating system. Acta Hortic. 2003, 609, 377–381. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Chatzieustratiou, E.; Constantopoulou, E.; Kapotis, G. Yield and Quality of Lettuce and Rocket Grown in Floating Culture System. Not. Bot. Horti Agrobot. 2016, 44, 603–612. [Google Scholar] [CrossRef] [Green Version]
- Sharma, N.; Acharya, S.; Kumar, K.; Singh, N.; Chaurasia, O.P. Hydroponics as an advanced technique for vegetable production: An overview. J. Soil Water Conserv. 2018, 17, 364–371. [Google Scholar] [CrossRef]
- Björkman, T. Dose and timing of brushing to control excessive hypocotyl elongation in cucumber transplants. HortTechnology 1999, 9, 40–42. [Google Scholar] [CrossRef] [Green Version]
- Radman, S.; Bedek, M.; Židovec, V.; Toth, N.; Benko, B.; Žutić, I. The influence of mechanical stress on the morphological properties of thyme and coriander. In Proceedings of the 54th Croatian & 14th International Symposium on Agriculture, Vodice, Croatia, 17–22 February 2019; pp. 297–301. [Google Scholar]
- Li, Z.G.; Gong, M. Mechanical Stimulation-Induced Cross-Adaptation in Plants: An Overview. J. Plant Biol. 2011, 54, 358–364. [Google Scholar] [CrossRef] [Green Version]
- Biddington, N.L. The effects of mechanically-induced stress in plants—A review. Plant Growth Regul. 1986, 4, 103–123. [Google Scholar] [CrossRef]
- Goodman, A.M.; Ennos, A.R. The effects of mechanical stimulation on the morphology and mechanics of maize roots grown in an aerated nutrient solution. Int. J. Plant Sci. 2001, 162, 691–696. [Google Scholar] [CrossRef]
- Benikhlef, L.; L’Haridon, F.; Abou-Mansour, E.; Serrano, M.; Binda, M.; Costa, A.; Lehmann, S.; Métraux, J.P. Perception of soft mechanical stress in Arabidopsis leaves activates disease resistance. BMC Plant Biol. 2013, 13, 133. [Google Scholar] [CrossRef] [Green Version]
- Saidi, I.; Ammar, S.; Demont-Caulet, N.; Thévenin, J.; Lapierre, C.; Bouzid, S.; Jouanin, L. Thigmomorphogenesis in Solanum lycopersicum, Morphological and biochemical responses in stem after mechanical stimulation. Plant Signal. Behav. 2010, 5, 122–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Isah, T. Stress and defense responses in plant secondary metabolites production. Biol. Res. 2019, 52, 1–25. [Google Scholar] [CrossRef] [Green Version]
- Potocka, I.; Szymanowska-Pułka, J. Morphological responses of plant roots to mechanical stress. Ann. Bot. 2018, 122, 711–723. [Google Scholar] [CrossRef]
- Latimer, J.G. Mechanical conditioning for control of growth and quality of vegetable transplants. HortScience 1991, 26, 1456–1461. [Google Scholar] [CrossRef] [Green Version]
- Latimer, J.G.; Beverly, R.B. Mechanical conditioning of greenhouse-grown transplants. HortTechnology 1993, 3, 412–413. [Google Scholar] [CrossRef]
- Puijalon, S.; Bouma, T.J.; Douady, C.J.; van Groenendael, J.; Anten, N.P.R.; Martel, E.; Bornette, G. Plant resistance to mechanical stress: Evidence of an avoidance–tolerance trade-off. New Phytol. 2011, 191, 1141–1149. [Google Scholar] [CrossRef] [PubMed]
- Braam, J. In touch: Plant responses to mechanical stimuli. New Phytol. 2005, 165, 373–389. [Google Scholar] [CrossRef] [PubMed]
- Latimer, J.G. Mechanical conditioning to control height. HortTechnology 1998, 8, 529–534. [Google Scholar] [CrossRef] [Green Version]
- Li, N.; Jia, Z.; Trush, M.A. Defining ROS in Biology and Medicine. React. Oxyg. Species 2016, 1, 9. [Google Scholar] [CrossRef] [Green Version]
- Ahanger, M.A.; Singh Tomar, N.; Tittal, M.; Argal, S.; Agarwal, R.M. Plant growth under water/salt stress: ROS production; antioxidants and significance of added potassium under such conditions. Physiol. Mol. Biol. Plants 2017, 23, 731–744. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Ullah, F.; Zhou, D.X.; Yi, M.; Zhao, Y. Mechanisms of ROS Regulation of Plant Development and Stress Responses. Front. Plant Sci. 2019, 10, 800. [Google Scholar] [CrossRef]
- Cuypers, A.; Vangronsveld, J.; Clisjters, H. The redox status of plant cells (AsA and GSH) is sensitive to zinc imposed oxidative stress in roots and primary leaves of Phaseolus vulgaris. Plant Physiol. Biochem. 2001, 39, 657–664. [Google Scholar] [CrossRef]
- Alam, M.M.; Nahar, K.; Hasanuzzaman, M.; Fujita, M. Alleviation of osmotic stress in Brassica napus, B. campestris and B. juncea by ascorbic acid application. Biol. Plant 2014, 58, 697–708. [Google Scholar] [CrossRef]
- Shafiq, S.; Akram, N.A.; Ashraf, M. Does exogenously-applied trehalose alter oxidative defense system in the edible part of radish (Raphanus sativus L.) under water-deficit conditions? Sci. Hortic. 2015, 185, 68–75. [Google Scholar] [CrossRef]
- El-Sayed, O.M.; El-Gammal, O.H.M.; Salama, A.S.M. Effect of ascorbic acid, proline and jasmonic acid foliar spraying on fruit set and yield of Manzanillo olive trees under salt stress. Sci. Hortic. 2014, 176, 32–37. [Google Scholar] [CrossRef]
- Shan, C.; Zhao, X. Effects of lanthanum on the ascorbate and glutathione metabolism of Vigna radiata seedlings under salt stress. Biol. Plant 2014, 58, 595–599. [Google Scholar] [CrossRef]
- Medina-Lozano, I.; Bertolín, J.R.; Díaz, A. Nutritional value of commercial and traditional lettuce (Lactuca sativa L.) and wild relatives: Vitamin C and anthocyanin content. Food Chem. 2021, 359, 129864. [Google Scholar] [CrossRef]
- van Treuren, R.; van Eekelen, H.D.L.M.; Wehrens, R.; de Vos, R.C.H. Metabolite variation in the lettuce gene pool: Towards healthier crop varieties and food. Metabolomics 2018, 14, 146. [Google Scholar] [CrossRef] [Green Version]
- Mieszczakowska-Frąc, M.; Celejewska, K.; Płocharski, W. Impact of Innovative Technologies on the Content of Vitamin C and Its Bioavailability from Processed Fruit and Vegetable Products. Antioxidants 2021, 10, 54. [Google Scholar] [CrossRef]
- Helaly, A.A.; Abdullah, H.M. Phytochemical Analysis and Yield Characterization of Eight Cichorium intybus L. Landraces. J. Hortic. Sci. Ornam. Plants 2017, 9, 39–51. [Google Scholar]
- Locato, V.; Cimini, S.; De Gara, L. Strategies to increase vitamin C in plants: From plant defense perspective to food biofortification. Front. Plant Sci. 2013, 4, 152. [Google Scholar] [CrossRef] [Green Version]
- Marchiosi, R.; dos Santos, W.D.; Polimeni Constantin, P.; de Lima, R.B.; Soares, A.R.; Finger-Teixeira, A.; Rodrigues Mota, T.; de Oliveira, D.M.; Foletto-Felipe, M.P.; Abrahão, J.; et al. Biosynthesis and metabolic actions of simple phenolic acids in plants. Phytochem. Rev. 2020, 19, 865–906. [Google Scholar] [CrossRef]
- Šamec, D.; Karalija, E.; Šola, I.; Vujčić Bok, V.; Salopek-Sondi, B. The Role of polyphenols in abiotic stress response: The influence of molecular structure. Plants 2021, 10, 118. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of phenylpropanoid pathway and the role of polyphenols in plants under abiotic stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bayazid, A.B.; Park, S.H.; Kim, J.G.; Lim, B.O. Green chicory leaf extract exerts anti-inflammatory effects through suppressing LPS-induced MAPK/NF-κB activation and hepatoprotective activity in vitro. Food Agric. Immunol. 2020, 31, 513–532. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.-W.; Lee, S.-H.; Asamenew, G.; Lee, M.-K.; Lee, S.; Park, J.J.; Choi, Y.; Lee, S.H. Study on Phenolic Compounds in Lettuce Samples Cultivated from Korea Using UPLC-DAD-QToF/MS. Korean J. Food Nutr. 2019, 32, 717–729. [Google Scholar]
- Zhou, W.; Liang, X.; Dai, P.; Chen, Y.; Zhang, Y.; Zhang, M.; Lu, L.; Jin, C.; Lin, X. Alteration of Phenolic Composition in Lettuce (Lactuca sativa L.) by Reducing Nitrogen Supply Enhances its Anti-Proliferative Effects on Colorectal Cancer Cells. Int. J. Mol. Sci. 2019, 20, 4205. [Google Scholar] [CrossRef] [Green Version]
- Vidal, V.; Laurent, S.; Charles, F.; Sallanon, H. Fine monitoring of major phenolic compounds in lettuce and escarole leaves during storage. J. Food Biochem. 2018, 43, e12726. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, V.; Shahzad, B.; Ramakrishnan, M.; Sidhu, G.P.S.; Bali, A.S.; Handa, N.; Kapoor, D.; Yadav, P.; Khanna, K.; et al. Photosynthetic Response of Plants Under Different Abiotic Stresses: A Review. J. Plant Growth Regul. 2020, 39, 509–531. [Google Scholar] [CrossRef]
- Žnidarčić, D.; Ban, D.; Šircelj, H. Carotenoid and chlorophyll composition of commonly consumed leafy vegetables in Mediterranean countries. Food Chem. 2011, 129, 1164–1168. [Google Scholar] [CrossRef]
- Derbie Assefa, A.; Choi, S.; Lee, J.-E.; Sung, J.-S.; Hur, O.-S.; Ro, N.-Y.; Lee, H.-S.; Jang, S.-W.; Rhee, J.-H. Identification and quantification of selected metabolites in differently pigmented leaves of lettuce (Lactuca sativa L.) cultivars harvested at mature and bolting stages. BMC Chem. 2019, 13, 56. [Google Scholar]
- Wink, M. Functions and Biotechnology of Plant Secondary Metabolites; Blackwell Publishing Ltd.: West Sussex, UK, 2010. [Google Scholar]
- Rasooli, I. Phytochemicals—Bioactivities and Impact on Health; InTech: Rijeka, Croatia, 2011. [Google Scholar]
- Giusti, M.M.; Wallace, T.C. Health-Promoting Components of Fruits and Vegetables in Human Health; Nutrients: Basel, Switzerland, 2017. [Google Scholar]
- Geneva, M.; Kostadinov, K.; Filipov, S.; Kirova, E.; Stancheva, I. Analysis of the antioxidant capacity of lettuce growth at different fertilizer regimes. Agric. Sci. 2021, 74, 145–154. [Google Scholar]
- Consultant Plant Nutrition in Horticulture. Available online: https://cdnmedia.eurofins.com/corporate-eurofins/media/12142795/160825_manual_nutrient_solutions_digital_en.pdf (accessed on 3 April 2019).
- Association of Officiating Analytical Chemists. Official Methods of Analysis: Method 2002, 17th ed.; AOAC International: Washington, DC, USA, 2002. [Google Scholar]
- Ough, C.S.; Amerine, M.A. Methods for Analysis of Musts and Wines; John Wiley & Sons: Washington, DC, USA, 1988. [Google Scholar]
- Holm, G. Chlorophyll mutations in barley. Acta Agric. Scand. 1954, 4, 457–471. [Google Scholar] [CrossRef]
- Wettstein, D.V. Chlorophyll-letale und der submikroskopische Formwechsel der Plastiden. Exp. Cell Res. 1957, 12, 427–506. [Google Scholar] [CrossRef]
- Miller, N.J.; Diplock, A.T.; Rice-Evans, C.; Davies, M.J.; Gopinathan, V.; Milner, A. A novel method for measuring antioxidant capacity and its application to monitoring the antioxidant status in premature neonates. Clin. Sci. 1993, 84, 407–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- SAS Version 9.3; SAS Institute Inc.: Cary, NC, USA, 2010.

| Treatment | AsA (mg/100 g fw) | TPC (mg GAE/100 g fw) | TFC (mg GAE/100 g fw) | TNFC (mg GAE/100 g fw) |
|---|---|---|---|---|
| 1st harvest | ||||
| L-MC01 | 15.53 ± 1.20 b | 51.38 ± 5.67 e | 35.59 ± 1.03 f | 15.77 ± 6.50 d |
| L-MC101 | 23.71 ± 3.20 a | 90.24 ± 0.82 d | 55.19 ± 0.47 d | 35.06 ± 1.27 c |
| L-MC201 | 24.39 ± 2.03 a | 102.46 ± 1.25 c | 66.72 ± 0.43 b | 35.74 ± 1.14 c |
| 2nd harvest | ||||
| L-MC02 | 16.45 ± 2.35 b | 107.47 ± 0.43 b | 44.30 ± 0.29 e | 90.34 ± 0.51 a |
| L-MC102 | 17.60 ± 2.52 b | 150.94 ± 1.52 a | 60.60± 0.1 c | 63.17 ± 1.36 b |
| L-MC202 | 15.90 ± 1.97 b | 86.89 ± 0.57 d | 70.75 ± 0.67 a | 16.13 ± 1.14 d |
| ANOVA | p ≤ 0.0008 | p ≤ 0.0001 | p ≤ 0.0001 | p ≤ 0.0001 |
| Treatment | Chl_a (mg/g) | Chl_b (mg/g) | TCh (mg/g) | TCA (mg/g) |
|---|---|---|---|---|
| 1st harvest | ||||
| L-MC01 | 0.2 ± 0.004 d | 0.16 ± 0.002 a | 0.36 ± 0.006 e | 0.15 ± 0.002 e |
| L-MC101 | 0.17 ± 0.001 e | 0.12 ± 0.002 b | 0.29 ± 0.002 f | 0.1 ± 0.001 f |
| L-MC201 | 0.28 ± 0.001 c | 0.15 ± 0.001 ab | 0.44 ± 0.001 d | 0.20 ± 0.001 d |
| 2nd harvest | ||||
| L-MC02 | 0.28 ± 0.002 c | 0.17 ± 0.001 a | 0.45 ± 0.002 c | 0.21 ± 0.001 c |
| L-MC102 | 0.37 ± 0.002 a | 0.17 ± 0.045 a | 0.57 ± 0.003 a | 0.27 ± 0.001 a |
| L-MC202 | 0.35 ± 0.001 b | 0.18 ± 0.001 a | 0.53 ± 0.002 b | 0.26 ± 0.001 b |
| ANOVA | p ≤ 0.0001 | p ≤ 0.0240 | p ≤ 0.0001 | p ≤ 0.0001 |
| Treatment | AsA (mg/100 g fw) | TPC (mg GAE/100 g fw) | TFC (mg GAE/100 g fw) | TNFC (mg GAE/100 g fw) |
|---|---|---|---|---|
| 1st harvest | ||||
| GC-MC01 | 36.52 ± 2.75 b | 104.85 ± 1.60 d | 42.33 ± 1.95 d | 62.51 ± 0.50 c |
| GC-MC101 | 40.97 ± 0.77 a | 79.42 ± 1.13 e | 26.80 ± 2.20 e | 52.61 ± 1.27 d |
| GC-MC201 | 34.45 ± 4.47 b | 50.03 ± 1.48 f | 16.92 ± 1.84 f | 33.09 ± 0.35 e |
| 2nd harvest | ||||
| GC-MC02 | 23.64 ± 1.97 c | 172.64 ± 0.28 b | 96.35 ± 0.72b | 76.28 ± 0.50 a |
| GC-MC102 | 25.31 ± 0.74 c | 161.06 ± 0.69 c | 92.45 ± 1.08c | 68.60 ± 0.39 b |
| GC-MC202 | 33.23 ± 0.75 b | 179.77 ± 0.62 a | 103.14 ± 1.33a | 76.63 ± 0.82 a |
| ANOVA | p ≤ 0.0001 | p ≤ 0.0001 | p ≤ 0.0001 | p ≤ 0.0001 |
| Treatment | Chl_a (mg/g) | Chl_b (mg/g) | TCh (mg/g) | TCA (mg/g) |
|---|---|---|---|---|
| 1st harvest | ||||
| GC-MC01 | 0.36 ± 0.001 e | 0.15 ± 0.001 f | 0.51 ± 0.001 b | 0.25 ± 0.001 e |
| GC-MC101 | 0.43 ± 0.001 d | 0.20 ± 0.001 d | 0.63 ± 0.002 b | 0.30 ± 0.001 d |
| GC-MC201 | 0.33 ± 0.001 f | 0.18 ± 0.001 e | 0.51 ± 0.001 b | 0.23 ± 0.001 f |
| 2nd harvest | ||||
| GC-MC02 | 0.61 ± 0.002 b | 0.29 ± 0.002 c | 0.89 ± 0.004 a | 0.42 ± 0.002 b |
| GC-MC102 | 0.71 ± 0.001 a | 0.36 a | 0.92 ± 0.26 a | 0.48 ± 0.02 a |
| GC-MC202 | 0.56 c | 0.30 b | 0.86 ± 0.001 a | 0.39 ± 0.001 c |
| ANOVA | p ≤ 0.0001 | p ≤ 0.0001 | p ≤ 0.0008 | p ≤ 0.0001 |
| Biogenic Element | Measuring Unit | Values |
|---|---|---|
| pH | mS/cm | 5.5 |
| EC | 2.5 | |
| Na | mmol/L | <6 |
| Cl | <6 | |
| HCO3 | <0.5 | |
| N-NH4 | mmol/L | <0.5 |
| K | 6 | |
| Ca | 6 | |
| Mg | 2 | |
| N-NO3 | mmol/L | 14 |
| S | 2 | |
| P | 2 | |
| Fe | μmol/L | 40 |
| Mn | 8 | |
| Zn | 8 | |
| B | 50 | |
| Cu | 1.5 | |
| Mo | 1.5 |
| Species | Brushing | Hydroponic System | Harvest Period | Treatment |
|---|---|---|---|---|
| Lettuce | 0 | FH | I. | L-MC01 |
| II. | L-MC102 | |||
| 10 | FH | I. | L-MC201 | |
| II. | L-MC02 | |||
| 20 | FH | I. | L-MC101 | |
| II. | L-MC202 | |||
| Green chicory | 0 | FH | I. | GC-MC01 |
| II. | GC-MC102 | |||
| 10 | FH | I. | GC-MC201 | |
| II. | GC-MC02 | |||
| 20 | FH | I. | GC-MC101 | |
| II. | GC-MC202 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šic Žlabur, J.; Radman, S.; Fabek Uher, S.; Opačić, N.; Benko, B.; Galić, A.; Samirić, P.; Voća, S. Plant Response to Mechanically-Induced Stress: A Case Study on Specialized Metabolites of Leafy Vegetables. Plants 2021, 10, 2650. https://doi.org/10.3390/plants10122650
Šic Žlabur J, Radman S, Fabek Uher S, Opačić N, Benko B, Galić A, Samirić P, Voća S. Plant Response to Mechanically-Induced Stress: A Case Study on Specialized Metabolites of Leafy Vegetables. Plants. 2021; 10(12):2650. https://doi.org/10.3390/plants10122650
Chicago/Turabian StyleŠic Žlabur, Jana, Sanja Radman, Sanja Fabek Uher, Nevena Opačić, Božidar Benko, Ante Galić, Paola Samirić, and Sandra Voća. 2021. "Plant Response to Mechanically-Induced Stress: A Case Study on Specialized Metabolites of Leafy Vegetables" Plants 10, no. 12: 2650. https://doi.org/10.3390/plants10122650










