Abundant Allelochemicals and the Inhibitory Mechanism of the Phenolic Acids in Water Dropwort for the Control of Microcystis aeruginosa Blooms
Abstract
:1. Introduction
2. Results
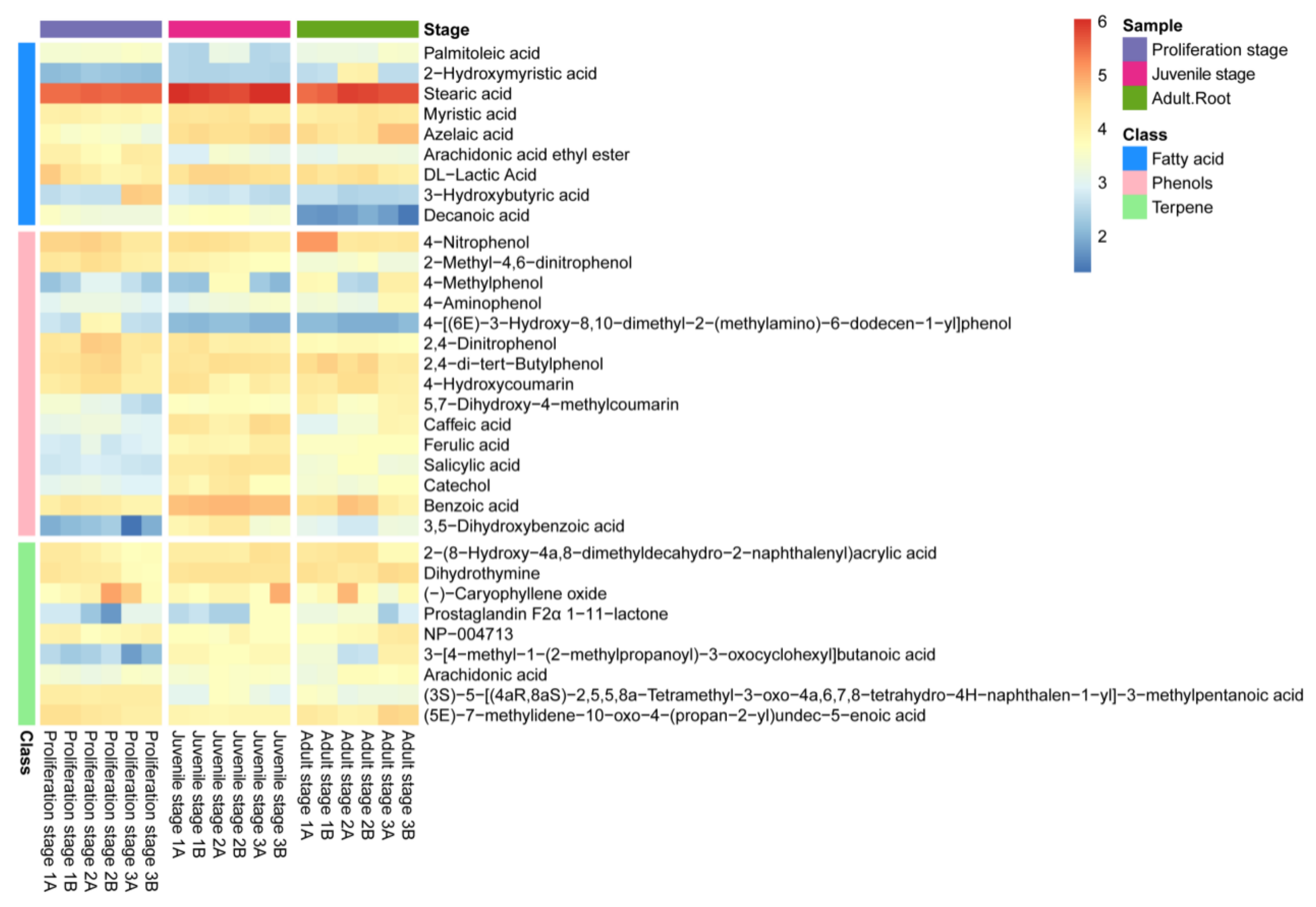
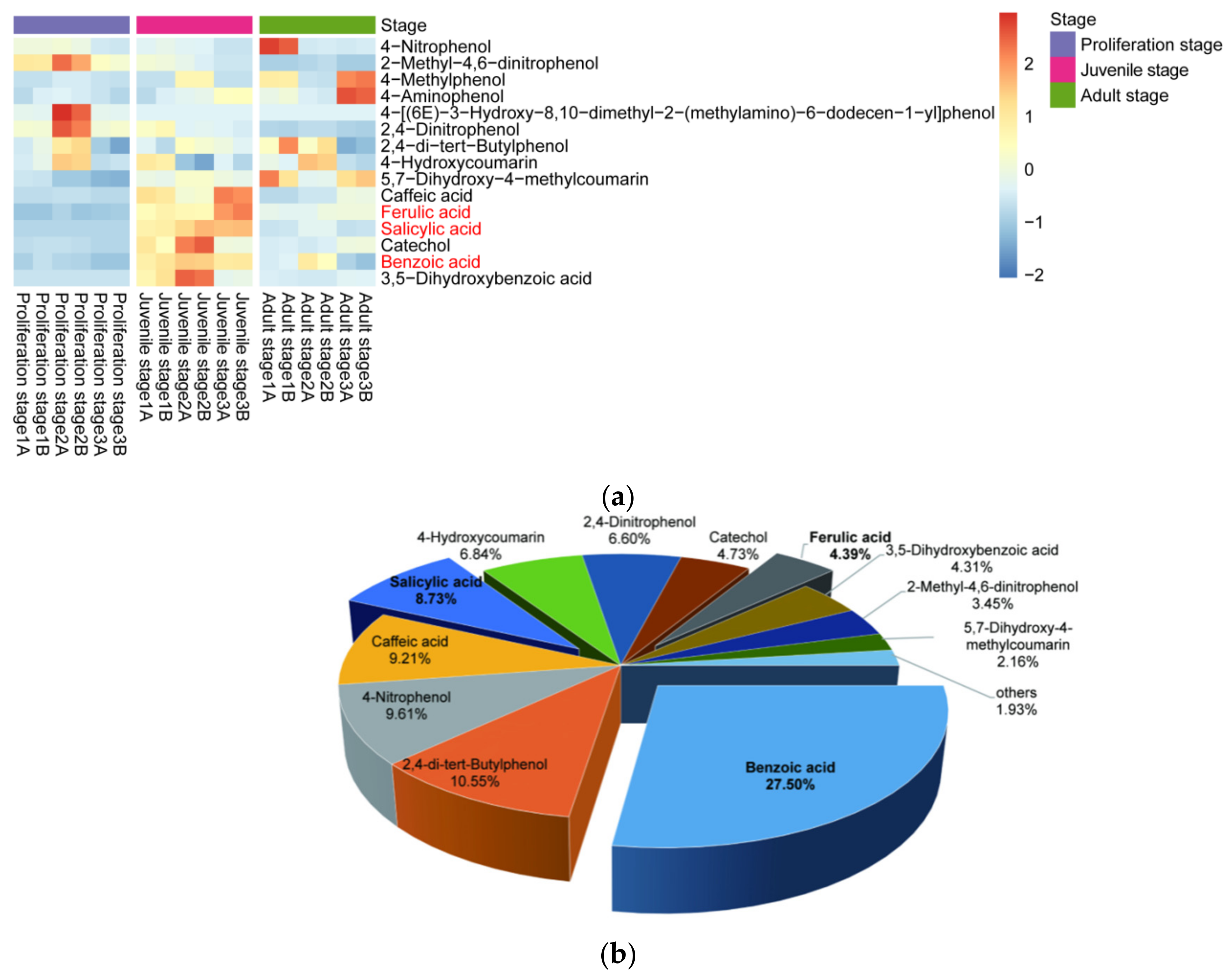
2.1. Inhibitory Effect of the Water Dropwort Culture Water on M. aeruginosa and the Identification of Metabolomics
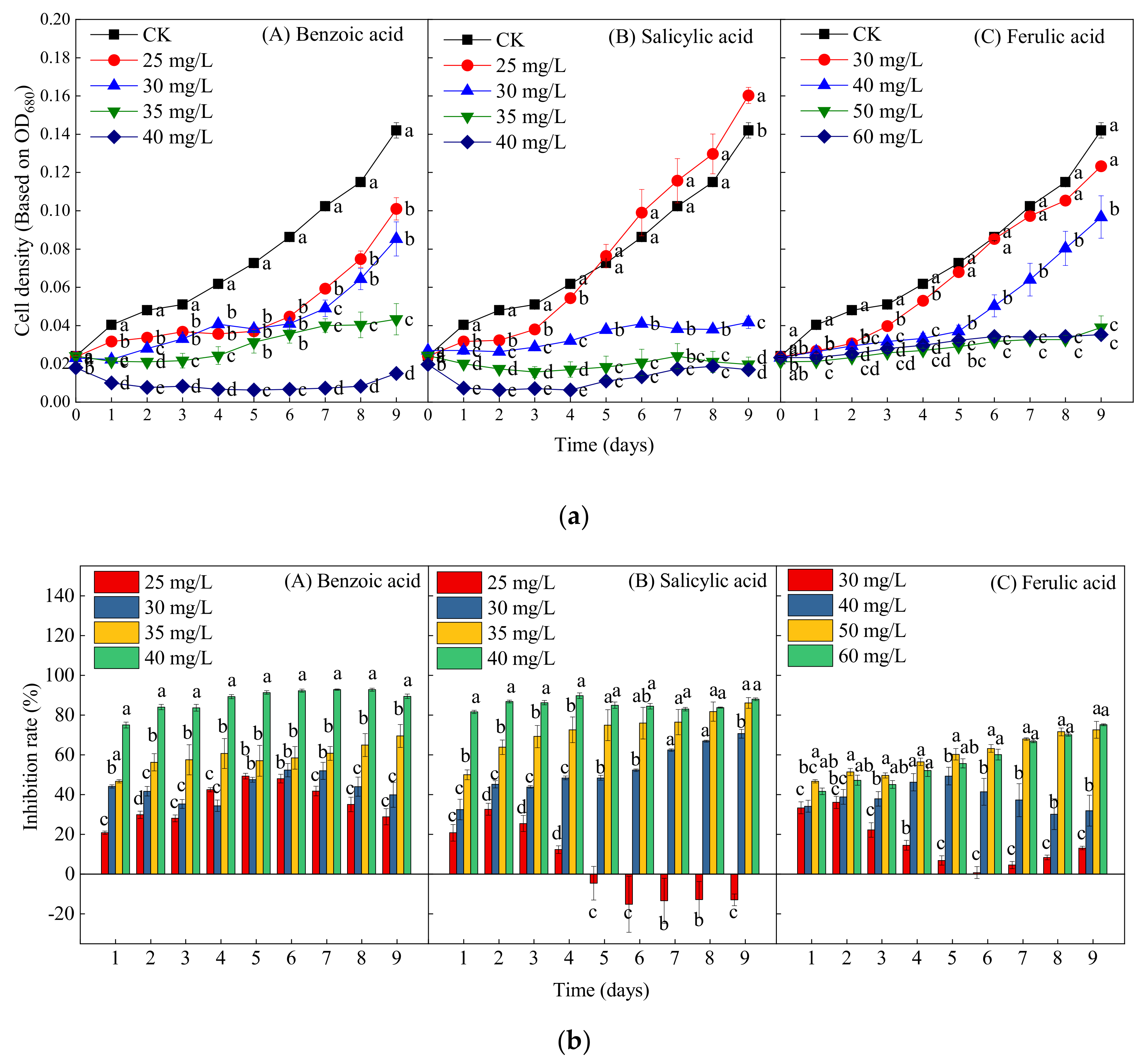
2.2. Effects of Different Phenolic Acids on the Growth of M. aeruginosa
2.3. Effects of Different Phenolic Acids on the Cell Morphology of M. aeruginosa
2.4. Effects of Different Phenolic Acids on the Chl-a Content in M. aeruginosa
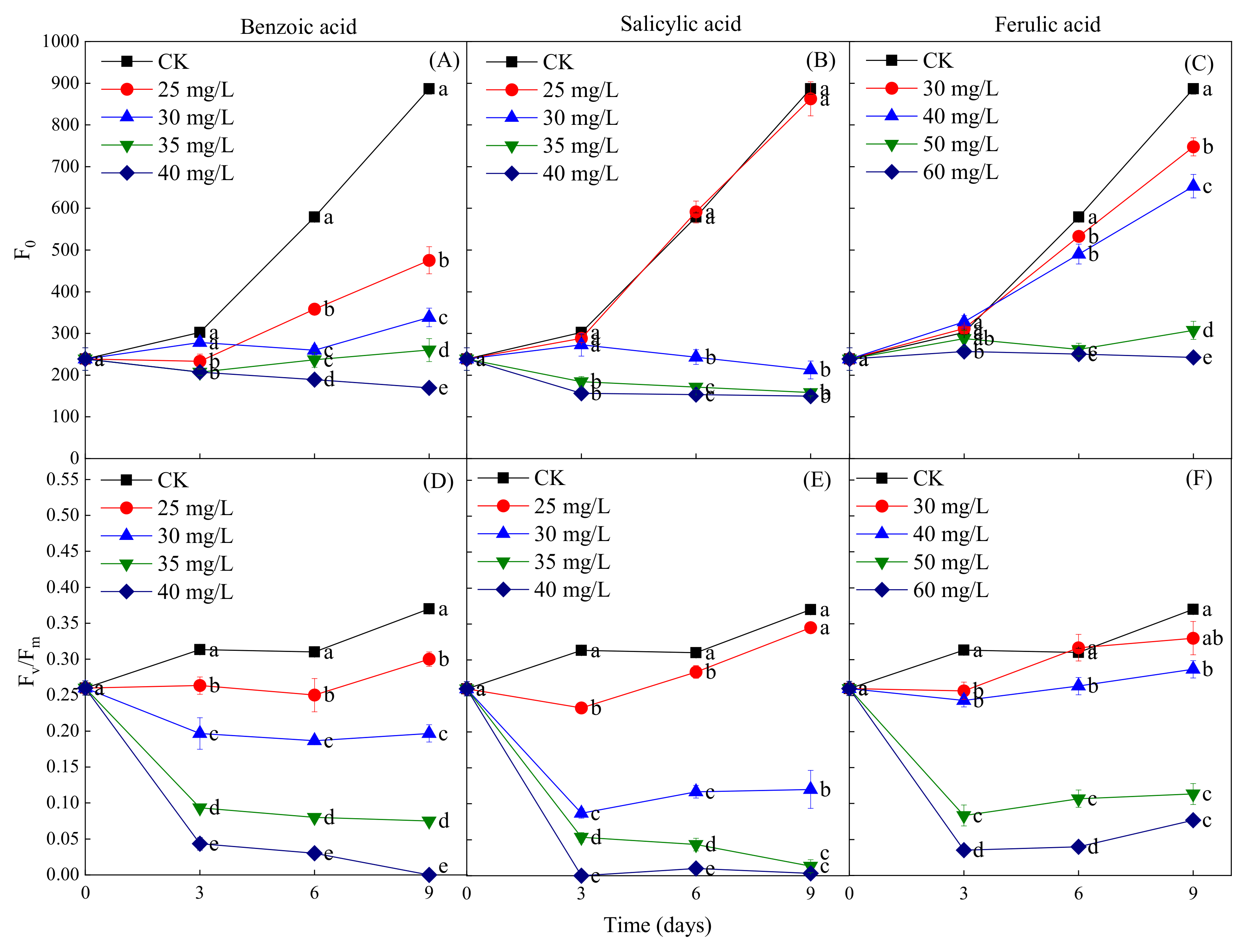
2.5. Effects of Different Phenolic Acids on the Fluorescence Parameters of M. aeruginosa
2.6. Effects of Different Phenolic Acids on the Antioxidant Enzyme Activity of M. aeruginosa
3. Discussion
4. Materials and Methods
4.1. Culture of M. aeruginosa
4.2. Preparation of Water Dropwort Culture Water
4.3. Sample Extraction for Water Dropwort
4.4. Metabolomics Determination of Allelochemicals
4.5. Inhibitory Effect of the Allelochemicals on the Growth of M. aeruginosa
4.5.1. Test 1: The Inhibitory Effect of the Water Dropwort Culture Water on the Growth of M. aeruginosa
4.5.2. Test 2: The Inhibitory Effect of Phenolic Acids on the Growth of M. aeruginosa
4.6. Cell Density and Chl-a
4.7. Fluorescence Parameters Fv/Fm and F0
4.8. Antioxidant Enzyme Activities
4.9. Cell Morphology Assays
4.10. Data Processing and Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Monchamp, M.E.; Spaak, P.; Domaizon, I.; Dubois, N.; Bouffard, D.; Pomati, F. Homogenization of lake cyanobacterial communities over a century of climate change and eutrophication. Nat. Ecol. Evol. 2018, 2, 317–324. [Google Scholar] [CrossRef] [Green Version]
- Vikrant, K.; Kim, K.H.; Ok, Y.S.; Tsang, D.C.W.; Tsang, Y.F.; Giri, B.S.; Singh, R.S. Engineered/designer biochar for the removal of phosphate in water and wastewater. Sci. Total Environ. 2018, 616, 1242–1260. [Google Scholar] [CrossRef] [PubMed]
- Hofer, U. Climate change boosts cyanobacteria. Nat. Rev. Microbiol. 2018, 16, 122–123. [Google Scholar] [CrossRef] [PubMed]
- Mohan, G.; Swathy, K.S.; Raffi, R.A.S. Fish mortality due to cyanobacterial bloom in freshwater pond, Cochin, Kerala. Message 2020, 9, 110–113. [Google Scholar] [CrossRef]
- Metcalf, J.S.; Banack, S.A.; Wessel, R.A.; Lester, M.; Pim, J.G.; Cassani, J.R.; Cox, P.A. Toxin analysis of freshwater cyanobacterial and marine harmful algal blooms on the west coast of Florida and implications for estuarine environments. Neurotoxic. Res. 2021, 39, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Skafi, M.; Duy, S.V.; Munoz, G.; Dinh, Q.T.; Simon, D.F.; Juneau, P.; Sauvé, S. Occurrence of microcystins, anabaenopeptins and other cyanotoxins in fish from a freshwater wildlife reserve impacted by harmful cyanobacterial blooms. Toxicon 2021, 194, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Peng, Y.; Zhang, Z.; Zhang, M.; Zhou, Y.; Duan, Z. Removal of Microcystis aeruginosa by ultrasound: Inactivation mechanism and release of algal organic matter. Ultrason. Sonochem. 2019, 56, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Serrà, A.; Pip, P.; Gómez, E.; Philippe, L. Efficient magnetic hybrid ZnO-based photocatalysts for visible-light-driven removal of toxic cyanobacteria blooms and cyanotoxins. Appl. Catal. B. 2020, 268, 118745. [Google Scholar] [CrossRef]
- Gong, H.; Chu, W.; Chen, M.; Wang, Q. A systematic study on photocatalysis of antipyrine: Catalyst characterization, parameter optimization, reaction mechanism a toxicity evolution to plankton. Water Res. 2017, 112, 167–175. [Google Scholar] [CrossRef]
- Wang, B.; Li, Y.; Zheng, J.; Hu, Y.; Wang, X.; Hu, B. Efficient removal of U (VI) from aqueous solutions using the magnetic biochar derived from the biomass of a bloom-forming cyanobacterium (Microcystis aeruginosa). Chemosphere 2020, 254, 126898. [Google Scholar] [CrossRef]
- Tsai, K.; Uzun, H.; Chen, H.; Karanfil, T.; Chow, A. Control wildfire-induced Microcystis aeruginosa blooms by copper sulfate: Trade-offs between reducing algal organic matter and promoting disinfection byproduct formation. Water Res. 2019, 158, 227–236. [Google Scholar] [CrossRef]
- Xuan, H.; Dai, X.; Li, J.; Zhang, X.; Yang, C.; Luo, F. A Bacillus sp strain with antagonistic activity against Fusarium graminearum kills Microcystis aeruginosa selectively. Sci. Total Environ. 2017, 583, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Ye, Q.; Zhang, F.; Shao, X.; Fan, Y.; Zhu, X.; Li, Y.; Yao, L.; Tian, Y.; Zheng, T.; et al. Flocculating properties and potential of Halobacillus sp. strain H9 for the mitigation of Microcystis aeruginosa blooms. Chemosphere 2019, 218, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Mecina, G.F.; Chia, M.A.; Cordeiro-Araújo, M.K.; Bittencourt-Oliveira, M.C.; Varela, R.M.; Torres, A.; González Molinillo, J.M.; Macías, F.A.; da Silva, R.M.G. Effect of flavonoids isolated from Tridax procumbens on the growth and toxin production of Microcystis aeruginos. Aquat. Toxicol. 2019, 211, 81–91. [Google Scholar] [CrossRef]
- Chiang, I.Z.; Huang, W.Y.; Wu, J.T. Allelochemicals of Botryococcus braunii (chlorophyceae) 1. J. Phycol. 2004, 40, 474–480. [Google Scholar] [CrossRef]
- Nakai, S.; Inoue, Y.; Hosomi, M.; Murakami, A. Growth inhibition of blue-green algae by allelopathic effects of macrophytes. Water Sci. Technol. 1999, 39, 47–53. [Google Scholar] [CrossRef]
- Neilen, A.D.; Hawker, D.W.; O’Brien, K.R.; Burford, M.A. Phytotoxic effects of terrestrial dissolved organic matter on a freshwater cyanobacteria and green algae species is affected by plant source and DOM chemical composition. Chemosphere 2017, 184, 969–980. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Li, C.; Xu, C.; Effiong, K.; Xiao, X. Understanding the inhibitory mechanism of antialgal allelochemical flavonoids from genetic variations: Photosynthesis, toxin synthesis and nutrient utility. Ecotoxicol. Environ. Saf. 2019, 177, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Hua, Q.; Liu, Y.G.; Yan, Z.L.; Zeng, G.M.; Liu, S.B.; Wang, W.J.; Tan, X.F.; Deng, J.Q.; Tang, X.; Wang, Q.P. Allelopathic effect of the rice straw aqueous extract on the growth of Microcystis aeruginosa. Ecotoxicol. Environ. Saf. 2018, 148, 953–959. [Google Scholar] [CrossRef]
- Tan, K.; Huang, Z.; Ji, R.; Qiu, Y.; Wang, Z.; Liu, J. A review of allelopathy on microalgae. Microbiology 2019, 165, 587–592. [Google Scholar] [CrossRef]
- Ma, H.; Huang, L.; Zhang, J.; Shi, D.; Yang, J. Optical properties of straw-derived dissolved organic matter and growth inhibition of Microcystis aeruginosa by straw-derived dissolved organic matter via photo-generated hydrogen peroxide. Environ. Pollut. 2018, 242, 760–768. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Zheng, Z.; Zhang, J.; Roger, S.F.; Luo, X. Evaluation of the use of eucalyptus to control algae bloom and improve water quality. Sci. Total Environ. 2019, 667, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Rice, E.L. Allelopathy; Academic Press: London, UK, 2012; pp. 1–23. [Google Scholar]
- Moosavi, A.; Afshari, R.T.; Asadi, A.; Gharineh, M.H. Allelopathic effects of aqueous extract of leaf stem and root of sorghum bicolor on seed germination and seedling growth of Vigna radiata L. Not. Sci. Biol. 2011, 3, 114–118. [Google Scholar] [CrossRef] [Green Version]
- Sodaeizadeh, H.; Rafieiolhossaini, M.; Havlík, J.; Van Damme, P. Allelopathic activity of different plant parts of Peganum harmala L. and identification of their growth inhibitors substances. Plant Growth Regul. 2009, 59, 227–236. [Google Scholar] [CrossRef]
- Mulderij, G.; Mau, B.; van Donk, E.; Gross, E.M. Allelopathic activity of Stratiotes aloides on phytoplankton-towards identification of allelopathic substances. Hydrobiologia 2007, 584, 89–100. [Google Scholar] [CrossRef] [Green Version]
- Feng, K.; Xu, Z.S.; Que, F.; Liu, J.X.; Wang, F.; Xiong, A.S. An R2R3-MYB transcription factor, OjMYB1, functions in anthocyanin biosynthesis in Oenanthe javanica. Planta 2018, 247, 301–315. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Wang, F.; Tan, H.W.; Li, M.Y.; Xu, Z.S.; Tan, G.F.; Xiong, A.S. De novo transcriptome assembly, gene annotation, marker development, and miRNA potential target genes validation under abiotic stresses in Oenanthe javanica. Mol. Genet. Genom. 2015, 290, 671–683. [Google Scholar] [CrossRef]
- Lu, C.; Li, X. A review of Oenanthe javanica (Blume) DC. as traditional medicinal plant and its therapeutic potential. Evid. Based Complement. Alternat. Med. 2019, 2019, 6495819. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.H.; Zhao, H.J.; Liu, J.X.; Li, B.; Chang, Y.J.; Yao, D.R. A new green model for the bioremediation and resource utilization of livestock wastewater. Int. J. Environ. Res. Public Health 2021, 18, 8634. [Google Scholar] [CrossRef]
- Gao, J.Q.; Yang, L.; Zhong, R.; Chen, Y.; Zhang, J.S.; Gao, J.L.; Cai, M.; Zhang, J.L. Comparison of nitrogen and phosphorus removal efficiency between two types of baffled vertical flow constructed wetlands planted with Oenanthe javanica. Water Sci. Technol. 2020, 81, 2023–2032. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Li, G.J.; Huang, X.F.; Ji, Q.; Zhou, K.; Hou, H.W.; Ke, W.D.; Yang, J.J. Phenotypic, nutritional, and antioxidant characterization of blanched Oenanthe javanica for preferable cultivar. Front. Plant Sci. 2021, 12, 231. [Google Scholar] [CrossRef]
- Zai, X.M.; Zhu, S.N.; Qin, P.; Yuan, Y.G.; Wu, X.H.; Zhou, W.Z. Allelopathy Effects of Oenanthe javanica Extracts on Scenedesmus obliquus. Bull. Bot. Res. 2011, 31, 735–757. [Google Scholar] [CrossRef]
- Wu, Z.B.; Zhang, Y.Y.; Liu, B.Y. Allelopathy of Aquatic Macrophytes on Phytoplankton; Science Press: Beijing, China, 2016; pp. 44–80. [Google Scholar]
- Qian, Y.P.; Li, X.T.; Tian, R.N. Effects of aqueous extracts from the rhizome of Pontederia cordata on the growth and interspecific competition of two algal species. Ecotoxicol. Environ. Saf. 2019, 168, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Yuan, R.; Li, Y.; Li, J.; Ji, S.; Wang, S.; Kong, F. The allelopathic effects of aqueous extracts from Spartina alterniflora on controlling the Microcystis aeruginosa blooms. Sci. Total Environ. 2020, 712, 136332. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Liu, R.; Liu, H.; Qu, J. Chlorination of Microcystis aeruginosa suspension: Cell lysis, toxin release and degradation. J. Hazard. Mater. 2012, 217, 279–285. [Google Scholar] [CrossRef]
- Sun, F.; Pei, H.Y.; Hu, W.R.; Ma, C.X. The lysis of Microcystis aeruginosa in AlCl3 coagulation and sedimentation processes. Chem. Eng. J. 2012, 193, 196–202. [Google Scholar] [CrossRef]
- Wang, N.; Ni, L.X.; Liu, X.Y. Study on algae inhibition mechanism of a novel sustained release Microparticles. Sichuan Environ. 2020, 39, 1–8. [Google Scholar] [CrossRef]
- Yan, H.; Wang, H.S.; Liu, X.L.; Yin, C.H.; Xu, Q.Q.; Lü, L.; Ma, W.B. Advances in the pathway and molecular mechanism for the biodegradation of microcystins. Environ. Sci. 2014, 35, 1205–1214. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Xiao, H.; Zhang, M.; Bao, T.; Luo, X.; Chen, S. Genotoxicity of microcystin-LR in mammalian cells: Implication from peroxynitrite produced by mitochondria. Ecotoxicol. Environ. Saf. 2020, 195, 110408. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.P.; Liu, F.; Luo, P.; Li, Z.H.; Zheng, L.G.; Wang, H.; Zou, D.S.; Wu, J.S. Allelopathic effects of Myriophyllum aquaticum on two cyanobacteria of Anabaena flos-aquae and Microcystis aeruginosa. Bull. Environ. Contam. Toxicol. 2017, 98, 556–561. [Google Scholar] [CrossRef]
- Wu, C.; Chang, X.X.; Dong, H.J.; Li, D.F.; Liu, J.Y. Allelopathic inhibitory effect of Myriophyllum aquaticum (VelL.) Verdc. on Microcystis aeruginosa and its physiological mechanism. Acta Ecol. Sin. 2008, 28, 2595–2603. [Google Scholar] [CrossRef]
- Wang, H. Study on allelopathic inhibition of alga of Acorus tatarinowii by cutting. Abstracts of papers. In Proceedings of the 9th Chinese Symposium on Plant Allelopathy, Yangling, China, 19 September 2019; p. 29, Professional Committee of Plant Allelopathy of China Society of Plant Protection. [Google Scholar]
- Zhang, S.J. Inhibitory Effect of Salvinia natans (L.) All. on Algae and Its Tolerance to the Stress of Eutrophication; Anhui Normal University: Wuhu, Anhui, 2015; pp. 1–9. [Google Scholar]
- Nakai, S.; Inoue, Y.; Hosomi, M.; Murakami, A. Myriophyllum spicatum-released allelopathic polyphenols inhibiting growth of blue-green algae Microcystis aeruginosa. Water Res. 2000, 34, 3026–3032. [Google Scholar] [CrossRef]
- Gross, E.M.; Meyer, H.; Schilling, G. Release and ecological impact of algicidal hydrolysable polyphenols in Myriophyllum spicatum. Phytochemistry 1996, 41, 133–138. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Zhou, Q.H.; Liu, B.Y.; Cheng, L.; Tian, Y.; Zhang, Y.Y.; Wu, Z.B. Programmed cell death in the cyanobacterium Microcystis aeruginosa induced by allelopathic effect of submerged macrophyte Myriophyllum spicatum in co-culture system. J. Appl. Phycol. 2016, 28, 2805–2814. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, B.; Wang, J.; He, F.; Liang, W.; Xu, D.; Zhang, L.; Wu, Z. Allelopathic effects of phenolic compounds released by Vallisneria spiralis on Microcystis aeruginosa. J. Lake Sci. 2011, 23, 761–766. [Google Scholar] [CrossRef] [Green Version]
- Hu, C.; Ge, F.; Zhang, S.; Liu, B.; Wang, J.; Gao, Y.; Wu, Z. Isolation of antialgal compounds from Potamogeton malaianus and algal inhibitory effects of common fatty acids. J. Lake Sci. 2010, 22, 569–576. [Google Scholar] [CrossRef]
- Huang, X.; Chong, Y.; Tang, Z.; Wu, Q. Analysis on antialgal allelochemicals in culture solutions of three submerged macrophytes. J. South China Agric. Univ. 2010, 31, 19–23. [Google Scholar]
- Li, F.M.; Hu, H.Y.; Chong, Y.X.; Men, Y.J.; Guo, M.T. Influence of EMA isolated from Phragmites communis on physiological characters of Microcystis aeruginosa. China Environ. Sci. (Chin. Ed.) 2007, 27, 377–381. [Google Scholar]
- Liu, J.S.; Chen, Z.L.; Yang, W.D.; Chen, W.F. Inhibitory mechanism of acetone extract from Eichhornia crassipes root on Alexandrium tamarense. Acta Sci. Circumstantiae 2006, 26, 815–820. [Google Scholar] [CrossRef]
- Xian, Q.; Chen, H.; Zou, H.; Yin, D.; Gong, H.; Qu, L. Allelopathic effects of four submerged macrophytes on Microcystis aeruginosa. J. Lake Sci. 2005, 17, 75–80. [Google Scholar] [CrossRef]
- Yu, S.C.; Jiang, Y.; Deng, H.Y.; Wang, X.Y.; Li, J.W.; Tong, Y.X.; Han, Z.P.; Zhao, M.X. Inhibition of reed lixivium on Microcystis aeruginosa growth. Freshw. Fish. 2013, 43, 66–70. [Google Scholar]
- Eigemann, F.; Hilt, S.; Schmitt-Jansen, M. Flow cytometry as a diagnostic tool for the effects of polyphenolic allelochemicals on phytoplankton. Aquat. Bot. 2013, 104, 5–14. [Google Scholar] [CrossRef]
- Wang, R.; Hua, M.; Yu, Y.; Zhang, M.; Xian, Q.M.; Yin, D.Q. Evaluating the effects of allelochemical ferulic acid on Microcystis aeruginosa by pulse-amplitude-modulated (PAM) fluorometry and flow cytometry. Chemosphere 2016, 147, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Xu, N.; Liu, J.; Tian, R. Inhibitory effects of Pontederia cordata on the growth of Microcystis aeruginosa. Water Sci. Technol. 2018, 2017, 99–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karasyova, T.A.; Klose, E.O.; Menzel, R.; Steinberg, C.E. Natural organic matter differently modulates growth of two closely related coccal green algal species. Environ. Sci. Pollut. R. 2007, 14, 88–93. [Google Scholar] [CrossRef]
- Mecina, G.F.; Dokkedal, A.L.; Saldanha, L.L.; Chia, M.A.; Cordeiro-Araújo, M.K.; Bittencourt-Oliveira, M.C.; da Silva, R.M.G. Response of Microcystis aeruginosa BCCUSP 232 to barley (Hordeum vulgare L.) straw degradation extract and fractions. Sci. Total Environ. 2017, 599, 1837–1847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, P.; Pei, H.; Hu, W.; Liu, Z.; Li, X.; Xu, H. Allelopathic effects of Ailanthus altissima extracts on Microcystis aeruginosa growth, physiological changes and microcystins release. Chemosphere 2015, 141, 219–226. [Google Scholar] [CrossRef]
- Zhang, T.; Zheng, C.; He, M.; Wu, A.; Nie, L. The inhibitory mechanism of linoleic acid on Microcystis aeruginosa. China Environ. Sci. 2009, 29, 419–424. [Google Scholar] [CrossRef]
- Yang, X.; Deng, S.; De Philippis, R.; Chen, L.; Hu, C.; Zhang, W. Chemical composition of volatile oil from Artemisia ordosica and its allelopathic effects on desert soil microalgae, Palmellococcus miniatus. Plant Physiol. Biochem. 2012, 51, 153–158. [Google Scholar] [CrossRef]
- Zhang, C.; Yi, Y.L.; Hao, K.; Liu, G.L.; Wang, G.X. Algicidal activity of Salvia miltiorrhiza Bung on Microcystis aeruginosa—Towards identification of algicidal substance and determination of inhibition mechanism. Chemosphere 2013, 93, 997–1004. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, M.; Yu, J.; Zhang, J.; Zhang, R.; Zhang, L.; Chen, G. Differences in growth, pigment composition and photosynthetic rates of two phenotypes Microcystis aeruginosa strains under high and low iron conditions. Biochem. Syst. Ecol. 2014, 55, 112–117. [Google Scholar] [CrossRef]
- Ni, L.X.; Jie, X.T.; Wang, P.F.; Li, S.Y.; Wang, G.X.; Li, Y.P.; Li, Y.; Acharya, K. Effect of linoleic acid sustained-release microspheres on Microcystis aeruginosa antioxidant enzymes activity and microcystins production and release. Chemosphere 2015, 121, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Wayne, W.C.; Gregory, L.B. Health impacts from cyanobacteria harmful algae blooms: Implications for the North American Great Lakes. Harmful Algae 2016, 54, 194–212. [Google Scholar] [CrossRef]
- Wang, X.; Miao, J.; Pan, L.; Li, Y.; Lin, Y.; Wu, J. Toxicity effects of p-choroaniline on the growth, photosynthesis, respiration capacity and antioxidant enzyme activities of a diatom, Phaeodactylum tricornutu. Ecotoxicol. Environ. Saf. 2019, 169, 654–661. [Google Scholar] [CrossRef] [PubMed]
- Jin, P.; Wang, H.; Huang, W.; Liu, W.; Fan, Y.; Miao, W. The allelopathic effect and safety evaluation of 3,4-dihydroxybenzalacetone on Microcystis aeruginosa. Pestic. Biochem. Phys. 2018, 147, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Kong, F.; Shi, X.; Yu, Y.; Zhang, M. Effects of UV-B radiation on microcystin production of a toxic strain of Microcystis aeruginosa and its competitiveness against a non-toxic strain. J. Hazard. Mater. 2015, 283, 447–453. [Google Scholar] [CrossRef]
- Chen, L.F.; Wang, Y.; Shi, L.L.; Zhao, J.C.; Wang, W.H. Identification of allelochemicals from pomegranate peel and their effects on Microcystis aeruginosa growth. Environ. Sci. Pollut. Res. 2019, 26, 22389–22399. [Google Scholar] [CrossRef]
- López-Rodas, V.; Marvá, F.; Costas, E.; Flores-Moya, A. Microalgal adaptation to a stressful environment (acidic, metal-rich mine waters) could be due to selection of pre-selective mutants originating in non-extreme environments. Environ. Exp. Bot. 2008, 64, 43–48. [Google Scholar] [CrossRef]
- Deenamo, N.; Kuyyogsuy, A.; Khompatara, K.; Chanwun, T.; Ekchaweng, K.; Churngchow, N. Salicylic acid induces resistance in rubber tree against Phytophthora palmivora. Int. J. Mol. Sci. 2018, 19, 1883. [Google Scholar] [CrossRef] [Green Version]
- Parvez, S.; Abbas, G.; Shahid, M.; Amjad, M.; Hussain, M.; Asad, S.A.; Imran, M.; Naeem, M.A. Effect of salinity on physiological, biochemical and photostabilizing attributes of two genotypes of quinoa (Chenopodium quinoa Willd.) exposed to arsenic stress. Ecotoxicol. Environ. Saf. 2020, 187, 189014. [Google Scholar] [CrossRef]
- Wang, Y.S.; Luo, S.Q.; Yang, Z.N.; Ye, H.T.; Ding, Q. Responses of Houttuynia cordata Thunb. to Tetracycline Stress. Med. Plant. 2021, 12, 35–43. [Google Scholar] [CrossRef]
- Zheng, H.; Sun, C.; Hou, X.; Wu, M.; Yao, Y.; Li, F. Pyrolysis of Arundo donax L. to produce pyrolytic vinegar and its effect on the growth of dinoflagellate Karenia brevis. Bioresour. Technol. 2018, 247, 273–281. [Google Scholar] [CrossRef]
- Rippka, R.; Deruelles, J.; Waterbury, J.B.; Herdman, M.; Stanier, R.Y. Generic assignments, strain histories and properties of pure cultures of cyanobacteria. Microbiology 1979, 111, 1–61. [Google Scholar] [CrossRef] [Green Version]
- Lv, L.; Zhang, X.; Qiao, J. Flocculation of low algae concentration water using polydiallyldimethylammonium chloride coupled with polysilicate aluminum ferrite. Environ. Technol. 2017, 39, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Q.; Mao, T.G.; Xi, B.D.; Zhang, L.Y.; Zhou, Q.H. KMnO4 pre-oxidation for Microcystis aeruginosa removal by a low dosage of flocculant. Ecol. Eng. 2015, 81, 298–300. [Google Scholar] [CrossRef]
- Rowan, K.S. Photosynthetic Pigments of Alga; Cambridge University Press: Cambridge, MA, USA, 1989; p. 334. [Google Scholar]
- Zhou, X.; Zhang, Y.; An, X.; De Philippis, R.; Ma, X.; Ye, C.; Chen, L. Identification of aqueous extracts from Artemisia ordosica and their allelopathic effects on desert soil algae. Chemoecology 2019, 29, 61–71. [Google Scholar] [CrossRef]
- Kitajima, M.; Butler, W.L. Quenching of chlorophyll fluorescence and primary photochemistry in chloroplasts by dibromothymoquinone. Biochim. Biophys. Acta 1975, 376, 105–115. [Google Scholar] [CrossRef]
- Araus, J.L.; Hogan, K.P. Leaf structure and patterns of photoinhibition in two neotropical palms in clearings and forest understory during the dry season. Am. J. Bot. 1994, 81, 726–738. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Giannoplities, C.N.; Ries, S.K. Superoxide dismutases: II. purification and quantitative relationship with water-soluble protein in seedlings. Plant Physiol. 1977, 59, 315–318. [Google Scholar] [CrossRef] [Green Version]
- Evans, J.J.; Alldridge, N.A. The distribution of peroxidases in extreme dwarf and normal tomato (Lycopersicon esculentum mill.). Phytochemistry 1965, 4, 499–503. [Google Scholar] [CrossRef]





| Component | Stock Solution (g/L) | Component | Stock Solution (g/L) |
|---|---|---|---|
| NaNO3 | 1.5 | Na2CO3 | 0.02 |
| K2HPO4·3H2O | 0.04 | H3BO4 | 0.00286 |
| MgSO4·7H2O | 0.075 | MnCl2·H2O | 0.00181 |
| CaCl·2H2O | 0.036 | ZnSO4·7H2O | 0.000222 |
| Citric acid | 0.006 | CuSO4·5H2O | 0.000079 |
| Ferric ammonium citrate | 0.006 | Na2MoO4·2H2O | 0.00039 |
| EDTA | 0.001 | Co(NO3)2·6H2O | 0.000049 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Chang, Y.; Sun, L.; Du, F.; Cui, J.; Liu, X.; Li, N.; Wang, W.; Li, J.; Yao, D. Abundant Allelochemicals and the Inhibitory Mechanism of the Phenolic Acids in Water Dropwort for the Control of Microcystis aeruginosa Blooms. Plants 2021, 10, 2653. https://doi.org/10.3390/plants10122653
Liu J, Chang Y, Sun L, Du F, Cui J, Liu X, Li N, Wang W, Li J, Yao D. Abundant Allelochemicals and the Inhibitory Mechanism of the Phenolic Acids in Water Dropwort for the Control of Microcystis aeruginosa Blooms. Plants. 2021; 10(12):2653. https://doi.org/10.3390/plants10122653
Chicago/Turabian StyleLiu, Jixiang, Yajun Chang, Linhe Sun, Fengfeng Du, Jian Cui, Xiaojing Liu, Naiwei Li, Wei Wang, Jinfeng Li, and Dongrui Yao. 2021. "Abundant Allelochemicals and the Inhibitory Mechanism of the Phenolic Acids in Water Dropwort for the Control of Microcystis aeruginosa Blooms" Plants 10, no. 12: 2653. https://doi.org/10.3390/plants10122653







