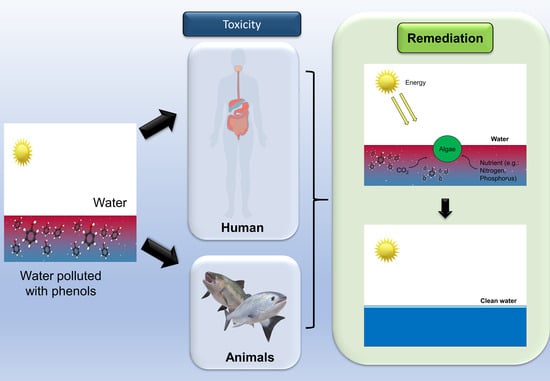
Potential Application of Algae in Biodegradation of Phenol: A Review and Bibliometric Study
Abstract
1. Introduction
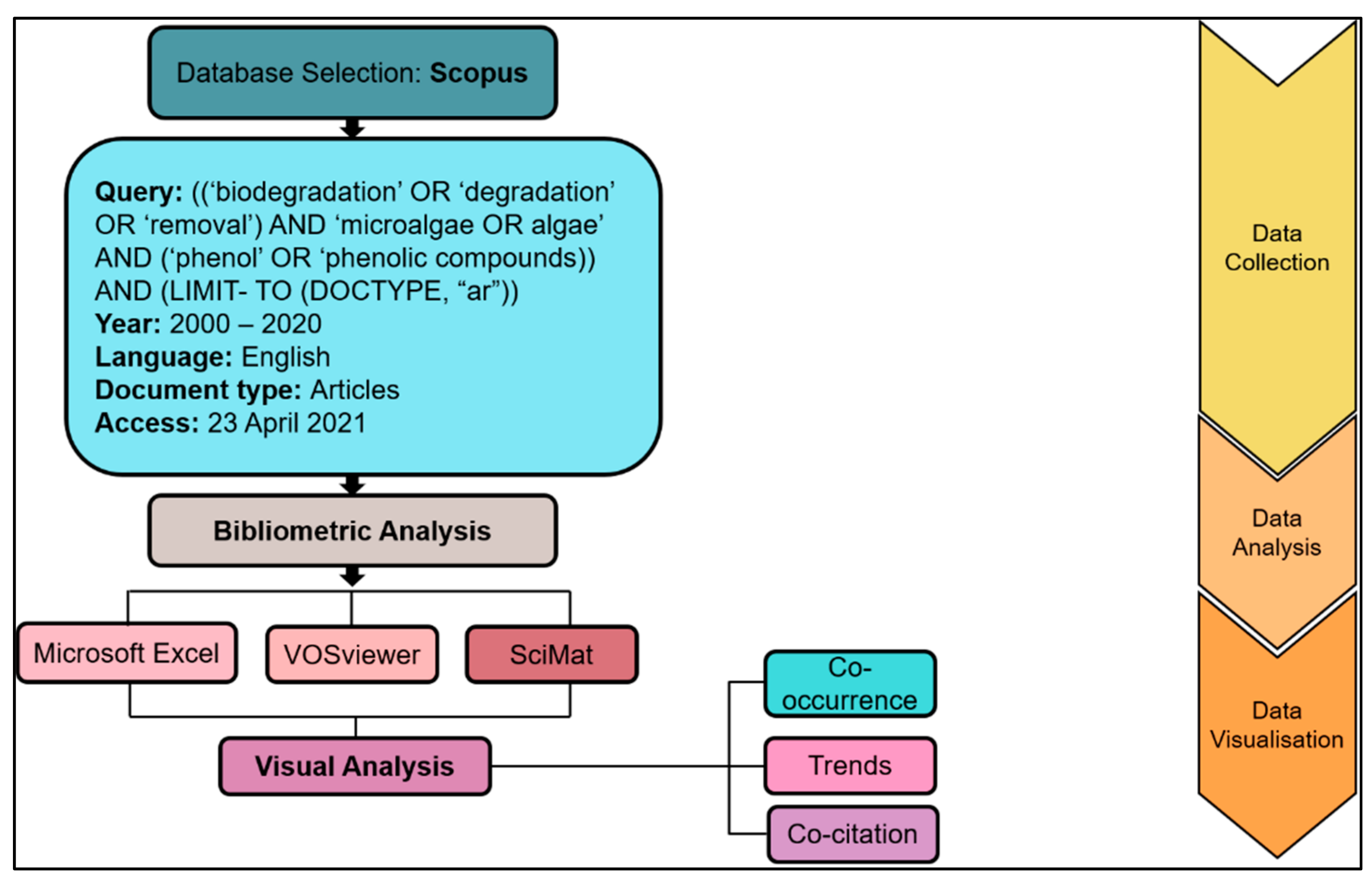
2. Bibliometric Analysis
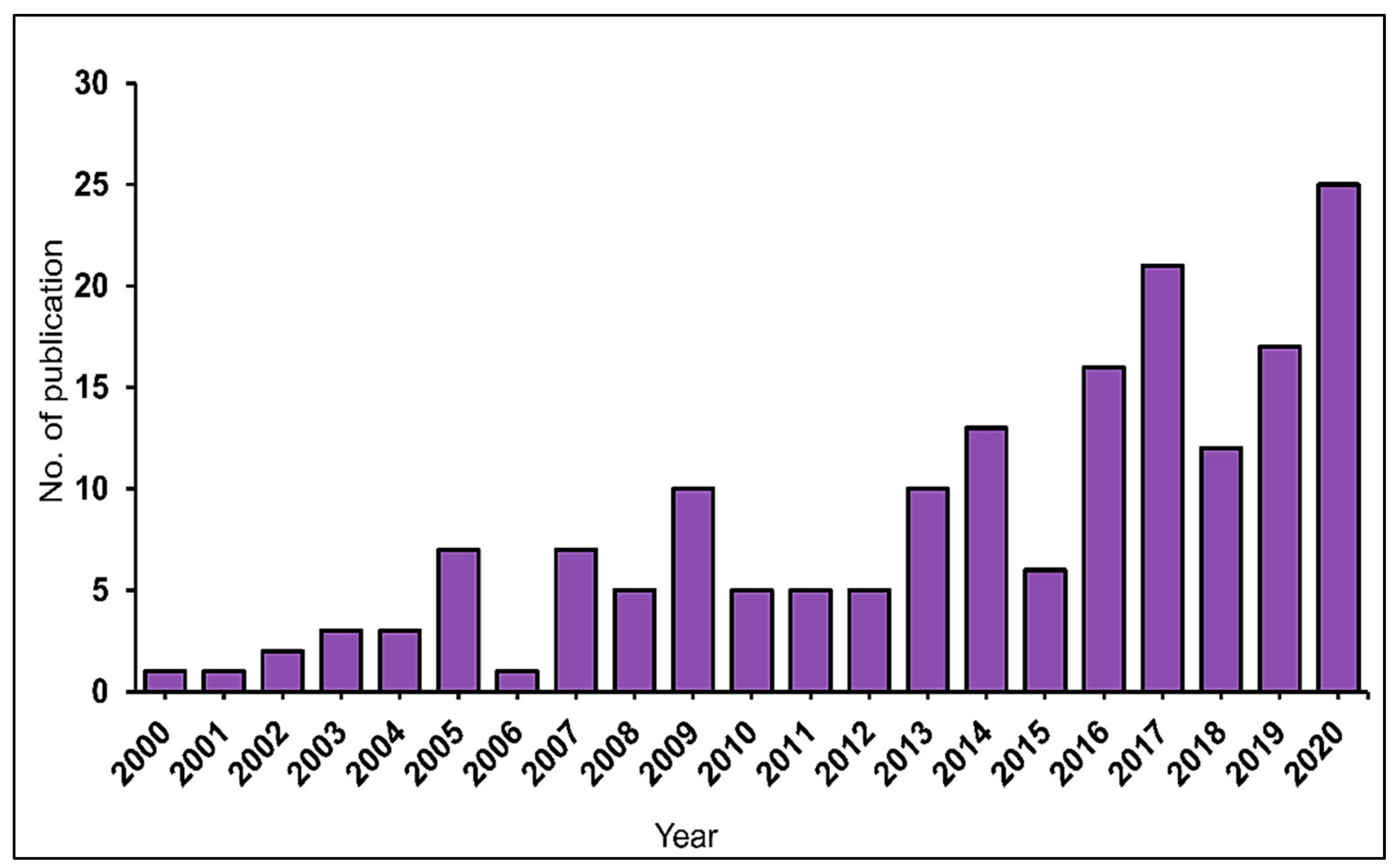
2.1. Trends in Publication
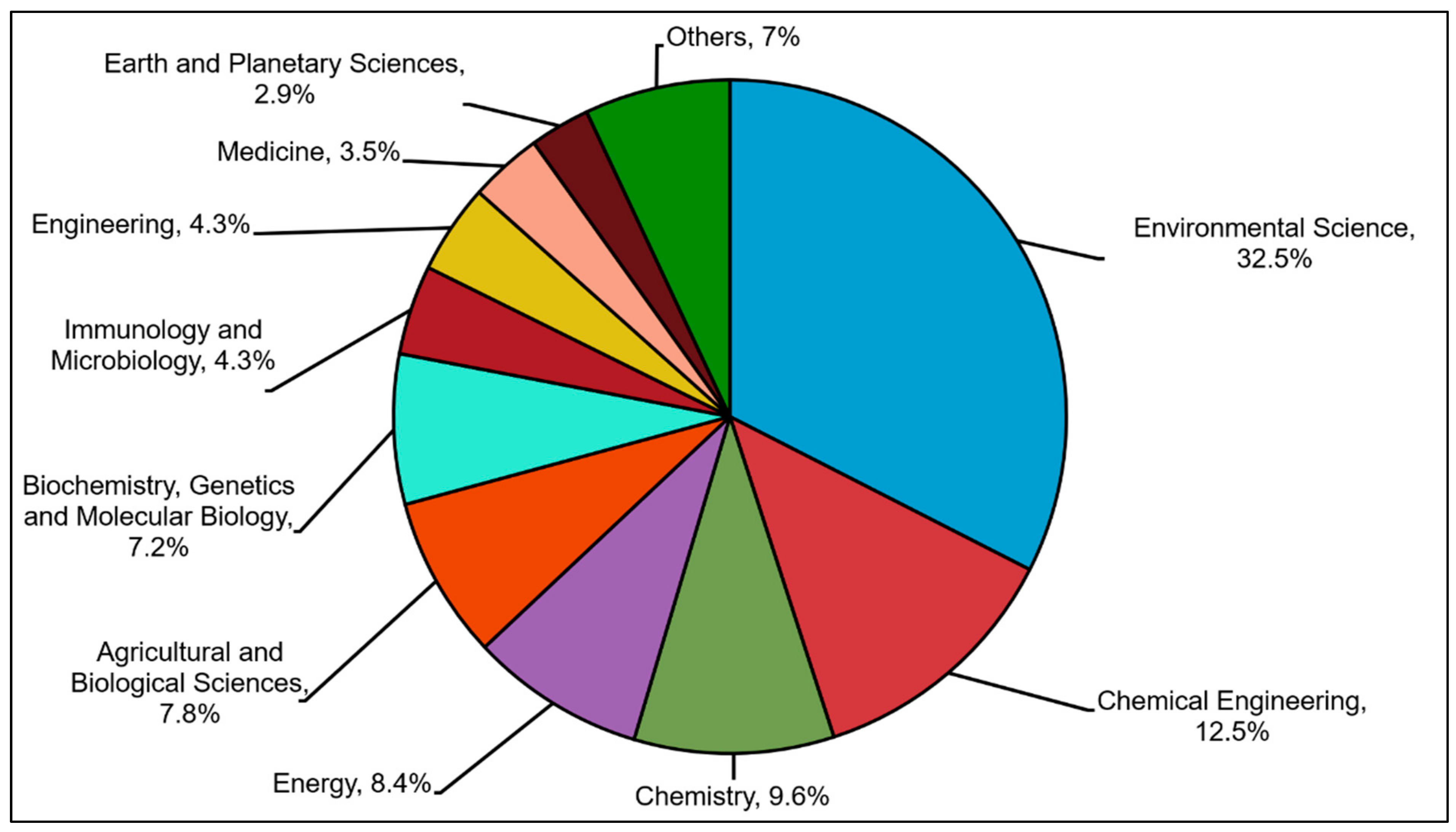
2.2. Analysis Based on Subject Areas
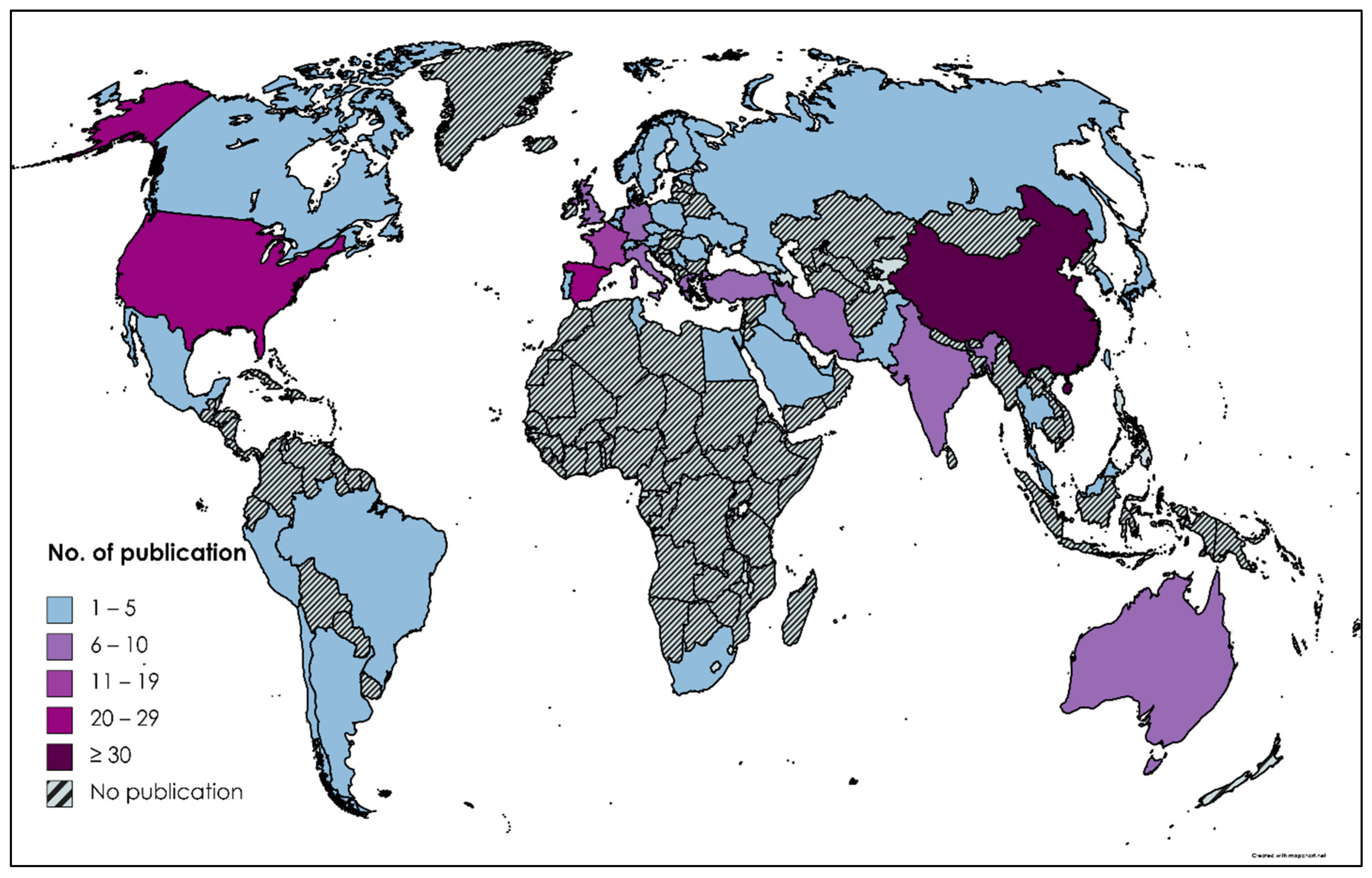
2.3. Countries with the Highest Work Published
3. Analysis Using SciMAT
3.1. Strategic Diagram
3.1.1. First Period (2000–2005)
3.1.2. Second Period (2006–2010)
3.1.3. Third Period (2011–2015)
3.1.4. Fourth Period (2016–2020)
3.2. Thematic Network- The Central Cluster of Each Period
3.2.1. First Period (2000–2005)
3.2.2. Second Period (2006–2010)
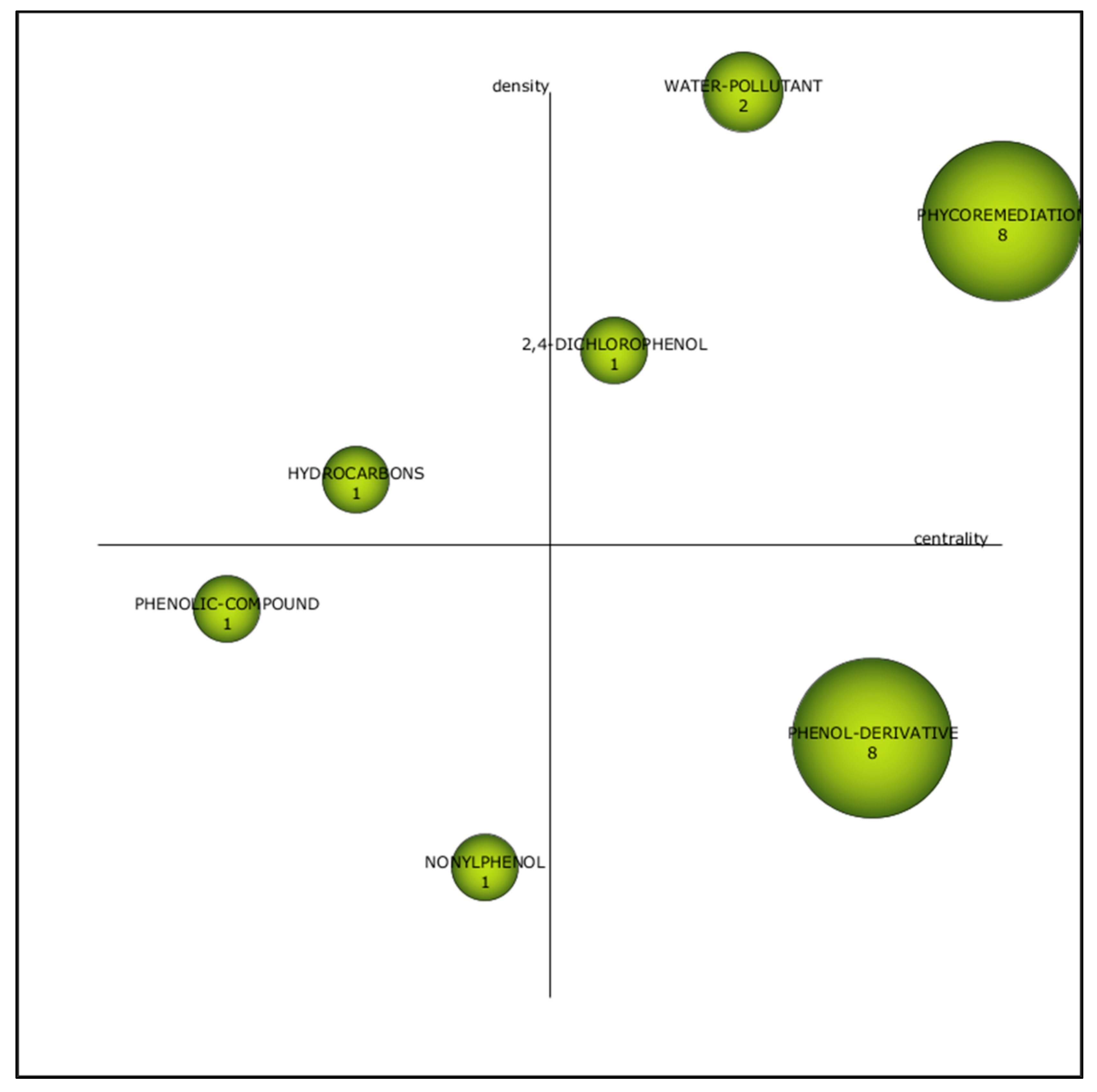
3.2.3. Third Period (2011–2015)
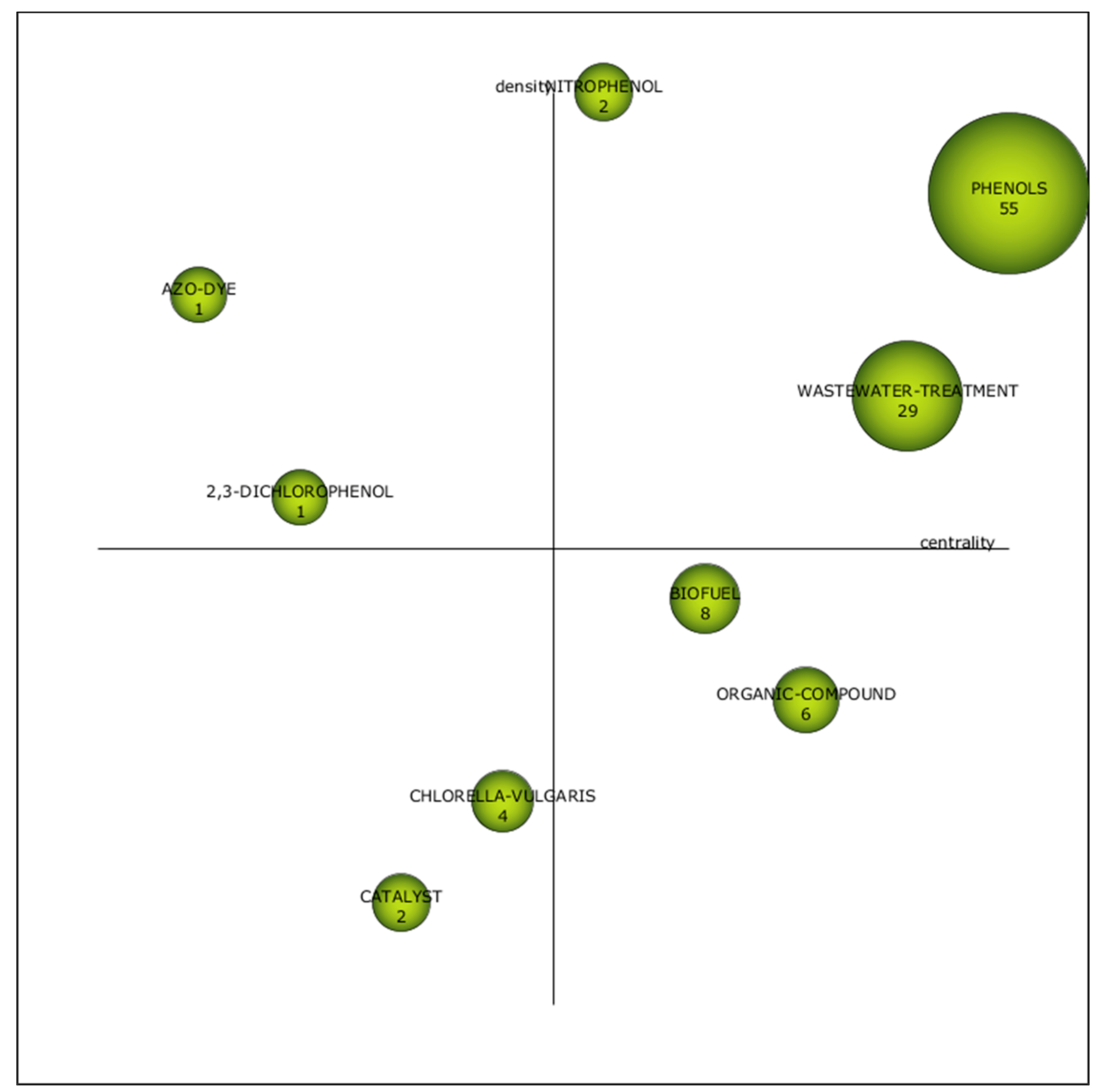
3.2.4. Fourth Period (2016–2020)
3.3. Evolution Map
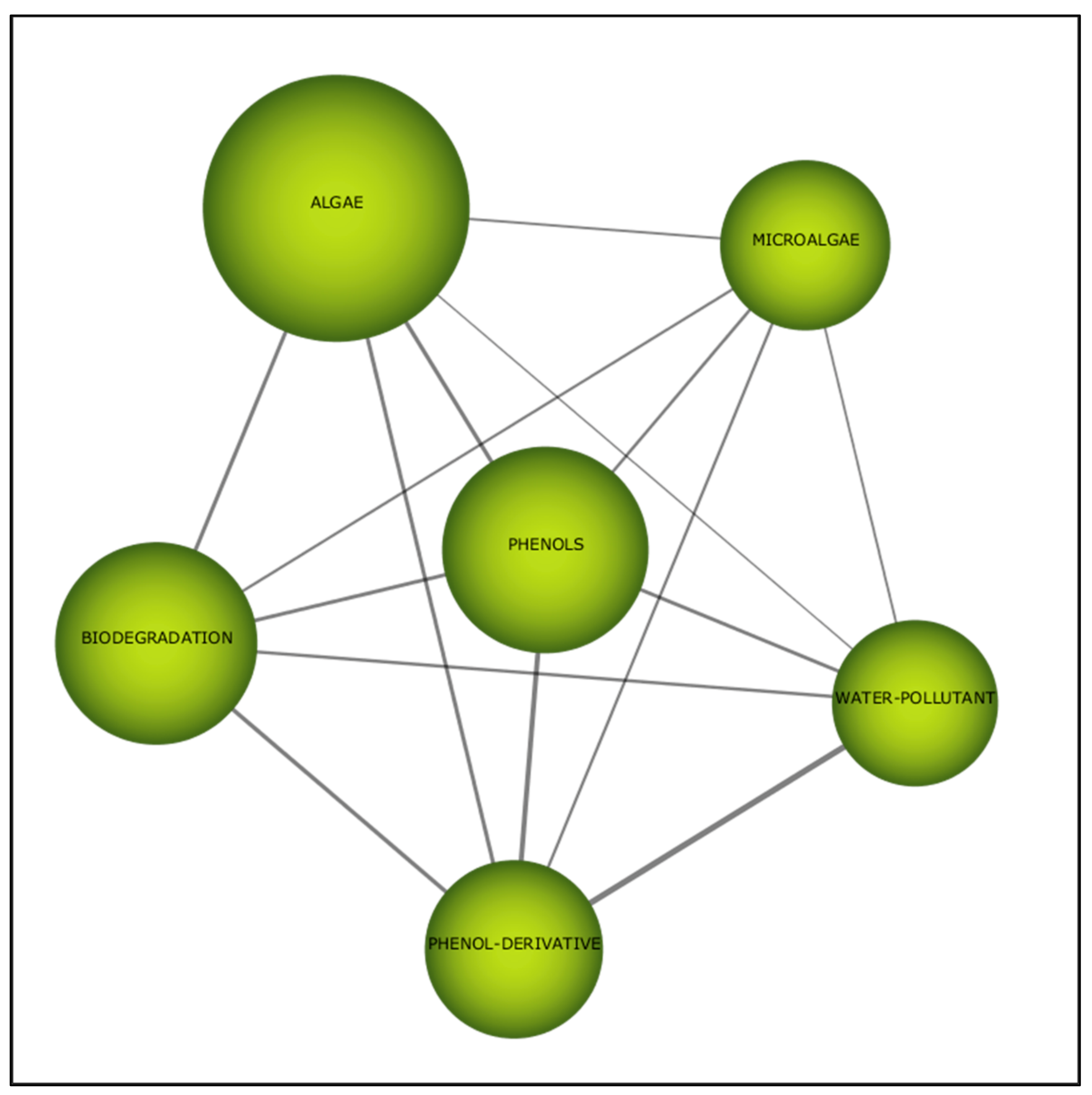
4. Visualisation Using VOSviewer-Keywords Visualisation
4.1. Phenols
4.1.1. Sources of Phenol
4.1.2. Toxicity
4.2. Algae
4.3. Phenol-Degrading Algae
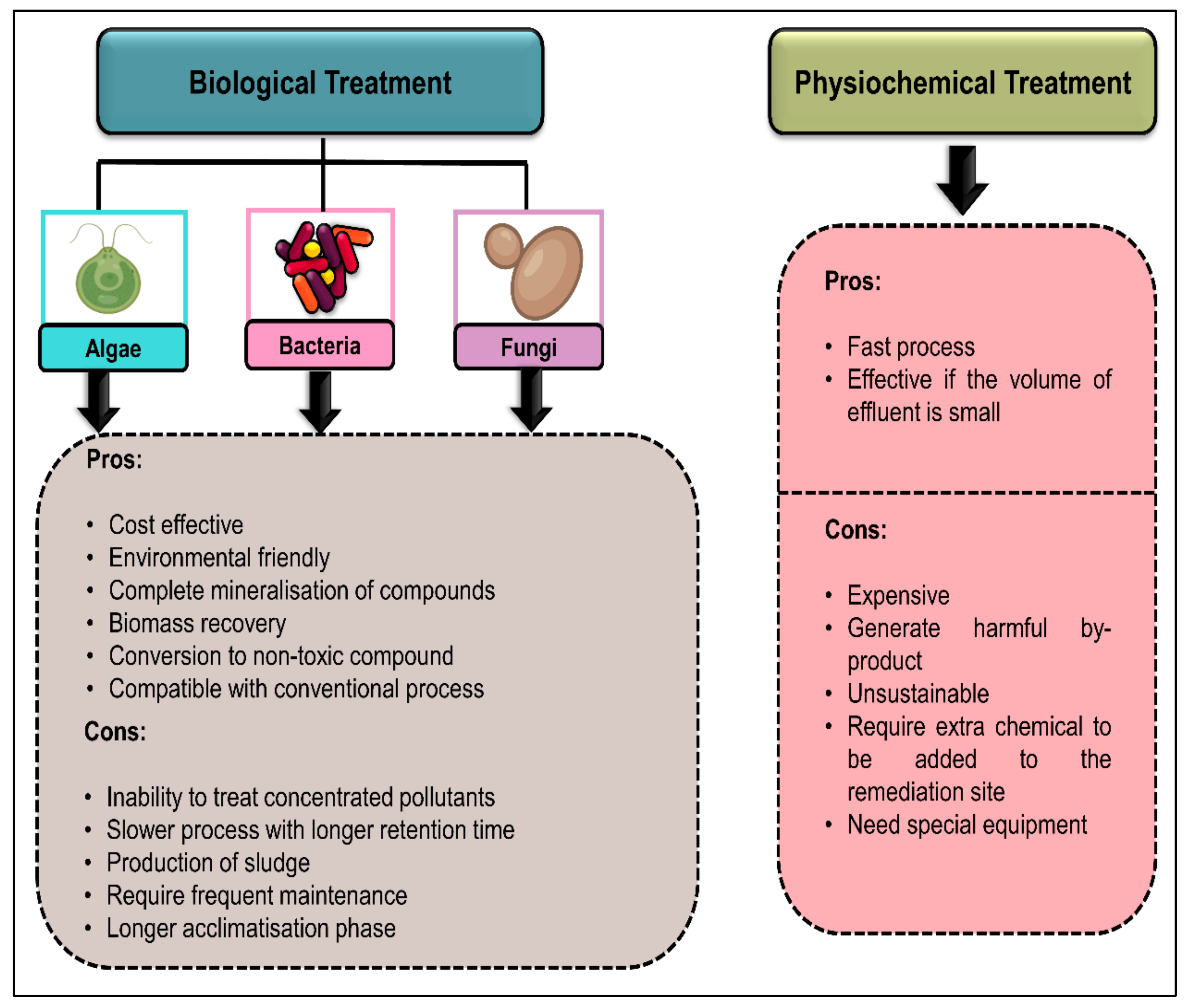
4.4. Insight into Biodegradation
4.4.1. Factors Affecting Phenol Degradation by Algae
4.4.2. Elucidation of Mechanism and Enzymatic Action on Phenol Degradation
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Duan, W.; Meng, F.; Lin, Y.; Wang, G. Toxicological Effects of Phenol on Four Marine Microalgae. Environ. Toxicol. Pharm. 2017, 52, 170–176. [Google Scholar] [CrossRef]
- Mahugo-Santana, C.; Sosa-Ferrera, Z.; Torres-Padrón, M.E.; Santana-Rodríguez, J.J. Analytical Methodologies for the Determination of Nitroimidazole Residues in Biological and Environmental Liquid Samples: A Review. Anal. Chim. Acta 2010, 665, 113–122. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Joo, H.S.; Yang, Y.H. Biowaste-to-Bioenergy Using Biological Methods—A Mini Review. Energy Convers. Manag. 2018, 177, 640–660. [Google Scholar] [CrossRef]
- Manaf, I.S.A.; Embong, N.H.; Khazaai, S.N.M.; Rahim, M.H.A.; Yusoff, M.M.; Lee, K.T.; Maniam, G.P. A Review for Key Challenges of the Development of Biodiesel Industry. Energy Convers. Manag. 2019, 185, 508–517. [Google Scholar] [CrossRef]
- Massalha, N.; Shaviv, A.; Sabbah, I. Modeling the Effect of Immobilization of Microorganisms on the Rate of Biodegradation of Phenol Under Inhibitory Conditions. Water Res. 2010, 44, 5252–5259. [Google Scholar] [CrossRef]
- Subramaniam, K.; Ahmad, S.A.; Shaharuddin, N.A. Mini Review on Phenol Biodegradation in Antarctica Using Native Microorganisms. Asia-Pac. J. Mol. Biol. Biotechnol. 2020, 28, 77–89. [Google Scholar] [CrossRef]
- Wu, Y.; He, J.; Yang, L. Evaluating Adsorption and Biodegradation Mechanisms During the Removal of Microcystin-RR by Periphyton. Environ. Sci. Technol. 2010, 44, 6319–6324. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Hu, Z.; Yang, L.; Graham, B.; Kerr, P.G. The Removal of Nutrients from Non-Point Source Wastewater by A Hybrid Bioreactor. Bioresour. Technol. 2011, 102, 2419–2426. [Google Scholar] [CrossRef]
- Phang, S.-M.; Chu, W.-L.; Rabiei, R. Phycoremediation. In Algae World; Springer: Dordrecht, The Nederland, 2015; pp. 357–389. [Google Scholar] [CrossRef]
- Krishnamoorthy, S.; Manickam, P. Phycoremediation of Industrial Wastewater: Challenges and Prospects. In Bioremediation for Environmental Sustainability; Elsevier: Amsterdam, The Netherlands, 2021; pp. 99–123. [Google Scholar] [CrossRef]
- Ke, L.; Wong, Y.S.; Tam, N.F.Y. Toxicity and removal of organic pollutants by microalgae: A Review. In Microalgae, Biotechnology, Microbiology, and Energy; Nova Science Publishers, Inc.: New York, NY, USA, 2012; pp. 101–140. [Google Scholar]
- Al-Jabri, H.; Das, P.; Khan, S.; Thaher, M.; AbdulQuadir, M. Treatment of Wastewaters by Microalgae and the Potential Applications of the Produced Biomass—A Review. Water 2020, 13, 27. [Google Scholar] [CrossRef]
- Laurens, L.M.L.; Chen-Glasser, M.; McMillan, J.D. A Perspective on Renewable Bioenergy from Photosynthetic Algae as Feedstock for Biofuels and Bioproducts. Algal Res. 2017, 24, 261–264. [Google Scholar] [CrossRef]
- Mahana, A.; Guliy, O.I.; Mehta, S.K. Accumulation and Cellular Toxicity of Engineered Metallic Nanoparticle in Freshwater Microalgae: Current Status and Future Challenges. Ecotoxicol. Environ. Saf. 2021, 208, 111662. [Google Scholar] [CrossRef]
- Van Schie, P.M.; Young, L.Y. Biodegradation of Phenol: Mechanisms and Applications. Bioremediation J. 2000, 4, 1–18. [Google Scholar] [CrossRef]
- Krastanov, A.; Alexieva, Z.; Yemendzhiev, H. Microbial Degradation of Phenol and Phenolic Derivatives. Eng. Life Sci. 2013, 13, 76–87. [Google Scholar] [CrossRef]
- Lee, H.C.; Lee, M.; Den, W. Spirulina maxima for Phenol Removal: Study on Its Tolerance, Biodegradability and Phenol-Carbon Assimilability. Water. Air. Soil Pollut. 2015, 226, 1–11. [Google Scholar] [CrossRef]
- Brar, A.; Kumar, M.; Vivekanand, V.; Pareek, N. Photoautotrophic Microorganisms and Bioremediation of Industrial Effluents: Current Status and Future Prospects. 3 Biotech. 2017, 7, 18. [Google Scholar] [CrossRef]
- Garrett, M.K.; Allen, M.D.B. Photosynthetic Purification of the Liquid Phase of Animal Slurry. Environ. Pollut. 1976, 10, 127–139. [Google Scholar] [CrossRef]
- Fallowfield, H.J.; Garrett, M.K. The Photosynthetic Treatment of Pig Slurry in Temperate Climatic Conditions: A Pilot-Plant Study. Agric. Wastes 1985, 12, 111–136. [Google Scholar] [CrossRef]
- Maza-Márquez, P.; Martinez-Toledo, M.V.; Fenice, M.; Andrade, L.; Lasserrot, A.; Gonzalez-Lopez, J. Biotreatment of Olive Washing Wastewater by a Selected Microalgal-Bacterial Consortium. Int. Biodeterior. Biodegrad. 2014, 88, 69–76. [Google Scholar] [CrossRef]
- Pritchard, A. Statistical Bibliography or Bibliometrics. J. Doc. 1969, 25, 348–349. [Google Scholar]
- Lim, Z.S.; Wong, R.R.; Wong, C.-Y.; Zulkharnain, A.; Shaharuddin, N.A.; Ahmad, S.A. Bibliometric Analysis of Research on Diesel Pollution in Antarctica and a Review on Remediation Techniques. Appl. Sci. 2021, 11, 1123. [Google Scholar] [CrossRef]
- Zakaria, N.N.; Convey, P.; Gomez-Fuentes, C.; Zulkharnain, A.; Sabri, S.; Shaharuddin, N.A.; Ahmad, S.A. Oil Bioremediation in the Marine Environment of Antarctica: A Review and Bibliometric Keyword Cluster Analysis. Microorganisms 2021, 9, 419. [Google Scholar] [CrossRef]
- Khalid, F.E.; Lim, Z.S.; Sabri, S.; Gomez-Fuentes, C.; Zulkharnain, A.; Ahmad, S.A. Bioremediation of Diesel Contaminated Marine Water by Bacteria: A Review and Bibliometric Analysis. J. Mar. Sci. Eng. 2021, 9, 155. [Google Scholar] [CrossRef]
- Zahri, K.N.M.; Zulkharnain, A.; Sabri, S.; Gomez-Fuentes, C.; Ahmad, S.A. Research Trends of Biodegradation of Cooking Oil in Antarctica from 2001 to 2021: A Bibliometric Analysis Based on the Scopus Database. Int. J. Environ. Res. Public Health 2021, 18, 2050. [Google Scholar] [CrossRef]
- Verasoundarapandian, G.; Wong, C.-Y.; Shaharuddin, N.A.; Gomez-Fuentes, C.; Zulkharnain, A.; Ahmad, S.A. A Review and Bibliometric Analysis on Applications of Microbial Degradation of Hydrocarbon Contaminants in Arctic Marine Environment at Metagenomic and Enzymatic Levels. Int. J. Environ. Res. Public Health 2021, 18, 1671. [Google Scholar] [CrossRef]
- Gorraiz, J.; Schloegl, C. A Bibliometric Analysis of Pharmacology and Pharmacy Journals: Scopus versus Web of Science. J. Inf. Sci. 2008, 34, 715–725. [Google Scholar] [CrossRef]
- Song, Y.; Hou, D.; Zhang, J.; O’Connor, D.; Li, G.; Gu, Q.; Li, S.; Liu, P. Environmental and Socio-Economic Sustainability Appraisal of Contaminated Land Remediation Strategies: A Case Study at a Mega-Site in China. Sci. Total Environ. 2018, 610–611, 391–401. [Google Scholar] [CrossRef]
- Cobo, M.J.; Lõpez-Herrera, A.G.; Herrera-Viedma, E.; Herrera, F. SciMAT: A New Science Mapping Analysis Software Tool. J. Am. Soc. Inf. Sci. Technol. 2012, 63, 1609–1630. [Google Scholar] [CrossRef]
- Tobon, S.; Ruiz-Alba, J.L.; García-Madariaga, J. Gamification and Online Consumer Decisions: Is the Game Over? Decis. Support Syst. 2020, 128, 113167. [Google Scholar] [CrossRef]
- Stephen, D.P.; Ayalur, K.B. Phycoremediation of Phenolic Effluent of a Coal Gasification Plant by Chlorella pyrenoidosa. Process Saf. Environ. Prot. 2017, 111, 31–39. [Google Scholar] [CrossRef]
- Mandal, A.; Das, S.K. Phenol Adsorption from Wastewater Using Clarified Sludge from Basic Oxygen Furnace. J. Environ. Chem. Eng. 2019, 7, 103259. [Google Scholar] [CrossRef]
- Caetano, M.; Valderrama, C.; Farran, A.; Cortina, J.L. Phenol Removal from Aqueous Solution by Adsorption and Ion Exchange Mechanisms onto Polymeric Resins. J. Colloid Interface Sci. 2009, 338, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Li, G.; Liu, D.; Wei, Y. Synthesis of Zeolite Y Promoted by Fenton’s Reagent and Its Application in Photo-Fenton-like Oxidation of Phenol. Solid State Sci. 2019, 91, 89–95. [Google Scholar] [CrossRef]
- Jiang, L.; Mao, X. Degradation of Phenol-Containing Wastewater Using an Improved Electro-Fenton Process. Int. J. Electrochem. Sci. 2012, 7, 4078–4088. [Google Scholar]
- Abdelaziz, A.; Mubarak, A.; Nosier, S.; Hussien, M. Treatment of Industrial Wastewater Containing Phenol Using the Electro-Fenton Technique in Gas Sparged Cell. Am. J. Environ. Eng. Sci. 2015, 2, 47–59. [Google Scholar]
- Rubalcaba, A.; Suárez-Ojeda, M.E.; Stüber, F.; Fortuny, A.; Bengoa, C.; Metcalfe, I.; Font, J.; Carrera, J.; Fabregat, A. Phenol Wastewater Remediation: Advanced Oxidation Processes Coupled to a Biological Treatment. Water Sci. Technol. 2007, 55, 221–227. [Google Scholar] [CrossRef]
- Rezakazemi, M.; Khajeh, A.; Mesbah, M. Membrane Filtration of Wastewater from Gas and Oil Production. Environ. Chem. Lett. 2018, 16, 367–388. [Google Scholar] [CrossRef]
- Mixa, A.; Staudt, C. Membrane-Based Separation of Phenol/Water Mixtures Using Ionically and Covalently Cross-Linked Ethylene-Methacrylic Acid Copolymers. Int. J. Chem. Eng. 2008, 2008, 1–12. [Google Scholar] [CrossRef]
- Achak, M.; Elayadi, F.; Boumya, W. Chemical Coagulation/Flocculation Processes for Removal of Phenolic Compounds from Olive Mill Wastewater: A Comprehensive Review. Am. J. Appl. Sci. 2019, 16, 59–91. [Google Scholar] [CrossRef]
- Rodriguez Arreola, A.; Sanchez Tizapa, M.; Zurita, F.; Pablo Morán-Lázaro, J.; Castañeda Valderrama, R.; Luis Rodríguez-López, J.; Carreon-Alvarez, A. Treatment of Tequila Vinasse and Elimination of Phenol by Coagulation-Flocculation Process Coupled with Heterogeneous Photocatalysis Using Titanium Dioxide Nanoparticles. Environ. Technol. 2018, 41, 1023–1033. [Google Scholar] [CrossRef]
- Patel, H.; Vashi, R.T. Treatment of Textile Wastewater by Adsorption and Coagulation. E-J. Chem. 2010, 7, 1468–1476. [Google Scholar] [CrossRef]
- García-Araya, J.F.; Beltrán, F.J.; Álvarez, P.; Masa, F.J. Activated Carbon Adsorption of Some Phenolic Compounds Present in Agroindustrial Wastewater. Adsorption 2003, 9, 107–115. [Google Scholar] [CrossRef]
- Zainuddin, Z.; Abdullah, L.C.; Choong, T. Equilibrium, Kinetics and Thermodynamic Studies: Adsorption of Remazol Black 5 on the Palm Kernel Shell Activated Carbon (PKS-AC). Eng. J. Sci. Res. 2009, 37, 67–76. [Google Scholar]
- Hamby, D.M. Site Remediation Techniques Supporting Environmental Restoration Activities—A Review. Sci. Total Environ. 1996, 191, 203–224. [Google Scholar] [CrossRef]
- Ksibi, M. Chemical Oxidation with Hydrogen Peroxide for Domestic Wastewater Treatment. Chem. Eng. J. 2006, 119, 161–165. [Google Scholar] [CrossRef]
- Pradeep, N.V.; Anupama, S.; Navya, K.; Shalini, H.N.; Idris, M.; Hampannavar, U.S. Biological Removal of Phenol from Wastewaters: A Mini Review. Appl. Water Sci. 2015, 5, 105–112. [Google Scholar] [CrossRef]
- Tengku-Mazuki, T.A.; Subramaniam, K.; Zakaria, N.N.; Convey, P.; Khalil, K.A.; Lee, G.L.Y.; Zulkharnain, A.; Shaharuddin, N.A.; Ahmad, S.A. Optimization of Phenol Degradation by Antarctic Bacterium Rhodococcus sp. Antarct. Sci. 2020, 32, 486–495. [Google Scholar] [CrossRef]
- Subramaniam, K.; Shaharuddin, N.A.; Tengku-Mazuki, T.A.; Zulkharnain, A.; Khalil, K.A.; Convey, P.; Ahmad, S.A. Statistical Optimisation for Enhancement of Phenol Biodegradation by the Antarctic Soil Bacterium Arthrobacter Sp. Strain AQ5-15 Using Response Surface Methodology. J. Environ. Biol. 2020, 41, 1560–1569. [Google Scholar] [CrossRef]
- Kong, W.; Yang, S.; Guo, B.; Wang, H.; Huo, H.; Zhang, A.; Niu, S. Growth Behavior, Glucose Consumption and Phenol Removal Efficiency of Chlorella vulgaris Under the Synergistic Effects of Glucose and Phenol. Ecotoxicol. Environ. Saf. 2019, 186, 109762. [Google Scholar] [CrossRef]
- Subashchandrabose, S.R.; Ramakrishnan, B.; Megharaj, M.; Venkateswarlu, K.; Naidu, R. Mixotrophic Cyanobacteria and Microalgae as Distinctive Biological Agents for Organic Pollutant Degradation. Environ. Int. 2013, 51, 59–72. [Google Scholar] [CrossRef]
- Wang, L.L.; Xue, C.; Wang, L.L.; Zhao, Q.; Wei, W.; Sun, Y. Strain Improvement of Chlorella sp. for Phenol Biodegradation by Adaptive Laboratory Evolution. Bioresour. Technol. 2016, 205, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A. Toxic Effect and Bioremediation of Oil Contamination in Algal Perspective. In Bioremediation for Environmental Sustainability; Elsevier: Amsterdam, The Netherlands, 2020; pp. 283–298. [Google Scholar] [CrossRef]
- Sharifi, A. Urban Sustainability Assessment: An Overview and Bibliometric Analysis. Ecological Indic. 2021, 121, 107102. [Google Scholar] [CrossRef]
- Cobo, M.J.; Jürgens, B.; Herrero-Solana, V.; Martínez, M.A.; Herrera-Viedma, E. Industry 4.0: A Perspective Based on Bibliometric Analysis. Procedia Comput. Sci. 2018, 139, 364–371. [Google Scholar] [CrossRef]
- Van Eck, N.J.; Waltman, L. Software Survey: VOSviewer, A Computer Program for Bibliometric Mapping. Scientometrics 2010, 84, 523–538. [Google Scholar] [CrossRef]
- Cobo, M.J.; López-Herrera, A.G.; Herrera-Viedma, E.; Herrera, F. Science Mapping Software Tools: Review, Analysis, and Cooperative Study Among Tools. J. Am. Soc. Inf. Sci. Technol. 2011, 62, 1382–1402. [Google Scholar] [CrossRef]
- Tyman, J.H. Chapter 1 Historical Aspects and Industrial Syntheses of Monohydric and Dihydric Phenols. Stud. Org. Chem. 1996, 52, 1–22. [Google Scholar] [CrossRef]
- Anku, W.W.; Mamo, M.A.; Govender, P.P. Phenolic Compounds in Water: Sources, Reactivity, Toxicity and Treatment Methods. In Phenolic Compounds: Natural Sources, Importance and Applications; IntechOpen: London, UK, 2017; pp. 419–443. [Google Scholar] [CrossRef]
- Mohanty, S.S. Microbial Degradation of Phenol:A Comparitive Study. Ph.D. Thesis, National Institute of Technology, Rourkela, India, January 2012. [Google Scholar]
- Aggelis, G.; Ehaliotis, C.; Nerud, F.; Stoychev, I.; Lyberatos, G.; Zervakis, G. Evaluation of White-Rot Fungi for Detoxification and Decolorization of Effluents from the Green Olive Debittering Process. Appl. Microbiol. Biotechnol. 2002, 59, 353–360. [Google Scholar] [CrossRef]
- Field, J.A.; Lettinga, G. Treatment and Detoxification of Aqueous Spruce Bark Extracts by Aspergillus niger. Water Sci. Technol. 1991, 24, 127–137. [Google Scholar] [CrossRef]
- Kadir, W.N.A.; Lam, M.K.; Uemura, Y.; Lim, J.W.; Lee, K.T. Harvesting and Pre-Treatment of Microalgae Cultivated in Wastewater for Biodiesel Production: A Review. Energy Convers. Manag. 2018, 171, 1416–1429. [Google Scholar] [CrossRef]
- Lellis, B.; Fávaro-Polonio, C.Z.; Pamphile, J.A.; Polonio, J.C. Effects of Textile Dyes on Health and the Environment and Bioremediation Potential of Living Organisms. Biotechnol. Res. Innov. 2019, 3, 275–290. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, X.; Zhou, Z. Impacts of Small-Scale Industrialized Swine Farming on Local Soil, Water and Crop Qualities in a Hilly Red Soil Region of Subtropical China. Int. J. Environ. Res. Public Health 2017, 14, 1524. [Google Scholar] [CrossRef]
- Sueoka, K.; Satoh, H.; Onuki, M.; Mino, T. Microorganisms Involved in Anaerobic Phenol Degradation in the Treatment of Synthetic Coke-Oven Wastewater Detected by RNA Stable-Isotope Probing: Research Letter. FEMS Microbiol. Lett. 2009, 291, 169–174. [Google Scholar] [CrossRef]
- Weber, M.; Weber, M. Phenols. In Phenolic Resins: A Century of Progress; Springer: Berlin/Heidelberg, Germany, 2010; pp. 9–23. [Google Scholar] [CrossRef]
- Hirano, K.; Asami, M. Phenolic Resins-100 Years of Progress and Their Future. React. Funct. Polym. 2013, 73, 256–269. [Google Scholar] [CrossRef]
- Gestermann, S.; Köppchen, W.; Krause, V.; Möthrath, M.; Pophusen, D.W.; Sandquist, A.; Zöllner, O. Polycarbonate and Its Blends for Car Body Parts (Polycarbonat Und Seine Blends Für Karosseriebauteile). ATZ Worldw. 2005, 107, 22–24. [Google Scholar] [CrossRef]
- Barlow, J.; Bornschein, R.; Brown, K.; Ann Johnson, J.P.; Scofield, L.; Belcher, S.M.; Professor, A.; Fenton, S.E.; Lamartiniere, C.A. Early Life Exposure and Breast Cancer Risk in Later Years; Fact Sheets; Breast Cancer & The Environment Centers, University of California: San Francisco, CA, USA, 2007. [Google Scholar]
- Downs, C.A.; Kramarsky-Winter, E.; Segal, R.; Fauth, J.; Knutson, S.; Bronstein, O.; Ciner, F.R.; Jeger, R.; Lichtenfeld, Y.; Woodley, C.M.; et al. Toxicopathological Effects of the Sunscreen UV Filter, Oxybenzone (Benzophenone-3), on Coral Planulae and Cultured Primary Cells and Its Environmental Contamination in Hawaii and the U.S. Virgin Islands. Arch. Environ. Contam. Toxicol. 2016, 70, 265–288. [Google Scholar] [CrossRef]
- Soto, M.; Falqué, E.; Domínguez, H. Relevance of Natural Phenolics from Grape and Derivative Products in the Formulation of Cosmetics. Cosmetics 2015, 2, 259–276. [Google Scholar] [CrossRef]
- Suslick, K.S. Kirk-Othmer Encyclopedia of Chemical Technology; Wiley: Hoboken, NJ, USA, 1998. [Google Scholar] [CrossRef]
- Badanthadka, M.; Mehendale, H.M. Chlorophenols. In Encyclopedia of Toxicology, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 896–899. [Google Scholar] [CrossRef]
- Hong, J.; Xu, X. Environmental Impact Assessment of Caprolactam Production—A Case Study in China. J. Clean. Prod. 2012, 27, 103–108. [Google Scholar] [CrossRef]
- Mohammadi, S.; Kargari, A.; Sanaeepur, H.; Abbassian, K.; Najafi, A.; Mofarrah, E. Phenol Removal from Industrial Wastewaters: A Short Review. Desalin. Water Treat. 2015, 53, 2215–2234. [Google Scholar] [CrossRef]
- García García, I.; Bonilla Venceslada, J.L.; Jiménez Peña, P.R.; Ramos Gómez, E. Biodegradation of Phenol Compounds in Vinasse Using Aspergillus terreus and Geotrichum candidum. Water Res. 1997, 31, 2005–2011. [Google Scholar] [CrossRef]
- Jusoh, N.; Razali, F. Microbial Consortia from Residential Wastewater for Bioremediation of Phenol in a Chemostat. J. Teknol. 2008, 4, 51–60. [Google Scholar] [CrossRef][Green Version]
- Wei, X.; Gilevska, T.; Wetzig, F.; Dorer, C.; Richnow, H.H.; Vogt, C. Characterization of Phenol and Cresol Biodegradation by Compound-Specific Stable Isotope Analysis. Environ. Pollut. 2016, 210, 166–173. [Google Scholar] [CrossRef]
- Gorini, F.; Bustaffa, E.; Coi, A.; Iervasi, G.; Bianchi, F. Bisphenols as Environmental Triggers of Thyroid Dysfunction: Clues and Evidence. Int. J. Environ. Res. Public Health 2020, 17, 2654. [Google Scholar] [CrossRef]
- Kim, J.J.; Kumar, S.; Kumar, V.; Lee, Y.M.; Kim, Y.S.; Kumar, V. Bisphenols as a Legacy Pollutant, and Their Effects on Organ Vulnerability. Int. J. Environ. Res. Public Health 2020, 17, 112. [Google Scholar] [CrossRef]
- Luo, Y.; Guo, W.; Ngo, H.H.; Nghiem, L.D.; Hai, F.I.; Zhang, J.; Liang, S.; Wang, X.C. A Review on the Occurrence of Micropollutants in the Aquatic Environment and Their Fate and Removal During Wastewater Treatment. Sci. Total Environ. 2014, 473, 619–641. [Google Scholar] [CrossRef]
- Kulkarni, S.J.; Kaware, J.P. Review on Research for Removal of Phenol from Wastewater. Int. J. Sci. Res. Publ. 2013, 3, 1–5. [Google Scholar]
- Menz, J.; Olsson, O.; Kümmerer, K. Antibiotic Residues in Livestock Manure: Does the EU Risk Assessment Sufficiently Protect Against Microbial Toxicity and Selection of Resistant Bacteria in the Environment? J. Hazard. Mater. 2019, 379, 120807. [Google Scholar] [CrossRef] [PubMed]
- Tišler, T.; Zagorc-Končan, J. Comparative Assessment of Toxicity of Phenol, Formaldehyde, and Industrial Wastewater to Aquatic Organisms. Water Air Soil Pollut. 1997, 97, 315–322. [Google Scholar] [CrossRef]
- Hammam, A.M.; Zaki, M.S.; Yousef, R.A.; Fawzi, O.M. Toxicity, Mutagenicity and Carcinogenicity of Phenols and Phenolic Compounds on Human and Living Organisms [A Review]. Adv. Environ. Biol. 2015, 9, 38–49. [Google Scholar]
- Pan, G.; Kurumada, K.I. Hybrid Gel Reinforced with Coating Layer for Removal of Phenol from Aqueous Solution. Chem. Eng. J. 2008, 138, 194–199. [Google Scholar] [CrossRef]
- Foszpańczyk, M.; Drozdek, E.; Gmurek, M.; Ledakowicz, S. Toxicity of Aqueous Mixture of Phenol and Chlorophenols Upon Photosensitized Oxidation Initiated by Sunlight or Vis-Lamp. Environ. Sci. Pollut. Res. 2018, 25, 34968–34975. [Google Scholar] [CrossRef]
- Hansch, C.; McKarns, S.C.; Smith, C.J.; Doolittle, D.J. Comparative QSAR Evidence for a Free-Radical Mechanism of Phenol-Induced Toxicity. Chem. Biol. Interact. 2000, 127, 61–72. [Google Scholar] [CrossRef]
- Oluwasanu, A.A. Fate and Toxicity of Chlorinated Phenols of Environmental Implications: A Review. Med. Anal. Chem. Int. J. 2018, 2, 000126. [Google Scholar] [CrossRef]
- Clayton, G.D.; Clayton, F.E. Patty’s Industrial Hygiene and Toxicology; John Wiley & Sons: Chichester, UK, 1981. [Google Scholar]
- Koriem, K.M.M.; Soliman, R.E. Chlorogenic and Caftaric Acids in Liver Toxicity and Oxidative Stress Induced by Methamphetamine. J. Toxicol. 2014, 2014, 1–10. [Google Scholar] [CrossRef]
- ATSDR. Medical Management Guidelined for Phenol; ATDSR: Atlanta, GA, USA, 2014.
- Kamijo, Y.; Soma, K.; Fukuda, M.; Asari, Y.; Ohwada, T. Rabbit Syndrome Following Phenol Ingestion. J. Toxicol. Clin. Toxicol. 1999, 37, 509–511. [Google Scholar] [CrossRef]
- ATSDR. Toxicological Profile for Phenol; ATDSR: Atlanta, GA, USA, 2008.
- Eisler, R. Arsenic Hazards to Fish, Wildlife, and Invertebrates: A Synoptic Review; Fish and Wildlife Service, US Department of the Interior: Washington, DC, USA, 1988.
- Michałowicz, J.; Duda, W. Phenols—Sources and Toxicity. Pol. J. Environ. Stud. 2007, 16, 347–362. [Google Scholar]
- Alvarez-Torrellas, S.; Martin-Martinez, M.; Gomes, H.T.; Ovejero, G.; García, J. Enhancement of p-nitrophenol Adsorption Capacity through N2 -thermal-based Treatment of Activated Carbons. Appl. Surf. Sci. 2017, 414, 424–434. [Google Scholar] [CrossRef]
- McDonald, T.A.; Holland, N.T.; Skibola, C.; Duramad, P.; Smith, M.T. Hypothesis: Phenol and Hydroquinone Derived Mainly from Diet and Gastrointestinal Flora Activity are Causal Factors in Leukemia. Leukemia 2001, 15, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Medinsky, M.A.; Kenyon, E.M.; Seaton, M.J.; Schlosser, P.M. Mechanistic Considerations in Benzene Physiological Model Development. Environ. Health Perspect. 1996, 104, 1399–1404. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer. Some Organic Solvents, Resin Monomers, and Related Compounds, Pigments, and Occupational Exposures in Paint Manufacture and Painting. IARC Monogr. Eval. Carcinog. Risks Hum. 1989, 47, 442. [Google Scholar]
- Villegas, L.G.C.; Mashhadi, N.; Chen, M.; Mukherjee, D.; Taylor, K.E.; Biswas, N. A Short Review of Techniques for Phenol Removal from Wastewater. Curr. Pollut. Rep. 2016, 2, 157–167. [Google Scholar] [CrossRef]
- Saha, N.C.; Bhunia, F.; Kaviraj, A. Toxicity of Phenol to Fish and Aquatic Ecosystems. Bull. Environ. Contam. Toxicol. 1999, 63, 195–202. [Google Scholar] [CrossRef]
- Ponepal, M.C.; Păunescu, A. Effect of Phenol Intoxication on Some Physiological Parameters of Perca fluviatilis and Pelophylax ridibundus. Curr. Trends Nat. Sci. 2014, 3, 82–87. [Google Scholar]
- Ferrando, M.D.; Andreu-Moliner, E. Effect of Lindane on the Blood of a Freshwater Fish. Bull. Environ. Contam. Toxicol. 1991, 47, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Babich, H.; Davis, D.L. Phenol: A Review of Environmental and Health Risks. Regul. Toxicol. Pharmacol. 1981, 1, 90–109. [Google Scholar] [CrossRef]
- Balakirski, G.; Krabbe, J.; Schettgen, T.; Rieg, A.; Schröder, T.; Spillner, J.; Kalverkamp, S.; Braunschweig, T.; Kintsler, S.; Krüger, I.; et al. Low Concentration of Phenol in Medical Solutions can Induce Bronchoconstriction and Toxicity in Murine, Rat and Human Lungs. Eur. Respir. J. 2018, 52, PA1059. [Google Scholar] [CrossRef]
- El-Shahawi, M.S.; Hamza, A.; Bashammakh, A.S.; Al-Saggaf, W.T. An Overview on the Accumulation, Distribution, Transformations, Toxicity and Analytical Methods for the Monitoring of Persistent Organic Pollutants. Talanta 2010, 80, 1587–1597. [Google Scholar] [CrossRef]
- Subrahmanyam, V.V.; Doane-Setzer, P.; Steinmetz, K.L.; Ross, D.; Smith, M.T. Phenol-induced Stimulation of Hydroquinone Bioactivation in Mouse Bone Marrow in vivo: Possible Implications in Benzene Myelotoxicity. Toxicology. 1990, 62, 107–116. [Google Scholar] [CrossRef]
- Schweigert, N.; Zehnder, A.J.B.; Eggen, R.I.L. Chemical Properties of Catechols and Their Molecular Modes of Toxic Action in Cells, from Microorganisms to Mammals. Mini Review. Environ. Microbiol. 2001, 3, 81–91. [Google Scholar] [CrossRef]
- Pryor, W.A.; Stone, K.; Zang, A.L.-Y.; Bermúdez, E. Fractionation of Aqueous Cigarette Tar Extracts: Fractions That Contain the Tar Radical Cause DNA Damage. Chem. Res. Toxicol. 1998, 11, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Goshman, L.M. Clinical Toxicology of Commercial Products, 5th ed. J. Pharm. Sci. 1985, 74, 1139. [Google Scholar] [CrossRef]
- Yang, C.-F.; Lee, C.-M. Enrichment, Isolation, and Characterization of Phenol-degrading Pseudomonas resinovorans strain P-1 and Brevibacillus sp. strain P-6. Int. Biodeterior. Biodegrad. 2007, 59, 206–210. [Google Scholar] [CrossRef]
- Schweigert, N.; Belkin, S.; Leong-Morgenthaler, P.; Zehnder, A.J.B.; Eggen, R.I.L. Combinations of Chlorocatechols and Heavy Metals Cause DNA Degradation in vitro but must not result in Increased Mutation Rates in vivo. Environ. Mol. Mutagen. 1999, 33, 202–210. [Google Scholar] [CrossRef]
- Igbinosa, E.O.; Odjadjare, E.E.; Chigor, V.N.; Igbinosa, I.H.; Emoghene, A.O.; Ekhaise, F.O.; Igiehon, N.O.; Idemudia, O.G. Toxicological Profile of Chlorophenols and Their Derivatives in the Environment: The Public Health Perspective. Sci. World J. 2013, 2013, 1–11. [Google Scholar] [CrossRef]
- Ge, T.; Han, J.; Qi, Y.; Gu, X.; Ma, L.; Zhang, C.; Naeem, S.; Huang, D. The Toxic Effects of Chlorophenols and Associated Mechanisms in Fish. Aquat. Toxicol. 2017, 184, 78–93. [Google Scholar] [CrossRef]
- Pereira, P.; Enguita, F.J.; Ferreira, J.; Leitão, A.L. DNA Damage Induced by Hydroquinone can be Prevented by Fungal Detoxification. Toxicol. Rep. 2014, 1, 1096–1105. [Google Scholar] [CrossRef]
- Atkinson, T.J. A Review of the Role of Benzene Metabolites and Mechanisms in Malignant Transformation: Summative Evidence for a Lack of Research in Nonmyelogenous Cancer types. Int. J. Hyg. Environ. Health 2009, 212, 1–10. [Google Scholar] [CrossRef]
- North, M.; Tandon, V.J.; Thomas, R.; Loguinov, A.; Gerlovina, I.; Hubbard, A.E.; Zhang, L.; Smith, M.T.; Vulpe, C.D. Genome-Wide Functional Profiling Reveals Genes Required for Tolerance to Benzene Metabolites in Yeast. PLoS ONE 2011, 6, e24205. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.T. Advances in Understanding Benzene Health Effects and Susceptibility. Annu. Rev. Public Health 2010, 31, 133–148. [Google Scholar] [CrossRef]
- Muñoz-De-Toro, M.; Markey, C.M.; Wadia, P.R.; Luque, E.H.; Rubin, B.S.; Sonnenschein, C.; Soto, A.M. Perinatal Exposure to Bisphenol-A Alters Peripubertal Mammary Gland Development in Mice. Endocrinology 2005, 146, 4138–4147. [Google Scholar] [CrossRef] [PubMed]
- Salaudeen, T.; Okoh, O.; Agunbiade, F.; Okoh, A. Fate and impact of phthalates in activated sludge treated municipal wastewater on the water bodies in the Eastern Cape, South Africa. Chemosphere 2018, 203, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Vom Saal, F.S.; Hughes, C. An Extensive New Literature Concerning Low-Dose Effects of Bisphenol A Shows the Need for a New Risk Assessment. Environ. Health Perspect. 2005, 113, 926–933. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, A.; Cofini, M.; Rigante, D.; Lucchetti, L.; Cipolla, C.; Penta, L.; Esposito, S. The Effect of Bisphenol A on Puberty: A Critical Review of the Medical Literature. Int. J. Environ. Res. Public Health 2017, 14, 1044. [Google Scholar] [CrossRef]
- Vom Saal, F.S.; Akingbemi, B.T.; Belcher, S.; Birnbaum, L.; Crain, D.A.; Eriksen, M.; Farabollini, F.; Guillette, L.J.; Hauser, R.; Heindel, J.J.; et al. Chapel Hill Bisphenol A Expert Panel Consensus Statement: Integration of Mechanisms, Effects in Animals and Potential to Impact Human Health at Current Levels of Exposure. Reprod. Toxicol. 2007, 24, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Liu, Y.; Chen, S.; Le, X.; Zhou, X.; Zhao, Z.; Ou, Y.; Yang, J. Reversible Immobilization of Laccase onto Metal-ion-chelated Magnetic Microspheres for Bisphenol A Removal. Int. J. Biol. Macromol. 2016, 84, 189–199. [Google Scholar] [CrossRef]
- Hou, J.; Dong, G.; Ye, Y.; Chen, V. Enzymatic Degradation of Bisphenol-A with Immobilized Laccase on TiO2 sol–gel coated PVDF membrane. J. Membr. Sci. 2014, 469, 19–30. [Google Scholar] [CrossRef]
- Garcia-Morales, R.; Rodríiguez-Delgado, M.; Gomez-Mariscal, K.; Orona-Navar, C.; Hernandez-Luna, C.E.H.; Torres, E.; Parra, R.; Cárdenas-Chávez, D.; Mahlknecht, J.; Ornelas-Soto, N. Biotransformation of Endocrine-Disrupting Compounds in Groundwater: Bisphenol A, Nonylphenol, Ethynylestradiol and Triclosan by a Laccase Cocktail from Pycnoporus sanguineus CS43. Water Air Soil Pollut. 2015, 226, 1–14. [Google Scholar] [CrossRef]
- Michalak, I.; Chojnacka, K. Algae as production systems of bioactive compounds. Eng. Life Sci. 2014, 15, 160–176. [Google Scholar] [CrossRef]
- Mironiuk, M.; Chojnacka, K. The Environmental Benefits Arising from the Use of Algae Biomass in Industry. In Algae Biomass: Characteristics and Applications; Springer: Cham, Switzerland, 2018; pp. 7–16. [Google Scholar] [CrossRef]
- Anastopoulos, I.; Kyzas, G. Progress in batch biosorption of heavy metals onto algae. J. Mol. Liq. 2015, 209, 77–86. [Google Scholar] [CrossRef]
- Ma, X.; Gao, M.; Gao, Z.; Wang, J.; Zhang, M.; Ma, Y.; Wang, Q. Past, Current, and Future Research on Microalga-derived Biodiesel: A Critical Review and Bibliometric Analysis. Environ. Sci. Pollut. Res. 2018, 25, 10596–10610. [Google Scholar] [CrossRef] [PubMed]
- Nagappan, S.; Kumar Verma, S. Co-production of Biodiesel and alpha-linolenic acid (omega-3 fatty acid) from Microalgae, Desmodesmus sp. MCC34. Energy Sour. Part A Recovery Util. Environ. Eff. 2018, 40, 2933–2940. [Google Scholar] [CrossRef]
- Nagappan, S.; Verma, S.K. Growth Model for Raceway Pond Cultivation of Desmodesmus sp. MCC34 Isolated from a Local Water Body. Eng. Life Sci. 2015, 16, 45–52. [Google Scholar] [CrossRef]
- Vergara, C.; Muñoz, R.; Campos, J.; Seeger, M.; Jeison, D. Influence of Light Intensity on Bacterial Nitrifying Activity in Algal-Bacterial Photobioreactors and Its Implications for Microalgae-based Wastewater Treatment. Int. Biodeterior. Biodegrad. 2016, 114, 116–121. [Google Scholar] [CrossRef]
- Mahdy, A.; Mendez, L.; Ballesteros, M.; González-Fernández, C. Algaculture Integration in Conventional Wastewater Treatment Plants: Anaerobic Digestion Comparison of Primary and Secondary Sludge with Microalgae Biomass. Bioresour. Technol. 2015, 184, 236–244. [Google Scholar] [CrossRef]
- Greenwell, H.C.; Laurens, L.M.L.; Shields, R.J.; Lovitt, R.W.; Flynn, K.J. Placing Microalgae on the Biofuels Priority List: A Review of the Technological Challenges. J. R. Soc. Interf. 2009, 7, 703–726. [Google Scholar] [CrossRef]
- Guldhe, A.; Kumari, S.; Ramanna, L.; Ramsundar, P.; Singh, P.; Rawat, I.; Bux, F. Prospects, Recent Advancements and Challenges of Different Wastewater Streams for Microalgal Cultivation. J. Environ. Manag. 2017, 203, 299–315. [Google Scholar] [CrossRef] [PubMed]
- Grandclément, C.; Seyssiecq, I.; Piram, A.; Wong-Wah-Chung, P.; Vanot, G.; Tiliacos, N.; Roche, N.; Doumenq, P. From the Conventional Biological Wastewater Treatment to Hybrid Processes, the Evaluation of Organic Micropollutant Removal: A Review. Water Res. 2017, 111, 297–317. [Google Scholar] [CrossRef] [PubMed]
- Roostaei, J.; Zhang, Y. Spatially Explicit Life Cycle Assessment: Opportunities and Challenges of Wastewater-based Algal BioFuels in the United States. Algal Res. 2017, 24, 395–402. [Google Scholar] [CrossRef]
- Harun, R.; Singh, M.; Forde, G.M.; Danquah, M.K. Bioprocess Engineering of Microalgae to Produce a Variety of Consumer Products. Renew. Sustain. Energy Rev. 2010, 14, 1037–1047. [Google Scholar] [CrossRef]
- Peng, F.-Q.; Ying, G.-G.; Yang, B.; Liu, S.; Lai, H.-J.; Liu, Y.-S.; Chen, Z.-F.; Zhou, G.J. Biotransformation of Progesterone and Norgestrel by Two Freshwater Microalgae (Scenedesmus obliquus and Chlorella pyrenoidosa): Transformation Kinetics and Products Identification. Chemosphere 2014, 95, 581–588. [Google Scholar] [CrossRef]
- El-Naas, M.H.; Al-Rub, F.A.; Ashour, I.; Al Marzouqi, M. Effect of Competitive Interference on the Biosorption of Lead(II) by Chlorella vulgaris. Chem. Eng. Process. Process. Intensif. 2007, 46, 1391–1399. [Google Scholar] [CrossRef]
- Sibi, G. Biosorption of Chromium from Electroplating and Galvanizing Industrial Effluents under Extreme Conditions using Chlorella vulgaris. Green Energy Environ. 2016, 1, 172–177. [Google Scholar] [CrossRef]
- De Andrade, C.J.; De Andrade, L.M. Microalgae for Bioremediation of Textile Wastewater: An Overview. MOJ Food Process. Technol. 2018, 6, 1. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Pramanik, S.K.; Gehlot, P.S.; Patel, H.; Gajaria, T.; Mishra, S.; Kumar, A. Process for Preparing Value-Added Products from Microalgae Using Textile Effluent through a Biorefinery Approach. ACS Sustain. Chem. Eng. 2017, 5, 10019–10028. [Google Scholar] [CrossRef]
- Cheng, D.L.; Ngo, H.H.; Guo, W.S.; Chang, S.W.; Nguyen, D.D.; Kumar, S.M. Microalgae Biomass from Swine Wastewater and its Conversion to Bioenergy. Bioresour. Technol. 2018, 275, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Bhola, V.; Swalaha, F.; Kumar, R.R.; Singh, M.; Bux, F. Overview of the Potential of Microalgae for CO2 Sequestration. Int. J. Environ. Sci. Technol. 2014, 11, 2103–2118. [Google Scholar] [CrossRef]
- Chen, G.; Zhao, L.; Qi, Y. Enhancing the Productivity of Microalgae Cultivated in Wastewater toward Biofuel Production: A Critical Review. Appl. Energy 2015, 137, 282–291. [Google Scholar] [CrossRef]
- Singh, V.; Tiwari, A.; Das, M. Phyco-remediation of Industrial Waste-water and Flue Gases with Algal-diesel Engenderment from Micro-algae: A review. Fuel 2016, 173, 90–97. [Google Scholar] [CrossRef]
- Thawechai, T.; Cheirsilp, B.; Louhasakul, Y.; Boonsawang, P.; Prasertsan, P. Mitigation of Carbon Dioxide by Oleaginous Microalgae for Lipids and Pigments Production: Effect of Light Illumination and Carbon Dioxide Feeding Strategies. Bioresour. Technol. 2016, 219, 139–149. [Google Scholar] [CrossRef]
- Hemalatha, M.; Venkata Mohan, S. Microalgae Cultivation as Tertiary Unit Operation for Treatment of Pharmaceutical Wastewater Associated with Lipid Production. Bioresour. Technol. 2016, 215, 117–122. [Google Scholar] [CrossRef]
- Kumar, D.; Singh, B. Algal Biorefinery: An Integrated Approach for Sustainable Biodiesel Production. Biomass Bioenerg. 2019, 131, 105398. [Google Scholar] [CrossRef]
- Ruiz, J.; Olivieri, G.; de Vree, J.; Bosma, R.; Willems, P.; Reith, J.H.; Eppink, M.H.M.; Kleinegris, D.M.M.; Wijffels, R.H.; Barbosa, M.J. Towards Industrial Products from Microalgae. Energy Environ. Sci. 2016, 9, 3036–3043. [Google Scholar] [CrossRef]
- Pugazhendhi, A.; Nagappan, S.; Bhosale, R.R.; Tsai, P.-C.; Natarajan, S.; Devendran, S.; Al-Haj, L.; Ponnusamy, V.K.; Kumar, G. Various Potential Techniques to Reduce the Water Footprint of Microalgal Biomass Production for Biofuel—A Review. Sci. Total. Environ. 2020, 749, 142218. [Google Scholar] [CrossRef]
- Michalak, I.; Chojnacka, K. Introduction: Toward Algae-Based Products. In Algae Biomass: Characteristics and Applications; Springer: Cham, Switzerland, 2018; pp. 1–5. [Google Scholar] [CrossRef]
- Banerjee, S.; Rout, S.; Banerjee, S.; Atta, A.; Das, D. Fe2O3 Nanocatalyst Aided Transesterification for Biodiesel Production from Lipid-intact Wet Microalgal Biomass: A Biorefinery Approach. Energy Convers. Manag. 2019, 195, 844–853. [Google Scholar] [CrossRef]
- Zhan, J.; Rong, J.; Wang, Q. Mixotrophic Cultivation, A Preferable Microalgae Cultivation Mode for Biomass/bioenergy Production, and Bioremediation, Advances and Prospect. Int. J. Hydrogen Energy 2017, 42, 8505–8517. [Google Scholar] [CrossRef]
- Lowrey, J.; Brooks, M.S.; McGinn, P.J. Heterotrophic and Mixotrophic Cultivation of Microalgae for Biodiesel Production in Agricultural Wastewaters and Associated Challenges—A Critical Review. Environ. Boil. Fishes 2014, 27, 1485–1498. [Google Scholar] [CrossRef]
- Razzak, S.; Hossain, M.M.; Lucky, R.A.; Bassi, A.S.; de Lasa, H. Integrated CO2 Capture, Wastewater Treatment and Biofuel Production by Microalgae Culturing—A Review. Renew. Sustain. Energy Rev. 2013, 27, 622–653. [Google Scholar] [CrossRef]
- Cuellar-Bermudez, S.P.; Garcia-Perez, J.S.; Rittmann, B.E.; Parra-Saldivar, R. Photosynthetic Bioenergy Utilizing CO2: An Approach on Flue Gases Utilization for Third Generation Biofuels. J. Clean. Prod. 2015, 98, 53–65. [Google Scholar] [CrossRef]
- Klekner, V.; Kosaric, N. Degradation of Phenolic Mixtures by Chlorella. Environ. Technol. 1992, 13, 503–506. [Google Scholar] [CrossRef]
- Kahru, A.; Maloverjan, A.; Sillak, H.; Põllumaa, L. The Toxicity and Fate of Phenolic Pollutants in the Contaminated Soils Associated with the Oil-shale Industry. Environ. Sci. Pollut. Res. 2002, 9, 27–33. [Google Scholar] [CrossRef]
- Tikoo, V.; Scragg, A.H.; Shales, S.W. Degradation of Pentachlorophenol by Microalgae. J. Chem. Technol. Biotechnol. 1997, 68, 425–431. [Google Scholar] [CrossRef]
- Semple, K.T.; Cain, R.B. Biodegradation of Phenols by the Alga Ochromonas danica. Appl. Environ. Microbiol. 1996, 62, 1265–1273. [Google Scholar] [CrossRef] [PubMed]
- Lutzu, G.A.; Zhang, W.; Liu, T. Feasibility of using Brewery Wastewater for Biodiesel Production and Nutrient Removal by Scenedesmus dimorphus. Environ. Technol. 2015, 37, 1568–1581. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Ye, Q.; Xu, J.; Yang, Z.; Zhou, J.; Cen, K. Improving Pollutants Removal by Microalgae Chlorella PY-ZU1 with 15% CO2 from Undiluted Anaerobic Digestion Effluent of Food Wastes with Ozonation Pretreatment. Bioresour. Technol. 2016, 216, 273–279. [Google Scholar] [CrossRef]
- Hirooka, T.; Akiyama, Y.; Tsuji, N.; Nakamura, T.; Nagase, H.; Hirata, K.; Miyamoto, K. Removal of Hazardous Phenols by Microalgae Under Photoautotrophic Conditions. J. Biosci. Bioeng. 2003, 95, 200–203. [Google Scholar] [CrossRef]
- Klekner, V.; Kosaric, N. Degradation of Phenols by Algae. Environ. Technol. 1992, 13, 493–501. [Google Scholar] [CrossRef]
- Tsuji, N.; Hirooka, T.; Nagase, H.; Hirata, K.; Miyamoto, K. Photosynthesis-dependent Removal of 2,4-dichlorophenol by Chlorella fusca var. vacuolata. Biotechnol. Lett. 2003, 25, 241–244. [Google Scholar] [CrossRef] [PubMed]
- Lima, S.A.C.; Raposo, M.F.J.; Castro, P.M.L.; Morais, R.M. Biodegradation of p-chlorophenol by a Microalgae Consortium. Water Res. 2004, 38, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.T.; Wong, Y.S.; Tam, N.F.Y. Removal and Biodegradation of Nonylphenol by Different Chlorella species. Mar. Pollut. Bull. 2011, 63, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Pinto, G.; Pollio, A.; Previtera, L.; Temussi, F. Biodegradation of Phenols by Microalgae. Biotechnol. Lett. 2002, 24, 2047–2051. [Google Scholar] [CrossRef]
- Nazos, T.T.; Mavroudakis, L.; Pergantis, S.A.; Ghanotakis, D.F. Biodegradation of Phenol by Chlamydomonas reinhardtii. Photosynth. Res. 2020, 144, 383–395. [Google Scholar] [CrossRef]
- Pinto, G.; Pollio, A.; Previtera, L.; Stanzione, M.; Temussi, F. Removal of Low Molecular Weight Phenols from Olive Oil Mill Wastewater using Microalgae. Biotechnol. Lett. 2003, 25, 1657–1659. [Google Scholar] [CrossRef]
- Das, B.; Mandal, T.K.; Patra, S. A Comprehensive Study on Chlorella pyrenoidosa for Phenol Degradation and its Potential Applicability as Biodiesel Feedstock and Animal Feed. Appl. Biochem. Biotechnol. 2015, 176, 1382–1401. [Google Scholar] [CrossRef] [PubMed]
- Baldiris-Navarro, I.; Sanchez-Aponte, J.; Gonzáalez-Delgado, A.; Realpe Jimeénez, A.R.; Acevedo-Morantes, M. Removal and Biodegradation of Phenol by the Freshwater Microalgae Chlorella vulgaris. Contemp. Eng. Sci. 2018, 11, 1961–1970. [Google Scholar] [CrossRef]
- Yi, T.; Shan, Y.; Huang, B.; Tang, T.; Wei, W.; Quinn, N.W. An Efficient Chlorella sp.-Cupriavidus necator Microcosm for Phenol Degradation and its Cooperation Mechanism. Sci. Total Environ. 2020, 743, 140775. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Meng, F.; Li, H.; Zhao, S.; Liu, Q.; Lin, Y.; Wang, G.; Wu, J. Biodegradation of Phenol by Isochrysis galbana Screened from Eight Species of Marine Microalgae: Growth Kinetic Models, Enzyme Analysis and Biodegradation Pathway. Environ. Boil. Fishes 2018, 31, 445–455. [Google Scholar] [CrossRef]
- Shashirekha, S.; Uma, L.; Subramanian, G. Phenol Degradation by the Marine Cyanobacterium Phormidium valderianum BDU 30501. J. Ind. Microbiol. Biotechnol. 1997, 19, 130–133. [Google Scholar] [CrossRef]
- Başaran Kankılıç, G.; Metin, A.; Aluç, Y. Investigation on Phenol Degradation Capability of Scenedesmus regularis: Influence of Process Parameters. Environ. Technol. 2018, 41, 1065–1073. [Google Scholar] [CrossRef]
- Wurster, M.; Mundt, S.; Hammer, E.; Schauer, F.; Lindequist, U. Extracellular Degradation of Phenol by the Cyanobacterium Synechococcus PCC 7002. Environ. Boil. Fishes. 2003, 15, 171–176. [Google Scholar] [CrossRef]
- Cheng, T.; Zhang, W.; Zhang, W.; Yuan, G.; Wang, H.; Liu, T. An Oleaginous Filamentous Microalgae Tribonema minus Exhibits High Removing Potential of Industrial Phenol Contaminants. Bioresour. Technol. 2017, 238, 749–754. [Google Scholar] [CrossRef] [PubMed]
- Hirooka, T.; Nagase, H.; Uchida, K.; Hiroshige, Y.; Ehara, Y.; Nishikawa, J.-I.; Nishihara, T.; Miyamoto, K.; Hirata, Z. Biodegradation of Bisphenol A and Disappearance of Its Estrogenic Activity by the Green Alga Chlorella Fusca Var. Vacuolata. Environ. Toxicol. Chem. 2005, 24, 1896–1901. [Google Scholar] [CrossRef]
- Ji, M.-K.; Kabra, A.N.; Choi, J.; Hwang, J.-H.; Kim, J.R.; Abou-Shanab, R.A.; Oh, Y.-K.; Jeon, B.-H. Biodegradation of Bisphenol A by the Freshwater Microalgae Chlamydomonas mexicana and Chlorella vulgaris. Ecol. Eng. 2014, 73, 260–269. [Google Scholar] [CrossRef]
- Gattullo, C.E.; Bährs, H.; Steinberg, C.E.; Loffredo, E. Removal of Bisphenol A by the Freshwater Green Alga Monoraphidium braunii and the Role of Natural Organic Matter. Sci. Total Environ. 2012, 416, 501–506. [Google Scholar] [CrossRef]
- Li, R.; Chen, G.-Z.; Tam, N.F.Y.; Luan, T.-G.; Shin, P.K.; Cheung, S.G.; Liu, Y. Toxicity of Bisphenol A and its Bioaccumulation and Removal by a Marine Microalga Stephanodiscus hantzschii. Ecotoxicol. Environ. Saf. 2009, 72, 321–328. [Google Scholar] [CrossRef]
- He, N.; Sun, X.; Zhong, Y.; Sun, K.; Liu, W.; Duan, S. Removal and Biodegradation of Nonylphenol by Four Freshwater Microalgae. Int. J. Environ. Res. Public Health 2016, 13, 1239. [Google Scholar] [CrossRef]
- Wang, L.; Xiao, H.; He, N.; Sun, D.; Duan, S. Biosorption and Biodegradation of the Environmental Hormone Nonylphenol by Four Marine Microalgae. Sci. Rep. 2019, 9, 5277. [Google Scholar] [CrossRef]
- Tiwari, B.; Sellamuthu, B.; Ouarda, Y.; Drogui, P.; Tyagi, R.D.; Buelna, G. Review on Fate and Mechanism of Removal of Pharmaceutical Pollutants from Wastewater using Biological Approach. Bioresour. Technol. 2017, 224, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, D.L.; Ralph, P.J. Microalgal Bioremediation of Emerging Contaminants—Opportunities and Challenges. Water Res. 2019, 164, 114921. [Google Scholar] [CrossRef] [PubMed]
- Poirier, S.; Bize, A.; Bureau, C.; Bouchez, T.; Chapleur, O. Community Shifts Within Anaerobic Digestion Microbiota Facing Phenol Inhibition: Towards Early Warning Microbial Indicators? Water Res. 2016, 100, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Chapleur, O.; Madigou, C.; Civade, R.; Rodolphe, Y.; Mazéas, L.; Bouchez, T. Increasing Concentrations of Phenol Progressively Affect Anaerobic Digestion of Cellulose and Associated Microbial Communities. Biogeochemistry 2015, 27, 15–27. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, S.; Kumar, S. Biodegradation Kinetics of Phenol and Catechol using Pseudomonas putida MTCC 1194. Biochem. Eng. J. 2005, 22, 151–159. [Google Scholar] [CrossRef]
- Priyadharshini, S.D.; Bakthavatsalam, A. Optimization of Phenol Degradation by the Microalga Chlorella pyrenoidosa using Plackett–Burman Design and Response Surface Methodology. Bioresour. Technol. 2016, 207, 150–156. [Google Scholar] [CrossRef]
- Nazos, T.T.; Kokarakis, E.J.; Ghanotakis, D.F. Metabolism of Xenobiotics by Chlamydomonas reinhardtii: Phenol Degradation under Conditions Affecting Photosynthesis. Photosynth. Res. 2016, 131, 31–40. [Google Scholar] [CrossRef]
- Megharaj, M.; Pearson, H.W.; Venkateswarlu, K. Effects of Phenolic Compounds on Growth and Metabolic Activities of Chlorella vulgaris and Scenedesmus bijugatus Isolated from Soil. Plant Soil. 1992, 140, 25–34. [Google Scholar] [CrossRef]
- Lika, K.; Papadakis, I. Modeling the Biodegradation of Phenolic Compounds by Microalgae. J. Sea Res. 2009, 62, 135–146. [Google Scholar] [CrossRef]
- Papazi, A.; Kotzabasis, K. Bioenergetic Strategy of Microalgae for the Biodegradation of Phenolic Compounds—Exogenously Supplied Energy and Carbon Sources Adjust the Level of Biodegradation. J. Biotechnol. 2007, 129, 706–716. [Google Scholar] [CrossRef]
- Liu, Y.; Dai, X.; Wei, J. Toxicity of the Xenoestrogen Nonylphenol and its Biodegradation by the Alga Cyclotella caspia. J. Environ. Sci. 2013, 25, 1662–1671. [Google Scholar] [CrossRef]
- Cho, K.; Lee, C.-H.; Ko, K.; Lee, Y.-J.; Kim, K.-N.; Kim, M.-K.; Chung, Y.-H.; Kim, D.; Yeo, I.-K.; Oda, T. Use of Phenol-induced Oxidative Stress Acclimation to Stimulate Cell Growth and Biodiesel Production by the Oceanic Microalga Dunaliella salina. Algal Res. 2016, 17, 61–66. [Google Scholar] [CrossRef]
- Nakai, S.; Inoue, Y.; Hosomi, M. Algal Growth Inhibition Effects and Inducement Modes by Plant-producing Phenols. Water Res. 2001, 35, 1855–1859. [Google Scholar] [CrossRef]
- Di Caprio, F.; Scarponi, P.; Altimari, P.; Iaquaniello, G.; Pagnanelli, F. The Influence of Phenols Extracted from Olive Mill Wastewater on the Heterotrophic and Mixotrophic Growth of Scenedesmus sp. J. Chem. Technol. Biotechnol. 2018, 93, 3619–3626. [Google Scholar] [CrossRef]
- Li, H.; Meng, F.; Wang, Y.; Lin, Y. Removal of Phenol by Isochrysis galbana in Seawater Under Varying Temperature and Light Intensity. J. Oceanol. Limnol. 2019, 38, 773–782. [Google Scholar] [CrossRef]
- Raven, J.A.; Geider, R.J. Temperature and Algal Growth. New Phytol. 1988, 110, 441–461. [Google Scholar] [CrossRef]
- Singh, N.K.; Patel, D.B. Microalgae for Bioremediation of Distillery Effluent; Springer: Dordrecht, The Nederlands, 2012; pp. 83–109. [Google Scholar] [CrossRef]
- Sample, K.T.; Cain, R.B.; Schmidt, S. Biodegradation of Aromatic Compounds by Microalgae. FEMS Microbiol. Lett. 1999, 170, 291–300. [Google Scholar] [CrossRef]
- Xiong, J.-Q.; Kurade, M.; Jeon, B.-H. Can Microalgae Remove Pharmaceutical Contaminants from Water? Trends Biotechnol. 2018, 36, 30–44. [Google Scholar] [CrossRef]
- Basha, K.M.; Rajendran, A.; Thangavelu, V. Recent Advances in the Biodegradation of Phenol: A Review. Asian J. Exp. Biol. Sci. 2010, 1, 219–234. [Google Scholar]
- Neujahr, H.Y.; Lindsjö, S.; Varga, J.M. Oxidation of Phenols by Cells and cell-free enzymes from Candida tropicalis. Antonie van Leeuwenhoek 1974, 40, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.-C.; Tsai, L.-D.; Li, Y.-K. An Isolated Candida albicans TL3 Capable of Degrading Phenol at Large Concentration. Biosci. Biotechnol. Biochem. 2005, 69, 2358–2367. [Google Scholar] [CrossRef]











| Cluster | h-Index | Centrality | Density |
|---|---|---|---|
| Phycoremediation | 2 | 0.50 | 1 |
| Phenol derivatives | 2 | 0.83 | 0.83 |
| Water Pollutant | 9 | 1 | 0.33 |
| Phenolic compound | 1 | 0.14 | 0.43 |
| Hydrocarbon | 1 | 0.29 | 0.57 |
| 2,4-dichlorophenol | 1 | 0.57 | 0.71 |
| Nonylphenol | 1 | 0.43 | 0.14 |
| Cluster | h-Index | Centrality | Density |
|---|---|---|---|
| Biological water treatment | 3 | 0.67 | 1 |
| Microalgae | 3 | 0.83 | 0.67 |
| Algae | 17 | 1 | 0.50 |
| Aliphatic compound | 1 | 0.17 | 0.83 |
| Diatom | 2 | 0.33 | 0.33 |
| Chlorella vulgaris | 2 | 0.50 | 0.17 |
| Cluster | h-Index | Centrality | Density |
|---|---|---|---|
| Algae | 18 | 1 | 1 |
| Water Pollutant | 4 | 0.62 | 0.88 |
| Pollutant removal | 5 | 0.88 | 0.75 |
| Wastewater | 4 | 0.75 | 0.25 |
| Organic compound | 1 | 0.25 | 0.50 |
| Dyes | 1 | 0.12 | 0.62 |
| Scenedesmus | 1 | 0.50 | 0.38 |
| 2-nitrophenol | 1 | 0.38 | 0.12 |
| Cluster | h-Index | Centrality | Density |
|---|---|---|---|
| Phenols | 19 | 1 | 0.89 |
| Nitrophenol | 1 | 0.56 | 1 |
| Biofuel | 6 | 0.67 | 0.44 |
| Wastewater treatment | 11 | 0.89 | 0.67 |
| Organic compound | 5 | 0.78 | 0.33 |
| Chlorella vulgaris | 4 | 0.44 | 0.22 |
| 2,3-dinitrophenol | 1 | 0.22 | 0.56 |
| Catalyst | 1 | 0.33 | 0.11 |
| Azo dye | 1 | 0.11 | 0.78 |
| Member | Weight |
|---|---|
| Phenols | 0.33 |
| Aromatic hydrocarbon | 0.33 |
| Scenedesmus | 0.33 |
| Algae | 0.25 |
| Microalgae | 0.33 |
| Member | Weight |
|---|---|
| Phenolic compound | 0.27 |
| Pollutant removal | 0.67 |
| Mixotroph | 0.33 |
| Phenol derivative | 0.22 |
| Scenedesmus | 0.33 |
| Member | Weight |
|---|---|
| Heavy metal | 0.27 |
| Catalyst | 0.10 |
| Water pollution | 0.36 |
| Nonylphenol | 0.17 |
| Phenol derivative | 0.22 |
| Member | Weight |
|---|---|
| Algae | 0.31 |
| Water pollutant | 0.27 |
| Phenol derivatives | 0.37 |
| Biodegradation | 0.28 |
| Microalgae | 0.23 |
| Keyword | Occurrence | Total Link Strength |
|---|---|---|
| Algae | 116 | 1008 |
| Phenols | 79 | 810 |
| Phenol derivatives | 59 | 705 |
| Biodegradation | 50 | 543 |
| Water pollutant, chemical | 42 | 574 |
| Green alga | 41 | 528 |
| Phenolic compounds | 37 | 447 |
| Biomass | 36 | 367 |
| Microalga | 35 | 436 |
| Chlorophyta | 35 | 443 |
| Pollution removal | 35 | 506 |
| Bioremediation | 34 | 421 |
| Degradation | 33 | 298 |
| Enzyme activity | 33 | 153 |
| Wastewater treatment | 27 | 337 |
| Biodegradation, environment | 25 | 328 |
| Industry/Sources | Compound | Used in Application | References |
|---|---|---|---|
| Agriculture | Phenol and acetone | Production of pesticides, fungicides, and herbicides in such 2,4-dichlorophenoxyacetic acid. | [67] |
| Monoisopropylamine products | Protection of crop and increase yield | ||
| Automotive | Phenolic resins | Manufacture filters, tires, insulation, and coating additives | [68,69] |
| Phenol | Generation of polycarbonate for automotive parts | [70] | |
| Nylon intermediates | Manufacture of thermoplastics and carpeting | ||
| Construction | Phenolic resin | Concrete forming, insulation, beams, moulding compounds | [68] |
| Bisphenol A | Plastic pipes | [71] | |
| Cosmetic | Benzophenone-3 | Sunscreen | [72] |
| Phenol | Used in chemical skin peels, formulation of lip balm | [73] | |
| Household | Phenol, Benzophenone-3 | Manufacture of soaps, paints, toys, lacquers, and perfumes | [71,74] |
| Food and beverage | Bisphenol A | Coating of cans, cups, and polycarbonate container | [71] |
| Pharmaceutical | Phenol | Antiseptic, slimicide, lotion, ointment, mouthwash, oral spray for treating sore throat | [60] |
| Plywood | Pentachlorophenol | As wood preservatives | [75] |
| Textile | Caprolactam and adipic acid (Intermediate of phenol) | Production of synthetic yarn | [76] |
| Compounds | Organism | Effects | Details | References |
|---|---|---|---|---|
| Phenol | Human | Blister and burn on the skin | Coagulation is associated with phenol and amino acid reaction in the keratin of the epidermis and collagen | [60,92] |
| Heart failure | Ingestion of high concentration of phenol (70 ml of 42–52% phenol) | [93,94,95] | ||
| Acute renal failure | Exposure to 40% of phenol in dichloromenthane | [96] | ||
| Necrosis | In contact with phenol solution (concentration of 1%) | [87,93,97,98] | ||
| Dry mouth and throat, dark urine, and diarrhoea | Via ingestion of a high concentration (10–240 mg/L) of phenol | [87,96,99] | ||
| DNA and chromosomal damage in leukaemia inhibit Topoisomerase and clonal selection process | Effect of benzene-related hematotoxicity | [100,101] | ||
| Cause anorexia, weight loss, headache, muscle pain, jaundice | Chronic toxicity due to vaporisation of phenol | [102,103] | ||
| Animal | Increase gill necrosis and mucus production | Interference with respiration | [104] | |
| Asphyxia | [105] | |||
| Destruction of erythrocytes | ||||
| Hypocholesterolaemia | Manifesting uptake of cholesterol in corticosteroidogenesis | [106] | ||
| Modify aquatic biotas such as algae and other microorganisms | A high concentration of phenol is lethal | [107] | ||
| Cause bronchoconstriction and adverse effects in rat | Low phenol concentration (0.1%) causes strong bronchoconstriction | [108] | ||
| Toxicity to bone marrow | Generation of free radical and electrophilic intermediates during peroxidase-dependent oxidation | [109,110] | ||
| Changes in skin, urogenital tracts, lungs and liver | Generated by lipid peroxidation which damages and eventually degrades the membrane of the cell | [87] | ||
| Catechol | Human | Acrylation | Due to the generation of hydrogen peroxide, superoxide, and hydroxyl radicals | [111,112] |
| Destruction of a particular protein in the body | The reaction between catechol with sulphydryl groups of both protein and glutathione | [111] | ||
| Disruption of electron transportation in energy-transducing membranes | Result of the tendency of phenol to oxidise quickly to quinone radical that is more reactive | [111] | ||
| Lead to death | The dose of 50–500 mg/kg of body weight | [87] | ||
| Chlorophenol | Human | Burns of mouth and throat, white necrotic lesion in the mouth, stomach, and oesophagus | Acute poisoning | [113] |
| Vomiting and headache | [114] | |||
| Injury to the digestive tract, liver, kidney, lungs, and skin | [115] | |||
| Hypotension and abdominal pain | Chronic toxicity | [87] | ||
| Suppress immune system | Through drinking of water or eating food containing chlorophenol | [93,98,116] | ||
| Hypothermia, pulse fluctuation, muscle weakness, and seizures | Exposure to concentrated phenol | [113] | ||
| Animal | Disturb organ and endocrine system in aquatic organism | Disruption of free radical metabolism, the immune response factor | [117] | |
| Inhibit cell growth and induce genetic mutation in fish | Low concentration elevates point mutation on the zebrafish genome | |||
| Hydroquinone | Human | Damaging chromosomes | Through the generation of reactive oxygen species (ROS) | [118,119,120,121] |
| Bisphenol A | Human | Alter development of the mammary gland | BPA is an oestrogen compound that can also interfere with androgen activity | [122,123] |
| Delay onset of puberty among girls | Mimicking oestrogen action | [124,125] | ||
| Metabolic disorder and abnormalities among babies | It is linked to a low dosage of BPA and estrogenic activity | [126,127,128] | ||
| Cause breast and prostate gland cancer | [126,127,128] | |||
| Animal | Cause mutation and retardation of the animal reproductive system | Accumulation of BPA in the environment | [129] | |
| 2,4-dimethylphenol | Human | Skin and eye irritation, asthma, anoxia, and eczemas | Due to the initiation of semiquinone and superoxide radicals, which harm the cell’s biomolecule | [102] |
| Compound | Phenol-Degrading Algae | Efficiency | References |
|---|---|---|---|
| Phenol | Ankistrodesmus braunii | Removal of over 70% of phenol from olive oil mill wastewater within 5 days. | [176] |
| Chlorella sp. | Degraded 1000 mg/L of phenol in less than 6 days. There is no rapid degradation observed at higher concentrations (3000 mg/L). | [170] | |
| Degrade 500–700 mg/L phenol within 7 days under continuous illumination. | [53] | ||
| Chlorella pyrenoidosa | Degrade up to 60% of phenol at all concentration. | [32] | |
| Degrade with maximum phenol concentration of 200 mg/L under optimal condition. | [177] | ||
| Chlorella vulgaris | Removed 98% at high phenol concentration (100 mg/L) after 4 days. | [178] | |
| Chlorella sp.-Cupriavidus necator | Could degrade phenol with the maximum concentration of 1200 mg/L within 60 h under optimal condition. | [179] | |
| Isochrysis galbana MACC/H59 | Complete degrade phenol at the concentration of 100 mg/L within 4 days. It also degrades 50 mg/L phenol within 2 days. Lower concentration stimulates growth. The maximum concentration that can be degraded is 200 mg/L. | [180] | |
| Phaeodactylum tricornutum MACC/B114 | Require 8 days to degrade 50 mg/L of phenol and 10 days for 100 mg/L. | [180] | |
| Phormodium valderianum BDU 30501 | They were grown in 50 mg/L of phenol concentration and removal of 38 mg/L within 7 days retention period. Inhibition of the growth occurs at the concentration of 100 mg/L | [181] | |
| Scenedesmus regularis | Remove 40% of phenol. The optimal phenol concentration is 30 mg/L. | [182] | |
| Scenedesmus quadricauda | Resistant to phenol, they degrade low molecular weight phenol found in olive oil mills wastewater through biotransformation. High removal of monophenol (over 50%) in the dark. | [176] | |
| Spirulina maxima | Removed 97.5% of phenol at phenol concentration of 50 mg/L within 24 h. | [17] | |
| Degraded 1000 mg/L of phenol after the adaptation period. | [170] | ||
| Synechococcus PCC 7002 | Degrade phenol concentration of 100 mg/L in 5 to 7 days under a non-photosynthetic condition in the dark. | [183] | |
| Tribonema minus | Highest removal (94.6%) at the concentration of 250 mg/L. | [184] | |
| 2,4-dinitrophenol (2,4-DNP) | Anabaena variabilis NIES 23 | Removed 86% 2,4-dinitrophenol with an initial concentration of 40 µM and cultivated for 72 h. | [169] |
| Chlorella sp. | Degrade 70 mg/L of 2,4-DNP in 20 days. | [170] | |
| Scenedesmus obliquus | Degrade 190 mg/L of 2,4-DNP. | ||
| Bisphenol A (BPA) | Chlorella fusca var vaculota | Able to remove most BPA in the range concentration of 10 to 80 µM for 168 h under continuous illumination. | [185] |
| Chlorella vulgaris | Biodegrade 23% of BPA at the concentration of 1 mg/L BPA. Rapid degradation occurs at this concentration. | [186] | |
| Chlamydomonas mexicana | Degrade 24% of BPA at the concentration of 1 mg/L. Increasing the concentration of BPA caused an increase in carbohydrates levels in the cells due to the stress effect. | ||
| Monoraphidium braunii | Removed 48% of BPA at the concentration of 4 mg/L. The growth inhibited at high concentrations. | [187] | |
| Stephanodiscus hantzschii | Removed 99% of BPA in media supplemented with 0.10 mg/L BPA after 16 days of treatment. The biodegradation activity decreases with increased BPA concentration. The algal growth and biodegradation activity inhibited at higher BPA concentrations. The cell reached the death phase earlier than the control. | [188] | |
| Nonylphenol (NP) | Ankistrodesmus acicularis | Removal rate of 83.77% after 120 h of exposure to different NP concentration (0.5–2.5 mg/L). | [189] |
| Chlorella vulgaris | Degraded over 80% of NP after 168 h. | [173] | |
| Platymonas subcordiformis | Removed 82.34% of NP of its initial concentration after 5 days of culture. | [190] | |
| p-chlorophenol | Chlorella vulgaris and Coenochloris pyrenoidosa (Microalgal consortium) | Remove p-chlorophenol under different light regimes. Able to degrade 50 mg/L of p-chlorophenol under 24 h light regime within 5 days. | [172] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radziff, S.B.M.; Ahmad, S.A.; Shaharuddin, N.A.; Merican, F.; Kok, Y.-Y.; Zulkharnain, A.; Gomez-Fuentes, C.; Wong, C.-Y. Potential Application of Algae in Biodegradation of Phenol: A Review and Bibliometric Study. Plants 2021, 10, 2677. https://doi.org/10.3390/plants10122677
Radziff SBM, Ahmad SA, Shaharuddin NA, Merican F, Kok Y-Y, Zulkharnain A, Gomez-Fuentes C, Wong C-Y. Potential Application of Algae in Biodegradation of Phenol: A Review and Bibliometric Study. Plants. 2021; 10(12):2677. https://doi.org/10.3390/plants10122677
Chicago/Turabian StyleRadziff, Syahirah Batrisyia Mohamed, Siti Aqlima Ahmad, Noor Azmi Shaharuddin, Faradina Merican, Yih-Yih Kok, Azham Zulkharnain, Claudio Gomez-Fuentes, and Chiew-Yen Wong. 2021. "Potential Application of Algae in Biodegradation of Phenol: A Review and Bibliometric Study" Plants 10, no. 12: 2677. https://doi.org/10.3390/plants10122677
APA StyleRadziff, S. B. M., Ahmad, S. A., Shaharuddin, N. A., Merican, F., Kok, Y.-Y., Zulkharnain, A., Gomez-Fuentes, C., & Wong, C.-Y. (2021). Potential Application of Algae in Biodegradation of Phenol: A Review and Bibliometric Study. Plants, 10(12), 2677. https://doi.org/10.3390/plants10122677