Vegetation Analysis and Environmental Relationships of Riverain Plants in the Aswan Reservoir, Egypt
Abstract
:1. Introduction
2. Results
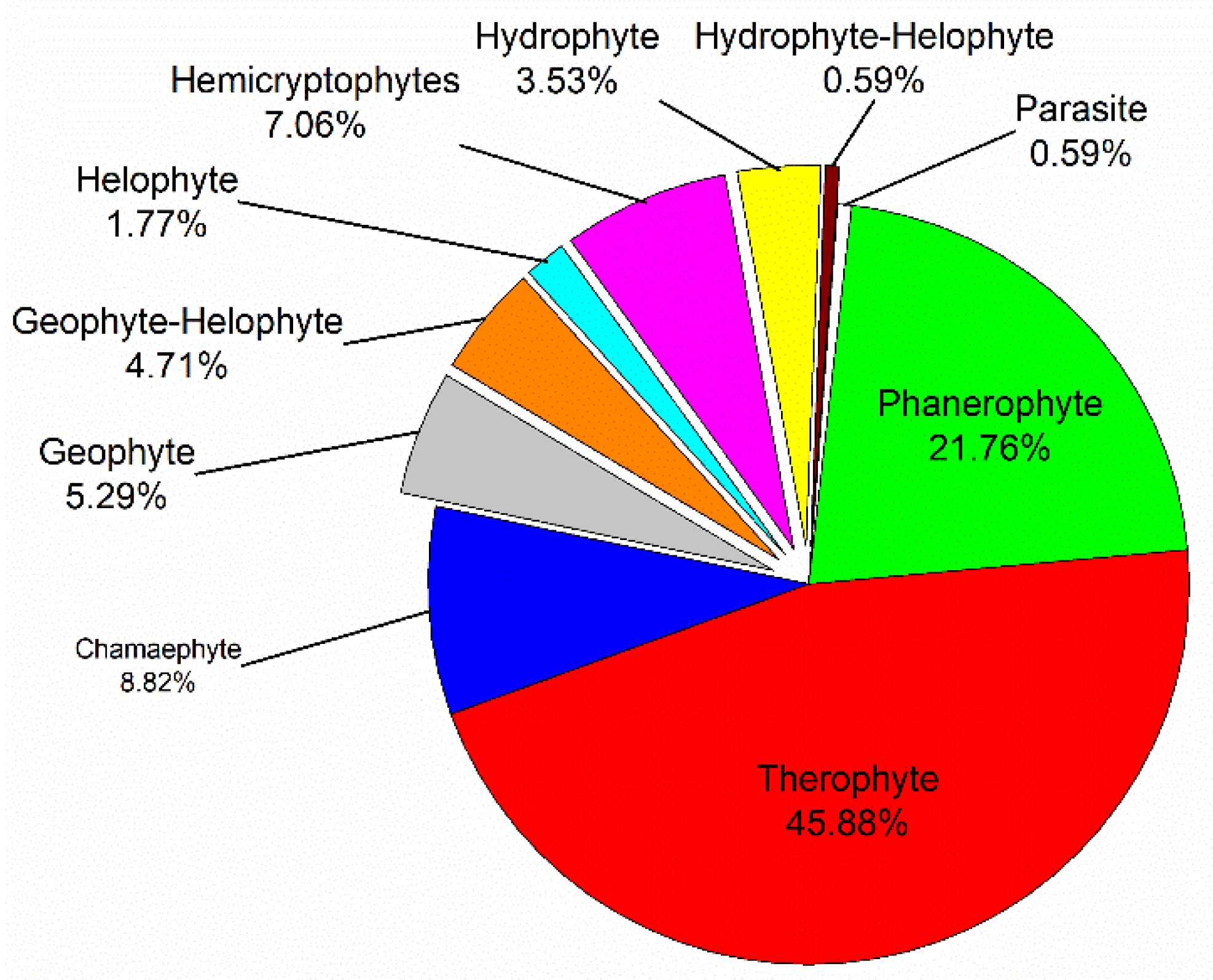
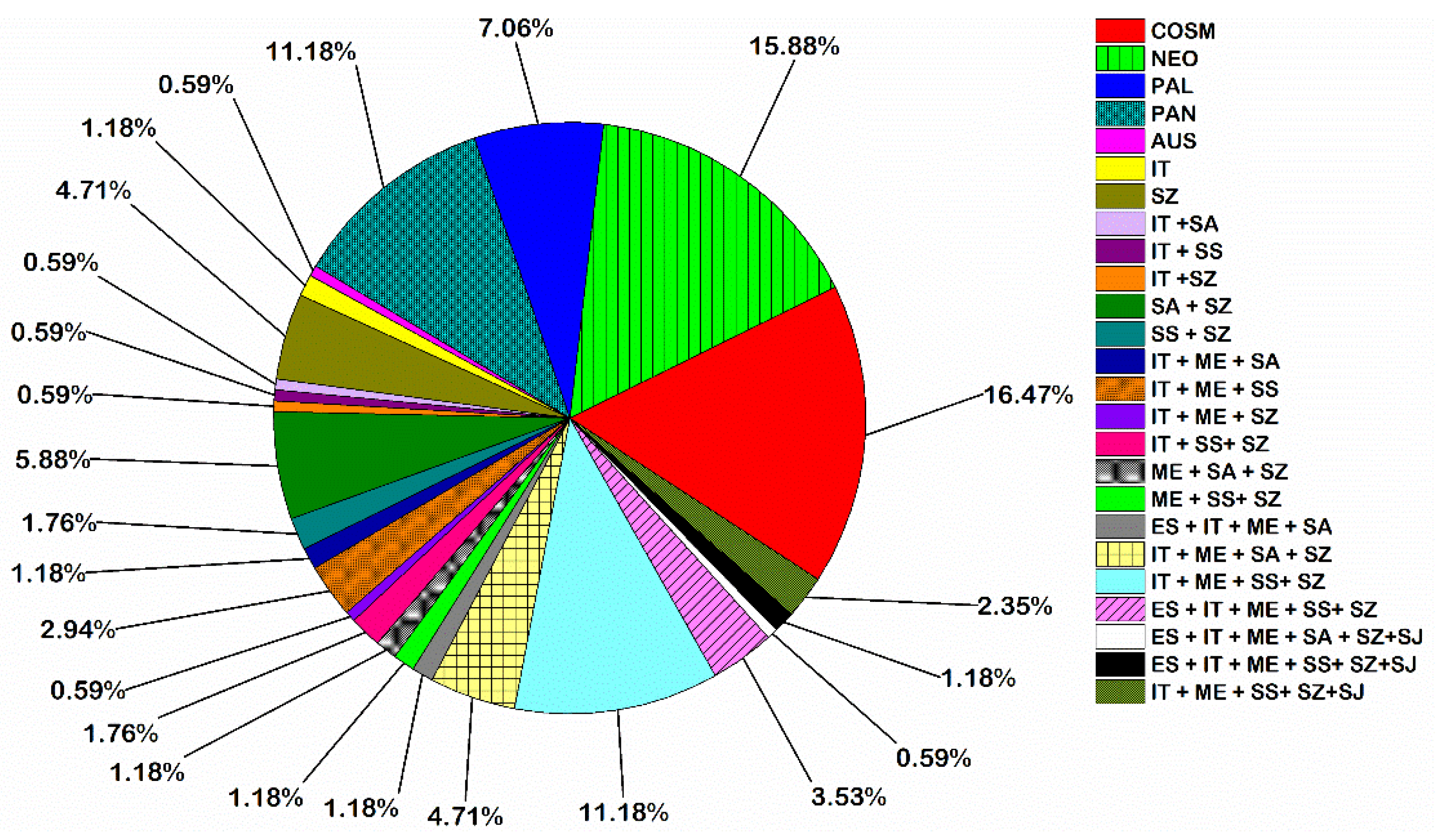
2.1. Floristic Composition
2.2. Distribution of Families among the Surveyed Locations
2.3. Similarity Coefficient between the Investigated Sites in the Aswan Reservoir Area
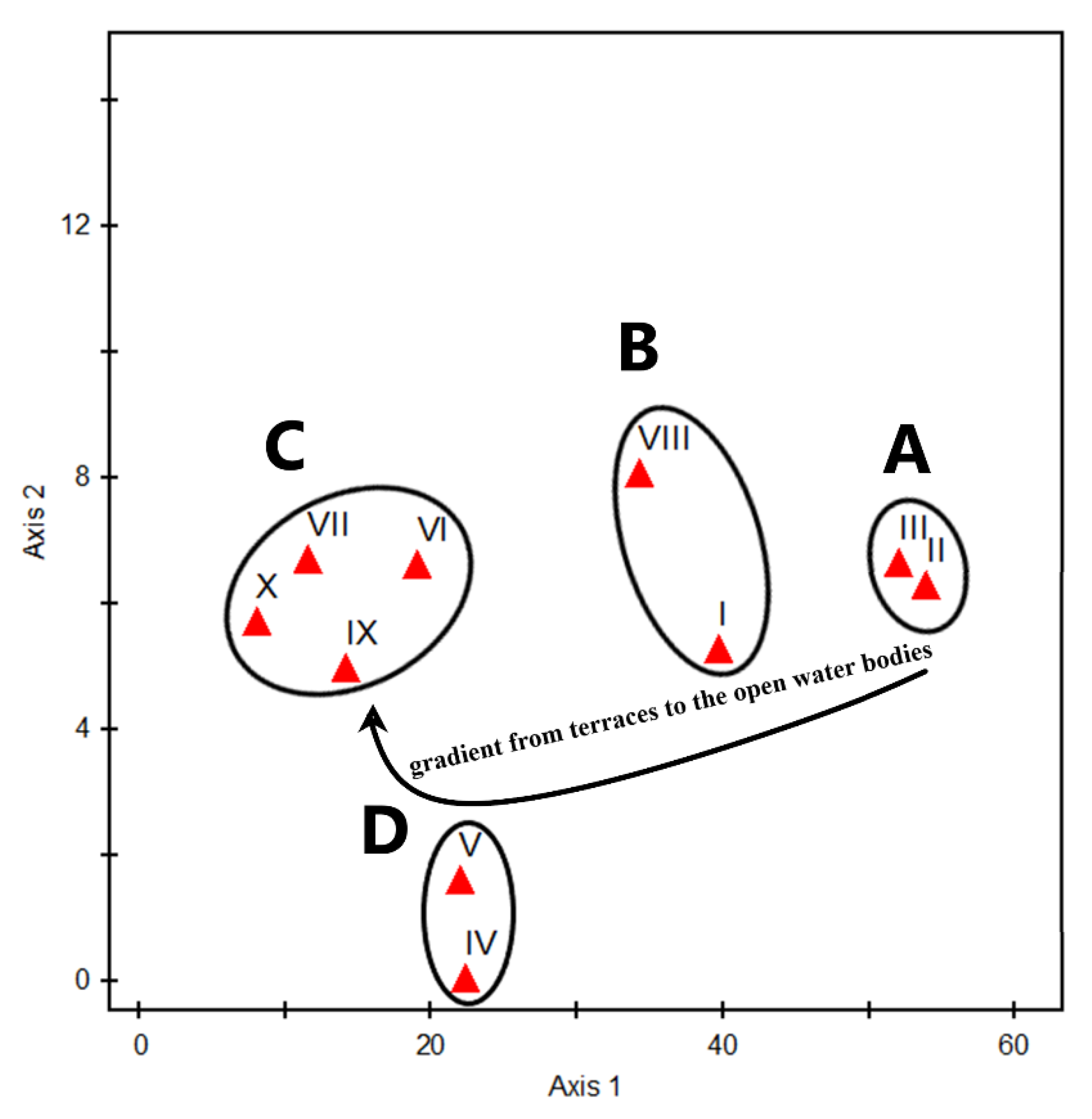
2.4. Vegetation Analysis
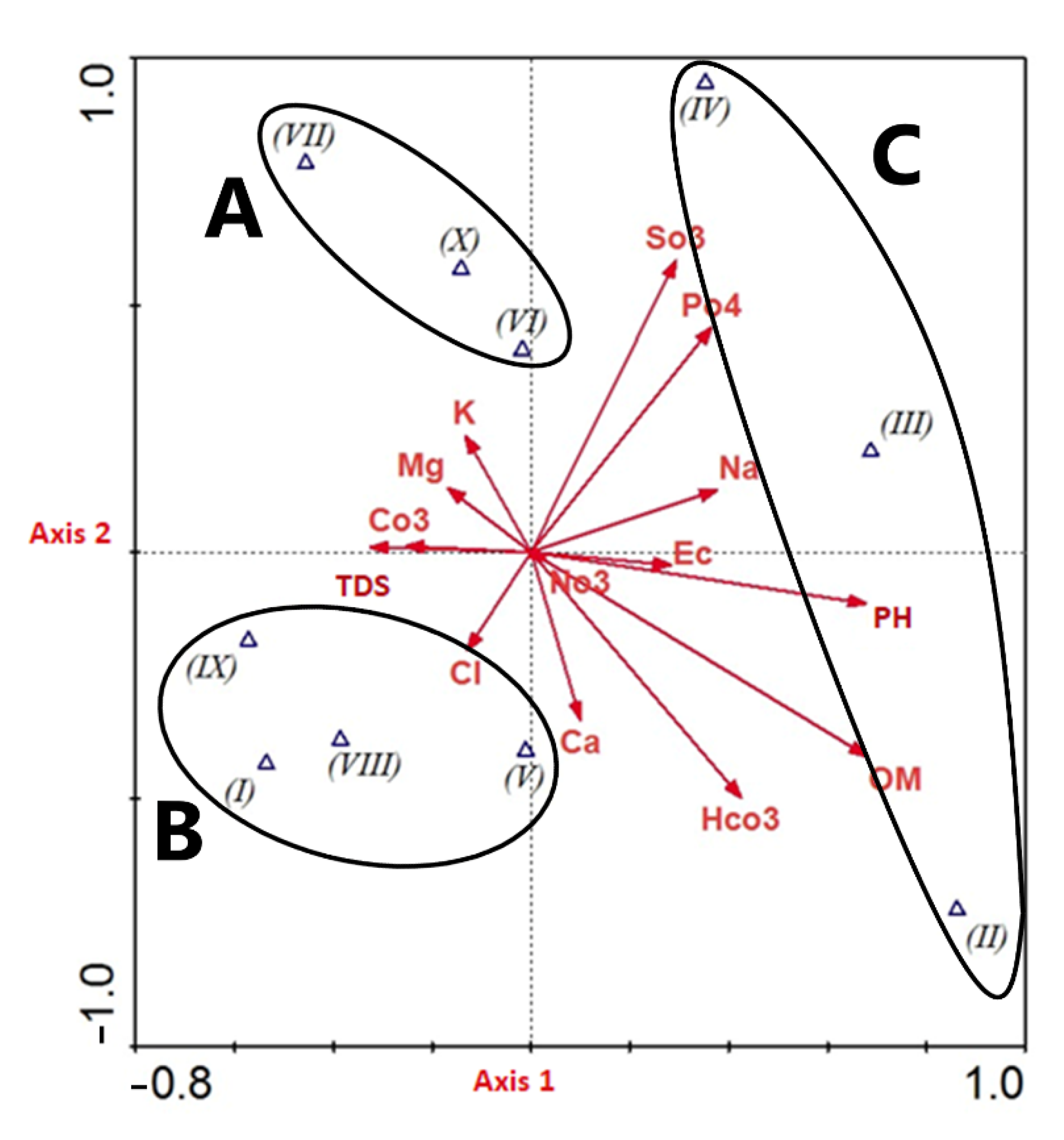
2.5. Plant Community and Soil Correlation
3. Discussion
4. Materials and Methods
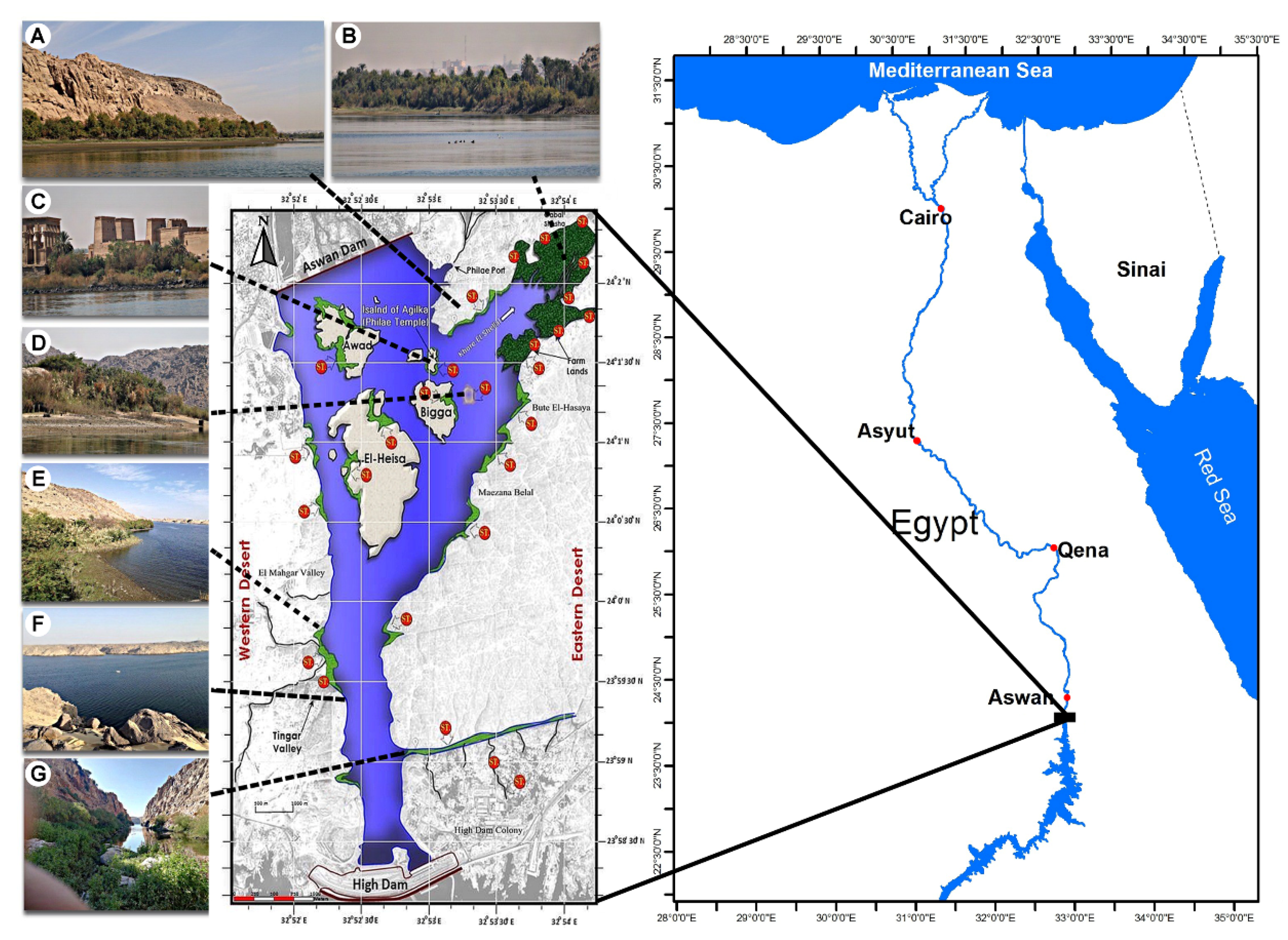
4.1. Study Area
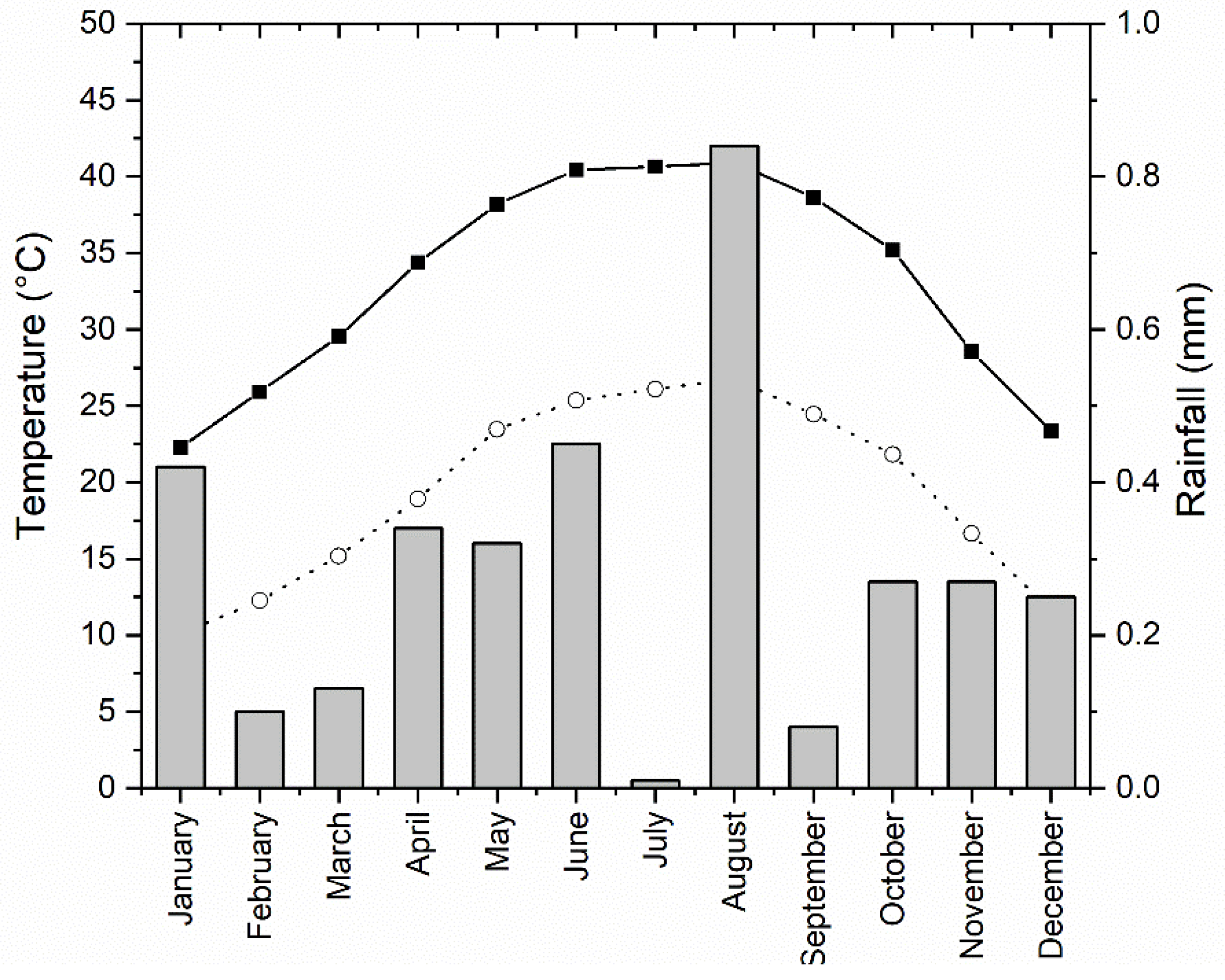
4.2. Water Fluctuation in Aswan Reservoir
4.3. Vegetation Sampling and Species Identification
4.4. Soil Sampling and Analyses
4.5. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cody, B. Undam It Impacts on Riparian Wetlands from the Removal of Small Dams. Ph.D. Thesis, Pennsylvania State University, Centre County, PA, USA, 2007. [Google Scholar]
- Catford, J.A.; Downes, B.J.; Gippel, C.J.; Vesk, P.A. Flow regulation reduces native plant cover and facilitates exotic invasion in riparian wetlands. J. Appl. Ecol. 2011, 48, 432–442. [Google Scholar] [CrossRef]
- Hopkinson, C.S.; Vallino, J.J. The relationships among man’s activities in watersheds and estuaries: A model of runoff effects on patterns of estuarine community metabolism. Estuaries 1995, 18, 598–621. [Google Scholar] [CrossRef]
- Kingsford, R.T.; Thomas, R.F. Destruction of wetlands and waterbird populations by dams and irrigation on the Murrumbidgee River in arid Australia. Environ. Manag. 2004, 34, 383–396. [Google Scholar] [CrossRef]
- Kuemmerlen, M.; Reichert, P.; Siber, R.; Schuwirth, N. Ecological assessment of river networks: From reach to catchment scale. Sci. Total Environ. 2019, 650, 1613–1627. [Google Scholar] [CrossRef]
- van Dam, R.A.; Finlayson, C.M.; Humphrey, C.L. Wetland risk assessment. Tech. Enhanc. Wetl. Invent. Monit. 1999, 147, 38–118. [Google Scholar]
- Shaltout, K.H.; Al-Sodany, Y.M. Vegetation analysis of Burullus Wetland: A RAMSAR site in Egypt. Wetl. Ecol. Manag. 2008, 16, 421–439. [Google Scholar] [CrossRef]
- El-Hadidi, M.N.; Kosinova, J.; El Hadidi, M.N.; Kosinova, J. Studies on the weed flora of cultivated land in Egypt. 1. Preliminary survey. Mitt. Bot. Staatssamml. Münch. 1971, 10, 354–367. [Google Scholar]
- Johnson, W.C. Riparian vegetation diversity along regulated rivers: Contribution of novel and relict habitats. Freshw. Biol. 2002, 47, 749–759. [Google Scholar] [CrossRef]
- Yousaf, A.; Shabbir, R.; Jabeen, A.; Erum, S.; Ahmad, S.S. LInkage between herbaceous vegetation and soil characteristics along rawal dam islamabad. J. Soil Sci. Plant Nutr. 2016, 16, 88–100. [Google Scholar] [CrossRef] [Green Version]
- Ghaemi, M.; Astaraei, A.R.; Emami, H.; Nassiri Mahalati, M.; Sanaeinejad, S.H. Determining soil indicators for soil sustainability assessment using principal component analysis of Astan Quds-east of Mashhad-Iran. J. Soil Sci. Plant Nutr. 2014, 14, 1005–1020. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.-J.; Li, W.-Q.; Yang, Y.-M.; Duan, D.-Y. Studies on the nutrient characteristics of soil and halophyte in coastal saline soil of Hebei Province. Chin. J. Eco-Agric. 2003, 11, 67–77. [Google Scholar]
- Khedr, A.-H.A. Vegetation zonation and management in the Damietta estuary of the River Nile. J. Coast. Conserv. 1998, 4, 79–86. [Google Scholar] [CrossRef]
- Kassas, M. The River Nile ecological system: A study towards an international programme. Biol. Conserv. 1971, 4, 19–25. [Google Scholar] [CrossRef]
- Sadek, N. Island development impacts on the Nile River morphology. Ain Shams Eng. J. 2013, 4, 25–41. [Google Scholar] [CrossRef] [Green Version]
- El-Hadidi, M.N.; Springuel, I. Plant life in Nubia, Egypt. I. Introduction. Plant communities of the Nile islands at Aswan. Taeckholmia 1978, 9, 103–109. [Google Scholar]
- Said, R. The River Nile: Geology, Hydrology and Utilization; Elsevier: Amsterdam, The Netherlands, 2013; p. 337. [Google Scholar]
- Karyabwite, D.R. Water Sharing in the Nile River Valley; UNEP/DEWA/Grid: Geneva, Switzerland, 2000. [Google Scholar]
- Petts, G.E. Long-term consequences of upstream impoundment. Environ. Conserv. 1980, 7, 325–332. [Google Scholar] [CrossRef]
- Bayley, P.B. The flood pulse advantage and the restoration of river-floodplain systems. Regul. Rivers Res. Manag. 1991, 6, 75–86. [Google Scholar] [CrossRef]
- Ligon, F.K.; Dietrich, W.E.; Trush, W.J. Downstream ecological effects of dams. Bioscience 1995, 45, 183–192. [Google Scholar] [CrossRef]
- Nilsson, C.; Berggren, K. Alterations of riparian ecosystems caused by river regulation. Bioscience 2000, 50, 783–792. [Google Scholar] [CrossRef]
- Stanford, J.A.; Ward, J.V.; Liss, W.J.; Frissell, C.A.; Williams, R.N.; Lichatowich, J.A.; Coutant, C.C. A general protocol for restoration of regulated rivers. Regul. Rivers Res. Manag. 1996, 12, 391–413. [Google Scholar] [CrossRef]
- Patten, D.T. Riparian ecosytems of semi-arid North America: Diversity and human impacts. Wetlands 1998, 18, 498–512. [Google Scholar] [CrossRef]
- Friedman, J.M.; Scott, M.L.; Auble, G.T. Water management and cottonwood forest dynamics along prairie streams. In Ecology and Conservation of Great Plains Vertebrates; Springer: Berlin/Heidelberg, Germany, 1997; pp. 49–71. [Google Scholar]
- Johnson, W.C. Woodland expansions in the Platte River, Nebraska: Patterns and causes. Ecol. Monogr. 1994, 64, 45–84. [Google Scholar] [CrossRef]
- Hunter, W.C.; Ohmart, R.D.; Anderson, B.W. Use of exotic saltcedar (Tamarix chinensis) by birds in arid riparian systems. Condor 1988, 90, 113–123. [Google Scholar] [CrossRef]
- Badry, M.O.; Radwan, T.A.A.; Ayed, F.A.A.; Sheded, M.G. Floristic Diversity of Riparian Plants in Aswan Reservoir at the Extreme South of the River Nile, Upper Egypt: A Closed Ecological System. Biosci. Biotechnol. Res. Asia 2019, 16, 595–609. [Google Scholar] [CrossRef]
- Shaheen, A.M. Weed Diversity of Newly Farmed Land on the Southern Border of Egypt (Eastern and Western Shores of Lake Nasser). Pak. J. Biol. Sci. 2002, 5, 802–806. [Google Scholar] [CrossRef] [Green Version]
- Ali, M.M. Aquatic and shoreline vegetation of Lake Nubia, Sudan. Acta Bot. Croat. 2004, 63, 101–111. [Google Scholar]
- El-Ghani, M.M.A.; Fawzy, A.M. Plant diversity around springs and wells in five oases of the western desert, Egypt. Int. J. Agric. Biol. 2006, 8, 249–255. [Google Scholar]
- Boulos, L. Flora of the Nile Region in Egyptian Nubia. Feddes Repert. 1966, 73, 184–215. [Google Scholar] [CrossRef]
- Sheded, M.G.; Hamed, S.T.; Badry, M.O. Vegetation analysis of six riverian islands in hyper-arid environments at Qena Governorate (Upper Egypt). Acta Bot. Hung. 2014, 56, 409–431. [Google Scholar] [CrossRef]
- Amer, W.; Soliman, A.; Hassan, W. Floristic composition of Nile islands in Middle Egypt with special reference to the species migration route. J. Am. Sci. 2015, 11, 14–23. [Google Scholar]
- Springuel, I.V. Studies on the Natural Vegetation of the Islands of the First Cataract at Aswan, Egypt. Ph.D. Thesis, Assiut University, Assiut Governorate, Egypt, 1981. [Google Scholar]
- Shaheen, A.M.; Sheded, M.G.; Hamed, I.; Hamada, F.A. Botanical diversity of the flora of some islands in the Egyptian Nubia. In Proceedings of the First International Conference on Strategy of the Egyptian Herbaria, Giza, Egypt, 9 March 2004; Egyptian Academy of Science and Technology: Cairo Governorate, Egypt, 2004; pp. 161–182. [Google Scholar]
- Ali, A.H. Ecology and Flora of Plants of the Nile Islands in the Area Between Aswan and Esna. Master’s Thesis, Aswan University, Qism Aswan, Egypt, 2014. [Google Scholar]
- Linder, H.P.; Lehmann, C.E.R.; Archibald, S.; Osborne, C.P.; Richardson, D.M. Global grass (Poaceae) success underpinned by traits facilitating colonization, persistence and habitat transformation. Biol. Rev. 2018, 93, 1125–1144. [Google Scholar] [CrossRef]
- Quezel, P. Analysis of the Flora of Mediterranean and Saharan Africa; Missouri Botanical Garden Press: St. Louis, MO, USA, 1978; Volume 65, pp. 479–534. [Google Scholar]
- Kassas, M. Ecological consequences of water development project, Keynote paper. In The Environmental Future 7; Polunin, N., Ed.; Macmillan: London, UK, 1972; pp. 215–246. [Google Scholar]
- Khedr, A.-H.H.; Cadotte, M.W.; El-Keblawy, A.; Lovett-Doust, J. Phylogenetic diversity and ecological features in the Egyptian flora. Biodivers. Conserv. 2002, 11, 1809–1824. [Google Scholar] [CrossRef]
- Boulos, L. Flora of Egypt Checklist—Revised Annotated Edition; Al Hadara Publishing: Cairo, Egypt, 2009; p. 410. [Google Scholar]
- Hassib, M. Distribution of Plant Communities in Egypt. Bull. Fac. of Sci. Fouad Univ. 1951, 29, 59–261. [Google Scholar]
- Mashaly, I.A.; El-Shahaby, O.A.; El-Ameir, Y.A. Floristic features of the canal bank habitats, Egypt. J. Environ. Sci. 2010, 39, 483–501. [Google Scholar]
- Faried, A.; Amro, A. Floristic and community structure of some irrigation and drainage canals in Assiut, Egypt. Taeckholmia 2016, 1, 1–20. [Google Scholar]
- Elkordy, A.; Elshikh, O.; Abdallah, N. Floristic diversity and vegetation analysis of riparian and aquatic plants of the canals in the Sohag Governorate, Egypt. Phytol. Black 2019, 25, 81–95. [Google Scholar]
- El Hadidi, M.N. Natural vegetation. In The Agriculture of Egypt; Craig, G., Ed.; Oxford University Press: Oxford, UK, 1993; pp. 39–62. [Google Scholar]
- Al-Robai, S.A.; Mohamed, H.A.; Howladar, S.M.; Ahmed, A.A. Vegetation structure and species diversity of Wadi Turbah Zahran, Albaha area, southwestern Saudi Arabia. Ann. Agric. Sci. 2017, 62, 61–69. [Google Scholar] [CrossRef]
- AlNafie, A.H. Phytogeography of Saudi Arabia. Saudi J. Biol. Sci. 2008, 15, 159–176. [Google Scholar]
- Alatar, A.; El-Sheikh, M.A.; Thomas, J. Vegetation analysis of Wadi Al-Jufair, a hyper-arid region in Najd, Saudi Arabia. Saudi J. Biol. Sci. 2012, 19, 357–368. [Google Scholar] [CrossRef] [Green Version]
- Ziaja, M.; Wójcik, T.; Wrzesień, M. Phytosociological Data in Assessment of Anthropogenic Changes in Vegetation of Rzeszów Reservoir. Sustainability 2021, 13, 9071. [Google Scholar] [CrossRef]
- Sadany, A. The sadness of the river. Dev. Environ. 1989, 33, 10–12. [Google Scholar]
- Shaltout, K.H.; El-Din, A.S.; El-Sheikh, M.A. Species richness and phenology of vegetation along irrigation canals and drains in the Nile Delta, Egypt. Vegetatio 1994, 112, 35–43. [Google Scholar] [CrossRef]
- Soliman, A.T.; Hamdy, R.S.; Hamed, A.B. Ludwigia Stolonifera (Guill. & Perr.) PH Raven, insight into its phenotypic plasticity, habitat diversity and associated species. Egypt. J. Bot. 2018, 58, 605–626. [Google Scholar]
- El-Amier, Y.A.; Zahran, M.A.; Al-Mamoori, S.O. Environmental changes along Damietta branch of the River Nile, Egypt. J. Environ. Sci. Mansoura Univ. 2015, 44, 235–255. [Google Scholar]
- Abdel Khalik, K.; Al-Gohary, I.; Al-Sodany, Y. Floristic composition and vegetation: Environmental relationships of Wadi Fatimah, Mecca, Saudi Arabia. Arid Land Res. Manag. 2017, 31, 316–334. [Google Scholar] [CrossRef]
- Shaltout, K.H.; El-Halawany, E.F.; El-Garawany, M.M. Coastal lowland vegetation of eastern Saudi Arabia. Biodivers. Conserv. 1997, 6, 1027–1040. [Google Scholar] [CrossRef]
- Sharaf-El-Din, A.; Shaltout, K.H. On the phytosociology of Wadi Araba in the Eastern Desert of Egypt. In Proceedings of the 4th Egyptian Conference of Botany, Ismaileyah, Egypt, 16–19 April 1985; pp. 1311–1325. [Google Scholar]
- Dasti, A.; Agnew, A.D.Q. The vegetation of Cholistan and Thai deserts, Pakistan. J. Arid Environ. 1994, 27, 193–208. [Google Scholar] [CrossRef]
- Traut, B.H. The role of coastal ecotones: A case study of the salt marsh/upland transition zone in California. J. Ecol. 2005, 93, 279–290. [Google Scholar] [CrossRef]
- Ayyad, M.A.; Ghabbour, S.I.; Goodall, D.W. Hot deserts of Egypt and the Sudan. Ecosyst. World 1986, 12, 149–202. [Google Scholar]
- Mueller-Dombois, D.; Ellenberg, H. Aims and Methods of Vegetation Ecology; John Wiley and Sons: New York, NY, USA, 1974; p. 547. [Google Scholar]
- POWO Plants of the World Online. Facilitated by the Royal Botanic Gardens, Kew. Available online: http://powo.science.kew.org/ (accessed on 27 September 2021).
- Raunkiaer, C. The Life Forms of Plants and Statistical Plant Geography; Clarendon Press: Oxford, UK, 1934; p. 632. [Google Scholar]
- Zohary, M. Flora Palaestina: Equisetaceae to Moringaceae; The Israel Academy of Science and Humanities: Jerusalem, Israel, 1966; Volume 1, p. 364. [Google Scholar]
- Wickens, G.E. The Flora of Jebel Marra (Sudan Republic) and Its Geographical Affinities: Kew Bulletin Additional Series V; HMSO: London, UK, 1976; p. 199. [Google Scholar]
- Zohary, M. Flora Palaestina: Platanaceae to Umbelliferae; Israel Academy of Science and Humanities: Jerusalem, Israel, 1972; Volume 2, p. 489. [Google Scholar]
- Thiers, B. Continuously Updated: Index Herbariorum: A global Directory of Public Herbaria and Associated Staff. Available online: http://sweetgum.nybg.org/science/ih/ (accessed on 1 August 2021).
- Kilmer, V.J.; Alexander, L.T. Methods of making mechanical analyses of soils. Soil Sci. 1949, 68, 15–24. [Google Scholar] [CrossRef]
- Sparks, D.L.; Helmke, P.A.; Page, A.L. Methods of Soil Analysis: Chemical Methods; SSSA: Madison, WI, USA, 1996. [Google Scholar]
- Williams, V.; Twine, S. Flame photometric method for sodium, potassium and calcium. Mod. Methods Plant Anal. 1960, 5, 3–5. [Google Scholar]
- Johnson, C.M.; Ulrich, A. Analytical methods for use in plant analysis. In Bulletin of the California Agricultural Experiment Station; California Agriculture Experiment Statistics: Berkeley, CA, USA, 1959. [Google Scholar]
- Allen, S.E. Chemical analysis. In Methods Plant Ecology; Blackwell Scientific: Hoboken, HJ, USA, 1986; pp. 285–344. [Google Scholar]
- Jackson, M.L. Soil Chemical Analysis Prentice; Hall India Private Limited: New Delhi, India, 1967. [Google Scholar]
- Black, C.A.; Evans, D.D.; Dinauer, R.C. Methods of Soil Analysis; American Society of Agronomy: Madison, WI, USA, 1965; Volume 9. [Google Scholar]
- Murphy, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Apha, A.-W. Standard Methods for the Examination of Water; Ignatius Press: San Francisco, CA, USA, 1998; Volume 21, p. 541. [Google Scholar]
- Sørenson, T. A method of establishing groups of equal amplitude in plant sociology based on similarity of species content and its application to analyses of the vegetation on Danish commons. Danske Vidensk. Selsk. Skr. 1948, 5, 1–34. [Google Scholar]
- Team, R.C. R: A Language and Environment for Statistical Computing; The R Foundation: Vienna, Austria, 2013. [Google Scholar]
- Sokal, R.R. A statistical method for evaluating systematic relationships. Kansas Univ. Sci. Bull. 1958, 38, 1409–1438. [Google Scholar]
- Henderson, P.A.; Seaby, R.M.H. Community Analysis Package (CAP) Version 1.2; Pisces Conservation Ltd.: Lymington, UK, 1999. [Google Scholar]
- Shaltout, K.H. On the diversity of the vegetation in the western Mediterranean coastal region of Egypt. In Proceedings of the 4th Egyptian Conference of Botany, Ismaileyah, Egypt, 16–19 April 1985; pp. 1355–1376. [Google Scholar]
- Frosini, B.V. Descriptive Measures of Ecological Diversity; Università cattolica del Sacro Cuore: Milan, Italy, 2003. [Google Scholar]
- Eilertsen, O.; Okland, R.H.; Okland, T.; Pedersen, O. Data manipulation and gradient length estimation in DCA ordination. J. Veg. Sci. 1990, 1, 261–270. [Google Scholar] [CrossRef]
- Ter Braak, C.J.; Smilauer, P. CANOCO Reference Manual and CanoDraw for Windows User’s Guide: Software for Canonical Community Ordination (Version 4.5); Microcomputer Power: Ithaca, NY, USA, 2002; Available online: https://edepot.wur.nl/405659 (accessed on 1 July 2020).
- dosSantos Coura, R.; Nardi, N.B. The state of the art of adeno-associated virus-based vectors in gene therapy. Virol. J. 2007, 4, 99. [Google Scholar] [CrossRef] [Green Version]
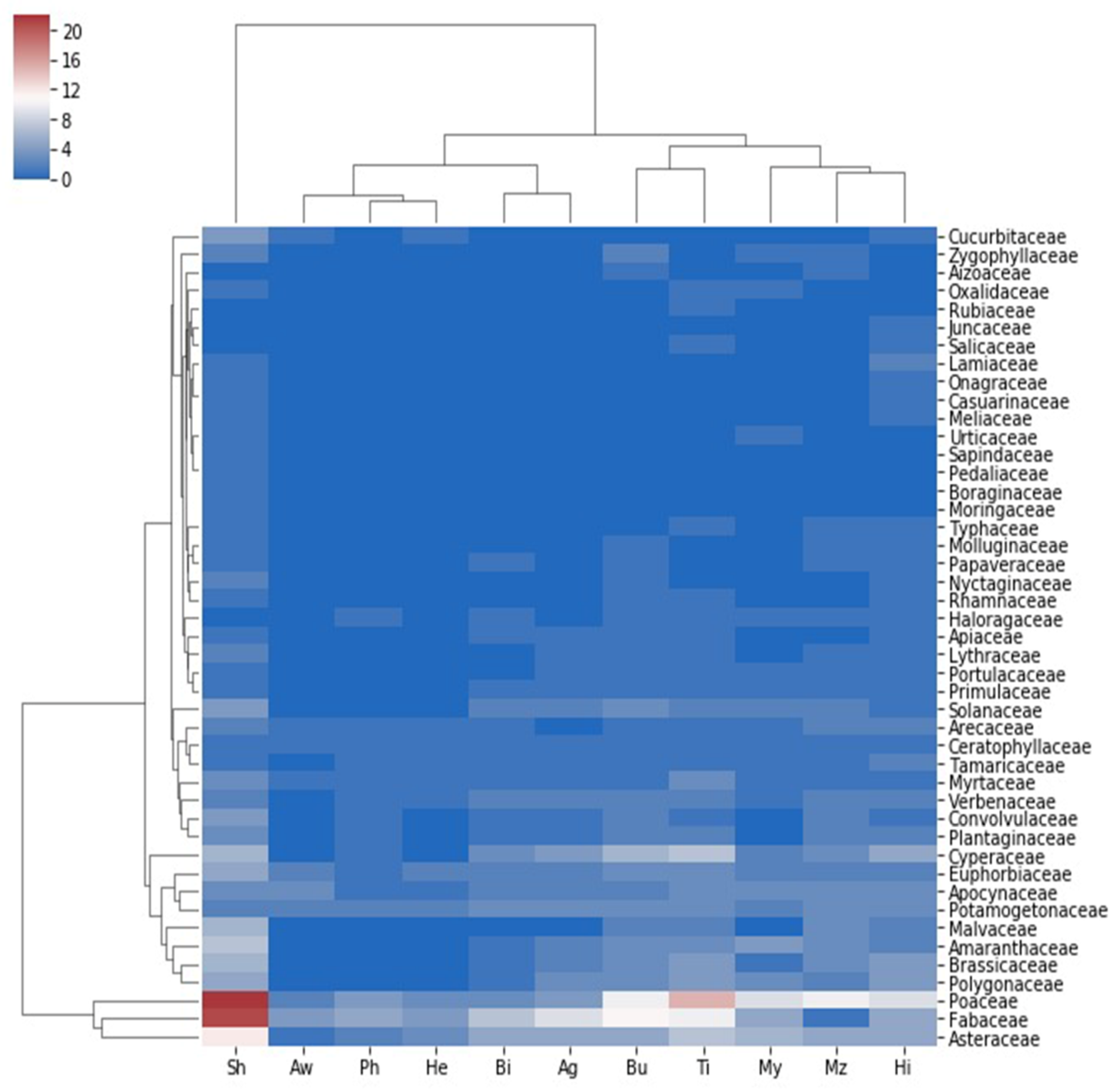

| Correlations | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sh | Bu | Mz | Hi | Ph | My | Ti | Aw | He | Bi | Ag | |
| Sh | |||||||||||
| Bu | −0.23 | ||||||||||
| Mz | 0.08 | 0.58 | |||||||||
| Hi | 0.21 | 0.81 ** | 0.31 | ||||||||
| Ph | −0.57 | 0.63 * | 0.61 * | 0.21 | |||||||
| My | −0.37 | −0.31 | −0.23 | −0.51 | −0.13 | ||||||
| Ti | 0.61 * | −0.42 | −0.25 | 0.12 | −0.47 | −0.16 | |||||
| Aw | −0.70 * | 0.43 | 0.38 | −0.02 | 0.67 * | 0.55 | −0.49 | ||||
| He | −0.69 * | 0.39 | 0.34 | −0.09 | 0.61 * | 0.60 | −0.56 | 0.90 ** | |||
| Bi | −0.22 | 0.04 | 0.06 | −0.30 | 0.07 | 0.47 | −0.62 * | 0.27 | 0.41 | ||
| Ag | −0.11 | −0.01 | 0.02 | −0.31 | −0.01 | 0.42 | −0.59 | 0.16 | 0.31 | 0.91 ** | |
| Edaphic Factors | I | II | III | IV | V | VI | VII | VIII | IX | X |
|---|---|---|---|---|---|---|---|---|---|---|
| PH | A: 7.43 ± 0.055 | 8.27 ± 0.053 | 8.09 ± 0.297 | 7.18 ± 0.04 | 7.45 ± 0 | 7.37 ± 0.207 | 7.77 ± 0.21 | 7.493 ± 0.159 | 7.67 ± 0.192 | 7.56 ± 0 |
| B: 7.45 ± 0.08 | 8.367 ± 0.071 | 8.117 ± 0.290 | 7.41 ± 0.02 | 7.415 ± 0.005 | 7.447 ± 0.106 | 7.675 ± 0.335 | 7.517 ± 0.061 | 7.33 ± 0.38 | 7.813 ± 0.058 | |
| TDS (mg/L) | A: 0.035 ± 0.005 | 0.02 ± 0.01 | 0.023 ± 0.006 | 0.015 ± 0.005 | 0.02 ± 0 | 0.023 ± 0.006 | 0.03 ± 0.01 | 0.023 ± 0.016 | 0.017 ± 0.01 | 0.05 ± 0 |
| B: 0.035 ± 0.005 | 0.02 ± 0.01 | 0.02 ± 0 | 0.02 ± 0.01 | 0.015 ± 0.005 | 0.023 ± 0.012 | 0.015 ± 0.005 | 0.02 ± 0.01 | 0.027 ± 0.01 | 0.037 ± 0.006 | |
| EC (µs/cm) | A: 122.5 ± 4.5 | 157 ± 49.1 | 159 ± 49.7 | 126 ± 1 | 103.5 ± 1.5 | 124.3 ± 21 | 117 ± 6 | 132.67 ± 2.89 | 101.3 ± 5.03 | 205 ± 0 |
| B: 99.95 ± 11.05 | 152.7 ± 55.4 | 147.3 ± 48.3 | 135 ± 14 | 106.5 ± 1.5 | 125.0 ± 21.1 | 113.67 ± 4.93 | 115.33 ± 5.69 | 88.3 ± 15.01 | 191.67 ± 2.89 | |
| Organic matter % | A: 1.04 ± 0.117 | 3.57 ± 1.29 | 1.215 ± 0.128 | 0.978 ± 0.006 | 0.637 ± 0.045 | 0.864 ± 0.1344 | 0.573 ± 0.181 | 0.749 ± 0.258 | 0.647 ± 0.23 | 0.869 ± 0 |
| B:0.035 ± 0.005 | 3.682 ± 1.429 | 1.445 ± 0.571 | 0.915 ± 0.039 | 0.682 ± 0.254 | 0.823 ± 0.11 | 0.599 ± 0.071 | 0.874 ± 0.282 | 0.704 ± 0.2 | 0.937 ± 0.02 | |
| Na+ (mg/g) | A: 16.02 ± 5.85 | 12.2 ± 1.77 | 26.31 ± 10.02 | 19.44 ± 0.999 | 29.9 ± 1.23 | 19.05 ± 5.18 | 8.603 ± 0.072 | 9.71 ± 2.48 | 10.96 ± 3.1 | 11.27 ± 0 |
| B: 13.44 ± 3.9 | 10.14 ± 2.45 | 23.57 ± 9.1 | 19.064 ± 1.72 | 25.015 ± 0.519 | 18.07 ± 5.59 | 8.141 ± 0.274 | 8.2 ± 1.94 | 11.10 ± 2.12 | 9.59 ± 0.403 | |
| K+ (mg/g) | A: 1.31 ± 0.566 | 0.79 ± 0.196 | 1.296 ± 0.379 | 1.036 ± 0.119 | 1.779 ± 0.0924 | 1.499 ± 0.382 | 1.27 ± 0.377 | 0.631 ± 0.21 | 1.27 ± 0.798 | 1.602 ± 0 |
| B: 1.16 ± 0.62 | 1.004 ± 0.233 | 1.057 ± 0.387 | 0.905 ± 0.096 | 1.4475 ± 0.0385 | 1.532 ± 0.492 | 0.589 ± 0.012 | 0.547 ± 0.121 | 1.17 ± 0.019 | 0.57 ± 0.004 | |
| Ca2+ (mg/g) | A:0.6 ± 0.1 | 0.833 ± 0.088 | 0.6 ± 0.033 | 0.633 ± 0 | 0.733 ± 0 | 0.622 ± 0.051 | 0.483 ± 0.05 | 0.624 ± 0.181 | 0.567 ± 0.1 | 0.467 ± 0 |
| B: 0.383 ± 0.05 | 0.656 ± 0.084 | 0.544 ± 0.157 | 0.533 ± 0.033 | 0.567 ± 0.167 | 0.444 ± 0.084 | 0.383 ± 0.05 | 0.518 ± 0.13 | 0.433 ± 0.1 | 0.264 ± 0.02 | |
| Mg2+ (mg/g) | A: 0.4 ± 0.08 | 0.347 ± 0.18 | 0.4 ± 0.08 | 0.42 ± 0.02 | 0.34 ± 0.06 | 0.413 ± 0.046 | 0.52 ± 0.04 | 0.427 ± 0.201 | 0.36 ± 0.069 | 0.44 ± 0 |
| B: 0.24 ± 0.04 | 0.213 ± 0.083 | 0.32 ± 0.183 | 0.3 ± 0.06 | 0.54 ± 0.34 | 0.24 ± 0.106 | 0.38 ± 0.1 | 0.334 ± 0.151 | 0.4 ± 0 | 0.367 ± 0.012 | |
| Cl−(mg/g) | A: 0.095 ± 0 | 0.063 ± 0.025 | 0.059 ± 0.021 | 0.065 ± 0.006 | 0.071 ± 0.024 | 0.063 ± 0.018 | 0.053 ± 0.006 | 0.079 ± 0.027 | 0.059 ± 0 | 0.047 ± 0 |
| B: 0.059 ± 0.012 | 0.059 ± 0.012 | 0.051 ± 0.014 | 0.065 ± 0.018 | 0.077 ± 0.005 | 0.071 ± 0.024 | 0.041 ± 0.006 | 0.055 ± 0.014 | 0.055 ± 0.03 | 0.054 ± 0.012 | |
| CO32− (mg/g) | A: 2.56 ± 0.019 | 1.44 ± 0.312 | 2.58 ± 0.287 | 1.92 ± 0.166 | 1.863 ± 0.1 | 1.757 ± 0.434 | 1.798 ± 0.14 | 2.078 ± 0.317 | 2.592 ± 0.45 | 2.438 ± 0 |
| B: 1.99 ± 0.337 | 1.716 ± 0.268 | 1.809 ± 0.151 | 1.75 ± 0.079 | 1.62 ± 0.279 | 1.267 ± 0.439 | 1.53 ± 0.143 | 1.66 ± 0.161 | 1.474 ± 0.42 | 2.04 ± 0.006 | |
| PO42− (mg/g) | A: 0.014 ± 0 | 0.033 ± 0.004 | 0.049 ± 0.026 | 0.036 ± 0.003 | 0.013 ± 0 | 0.048 ± 0.035 | 0.043 ± 0.002 | 0.016 ± 0.003 | 0.023 ± 0.01 | 0.031 ± 0 |
| B: 0.008 ± 0.005 | 0.028 ± 0.006 | 0.04 ± 0.026 | 0.026 ± 0.005 | 0.029 ± 0 | 0.045 ± 0.031 | 0.023 ± 0.014 | 0.015 ± 0.004 | 0.027 ± 0.01 | 0.04 ± 0.003 | |
| NO3− (mg/g) | A: 0.19 ± 0.025 | 0.166 ± 0.005 | 0.196 ± 0.024 | 0.167 ± 0.041 | 0.139 ± 0.0137 | 0.143 ± 0.016 | 0.163 ± 0.008 | 0.196 ± 0.045 | 0.127 ± 0.05 | 0.165 ± 0 |
| B: 0.171 ± 0.015 | 0.162 ± 0.049 | 0.161 ± 0.018 | 0.157 ± 0.029 | 0.14 ± 0.007 | 0.142 ± 0.021 | 0.119 ± 0.011 | 0.146 ± 0.02 | 0.105 ± 0.02 | 0.138 ± 0.03 | |
| SO42− (mg/g) | A: 0.091 ± 0.025 | 0.034 ± 0.027 | 0.225 ± 0.045 | 0.268 ± 0.04 | 0.125 ± 0 | 0.167 ± 0.057 | 0.068 ± 0.003 | 0.0527 ± 0.01 | 0.059 ± 0.03 | 0.164 ± 0 |
| B: 0.091 ± 0.02 | 0.0341 ± 0.03 | 0.2252 ± 0.045 | 0.243 ± 0.066 | 0.125 ± 0 | 0.167 ± 0.057 | 0.068 ± 0.003 | 0.053 ± 0.01 | 0.041 ± 0.03 | 0.15 ± 0.015 | |
| Soil texture | Sand loamy sand | sandy clay loam clay loam | clay loam | sand | sand | sand | sand | sand | sandy loam | sand |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abbas, A.M.; Ayed, F.A.A.; Sheded, M.G.; Alrumman, S.A.; Radwan, T.A.A.; Badry, M.O. Vegetation Analysis and Environmental Relationships of Riverain Plants in the Aswan Reservoir, Egypt. Plants 2021, 10, 2712. https://doi.org/10.3390/plants10122712
Abbas AM, Ayed FAA, Sheded MG, Alrumman SA, Radwan TAA, Badry MO. Vegetation Analysis and Environmental Relationships of Riverain Plants in the Aswan Reservoir, Egypt. Plants. 2021; 10(12):2712. https://doi.org/10.3390/plants10122712
Chicago/Turabian StyleAbbas, Ahmed M., Fatma A. A. Ayed, Mohamed G. Sheded, Sulaiman A. Alrumman, Tarek A. A. Radwan, and Mohamed O. Badry. 2021. "Vegetation Analysis and Environmental Relationships of Riverain Plants in the Aswan Reservoir, Egypt" Plants 10, no. 12: 2712. https://doi.org/10.3390/plants10122712








