Biomass and Species Diversity of Different Alpine Plant Communities Respond Differently to Nitrogen Deposition and Experimental Warming
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites and Experimental Treatment
2.2. Experimental Design
2.3. Plant Productivity and Diversity Calculation
2.4. Statistical Analysis
3. Results
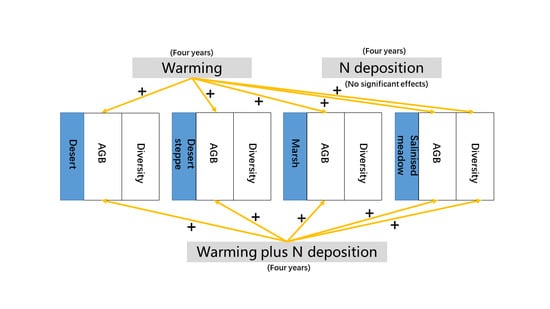
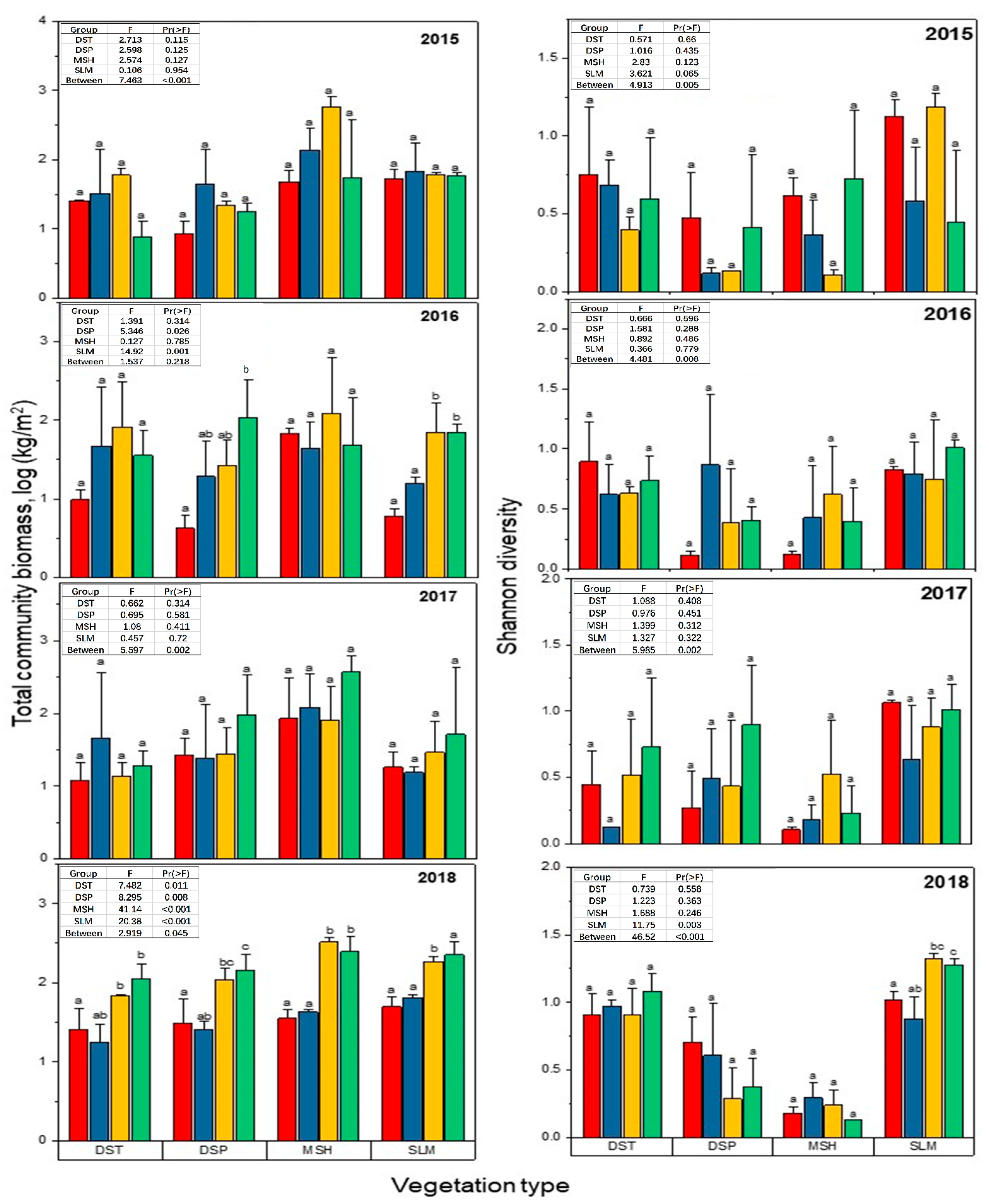
3.1. Effects of N, W and WN on Aboveground Plant Biomass and Plant Diversity
3.2. Interactions between Experimental Treatment and Sampling Year on Plant Community
3.3. Relationship among Plant Biomass, Coverage, Evenness, Height, Richness and Diversity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- IPCC Climate Change: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovenmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013. [Google Scholar]
- Vitousek, P.M.; Howarth, R.W. Nitrogen Limitation on Land and in the Sea: How Can It Occur? Biogeochemistry 1991, 13, 87–115. [Google Scholar] [CrossRef]
- Hutchison, J.S.; Henry, H.A.L. Additive effects of warming and increased nitrogen deposition in a temperate old field: Plant productivity and the importance of winter. Ecosystems 2010, 13, 661–672. [Google Scholar] [CrossRef]
- Zhang, M.; Song, C.; Kühn, P.; Shi, Y.; He, J.-S.; Scholten, T.; Baumann, F.; Geng, Y. Increasing temperature reduces the coupling between available nitrogen and phosphorus in soils of Chinese grasslands. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Guntiñas, M.E.; Leirós, M.C.; Trasar-cepeda, C.; Gil-sotres, F. Effects of moisture and temperature on net soil nitrogen mineralization: A laboratory study. Eur. J. Soil Biol. 2012, 48, 73–80. [Google Scholar] [CrossRef]
- Zeller, V.; Bahn, M.; Aichner, M.; Tappeiner, U. Impact of land-use change on nitrogen mineralization in subalpine grasslands in the Southern Alps. Biol. Fertil. Soils 2000, 31, 441–448. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, F.; Gao, Q.; Mao, R.; Liu, X. Impact of land-use types on soil nitrogen net mineralization in the sandstorm and water source area of Beijing, China. CATENA 2010, 82, 15–22. [Google Scholar] [CrossRef]
- Bedard-Haughn, A.; Matson, A.L.; Pennock, D.J. Land use effects on gross nitrogen mineralization, nitrification, and N2O emissions in ephemeral wetlands. Soil Biol. Biochem. 2006, 38, 3398–3406. [Google Scholar] [CrossRef]
- Boutin, M.; Corcket, E.; Alard, D.; Villar, L.; Corriol, G.; Lamaze, T.; Blaix, C. Nitrogen deposition and climate change have increased vascular plant species richness and altered the composition of grazed subalpine grasslands J. Ecol. 2017, 1199–1209. [Google Scholar] [CrossRef]
- Standish, R.J.; Fontaine, J.B.; Harris, R.J.; Stock, W.D.; Hobbs, R.J. Interactive effects of altered rainfall and simulated nitrogen deposition on seedling establishment in a global biodiversity hotspot. Oikos 2012, 121, 2014–2025. [Google Scholar] [CrossRef]
- Zhang, T.; Guo, R.; Gao, S.; Guo, J.; Sun, W. Responses of Plant Community Composition and Biomass Production to Warming and Nitrogen Deposition in a Temperate Meadow Ecosystem. PLoS ONE 2015, 10, e0123160. [Google Scholar] [CrossRef]
- Zavaleta, E.S.; Shaw, M.R.; Chiariello, N.R.; Mooney, H.A.; Field, C.B. Additive effects of simulated climate changes, elevated CO2, and nitrogen deposition on grassland diversity. Proc. Natl. Acad. Sci. USA 2003, 100, 7650–7654. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lü, X.; Isbell, F.; Stevens, C.; Han, X.; He, N.; Zhang, G.; Yu, Q.; Huang, J.; Han, X. Rapid plant species loss at high rates and at low frequency of N addition in temperate steppe. Glob. Chang. Biol. 2014, 20, 3520–3529. [Google Scholar] [CrossRef] [PubMed]
- Simkin, S.M.; Allen, E.B.; Bowman, W.D.; Clark, C.M.; Belnap, J.; Brooks, M.L.; Cade, B.S.; Collins, S.L.; Geiser, L.H.; Gilliam, F.S.; et al. Conditional vulnerability of plant diversity to atmospheric nitrogen deposition across the United States. Proc. Natl. Acad. Sci. USA 2016, 113, 4086–4091. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; He, H.L.; Gao, Q.; Zhao, C.Z.; Zhao, W.Q.; Yin, C.Y.; Chen, X.; Ma, Z.L.; Li, D.D.; Sun, D.D.; et al. Effects of short-term N addition on plant biomass allocation and C and N pools of theSibiraea angustatascrub ecosystem. Eur. J. Soil Sci. 2017, 68, 212–220. [Google Scholar] [CrossRef]
- Bobbink, R.; Hornung, M.; Roelofs, J.G.M. The effects of air-borne nitrogen pollutants on species diversity in natural and semi-natural European vegetation. J. Ecol. 1998, 86, 717–738. [Google Scholar] [CrossRef]
- Roth, T.; Kohli, L.; Rihm, B.; Amrhein, V.; Achermann, B. Nitrogen deposition and multi-dimensional plant diversity at the landscape scale. R. Soc. Open Sci. 2015, 2, 150017. [Google Scholar] [CrossRef]
- Thomas, J.A. Comparative Losses of British Butterflies, Birds, and Plants and the Global Extinction Crisis. Science 2004, 303, 1879–1881. [Google Scholar] [CrossRef] [PubMed]
- Stevens, C.J.; Dise, N.B.; Gowing, D.J.G.; Mountford, J.O. Loss of forb diversity in relation to nitrogen deposition in the UK: Regional trends and potential controls. Glob. Chang. Biol. 2006, 12, 1823–1833. [Google Scholar] [CrossRef]
- Tian, H.; Melillo, J.; Lu, C.; Kicklighter, D.; Liu, M.; Ren, W.; Xu, X.; Chen, G.; Zhang, C.; Pan, S.; et al. China's terrestrial carbon balance: Contributions from multiple global change factors. Glob. Biogeochem. Cycles 2011, 25. [Google Scholar] [CrossRef]
- Sala, O.E.; Kinzig, A.; Leemans, R.; Lodge, D.M.; Mooney, H.A.; Oesterheld, M.; Poff, N.L.; Sykes, M.T.; Walker, B.H.; Walker, M.; et al. Biodiversity—Global biodiversity scenarios for the year 2100. Science 2000, 287, 1770–1774. [Google Scholar] [CrossRef]
- Hoffmann, M.; Hilton-Taylor, C.; Angulo, A.; Böhm, M.; Brooks, T.M.; Butchart, S.H.M.; Carpenter, K.E.; Chanson, J.; Collen, B.; Cox, N.A.; et al. The Impact of Conservation on the Status of the World’s Vertebrates. Science 2010, 330, 1503–1509. [Google Scholar] [CrossRef]
- He, K.; Qi, Y.; Huang, Y.; Chen, H.; Sheng, Z.; Xu, X.; Duan, L. Response of aboveground biomass and diversity to nitrogen addition—A five-year experiment in semi-arid grassland of Inner Mongolia, China. Sci. Rep. 2016, 6, 31919. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Dong, S.; Li, S.; Xiao, J.; Han, Y.; Yang, M.; Zhang, J.; Gao, X.; Xu, Y.; Li, Y.; et al. Effects of simulated N deposition on photosynthesis and productivity of key plants from different functional groups of alpine meadow on Qinghai-Tibetan plateau. Environ. Pollut. 2019, 251, 731–737. [Google Scholar] [CrossRef] [PubMed]
- Verma, P.; Sagar, R.; Verma, H.; Verma, P.; Singh, D.K. Changes in species composition, diversity and biomass of herbaceous plant traits due to N amendment in a dry tropical environment of India. J. Plant Ecol. 2014, 8, 321–332. [Google Scholar] [CrossRef]
- Wan, S.; Hui, D.; Wallace, L.; Luo, Y. Direct and indirect effects of experimental warming on ecosystem carbon processes in a tallgrass prairie. Glob. Biogeochem. Cycles 2005, 19, 1–13. [Google Scholar] [CrossRef]
- Klein, J.A.; Harte, J.; Zhao, X.-Q. Experimental Warming, Not Grazing, Decreases Rangeland Quality On The Tibetan Plateau. Ecol. Appl. 2007, 17, 541–557. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Sherry, R.A.; Niu, S.; Li, D.; Luo, Y. Net primary productivity and rain-use efficiency as affected by warming, altered precipitation, and clipping in a mixed-grass prairie. Glob. Chang. Biol. 2013, 19, 2753–2764. [Google Scholar] [CrossRef]
- Lu, C.; Tian, H. Spatial and temporal patterns of nitrogen deposition in China: Synthesis of observational data. J. Geophys. Res. Space Phys. 2007, 112. [Google Scholar] [CrossRef]
- Dong, S.; Sherman, R. Enhancing the resilience of coupled human and natural systems of alpine rangelands on the Qinghai-Tibetan Plateau. Rangel. J. 2015, 37. [Google Scholar] [CrossRef]
- Li, Y.; Dong, S.; Liu, S.; Zhou, H.; Gao, Q.; Cao, G.; Wang, X.; Su, X.; Zhang, Y.; Tang, L.; et al. Seasonal changes of CO2, CH4 and N2O fluxes in different types of alpine grassland in the Qinghai-Tibetan Plateau of China. Soil Biol. Biochem. 2014, 80, 306–314. [Google Scholar] [CrossRef]
- Fu, G.; Shen, Z.-X. Response of Alpine Plants to Nitrogen Addition on the Tibetan Plateau: A Meta-analysis. J. Plant Growth Regul. 2016, 35, 974–979. [Google Scholar] [CrossRef]
- Han, Y.; Dong, S.; Zhao, Z.; Sha, W.; Li, S.; Shen, H.; Xiao, J.; Zhang, J.; Wu, X.; Jiang, X.; et al. Response of soil nutrients and stoichiometry to elevated nitrogen deposition in alpine grassland on the Qinghai-Tibetan Plateau. Geoderma 2019, 343, 263–268. [Google Scholar] [CrossRef]
- Dong, S.; Shang, Z.; Gao, J.; Boone, R.B. Enhancing sustainability of grassland ecosystems through ecological restoration and grazing management in an era of climate change on Qinghai-Tibetan Plateau. Agric. Ecosyst. Environ. 2019, 287, 106684. [Google Scholar] [CrossRef]
- Fu, G.; Zhang, X.; Zhang, Y.; Shi, P.; Li, Y.; Zhou, Y.; Yang, P.; Shen, Z. Experimental warming does not enhance gross primary production and above-ground biomass in the alpine meadow of Tibet. J. Appl. Remote. Sens. 2013, 7, 073505. [Google Scholar] [CrossRef]
- Gao, Q.; Guo, Y.; Xu, H.; Ganjurjav, H.; Li, Y.; Wan, Y.; Qin, X.; Ma, X.; Liu, S. Climate change and its impacts on vegetation distribution and net primary productivity of the alpine ecosystem in the Qinghai-Tibetan Plateau. Sci. Total. Environ. 2016, 554–555, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.F.; Li, F.; Zhou, G.Y.; Fang, K.; Zhang, D.Y.; Li, C.B.; Yang, G.B.; Wang, G.Q.; Wang, J.; Yang, Y.H. Linkages of plant stoichiometry to ecosystem production and carbon flfluxes with increasing nitrogen inputs in an alpine steppe. Global Chang. Biol. 2017, 23, 5249–5259. [Google Scholar] [CrossRef]
- Zong, N.; Chai, X.; Shi, P.L.; Yang, X.C. Effffects of warming and nitrogen addition on plant photosynthate partitioning in an Alpine Meadow on the Tibetan Plateau. J. Plant Growth Regul. 2018, 37, 803–812. [Google Scholar] [CrossRef]
- Zhang, Y.; Dong, S.; Gao, Q.; Ganjurjav, H.; Wang, X.; Geng, W. “Rare biosphere” plays important roles in regulating soil available nitrogen and plant biomass in alpine grassland ecosystems under climate changes. Agric. Ecosyst. Environ. 2018, 279, 187–193. [Google Scholar] [CrossRef]
- Wu, H.; Guo, Z.; Peng, C. Land use induced changes of organic carbon storage in soils of China. Glob. Chang. Biol. 2003, 9, 305–315. [Google Scholar] [CrossRef]
- Yu, C.; Zhang, Y.; Claus, H.; Zeng, R.; Zhang, X.; Wang, J. Ecological and Environmental Issues Faced by a Developing Tibet. Environ. Sci. Technol. 2012, 46, 1979–1980. [Google Scholar] [CrossRef]
- Fu, Y.-H.; Lu, R.-Y.; Guo, D. Changes in surface air temperature over China under the 1.5 and 2.0 °C global warming targets. Adv. Clim. Chang. Res. 2018, 9, 112–119. [Google Scholar] [CrossRef]
- Marion, G.M.; Henry, G.H.R.; Freckman, D.W.; Johnstone, J.; Jones, G.; Jones, M.H.; Lévesque, E.; Molau, U.; Mølgaard, P.; Parsons, A.N.; et al. Open-top designs for manipulating field temperature in high-latitude ecosystems. Glob. Chang. Biol. 1997, 3, 20–32. [Google Scholar] [CrossRef]
- Samson, M.; Słowińska, S.; Słowiński, M.; Lamentowicz, M.; Barabach, J.; Harenda, K.; Zielińska, M.; Robroek, B.; Jassey, V.; Buttler, A.; et al. The Impact of Experimental Temperature and Water Level Manipulation on Carbon Dioxide Release in a Poor Fen in Northern Poland. Wetlands 2018, 38, 551–563. [Google Scholar] [CrossRef]
- Lamentowicz, M.; Słowińska, S.; Słowiński, M. Combining short-term manipulative experiments with long-term palaeoecological investigations at high resolution to assess the response of Sphagnum peatlands to drought, fire and warming. Mires Peat 2016, 18, 1–17. [Google Scholar] [CrossRef]
- Shaver, G.R.; Canadell, J.; Chapin, F.S.; Gurevitch, J.; Harte, J.; Henry, G.H.R.; Ineson, P.; Jonasson, S.; Melillo, J.M.; Pitelka, L.F.; et al. Global Warming and Terrestrial Ecosystems: A Conceptual Framework for Analysis. BioScience 2000, 50, 871–882. [Google Scholar] [CrossRef]
- Ren, J.Z. Methods in Grassland Science; China Agricultural Press: Beijing, China, 1998. [Google Scholar]
- Shannon, C.E.; Weaver, W. The Mathematical Theory of Communication; The University of Illinois Press: Urbana, IL, USA, 1963. [Google Scholar]
- Raiesi, F.; Riahi, M. The influence of grazing exclosure on soil C stocks and dynamics, and ecological indicators in upland arid and semi-arid rangelands. Ecol. Indic. 2014, 41, 145–154. [Google Scholar] [CrossRef]
- Song, L.; Bao, X.; Liu, X.; Zhang, F. Impact of nitrogen addition on plant community in a semi-arid temperate steppe in China. J. Arid. Land 2012, 4, 3–10. [Google Scholar] [CrossRef][Green Version]
- Gill, R.A. The influence of 3-years of warming and N-deposition on ecosystem dynamics is small compared to past land use in subalpine meadows. Plant Soil 2013, 374, 197–210. [Google Scholar] [CrossRef]
- Botkin, D.B.; Saxe, H.; Araújo, M.B.; Betts, R.; Bradshaw, R.; Cedhagen, T.; Chesson, P.; Dawson, T.; Etterson, J.; Faith, D.P.; et al. Forecasting the Effects of Global Warming on Biodiversity. BioScience 2007, 57, 227–236. [Google Scholar] [CrossRef]
- Chapin, F.S.; Shaver, G.R.; Giblin, A.E.; Nadelhoffer, K.J.; Laundre, J.A. Responses of Arctic Tundra to Experimental and Observed Changes in Climate. Ecology 1995, 76, 694–711. [Google Scholar] [CrossRef]
- Klein, J.A.; Harte, J.; Zhao, X.-Q. Experimental warming causes large and rapid species loss, dampened by simulated grazing, on the Tibetan Plateau. Ecol. Lett. 2004, 7, 1170–1179. [Google Scholar] [CrossRef]
- Zhang, Y.; Dong, S.K.; Gao, Q.Z.; Liu, S.L.; Zhou, H.K.; Ganjurjav, H.; Wang, X.X. Climate change and human activities altered the diversity and composition of soil microbial community in alpine grasslands of the Qinghai-Tibetan Plateau. Sci. Total Environ. 2016, 562, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Engel, E.C.; Weltzin, J.F.; Norby, R.J.; Classen, A.T. Responses of an old-field plant community to interacting factors of elevated [CO2], warming, and soil moisture. J. Plant Ecol. 2009, 2, 1–11. [Google Scholar] [CrossRef]
- Pennings, S.C.; Clark, C.M.; Cleland, E.E.; Collins, S.L.; Gough, L.; Gross, K.L.; Milchunas, D.G.; Suding, K.N. Do individual plant species show predictable responses to nitrogen addition across multiple experiments? OIKOS 2005, 3, 547–555. [Google Scholar] [CrossRef]
- Jiang, Z.-Y.; Li, X.-Y.; Wu, H.-W.; Zhang, S.-Y.; Zhao, G.-Q.; Wei, J.-Q. Linking spatial distributions of the patchy grass Achnatherum splendens with dynamics of soil water and salt using electromagnetic induction. CATENA 2017, 149, 261–272. [Google Scholar] [CrossRef]
- Li, Y.; Dong, S.; Liu, S.; Su, X.; Wang, X.; Zhang, Y.; Zhao, Z.; Gao, X.; Li, S.; Tang, L. Relationships between plant diversity and biomass production of alpine grasslands are dependent on the spatial scale and the dimension of biodiversity. Ecol. Eng. 2018, 127, 375–382. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, X.; Ou, Y.; Jia, H.; Li, J.; Shi, C.; Liu, Y. Variations in soil δ13C with alpine meadow degradation on the eastern Qinghai–Tibet Plateau. Geoderma 2018, 338, 178–186. [Google Scholar] [CrossRef]
- Zhang, L.; Cui, G.; Shen, W.; Liu, X. Cover as a simple predictor of biomass for two shrubs in Tibet. Ecol. Indic. 2016, 64, 266–271. [Google Scholar] [CrossRef]
- Liu, M.; Liu, G.; Gong, L.; Wang, D.; Sun, J. Relationships of Biomass with Environmental Factors in the Grassland Area of Hulunbuir, China. PLoS ONE 2014, 9, e102344. [Google Scholar] [CrossRef]
- Dorji, T.; Moe, S.R.; Klein, J.A.; Totland, Ø. Plant Species Richness, Evenness, and Composition along Environmental Gradients in an Alpine Meadow Grazing Ecosystem in Central Tibet, China. Arct. Antarct. Alp. Res. 2014, 46, 308–326. [Google Scholar] [CrossRef]

| Factors | Desert | Marsh | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Biomass | Coverage | Height | Richness | Shannon | Pielou | Biomass | Coverage | Height | Richness | Shannon | Pielou | |
| Year | 1.303 | 3.646 * | 7.118 *** | 5.376 ** | 5.566 ** | 8.747 *** | 1.278 | 5.589 ** | 1.609 | 2.102 | 1.943 | 1.850 |
| TRT | 1.966 | 5.228 ** | 2.935 * | 0.398 | 1.086 | 1.781 | 5.151 ** | 4.045 * | 0.311 | 1.309 | 0.469 | 0.706 |
| Year × TRT | 1.687 | 4.504 *** | 1.843 | 1.807 | 0.737 | 0.695 | 1.680 | 1.141 | 3.903 ** | 1.539 | 1.868 | 1.420 |
| Factors | Desert steppe | Salinised meadow | ||||||||||
| Biomass | Coverage | Height | Richness | Shannon | Pielou | Biomass | Coverage | Height | Richness | Shannon | Pielou | |
| Year | 3.445 * | 7.441 *** | 1.107 | 5.576 ** | 0.918 | 2.204 + | 8.827 *** | 3.808 * | 2.711 + | 15.317 *** | 2.719 | 2.751 + |
| TRT | 6.583 ** | 5.985 ** | 1.401 | 0.580 | 0.885 | 1.016 | 6.786 ** | 0.396 | 1.782 | 1.936 | 3.056 * | 2.684 + |
| Year × TRT | 1.588 | 2.997 * | 0.859 | 1.967 + | 1.314 | 0.887 | 1.261 | 0.908 | 0.459 | 1.851 | 1.951 | 1.973 + |
| Variables | Biomass | Coverage | Height | Richness | Shannon | Pielou |
|---|---|---|---|---|---|---|
| Biomass | 1.00 | |||||
| Coverage | 0.45 ** | 1.00 | ||||
| Height | 0.41 ** | 0.65 ** | 1.00 | |||
| Richness | 0.31 ** | 0.59 ** | 0.43 ** | 1.00 | ||
| Shannon | −0.12 | −0.07 | 0.27 ** | −0.13 | 1.00 | |
| Pielou | −0.13 | −0.10 | 0.27 ** | −0.19 ** | 0.90 ** | 1.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwaku, E.A.; Dong, S.; Shen, H.; Li, W.; Sha, W.; Su, X.; Zhang, Y.; Li, S.; Gao, X.; Liu, S.; et al. Biomass and Species Diversity of Different Alpine Plant Communities Respond Differently to Nitrogen Deposition and Experimental Warming. Plants 2021, 10, 2719. https://doi.org/10.3390/plants10122719
Kwaku EA, Dong S, Shen H, Li W, Sha W, Su X, Zhang Y, Li S, Gao X, Liu S, et al. Biomass and Species Diversity of Different Alpine Plant Communities Respond Differently to Nitrogen Deposition and Experimental Warming. Plants. 2021; 10(12):2719. https://doi.org/10.3390/plants10122719
Chicago/Turabian StyleKwaku, Emmanuella A., Shikui Dong, Hao Shen, Wei Li, Wei Sha, Xukun Su, Yong Zhang, Shuai Li, Xiaoxia Gao, Shiliang Liu, and et al. 2021. "Biomass and Species Diversity of Different Alpine Plant Communities Respond Differently to Nitrogen Deposition and Experimental Warming" Plants 10, no. 12: 2719. https://doi.org/10.3390/plants10122719
APA StyleKwaku, E. A., Dong, S., Shen, H., Li, W., Sha, W., Su, X., Zhang, Y., Li, S., Gao, X., Liu, S., Shi, J., Li, X., Liu, Q., & Zhao, Z. (2021). Biomass and Species Diversity of Different Alpine Plant Communities Respond Differently to Nitrogen Deposition and Experimental Warming. Plants, 10(12), 2719. https://doi.org/10.3390/plants10122719