Identification of microRNAs Responding to Aluminium, Cadmium and Salt Stresses in Barley Roots
Abstract
:1. Introduction
2. Results
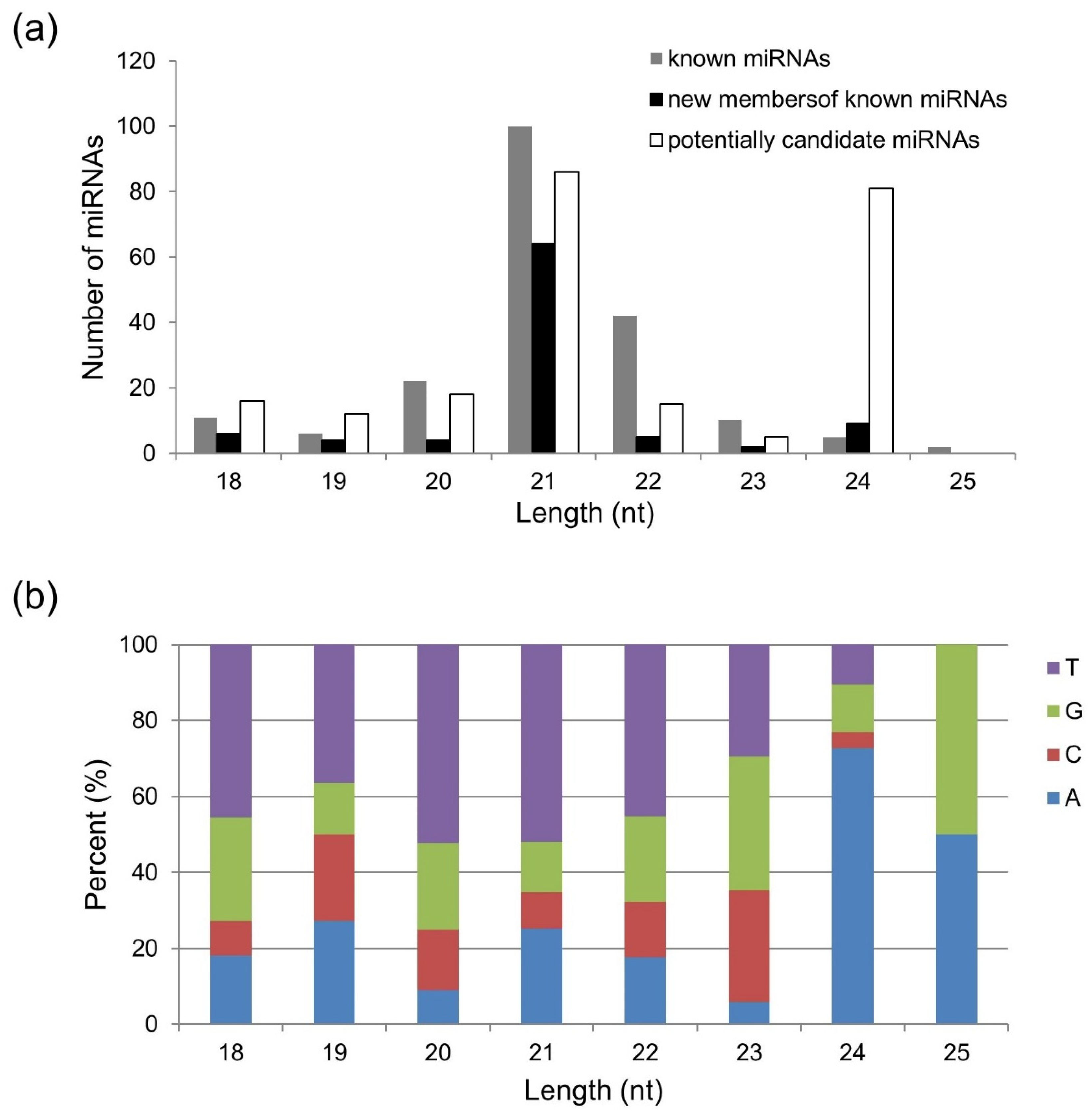
2.1. Identification of Conserved and Novel miRNAs in Golden Promise
2.2. Identification of miRNAs Responding to Al, Cd and Salt Stresses
2.3. Characterization of Target Genes for miRNAs
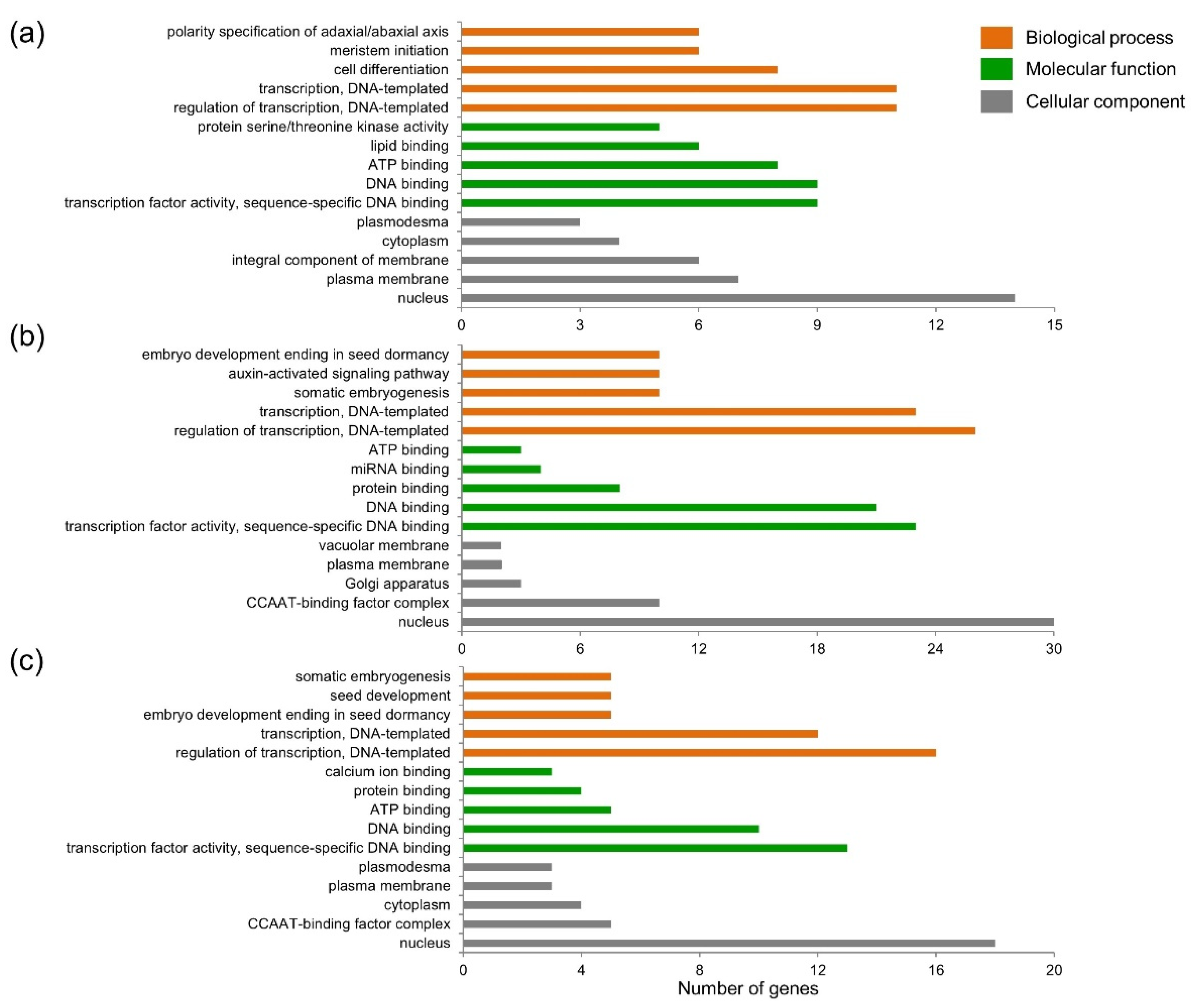
2.4. GO Analysis of Target Genes Regulated by Differently Expressed miRNAs
3. Discussion
3.1. miRNAs Specifically Responding to Al Stress
3.2. miRNAs Specifically Responding to Cd Stress
3.3. miRNAs Specifically Responding to Salt Stress
3.4. miRNAs Responding to Various Abiotic Stresses
4. Materials and Methods
4.1. Plant Culture and Stress Treatment
4.2. Construction of Small RNA and Degradome Libraries
4.3. Data Processing of Sequencing
4.4. Characterization of miRNAs Responding to Al, Cd and Salt Stresses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. Status of Research and Application of Crop Biotechnologies in Developing Countries. 2004. Available online: https://www.fao.org/3/y5800e/Y5800E00.htm (accessed on 31 August 2004).
- Kochian, L.V.; Pineros, M.A.; Liu, J.; Magalhaes, J.V. Plant adaptation to acid soils: The molecular basis for crop aluminum resistance. Annu. Rev. Plant Biol. 2015, 66, 571–598. [Google Scholar] [CrossRef]
- Dai, Z.; Zhang, X.; Tang, C.; Muhammad, N.; Wu, J.; Brookes, P.C.; Xu, J. Potential role of biochars in decreasing soil acidification—A critical review. Sci. Total Environ. 2017, 581, 601–611. [Google Scholar] [CrossRef]
- Ma, J.F. Syndrome of aluminum toxicity and diversity of aluminum resistance in higher plants. Int. Rev. Cytol. 2007, 264, 225–252. [Google Scholar] [PubMed]
- Rai, P.K.; Lee, S.S.; Zhang, M.; Tsang, Y.F.; Kim, K.-H. Heavy metals in food crops: Health risks, fate, mechanisms, and management. Environ. Int. 2019, 125, 365–385. [Google Scholar] [CrossRef]
- Rodriguez-Serrano, M.; Romero Puertas, M.C.; Pazmino, D.M.; Testillano, P.S.; Risueno, M.C.; del Rio, L.A.; Sandalio, L.M. Cellular response of pea plants to cadmium toxicity: Cross talk between reactive oxygen species, nitric oxide, and calcium. Plant Physiol. 2009, 150, 229–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rengasamy, P. Soil processes affecting crop production in salt-affected soils. Funct. Plant Biol. 2010, 37, 613–620. [Google Scholar] [CrossRef]
- Munns, R. Genes and salt tolerance: Bringing them together. New Phytol. 2005, 167, 645–663. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Wu, D.Z.; Ali, S.; Cai, S.G.; Dai, F.; Jin, X.L.; Wu, F.B.; Zhang, G.P. Evaluation of salinity tolerance and analysis of allelic function of HvHKT1 and HvHKT2 in Tibetan wild barley. Theor. Appl. Genet. 2011, 122, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.G.; Wu, D.Z.; Jabeen, Z.; Huang, Y.Q.; Huang, Y.C.; Zhang, G.P. Genome-wide association analysis of aluminum tolerance in cultivated and Tibetan wild barley. PLoS ONE 2013, 8, e69776. [Google Scholar] [CrossRef] [Green Version]
- Wu, D.Z.; Sato, K.; Ma, J.F. Genome-wide association mapping of cadmium accumulation in different organs of barley. New Phytol. 2015, 208, 817–829. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.L. MicroRNAs and their diverse functions in plants. Plant Mol. Biol. 2012, 80, 17–36. [Google Scholar] [CrossRef] [PubMed]
- Bian, H.W.; Xie, Y.K.; Guo, F.; Han, N.; Ma, S.Y.; Zeng, Z.H.; Wang, J.H.; Yang, Y.N.; Zhu, M.Y. Distinctive expression patterns and roles of the miRNA393/TIR1 homolog module in regulating flag leaf inclination and primary and crown root growth in rice (Oryza sativa). New Phytol. 2012, 196, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Houston, K.; McKim, S.M.; Comadran, J.; Bonar, N.; Druka, I.; Uzrek, N.; Cirillo, E.; Guzy-Wrobelska, J.; Collins, N.C.; Halpin, C.; et al. Variation in the interaction between alleles of HvAPETALA2 and microRNA172 determines the density of grains on the barley inflorescence. Proc. Natl. Acad. Sci. USA 2013, 110, 16675–16680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nair, S.K.; Wang, N.; Turuspekov, Y.; Pourkheirandish, M.; Sinsuwongwat, S.; Chen, G.; Sameri, M.; Tagiri, A.; Honda, I.; Watanabe, Y.; et al. Cleistogamous flowering in barley arises from the suppression of microRNA-guided HvAP2 mRNA cleavage. Proc. Natl. Acad. Sci. USA 2010, 107, 490–495. [Google Scholar] [CrossRef] [Green Version]
- Xia, K.F.; Wang, R.; Ou, X.J.; Fang, Z.M.; Tian, C.G.; Duan, J.; Wang, Y.Q.; Zhang, M.Y. OsTIR1 and OsAFB2 downregulation via osmiR393 overexpression leads to more tillers, early flowering and less tolerance to salt and drought in rice. PLoS ONE 2012, 7, e30039. [Google Scholar] [CrossRef]
- Gao, P.; Bai, X.; Yang, L.; Lv, D.; Li, Y.; Cai, H.; Ji, W.; Guo, D.; Zhu, Y. Over-expression of osa-MIR396c decreases salt and alkali stress tolerance. Planta 2010, 231, 991–1001. [Google Scholar] [CrossRef]
- Bukhari, S.A.H.; Shang, S.H.; Zhang, M.; Zheng, W.T.; Zhang, G.P.; Wang, T.Z.; Shamsi, I.H.; Wu, F.B. Genome-wide identification of chromium stress-responsive microRNAs and their target genes in tobacco (Nicotiana tabacum) roots. Environ. Toxicol. Chem. 2015, 34, 2573–2582. [Google Scholar] [CrossRef]
- Ding, Y.F.; Gong, S.H.; Wang, Y.; Wang, F.J.; Bao, H.X.G.D.L.; Sun, J.W.; Cai, C.; Yi, K.K.; Chen, Z.X.; Zhu, C. MicroRNA166 modulates cadmium tolerance and accumulation in rice. Plant Physiol. 2018, 177, 1691–1703. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.H.; Wu, L.Y.; Fu, L.B.; Shen, Q.F.; Kuang, L.H.; Wu, D.Z.; Zhang, G.P. Genotypic difference of cadmium tolerance and the associated microRNAs in wild and cultivated barley. Plant Growth Regul. 2019, 87, 389–401. [Google Scholar] [CrossRef]
- Wu, L.Y.; Yu, J.H.; Shen, Q.F.; Huang, L.; Wu, D.Z.; Zhang, G.P. Identification of microRNAs in response to aluminum stress in the roots of Tibetan wild barley and cultivated barley. BMC Genom. 2018, 19, 560. [Google Scholar] [CrossRef] [Green Version]
- Kuang, L.H.; Shen, Q.F.; Wu, L.Y.; Yu, J.H.; Fu, L.B.; Wu, D.Z.; Zhang, G.P. Identification of microRNAs responding to salt stress in barley by high-throughput sequencing and degradome analysis. Environ. Exp. Bot. 2019, 160, 59–70. [Google Scholar] [CrossRef]
- Huang, L.; Kuang, L.; Wu, L.; Shen, Q.; Han, Y.; Jiang, L.; Wu, D.; Zhang, G. The HKT transporter HvHKT1;5 negatively regulates salt tolerance. Plant Physiol. 2020, 182, 584–596. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Yamaji, N.; Yamane, M.; Kashino-Fujii, M.; Sato, K.; Ma, J.F. The HvNramp5 transporter mediates uptake of cadmium and manganese, but not iron. Plant Physiol. 2016, 172, 1899–1910. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.; Guo, Y.; Cai, S.; Kuang, L.; Shen, Q.; Wu, D.; Zhang, G. The zinc finger transcription factor ATF1 regulates aluminum tolerance in barley. J. Exp. Bot. 2020, 71, 6512–6523. [Google Scholar] [CrossRef] [PubMed]
- Hawker, N.P.; Bowman, J.L. Roles for class III HD-Zip and KANADI genes in Arabidopsis root development. Plant Physiol. 2004, 135, 2261–2270. [Google Scholar] [CrossRef] [Green Version]
- Prigge, M.J.; Otsuga, D.; Alonso, J.M.; Ecker, J.R.; Drews, G.N.; Clark, S.E. Class III homeodomain-leucine zipper gene family members have overlapping, antagonistic, and distinct roles in Arabidopsis development. Plant Cell 2005, 17, 61–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.S.; Zhang, H.; Srivastava, A.K.; Pan, Y.J.; Bai, J.J.; Fang, J.J.; Shi, H.Z.; Zhu, J.K. Knock-down of rice microRNA166 confers drought resistance by causing leaf rolling and altering stem xylem development. Plant Physiol. 2018, 176, 2082–2094. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Wang, T.Z.; Zhao, M.G.; Tian, Q.Y.; Zhang, W.H. Identification of aluminum-responsive microRNAs in Medicago truncatula by genome-wide high-throughput sequencing. Planta 2012, 235, 375–386. [Google Scholar] [CrossRef]
- Tichtinsky, G.; Vanoosthuyse, V.; Cock, J.M.; Gaude, T. Making inroads into plant receptor kinase signalling pathways. Trends Plant Sci. 2003, 8, 231–237. [Google Scholar] [CrossRef]
- Osakabe, Y.; Maruyama, K.; Seki, M.; Satou, M.; Shinozaki, K.; Yamaguchi Shinozaki, K. Leucine-rich repeat receptor-like kinase1 is a key membrane-bound regulator of abscisic acid early signaling in Arabidopsis. Plant Cell 2005, 17, 1105–1119. [Google Scholar] [CrossRef] [Green Version]
- Fan, W.; Xu, J.M.; Wu, P.; Yang, Z.X.; Lou, H.Q.; Chen, W.W.; Jin, J.F.; Zheng, S.J.; Yang, J.L. Alleviation by abscisic acid of Al toxicity in rice bean is not associated with citrate efflux but depends on ABI5-mediated signal transduction pathways. J. Integr. Plant Biol. 2019, 61, 140–154. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, I.M.; Nadira, U.A.; Cao, F.; He, X.; Zhang, G.; Wu, F. Physiological and molecular analysis on root growth associated with the tolerance to aluminum and drought individual and combined in Tibetan wild and cultivated barley. Planta 2016, 243, 973–985. [Google Scholar] [CrossRef]
- Kopittke, P.M. Role of phytohormones in aluminium rhizotoxicity. Plant Cell Environ. 2016, 39, 2319–2328. [Google Scholar] [CrossRef]
- Hu, Y.F.; Zhou, G.; Na, X.F.; Yang, L.; Nan, W.B.; Liu, X.; Zhang, Y.Q.; Li, J.L.; Bi, Y.R. Cadmium interferes with maintenance of auxin homeostasis in Arabidopsis seedlings. J. Plant Physiol. 2013, 170, 965–975. [Google Scholar] [CrossRef]
- Zhu, X.F.; Wang, Z.W.; Dong, F.; Lei, G.J.; Shi, Y.Z.; Li, G.X.; Zheng, S.J. Exogenous auxin alleviates cadmium toxicity in Arabidopsis thaliana by stimulating synthesis of hemicellulose 1 and increasing the cadmium fixation capacity of root cell walls. J. Hazard. Mater. 2013, 263, 398–403. [Google Scholar] [CrossRef]
- Zhu, Q.K.; Shao, Y.M.; Ge, S.T.; Zhang, M.M.; Zhang, T.S.; Hu, X.T.; Liu, Y.D.; Walker, J.; Zhang, S.Q.; Xu, J. A MAPK cascade downstream of IDA-HAE/HSL2 ligand-receptor pair in lateral root emergence. Nat. Plants 2019, 5, 414–423. [Google Scholar] [CrossRef]
- Enders, T.A.; Frick, E.M.; Strader, L.C. An Arabidopsis kinase cascade influences auxin-responsive cell expansion. Plant J. 2017, 92, 68–81. [Google Scholar] [CrossRef] [PubMed]
- Magome, H.; Yamaguchi, S.; Hanada, A.; Kamiya, Y.; Oda, K. The DDF1 transcriptional activator upregulates expression of a gibberellin-deactivating gene, GA2ox7, under high-salinity stress in Arabidopsis. Plant J. 2008, 56, 613–626. [Google Scholar] [CrossRef]
- Shan, C.; Mei, Z.L.; Duan, J.L.; Chen, H.Y.; Feng, H.F.; Cai, W.M. OsGA2ox5, a gibberellin metabolism enzyme, is involved in plant growth, the root gravity response and salt stress. PLoS ONE 2014, 9, e87110. [Google Scholar]
- Li, P.; Li, Y.J.; Zhang, F.J.; Zhang, G.Z.; Jiang, X.Y.; Yu, H.M.; Hou, B.K. The Arabidopsis UDP-glycosyltransferases UGT79B2 and UGT79B3, contribute to cold, salt and drought stress tolerance via modulating anthocyanin accumulation. Plant J. 2017, 89, 85–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pourcel, L.; Routaboul, J.M.; Cheynier, V.; Lepiniec, L.; Debeaujon, I. Flavonoid oxidation in plants: From biochemical properties to physiological functions. Trends Plant Sci. 2007, 12, 29–36. [Google Scholar] [CrossRef]
- Yang, Z.B.; Geng, X.Y.; He, C.M.; Zhang, F.; Wang, R.; Horst, W.J.; Ding, Z.J. TAA1-regulated local auxin biosynthesis in the root-apex transition zone mediates the aluminum-induced inhibition of root growth in Arabidopsis. Plant Cell 2014, 26, 2889–2904. [Google Scholar] [CrossRef] [Green Version]
- Yu, Q.Y.; An, L.J.; Li, W.L. The CBL-CIPK network mediates different signaling pathways in plants. Plant Cell Rep. 2014, 33, 203–214. [Google Scholar] [CrossRef]
- Ligaba-Osena, A.; Fei, Z.J.; Liu, J.P.; Xu, Y.M.; Shaff, J.; Lee, S.C.; Luan, S.; Kudla, J.; Kochian, L.; Pineros, M. Loss-of-function mutation of the calcium sensor CBL1 increases aluminum sensitivity in Arabidopsis. New Phytol. 2017, 214, 830–841. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.Y.; Xu, Z.S.; Chen, Y.; He, G.Y.; Yang, G.X.; Chen, M.; Li, L.C.; Ma, Y.Z. A novel role for Arabidopsis CBL1 in affecting plant responses to glucose and gibberellin during germination and seedling development. PLoS ONE 2013, 8, e56412. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Guo, G.H.; Guo, W.W.; Guo, G.G.; Tong, D.; Ni, Z.F.; Sun, Q.X.; Yao, Y.Y. miRNA164-directed cleavage of ZmNAC1 confers lateral root development in maize (Zea mays L.). BMC Plant Biol. 2012, 12, 220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, W.C.; Li, Z.L.; Fan, J.W.; Hu, C.L.; Yang, R.; Qi, X.; Chen, H.; Zhao, F.K.; Wang, S.H. Identification of jasmonic acid-associated microRNAs and characterization of the regulatory roles of the miR319/TCP4 module under root-knot nematode stress in tomato. J. Exp. Bot. 2015, 66, 4653–4667. [Google Scholar] [CrossRef]
- Kaur, H.; Sharma, P.; Sirhindi, G. Sugar accumulation and its regulation by jasmonic acid in Brassica napus L. under salt stress. J. Stress Physiol. Biochem. 2013, 9, 53–64. [Google Scholar]
- Lei, G.J.; Sun, L.; Sun, Y.; Zhu, X.F.; Li, G.X.; Zheng, S.J. Jasmonic acid alleviates cadmium toxicity in Arabidopsis via suppression of cadmium uptake and translocation. J. Integr. Plant Biol. 2020, 62, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Bazin, J.; Khan, G.A.; Combier, J.P.; Bustos-Sanmamed, P.; Manuel Debernardi, J.; Rodriguez, R.; Sorin, C.; Palatnik, J.; Hartmann, C.; Crespi, M.; et al. miR396 affects mycorrhization and root meristem activity in the legume Medicago truncatula. Plant J. 2013, 74, 920–934. [Google Scholar] [CrossRef]
- Ma, Z.R.; Coruh, C.; Axtell, M.J. Arabidopsis lyrata small RNAs: Transient MIRNA and small interfering RNA loci within the Arabidopsis genus. Plant Cell 2010, 22, 1090–1103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Addo-Quaye, C.; Miller, W.; Axtell, M.J. CleaveLand: A pipeline for using degradome data to find cleaved small RNA targets. Bioinformatics 2009, 25, 130–131. [Google Scholar] [CrossRef] [PubMed]



| miRNA Name | Al a | Cd b | Salt c | Target Rranscript | Annotation | Degradome Detection d |
|---|---|---|---|---|---|---|
| ata-miR156a-3p | 0.20 | 0.05 | 0.85 | HORVU3Hr1G072810.1 | Gibberellin 2-oxidase | + |
| HORVU1Hr1G078160.1 | P-loop containing nucleoside triphosphate hydrolases superfamily | + | ||||
| ata-miR156b-3p | 1.15 | 0.71 | 1.95 | HORVU6Hr1G028980.8 | Cinnamoyl coa reductase 1 | - |
| ata-miR156d-3p | −0.10 | 0.35 | 1.26 | HORVU5Hr1G042740.2 | UDP-Glycosyltransferase superfamily | + |
| hvu-MIR159a-5p | −0.44 | 0.56 | 0.38 | HORVU1Hr1G088510.1 | Mitogen-activated protein kinase 16 | - |
| osa-miR319a-3p.2-3p | −0.35 | 1.79 | 1.09 | HORVU2Hr1G060120.1 | TCP4 | + |
| HORVU5Hr1G103400.1 | TCP4 | + | ||||
| ata-miR160a-5p | −0.44 | −0.65 | −0.20 | HORVU2Hr1G089670.2 | ARF10 | + |
| HORVU2Hr1G089660.7 | ARF10 | + | ||||
| HORVU7Hr1G101270.6 | ARF16 | + | ||||
| HORVU6Hr1G026750.1 | ARF18 | + | ||||
| HORVU1Hr1G041770.6 | ARF22 | + | ||||
| ata-miR164a-5p | −0.39 | −1.32 | −1.13 | HORVU2Hr1G080460.8 | NAC domain protein | + |
| HORVU7Hr1G072670.3 | NAC domain containing protein 1 | + | ||||
| HORVU5Hr1G011650.2 | NAC domain containing protein 1 | + | ||||
| HORVU5Hr1G041400.1 | Phytosulfokine 2 | + | ||||
| hvu-miR166a | −0.82 | 0.30 | 0.01 | HORVU5Hr1G010650.1 | Homeobox-leucine zipper family | + |
| HORVU5Hr1G061410.29 | Homeobox-leucine zipper HOX10 | + | ||||
| HORVU0Hr1G010250.3 | Homeobox-leucine zipper HOX32 | + | ||||
| HORVU1Hr1G041790.2 | Homeobox-leucine zipper family | + | ||||
| ata-miR166a-3p | −0.54 | 0.06 | 0.22 | HORVU1Hr1G041790.2 | Homeobox-leucine zipper family | + |
| HORVU5Hr1G010650.1 | Homeobox-leucine zipper family | + | ||||
| ata-miR166a-5p | −0.71 | 1.06 | −0.99 | HORVU1Hr1G076940.1 | Nucleotide-diphospho-sugar transferase family | - |
| HORVU6Hr1G005350.2 | GPI mannosyltransferase 3 | - | ||||
| ata-miR167a-5p | −0.63 | −0.84 | −0.10 | HORVU2Hr1G121110.32 | ARF6 | + |
| ata-miR167b-3p | −0.92 | 1.17 | 0.13 | HORVU1Hr1G075520.2 | Jacalin-related lectin 3 | - |
| ata-miR167b-5p | 0.58 | −0.26 | −0.33 | HORVU2Hr1G059280.1 | SWI/SNF complex subunit SWI3C | - |
| HORVU2Hr1G059130.1 | SWI/SNF complex subunit SWI3C | - | ||||
| tae-miR167c-5p | 0.64 | 1.20 | 0.20 | HORVU1Hr1G077630.2 | Ubiquitin carboxyl-terminal hydrolase 25 | - |
| HORVU2Hr1G059280.1 | SWI/SNF complex subunit SWI3C | - | ||||
| HORVU2Hr1G059130.1 | SWI/SNF complex subunit SWI3C | - | ||||
| ata-miR167f-3p | 0.29 | 1.69 | 1.79 | HORVU4Hr1G016990.3 | Cysteine desulfurase | - |
| hvu-miR168-3p | −0.71 | 0.19 | −0.68 | HORVU5Hr1G037570.4 | Receptor-like protein kinase | - |
| HORVU4Hr1G031620.1 | 14-3-3 protein beta/alpha-A | - | ||||
| hvu-miR168-5p | −0.55 | −0.16 | −1.02 | HORVU1Hr1G055570.4 | WD repeat-containing protein WRAP73 | + |
| HORVU2Hr1G105050.1 | Protein of unknown function (DUF581) | + | ||||
| ata-miR169a-3p | 0.58 | −2.90 | −6.21 | HORVU4Hr1G087430.2 | Unknown | - |
| ata-miR169c-5p | −0.37 | −2.27 | −∞ | HORVU5Hr1G092700.17 | NF-YA10 | + |
| HORVU4Hr1G075830.4 | NF-YA3 | + | ||||
| HORVU6Hr1G081080.12 | NF-YA5 | + | ||||
| HORVU2Hr1G032130.27 | NF-YA5 | + | ||||
| HORVU2Hr1G032130.6 | NF-YA5 | + | ||||
| ata-miR169i-5p | 0.42 | −2.04 | −2.89 | HORVU5Hr1G092700.17 | NF-YA10 | + |
| HORVU4Hr1G075830.4 | NF-YA3 | + | ||||
| HORVU6Hr1G081080.12 | NF-YA5 | + | ||||
| HORVU2Hr1G032130.27 | NF-YA5 | + | ||||
| HORVU2Hr1G032130.6 | NF-YA5 | + | ||||
| hvu-miR171-3p | 0.28 | 0.72 | −1.31 | HORVU6Hr1G063650.1 | GRAS | + |
| HORVU1Hr1G053510.1 | GRAS | + | ||||
| ata-miR171a-3p | −0.36 | 0.09 | −1.40 | HORVU4Hr1G061310.1 | GRAS | + |
| ata-miR171a-5p | −1.42 | 0.62 | −1.08 | HORVU2Hr1G076620.7 | T-complex protein 11 | + |
| ata-miR172b-3p | −0.63 | −0.10 | −0.17 | HORVU5Hr1G112440.1 | Ethylene-responsive TF10 | + |
| HORVU1Hr1G011800.24 | AP2-like ethylene-responsive TF | + | ||||
| ata-miR172b-5p | −0.19 | −1.45 | −0.03 | HORVU7Hr1G106280.1 | ARF6 | + |
| HORVU6Hr1G088570.2 | Clathrin interactor EPSIN 2 | + | ||||
| ata-miR390-5p | −1.86 | 0.10 | 0.23 | HORVU7Hr1G007520.1 | LRR-RLK | - |
| HORVU1Hr1G043790.1 | LRR-RLK | - | ||||
| HORVU2Hr1G091840.16 | RLK2 | - | ||||
| HORVU2Hr1G124010.6 | RLK | - | ||||
| ata-miR393-5p | −0.16 | 1.02 | 0.03 | HORVU2Hr1G070800.3 | HvAFB | + |
| HORVU1Hr1G021550.4 | HvTIR1 | + | ||||
| ata-miR394-5p | −1.18 | 0.83 | −0.63 | HORVU1Hr1G043940.3 | Protein TIC110, chloroplastic | + |
| HORVU6Hr1G018370.1 | Calnexin 1 | + | ||||
| ata-miR396e-5p | 0.51 | 0.62 | −1.36 | HORVU7Hr1G008680.14 | GRF5 | + |
| HORVU4Hr1G010080.6 | GRF6 | + | ||||
| HORVU4Hr1G003440.12 | GRF9 | + | ||||
| ata-miR1432-5p | −1.56 | 0.00 | 0.84 | HORVU1Hr1G094160.1 | Calmodulin like 43 | + |
| HORVU5Hr1G111520.1 | EF hand calcium-binding protein family | + | ||||
| tae-MIR9662a-5p | −0.38 | 1.16 | 0.63 | HORVU5Hr1G123930.2 | Beta-fructofuranosidase, insoluble isoenzyme 3 | - |
| HORVU2Hr1G100080.7 | Protein strawberry notch homolog 1 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuang, L.; Yu, J.; Shen, Q.; Fu, L.; Wu, L. Identification of microRNAs Responding to Aluminium, Cadmium and Salt Stresses in Barley Roots. Plants 2021, 10, 2754. https://doi.org/10.3390/plants10122754
Kuang L, Yu J, Shen Q, Fu L, Wu L. Identification of microRNAs Responding to Aluminium, Cadmium and Salt Stresses in Barley Roots. Plants. 2021; 10(12):2754. https://doi.org/10.3390/plants10122754
Chicago/Turabian StyleKuang, Liuhui, Jiahua Yu, Qiufang Shen, Liangbo Fu, and Liyuan Wu. 2021. "Identification of microRNAs Responding to Aluminium, Cadmium and Salt Stresses in Barley Roots" Plants 10, no. 12: 2754. https://doi.org/10.3390/plants10122754
APA StyleKuang, L., Yu, J., Shen, Q., Fu, L., & Wu, L. (2021). Identification of microRNAs Responding to Aluminium, Cadmium and Salt Stresses in Barley Roots. Plants, 10(12), 2754. https://doi.org/10.3390/plants10122754







