Characterization of Root System Architecture Traits in Diverse Soybean Genotypes Using a Semi-Hydroponic System
Abstract
:1. Introduction
2. Results
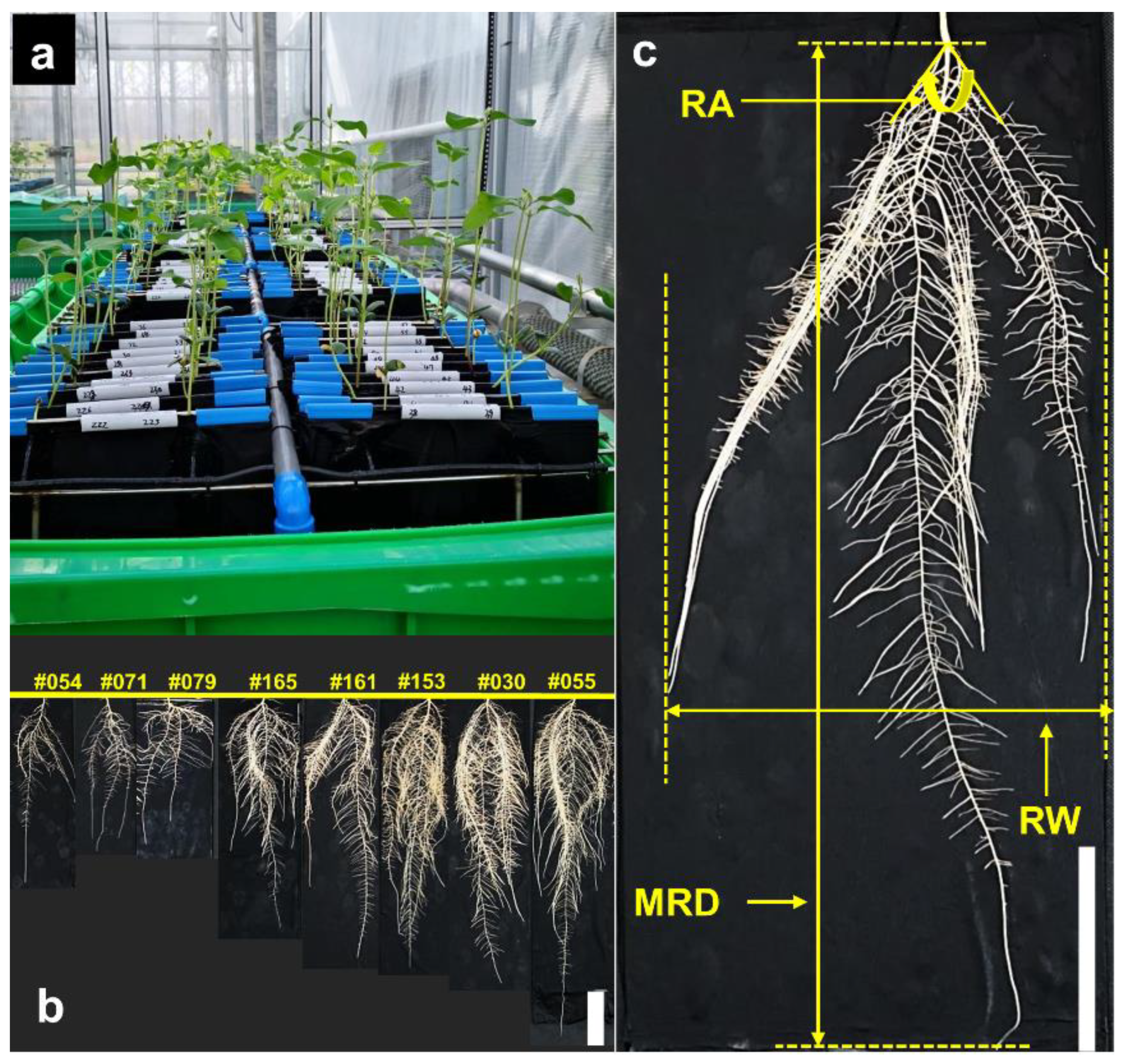
2.1. Adopting a Semi-Hydroponic System to Characterize 171 Soybean Accessions
2.1.1. Global Traits
| Traits | Abbreviation | Description | Units |
|---|---|---|---|
| Global traits (traits at the whole plant level) | |||
| Maximal root depth | MRD | Longest seminal root length, i.e., rooting depth | cm |
| Root width | RW | Maximal horizontal extent of a root system (Figure 1c) | cm |
| Root angle | RA | Growth angle between two outer lateral roots (Figure 1c) | Degree |
| Total root length | RL | Total root length per plant | cm |
| Root diameter | RD | Average diameter of root system per plant | mm |
| Root mass | RM | Total dry mass of roots per plant | mg |
| Specific root length | SRL | Total root length divided by root dry mass | cm mg−1 |
| Root tissue density | RTD | Total root dry mass divided by root volume | mg cm−3 |
| Root–shoot mass ratio | RSM | Total root dry mass divided by shoot dry mass | |
| Leaf number | LN | Number of leaves per plant | |
| Hypocotyl length | HL | Length from cotyledon node to the origin on the primary root | cm |
| Shoot height | SH | Shoot height (measured at its height from cotyledon node to plant growth point) | cm |
| Shoot mass | SM | Total shoot dry mass per plant | mg |
| Local traits (traits at local level including ratios) | |||
| Root length—upper | RL-upper | Root length in upper 0–20 cm soil layer | cm |
| Root length—lower | RL-lower | Root length below 20 cm depth | cm |
| Root length in diameter fine | RL-fine | Root length of ‘fine roots’ (in diameter class < 0.5 mm) | cm |
| Root length in diameter coarse | RL-coarse | Root length of ‘coarse roots’ (diameter class ≥ 0.5 mm) | cm |
| Root length ratio | RLR-upper/lower | Root length in top 20 cm soil layer divided by in root length below 20 cm soil depth | cm |
2.1.2. Local Traits
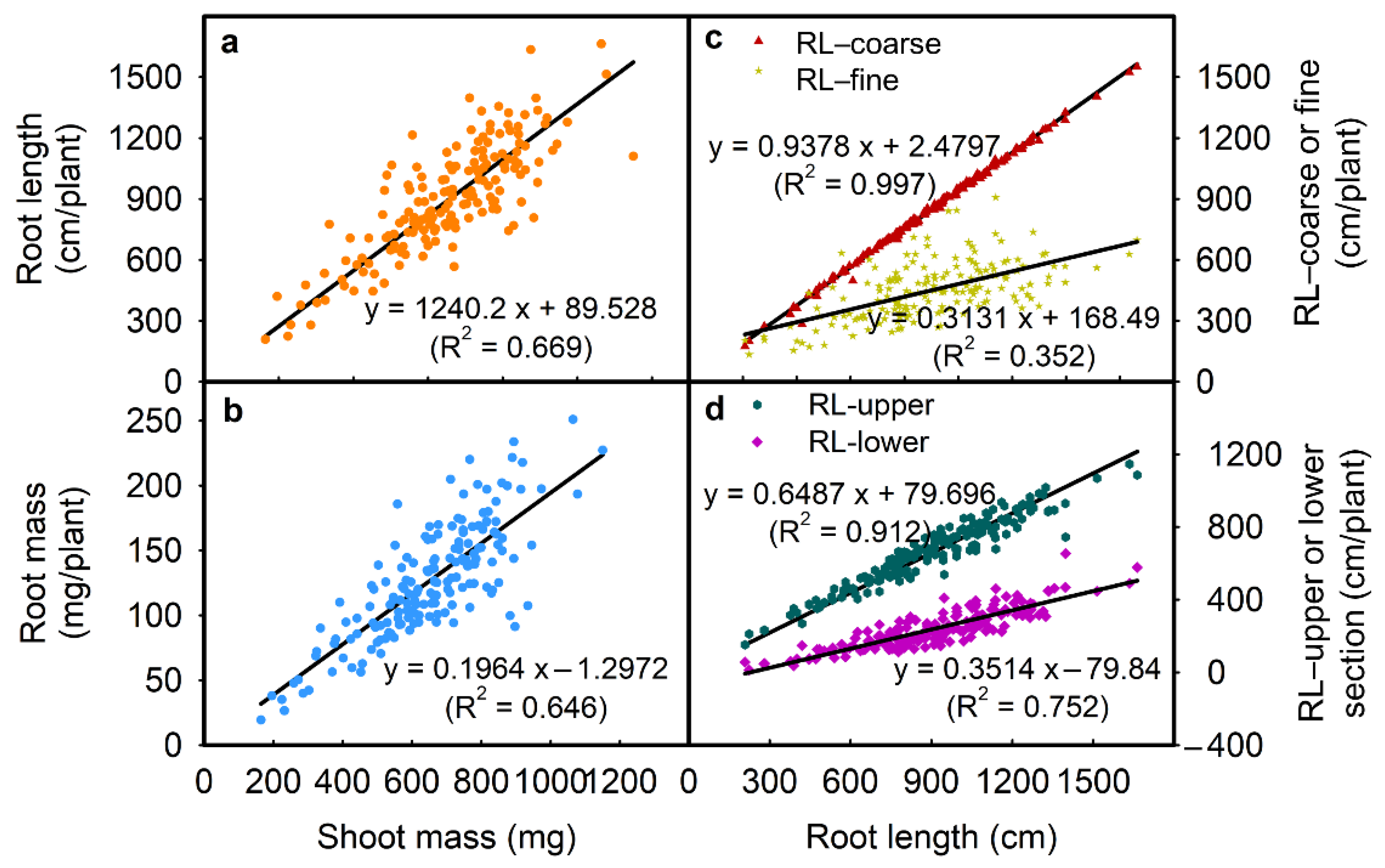
2.1.3. Correlation among Traits
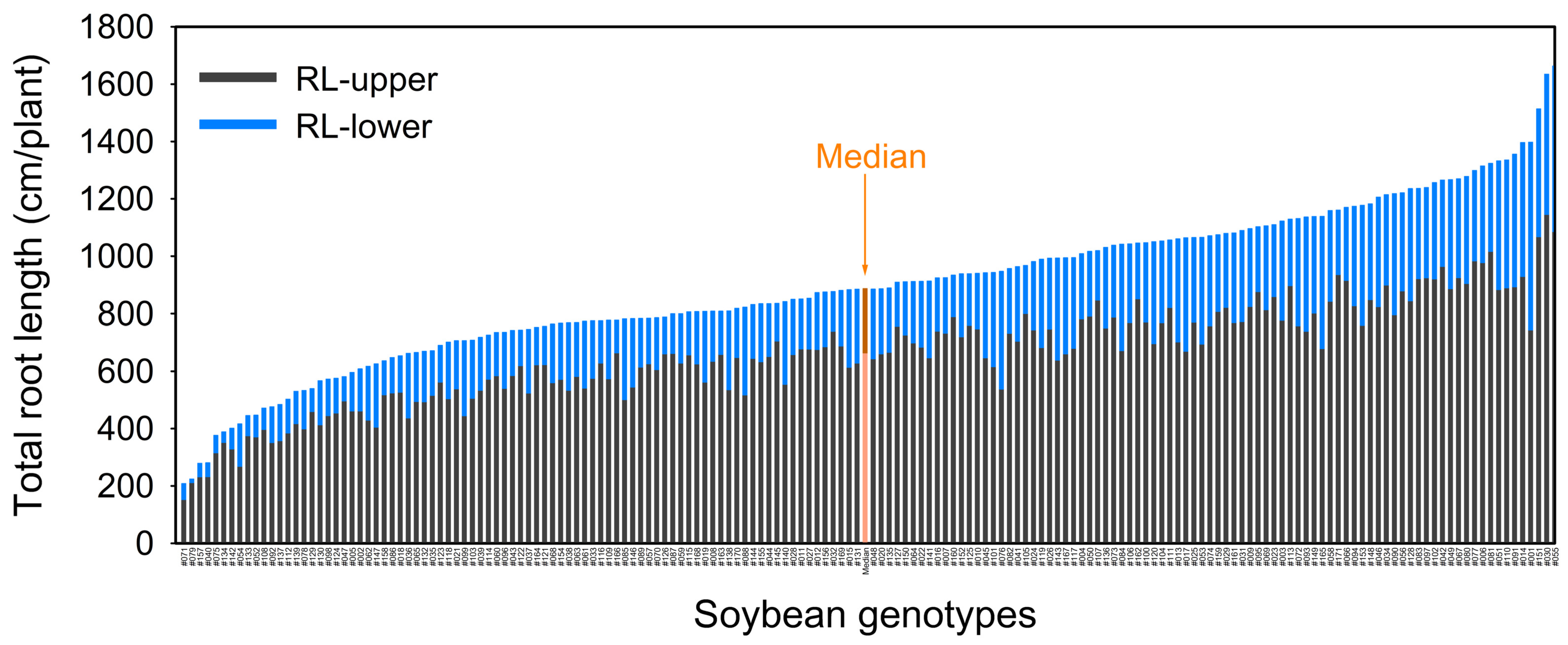
2.1.4. Root Trait Variability
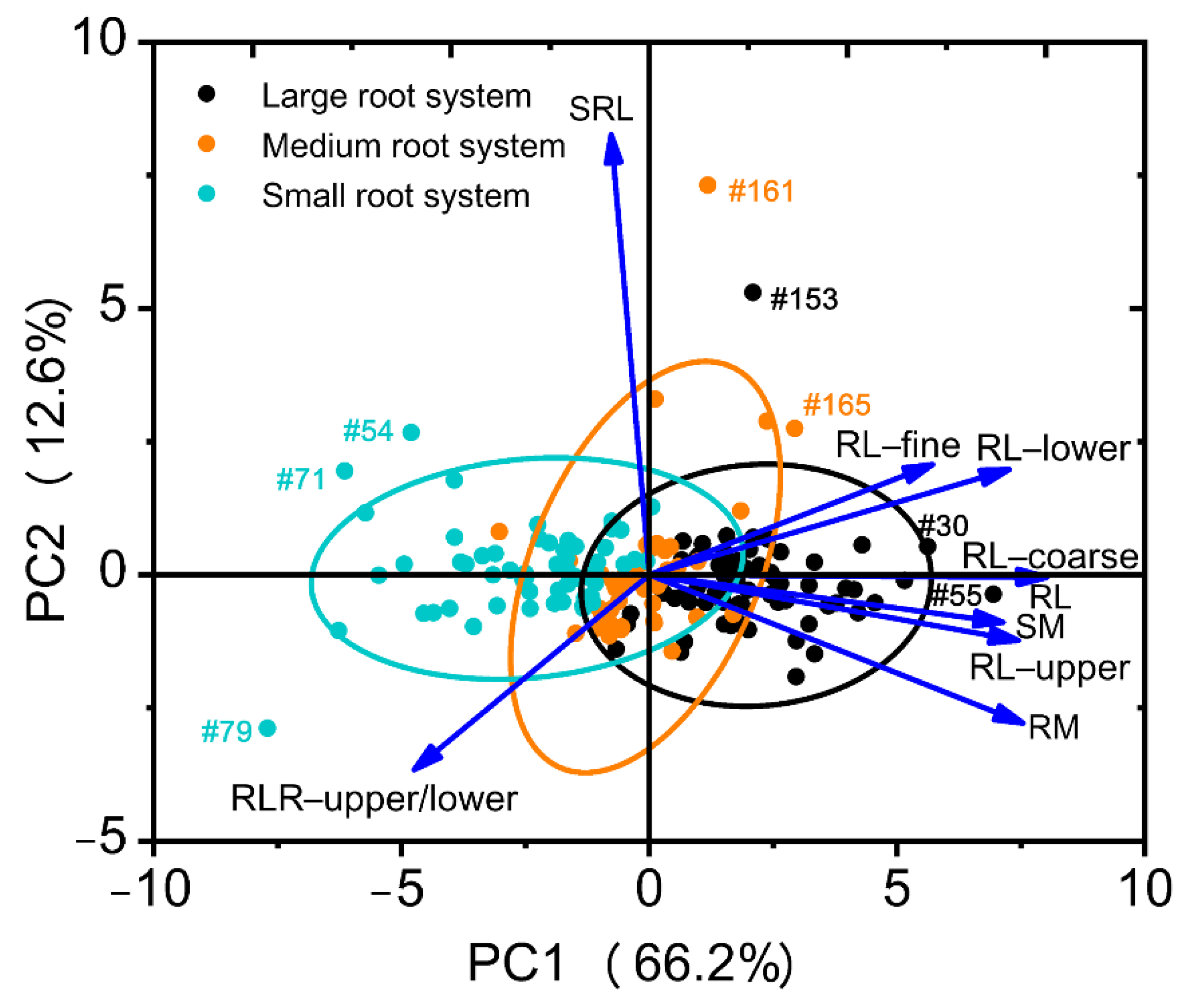
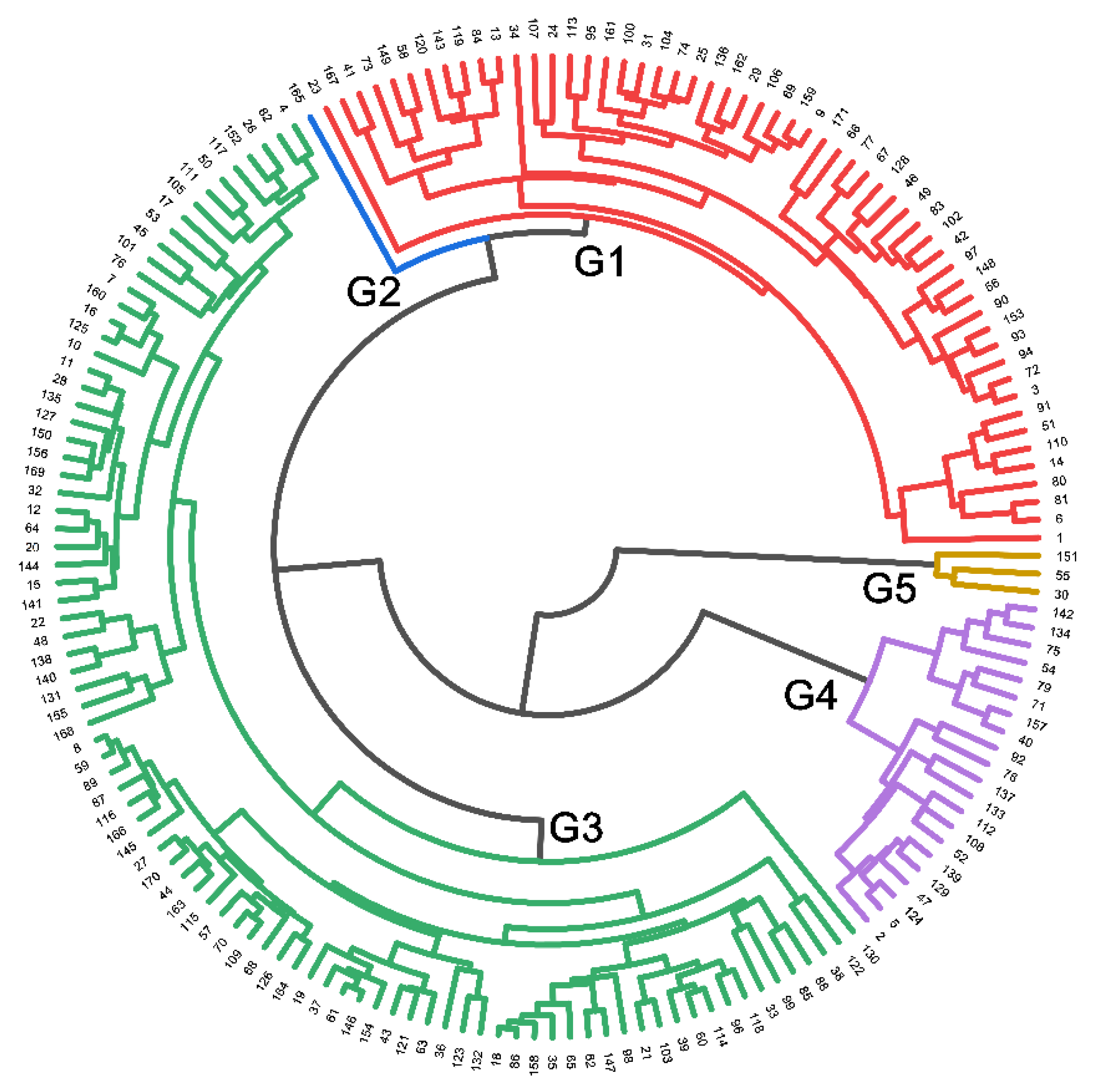
2.1.5. Genotype Homogeneous Grouping Based on Root Trait Variation
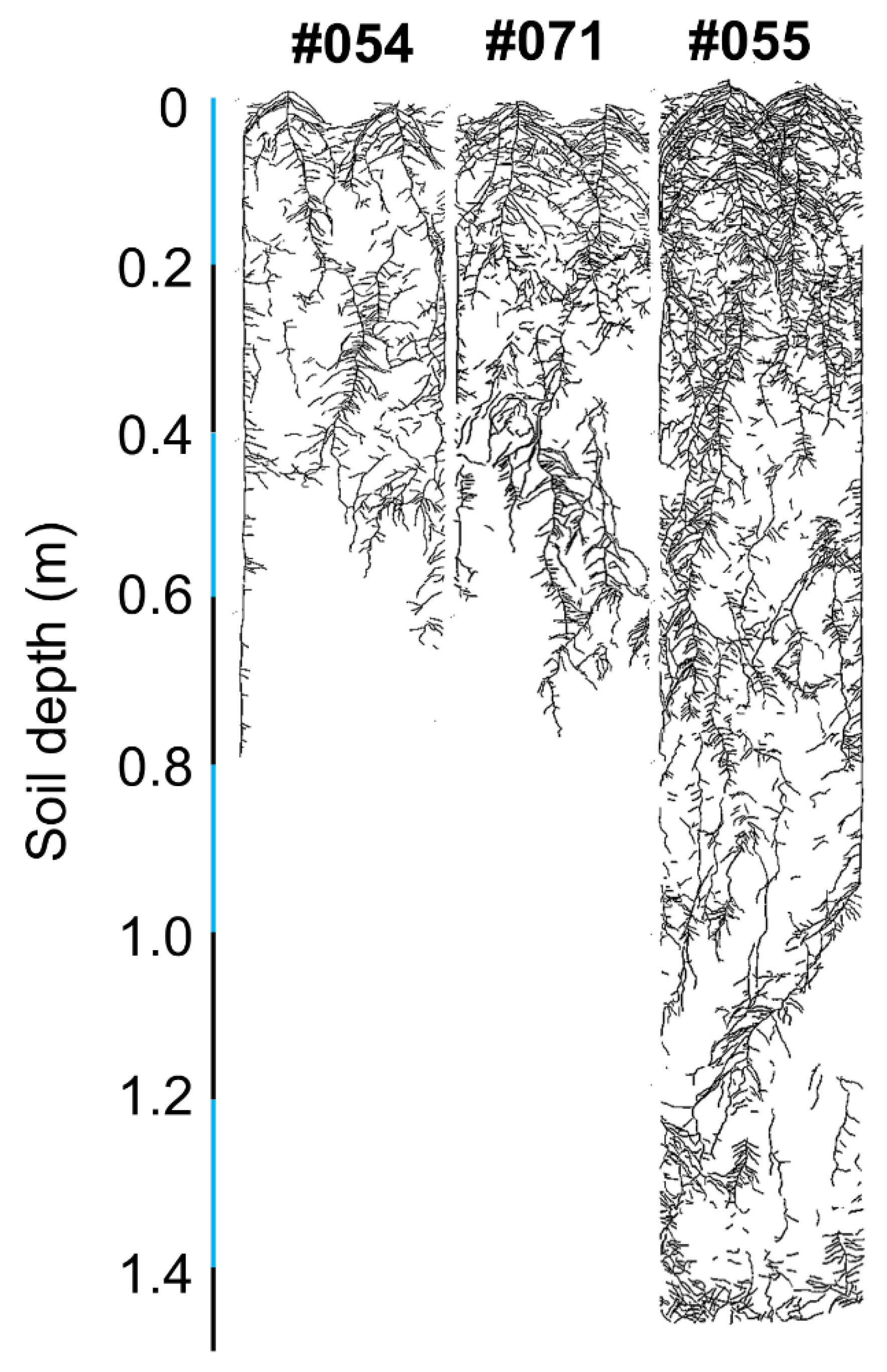
2.2. Validation of Root Characters in Rhizoboxes
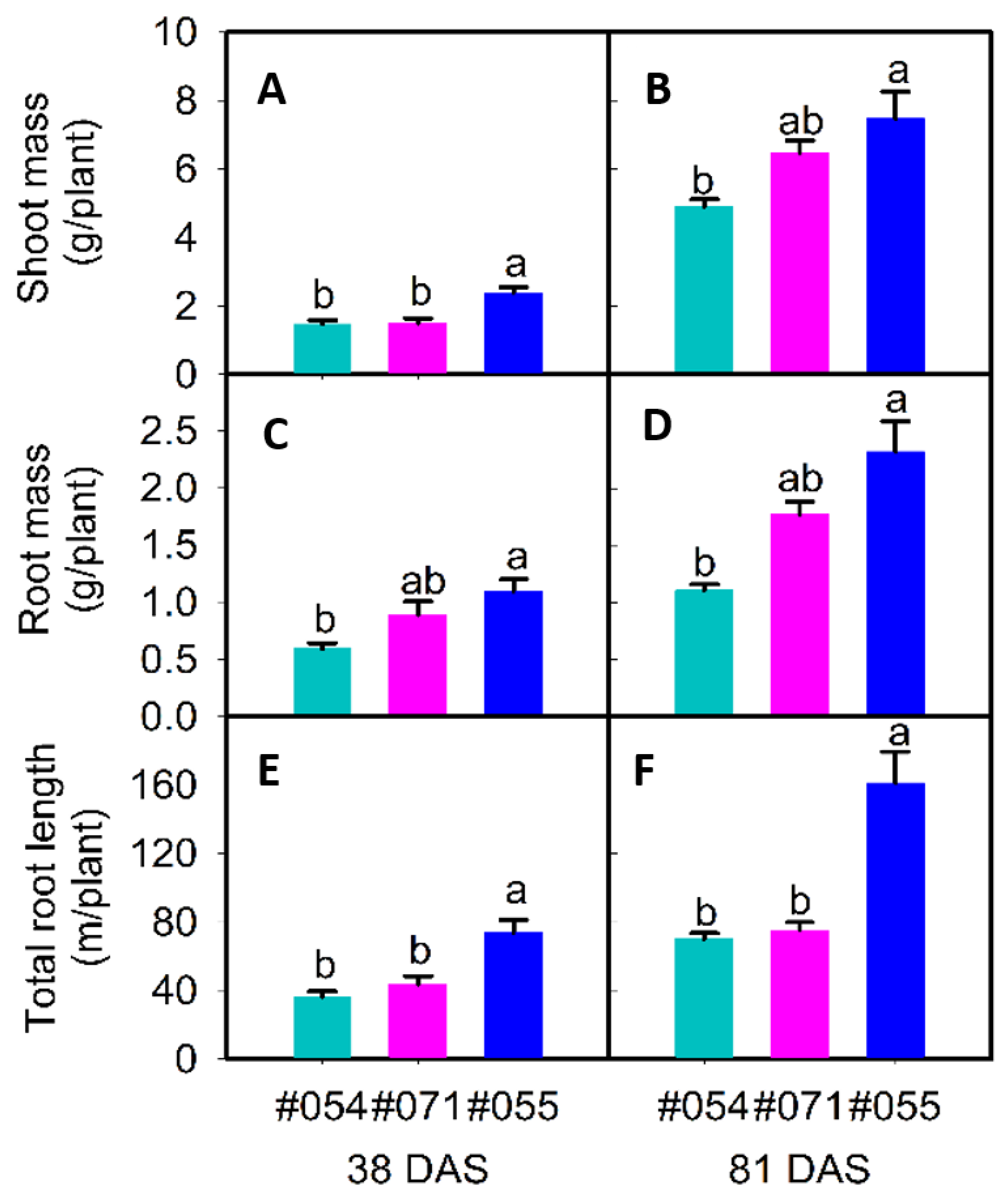
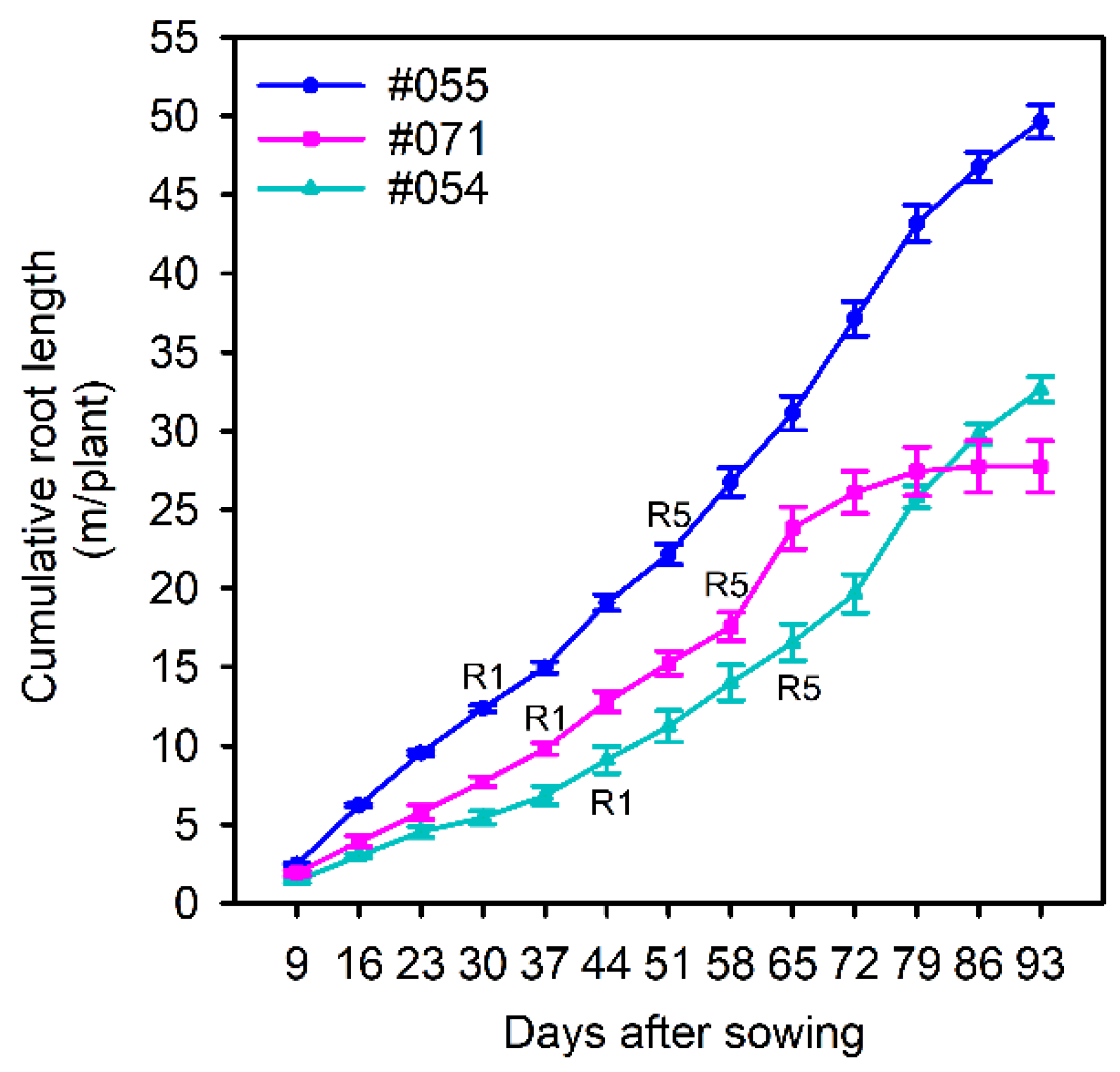
2.2.1. Phenotypes in Rhizoboxes
2.2.2. Correlations in Root and Growth Parameters between Experiments
3. Discussion
3.1. Variation among Root Architecture Traits
3.2. Genotype Selection Based on Root Trait Implications for Soybean Breeding
3.3. Validation of Root Characters in Soil
4. Materials and Methods
4.1. Soybean Genotypes
4.2. Experimental Design, Layout, and Maintenance
4.2.1. The Semi-Hydroponic Phenotyping System
4.2.2. Soil-Filled Rhizoboxes
4.3. Measurements and Assessments
4.3.1. The Semi-Hydroponic Phenotyping System
4.3.2. Soil-Filled Rhizoboxes
4.4. Root Scanning and Root Image Analysis
4.5. Data Treatment and Analysis
5. Conclusions
Supplementary Materials
 ) or bottom 15 (small values, indicated by
) or bottom 15 (small values, indicated by  ) among 171 genotypes from the phenotyping experiment for total root length (RL), with some also ranked in the top/bottom 15 for other traits. Figure S1: Root mass (RM; a), total root length (RL; b), root length in diameter fine (RL-fine; c), and root length in diameter coarse (RL-coarse; d) for genotypes in three source categories NHHR (68 genotypes), SHHR (75 genotypes), and PL (28 genotypes) used in the phenotyping experiment. NHHR, northern Huaihe River region; SHHR, southern Huaihe River region; PL, parental lines. The 171 genotypes were grown in a semi-hydroponic phenotyping platform 39 days after sowing. Significant differences are shown for the three source categories (p ≤ 0.05). The whiskers, box, and dot are determined by the 5th and 95th percentiles, 25th and 75th percentiles, and the 1st and 99th percentiles, respectively. The line and dashed inside the box marks are the median and mean, respectively, Figure S2: Principal component analysis of nine selected traits with CVs ≥ 0.3 across 171 soybean genotypes grown in a semi-hydroponic phenotyping platform 39 days after sowing. The position of each trait is shown for PC1 vs. PC2 representing 78.6% of the total variability. The genotypes with extreme traits (outliers) are indicated by genotype number. NHHR, northern Huaihe River region; SHHR, southern Huaihe River region; PL, parental lines.
) among 171 genotypes from the phenotyping experiment for total root length (RL), with some also ranked in the top/bottom 15 for other traits. Figure S1: Root mass (RM; a), total root length (RL; b), root length in diameter fine (RL-fine; c), and root length in diameter coarse (RL-coarse; d) for genotypes in three source categories NHHR (68 genotypes), SHHR (75 genotypes), and PL (28 genotypes) used in the phenotyping experiment. NHHR, northern Huaihe River region; SHHR, southern Huaihe River region; PL, parental lines. The 171 genotypes were grown in a semi-hydroponic phenotyping platform 39 days after sowing. Significant differences are shown for the three source categories (p ≤ 0.05). The whiskers, box, and dot are determined by the 5th and 95th percentiles, 25th and 75th percentiles, and the 1st and 99th percentiles, respectively. The line and dashed inside the box marks are the median and mean, respectively, Figure S2: Principal component analysis of nine selected traits with CVs ≥ 0.3 across 171 soybean genotypes grown in a semi-hydroponic phenotyping platform 39 days after sowing. The position of each trait is shown for PC1 vs. PC2 representing 78.6% of the total variability. The genotypes with extreme traits (outliers) are indicated by genotype number. NHHR, northern Huaihe River region; SHHR, southern Huaihe River region; PL, parental lines.Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Valliyodan, B.; Ye, H.; Song, L.; Murphy, M.; Shannon, J.G.; Nguyen, H.T. Genetic diversity and genomic strategies for improving drought and waterlogging tolerance in soybeans. J. Exp. Bot. 2017, 68, 1835–1849. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.Q.; He, J.B.; Wang, Y.F.; Xing, G.N.; Li, Y.; Yang, S.P.; Zhao, T.J.; Gai, J.Y. Geographic differentiation and phylogeographic relationships among world soybean populations. Crop J. 2020, 8, 260–272. [Google Scholar] [CrossRef]
- Li, S.Y.; Wang, W.B.; Cao, Y.Q.; Wang, C.L.; Yan, C.J.; Dong, L.J.; Wu, L.S.; Xie, F.T.; Song, S.H. How root traits would be affected by soybean yield improvement? An examination of historical cultivars grafted with record-yield cultivar scion. Plant Soil 2019, 439, 19–30. [Google Scholar] [CrossRef]
- Rosolem, C.A.; Neto, L.O.; Costa, V.E.; Grassmann, C.D. Ruzigrass root persistence and soybean root growth. Plant Soil 2019, 442, 333–341. [Google Scholar] [CrossRef]
- Schmidt, J.; Messmer, M.; Wilbois, K.P. Beneficial microorganisms for soybean (Glycine max (L.) Merr), with a focus on low root-zone temperatures. Plant Soil 2015, 397, 411–445. [Google Scholar] [CrossRef]
- Chen, W.; Yao, Q.M.; Patil, G.B.; Agarwal, G.; Deshmukh, R.K.; Lin, L.; Wang, B.; Wang, Y.Q.; Prince, S.J.; Song, L.; et al. Identification and comparative analysis of differential gene expression in soybean leaf tissue under drought and flooding stress revealed by RNA-Seq. Front. Plant Sci. 2016, 7, 1044. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.J.; Zeng, A.L.; Chen, P.Y.; Hummer, W.; Mokua, J.; Shannon, J.G.; Nguyen, H.T. Evaluation and development of flood-tolerant soybean cultivars. Plant Breed. 2017, 136, 913–923. [Google Scholar] [CrossRef]
- Seminario, A.; Song, L.; Zulet, A.; Nguyen, H.T.; Gonzalez, E.M.; Larrainzar, E. Drought stress causes a reduction in the biosynthesis of ascorbic acid in soybean plants. Front. Plant Sci. 2017, 8, 1042. [Google Scholar] [CrossRef] [Green Version]
- Jin, T.; Sun, Y.Y.; Shan, Z.; He, J.B.; Wang, N.; Gai, J.Y.; Li, Y. Natural variation in the promoter of GsERD15B affects salt tolerance in soybean. Plant Biotechnol. J. 2020, 19, 1155–1169. [Google Scholar] [CrossRef]
- Ge, Y.; Li, Y.; Zhu, Y.M.; Bai, X.; Lv, D.K.; Guo, D.J.; Ji, W.; Cai, H. Global transcriptome profiling of wild soybean (Glycine soja) roots under NaHCO3 treatment. BMC Plant Biol. 2010, 10, 153. [Google Scholar] [CrossRef] [Green Version]
- Foyer, C.H.; Nguyen, H.T.; Lam, H.M. Legumes-The art and science of environmentally sustainable agriculture. Plant Cell Environ. 2019, 42, 1–5. [Google Scholar] [CrossRef]
- Rehman, H.M.; Cheung, W.L.; Wong, K.S.; Xie, M.; Luk, C.Y.; Wong, F.L.; Li, M.W.; Tsai, S.N.; To, W.T.; Chan, L.Y.; et al. High-throughput mass spectrometric analysis of the whole proteome and secretome from Sinorhizobium fredii strains CCBAU25509 and CCBAU45436. Front. Microbiol. 2019, 10, 2569. [Google Scholar] [CrossRef]
- Zhao, H.Y.; Yang, A.H.; Kong, L.J.; Xie, F.T.; Wang, H.Y.; Ao, X. Proteome characterization of two contrasting soybean genotypes in response to different phosphorus treatments. AoB Plants 2021, 13, plab019. [Google Scholar] [CrossRef]
- Zhu, J.M.; Ingram, P.A.; Benfey, P.N.; Elich, T. From lab to field, new approaches to phenotyping root system architecture. Curr. Opin. Plant Biol. 2011, 14, 310–317. [Google Scholar] [CrossRef]
- Sun, X.C.; Ren, W.; Wang, P.; Chen, F.J.; Yuan, L.X.; Pan, Q.C.; Mi, G.H. Evaluation of maize root growth and genome-wide association studies of root traits in response to low nitrogen supply at seedling emergence. Crop J. 2021, 9, 794–804. [Google Scholar] [CrossRef]
- Mi, G.H.; Chen, F.J.; Yuan, L.X.; Zhang, F. Ideotype root system architecture for maize to achieve high yield and resource use efficiency in intensive cropping systems. Adv. Agron. 2016, 139, 73–97. [Google Scholar]
- van der Bom, F.J.T.; Williams, A.; Bell, M.J. Root architecture for improved resource capture: Trade-offs in complex environments. J. Exp. Bot. 2020, 71, 5752–5763. [Google Scholar] [CrossRef]
- Lynch, J.P.; Strock, C.F.; Schneider, H.M.; Sidhu, J.S.; Ajmera, I.; Galindo-Castañeda, T.; Klein, S.P.; Hanlon, M.T. Root anatomy and soil resource capture. Plant Soil 2021, 466, 21–63. [Google Scholar] [CrossRef]
- Yang, W.N.; Feng, H.; Zhang, X.H.; Zhang, J.; Doonan, J.H.; Batchelor, W.D.; Xiong, L.Z.; Yan, J.B. Crop phenomics and high-throughput phenotyping: Past decades, current challenges, and future perspectives. Mol. Plant 2020, 13, 187–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, P.; Wang, J.L.; Guo, X.Y.; Yang, W.N.; Zhao, C.J. High-throughput phenotyping: Breaking through the bottleneck in future crop breeding. Crop J. 2021, 9, 633–645. [Google Scholar] [CrossRef]
- Prince, S.J.; Valliyodan, B.; Ye, H.; Yang, M.; Tai, S.S.; Hu, W.S.; Murphy, M.; Durnell, L.A.; Song, L.; Joshi, T.; et al. Understanding genetic control of root system architecture in soybean: Insights into the genetic basis of lateral root number. Plant Cell Environ. 2019, 42, 212–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hina, A.; Cao, Y.C.; Song, S.Y.; Li, S.G.; Sharmin, R.A.; Elattar, M.A.; Bhat, J.A.; Zhao, T.J. High-resolution mapping in two RIL populations refines major “QTL Hotspot” regions for seed size and shape in soybean (Glycine max L.). Int. J. Mol. Sci. 2020, 21, 1040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Xu, M.G.; Hina, A.; Kong, J.J.; Gai, J.Y.; He, X.H.; Zhao, T.J. Seed storability of summer-planting soybeans under natural and artificial aging conditions. Legum. Res. 2019, 42, 250–259. [Google Scholar] [CrossRef]
- Chang, F.G.; Guo, C.Y.; Sun, F.L.; Zhang, J.S.; Wang, Z.L.; Kong, J.J.; He, Q.Y.; Sharmin, R.A.; Zhao, T.J. Genome-wide association studies for dynamic plant height and number of nodes on the main stem in summer sowing soybeans. Front. Plant Sci. 2018, 9, 1184. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.C.; Li, S.G.; He, X.H.; Chang, F.G.; Kong, J.J.; Gai, J.Y.; Zhao, T.J. Mapping QTLs for plant height and flowering time in a Chinese summer planting soybean RIL population. Euphytica 2017, 213, 39. [Google Scholar] [CrossRef]
- Pan, L.Y.; He, J.B.; Zhao, T.J.; Xing, G.N.; Wang, Y.F.; Yu, D.Y.; Chen, S.Y.; Gai, J.Y. Efficient QTL detection of flowering date in a soybean RIL population using the novel restricted two-stage multi-locus GWAS procedure. Theor. Appl. Genet. 2018, 131, 2581–2599. [Google Scholar] [CrossRef]
- Cao, Y.C.; Li, S.G.; Chen, G.L.; Wang, Y.F.; Bhat, J.A.; Karikari, B.; Kong, J.J.; Gai, J.Y.; Zhao, T.J. Deciphering the genetic architecture of plant height in soybean using two RIL populations sharing a common M8206 parent. Plants 2019, 8, 373. [Google Scholar] [CrossRef] [Green Version]
- Chang, F.G.; Lv, W.H.; Lv, P.Y.; Xiao, Y.T.; Yan, W.L.; Chen, S.; Zheng, L.Y.; Xie, P.; Wang, L.; Karikari, B.; et al. Exploring genetic architecture for pod-related traits in soybean using image-based phenotyping. Mol. Breed. 2021, 41, 28–49. [Google Scholar] [CrossRef]
- Khan, M.A.; Tong, F.; Wang, W.B.; He, J.B.; Zhao, T.J.; Gai, J.Y. Analysis of QTL-allele system conferring drought tolerance at seedling stage in a nested association mapping population of soybean (Glycine max (L.) Merr.) using a novel GWAS procedure. Planta 2018, 248, 947–962. [Google Scholar] [CrossRef]
- Korir, P.C.; Zhang, J.; Wu, K.; Zhao, T.; Gai, J. Association mapping combined with linkage analysis for aluminum tolerance among soybean cultivars released in Yellow and Changjiang River Valleys in China. Theor. Appl. Genet. 2013, 126, 1659–1675. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; Dunbabin, V.M.; Diggle, A.J.; Siddique, K.H.M.; Rengel, Z. Development of a novel semi-hydroponic phenotyping system for studying root architecture. Funct. Plant Biol. 2011, 38, 355–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.L.; Dunbabin, V.M.; Postma, J.A.; Diggle, A.J.; Palta, J.A.; Lynch, J.P.; Siddique, K.H.M.; Rengel, Z. Phenotypic variability and modelling of root structure of wild Lupinus angustifolius genotypes. Plant Soil 2011, 348, 345–364. [Google Scholar] [CrossRef]
- Chen, Y.L.; Dunbabin, V.M.; Diggle, A.J.; Siddique, K.H.M.; Rengel, Z. Assessing variability in root traits of wild Lupinus angustifolius germplasm: Basis for modelling root system structure. Plant Soil 2012, 354, 141–155. [Google Scholar] [CrossRef]
- Chen, Y.L.; Shan, F.C.; Nelson, M.N.; Siddique, K.H.M.; Rengel, Z. Root trait diversity, molecular marker diversity, and trait-marker associations in a core collection of Lupinus angustifolius. J. Exp. Bot. 2016, 67, 3683–3697. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Ghanem, M.E.; Siddique, K.H.M. Characterising root trait variability in chickpea (Cicer arietinum L.) germplasm. J. Exp. Bot. 2017, 68, 1987–1999. [Google Scholar]
- Qiao, S.; Fang, Y.; Wu, A.; Xu, B.; Zhang, S.; Deng, X.; Djalovic, I.; Siddique, K.H.M.; Chen, Y. Dissecting root trait variability in maize genotypes using the semi-hydroponic phenotyping platform. Plant Soil 2019, 439, 75–90. [Google Scholar] [CrossRef]
- Chen, Y.; Palta, J.; Prasad, P.V.V.; Siddique, K.H.M. Phenotypic variability in bread wheat root systems at the early vegetative stage. BMC Plant Biol. 2020, 20, 185. [Google Scholar] [CrossRef] [PubMed]
- Halder, T.; Liu, H.; Chen, Y.; Yan, G.J.; Siddique, K.H.M. Identification of candidate genes for root traits using genotype-phenotype association analysis of near-isogenic lines in hexaploid wheat (Triticum aestivum L.). Int. J. Mol. Sci. 2021, 22, 3579. [Google Scholar] [CrossRef]
- Wang, J.D.; Chen, Y.; Zhang, Y.E.; Zhang, Y.C.; Ai, Y.C.; Feng, Y.P.; Moody, D.; Diggle, A.; Damon, P.; Rengel, Z. Phenotyping and validation of root morphological traits in barley (Hordeum vulgare L.). Agron. J. 2021, 11, 1583. [Google Scholar] [CrossRef]
- Kuijken, R.C.; van Eeuwijk, F.A.; Marcelis, L.F.; Bouwmeester, H.J. Root phenotyping: From component trait in the lab to breeding. J. Exp. Bot. 2015, 66, 5389–5401. [Google Scholar] [CrossRef] [Green Version]
- Falk, K.G.; Jubery, T.Z.; O’Rourke, J.A.; Singh, A.; Sarkar, S.; Ganapathysubramanian, B.; Singh, A.K. Soybean root system architecture trait study through genotypic, phenotypic, and shape-based clusters. Plant Phenomics 2020, 2020, 1925495. [Google Scholar] [CrossRef]
- Wen, Z.H.; Li, H.B.; Shen, Q.; Tang, X.M.; Xiong, C.Y.; Li, H.G.; Pang, J.Y.; Ryan, M.H.; Lambers, H.; Shen, J. Tradeoffs among root morphology, exudation and mycorrhizal symbioses for phosphorus-acquisition strategies of 16 crop species. New Phytol. 2019, 223, 882–895. [Google Scholar] [CrossRef]
- Lynch, J.P. Root phenotypes for improved nutrient capture: An underexploited opportunity for global agriculture. New Phytol. 2019, 223, 548–564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zobel, R.W.; Kinraide, T.B.; Baligar, V.C. Fine root diameters can change in response to changes in nutrient concentrations. Plant Soil 2007, 297, 243–254. [Google Scholar] [CrossRef]
- Xiong, R.T.; Liu, S.; Considine, M.J.; Siddique, K.H.M.; Lam, H.M.; Chen, Y. Root system architecture, physiological and transcriptional traits of soybean (Glycine max L.) in response to water deficit: A review. Physiol. Plant 2021, 172, 405–418. [Google Scholar] [CrossRef]
- Yang, X.H.; Wu, Z.P.; Zhang, G.D. Evolution of root characters of soybean varieties developed in different years. Agric. Sci. China 2002, 1, 71–75. [Google Scholar]
- Mitchell, R.L.; Russell, W.J. Root development and rooting patterns of soybean (Glycine max (L.) Merrill) evaluated under field conditions. Agron. J. 1971, 63, 313–316. [Google Scholar] [CrossRef]
- Araki, H.; Morita, S.; Tatsumi, J.; Iijima, M. Physiol-morphological analysis on axile root growth in upland rice. Plant Prod. Sci. 2002, 5, 286–293. [Google Scholar] [CrossRef] [Green Version]
- Rincon, C.A.; Raper, C.D.J.; Patterson, R.P. Genotypic differences in root anatomy affecting water movement through roots of soybean. Int. J. Plant Sci. 2003, 164, 543–551. [Google Scholar] [CrossRef]
- Xavier, L.J.C.; Germida, J.J. Selective interactions between arbuscular mycorrhizal fungi and Rhizobium leguminosarum bv. viceae enhance pea yield and nutrition. Biol. Fertil. Soils 2003, 37, 261–267. [Google Scholar] [CrossRef]
- Wang, X.; Pan, Q.; Chen, F.; Yan, X.; Liao, H. Effects of co-inoculation with arbuscular mycorrhizal fungi and rhizobia on soybean growth as related to root architecture and availability of N and P. Mycorrhiza 2011, 21, 173–181. [Google Scholar] [CrossRef]
- Jitsuyama, Y. Morphological root responses of soybean to rhizosphere hypoxia reflect waterlogging tolerance. Can. J. Plant Sci. 2015, 95, 999–1005. [Google Scholar] [CrossRef]
- Suriyagoda, L.D.B.; Lambers, H.; Renton, M.; Ryan, M.H. Growth, carboxylate exudates and nutrient dynamics in three herbaceous perennial plant species under low, moderate and high phosphorus supply. Plant Soil 2012, 358, 105–117. [Google Scholar] [CrossRef]
- Fan, J.W.; Du, Y.L.; Turner, N.C.; Wang, B.R.; Fang, Y.; Xi, Y.; Guo, X.R.; Li, F.M. Changes in root morphology and physiology to limited phosphorus and moisture in a locally-selected cultivar and an introduced cultivar of Medicago sativa L. growing in alkaline soil. Plant Soil 2015, 392, 215–226. [Google Scholar] [CrossRef]
- Wu, A.J.; Fang, Y.; Liu, S.; Wang, H.; Xu, B.C.; Zhang, S.Q.; Deng, X.P.; Palta, J.A.; Siddique, K.H.M.; Chen, Y.L. Root morphology and rhizosheath acid phosphatase activity in legume and graminoid species respond differently to low phosphorus supply. Rhizosphere 2021, 19, 100391. [Google Scholar] [CrossRef]
- Tjoelker, M.G.; Craine, J.M.; Wedin, D.; Reich, P.B.; Tilman, D. Linking leaf and root trait syndromes among 39 grassland and savannah species. New Phytol. 2005, 167, 493–508. [Google Scholar] [CrossRef] [PubMed]
- Roumet, C.; Urcelay, C.; Diaz, S. Suites of root traits differ between annual and perennial species growing in the field. New Phytol. 2006, 170, 357–368. [Google Scholar] [CrossRef]
- Vandamme, E.; Renkens, M.; Pypers, P.; Smolders, E.; Vanlauwe, B.; Merckx, R. Root hairs explain P uptake efficiency of soybean genotypes grown in a P-deficient Ferralsol. Plant Soil 2013, 369, 269–282. [Google Scholar] [CrossRef]
- Zhang, X.M. Growth characteristics and cultivation techniques of a new soybean variety Sidou 520. Seed Ind. Guide 2010, 3, 27–28. (In Chinese) [Google Scholar]
- Zheng, L. Characteristics and high-yield cultivation techniques of Xudou 13. Xiandai Nongye Keji 2008, 19, 243–244. (In Chinese) [Google Scholar]
- Zhou, C.Z.; He, L.G.; Lin, H.M. Characteristics and high-yielding cultivation techniques of a new soybean variety Huaidou 10. Bull. Agric. Sci. Technol. 2008, 8, 139–140, (In Chinese with English abstract). [Google Scholar]
- Falk, K.G.; Jubery, T.Z.; Mirnezami, S.V.; Parmley, K.A.; Sarkar, S.; Singh, A.; Ganapathysubramanian, B.; Singh, A.K. Computer vision and machine learning enabled soybean root phenotyping pipeline. Plant Methods 2020, 16, 5. [Google Scholar] [CrossRef] [PubMed]
- Xiong, D.J.; Zhao, T.J.; Gai, J.Y. Parental analysis of soybean cultivars released in China. Sci. Agric. Sin. 2008, 41, 2589–2598, (In Chinese with English abstract). [Google Scholar]
- Wang, Y.D.; Xu, M.G.; Zhang, Y.J.; Weng, Y.Y.; Li, X.Y.; Kong, J.J.; Zhao, T.J.; He, X.H. Identification of drought-tolerance of soybean germplasms from Yangtze and Huaihe River Valleys at seedling stage. Soybean Sci. 2017, 36, 669–678, (In Chinese with English abstract). [Google Scholar]
- Wang, X.; Wang, Z.B.; Xu, Z.J. Breeding and cultivation techniques of a new soybean variety Xudou 16. Soybean Sci. Technol. 2011, 6, 64–65, (In Chinese with English abstract). [Google Scholar]
- Chen, Y.L.; Palta, J.; Clements, J.; Buirchell, B.; Siddique, K.H.M.; Rengel, Z. Root architecture alteration of narrow-leafed lupin and wheat in response to soil compaction. Field Crops Res. 2014, 165, 61–70. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Zhou, T.; Siddique, K.H.M. Method for characterization of root traits in chickpea germplasm for legume genomics and breeding. In Legume Genomics; Jain, M., Garg, R., Eds.; Humana: New York, NY, USA, 2020; Volume 2017, pp. 269–275. [Google Scholar]
- Jolliffe, I.T.; Cadima, J. Principal component analysis: A review and recent developments. Philos. Trans. R. Soc. A 2016, 374, 20150202. [Google Scholar] [CrossRef]

| Trait | Minimum | Maximum | Median | Mean | Std. Dev. | CV | p Value |
|---|---|---|---|---|---|---|---|
| MRD | 28.8 | 65.0 | 47.5 | 46.6 | 7.37 | 0.16 | <0.001 |
| RW | 9.57 | 20.5 | 14.7 | 14.9 | 2.70 | 0.18 | <0.001 |
| RA | 63.0 | 160 | 109 | 109 | 28.0 | 0.26 | <0.001 |
| RL | 208 | 1663 | 885 | 898 | 331 | 0.37 | <0.001 |
| RD | 0.20 | 0.47 | 0.42 | 0.42 | 0.03 | 0.07 | <0.001 |
| RM | 19.4 | 251 | 124 | 127 | 55.8 | 0.44 | <0.001 |
| SRL | 5.27 | 25.6 | 7.33 | 7.72 | 3.81 | 0.49 | 0.64 |
| RTD | 98.0 | 219 | 141 | 143 | 40.2 | 0.28 | 0.91 |
| RSM | 0.11 | 0.32 | 0.19 | 0.19 | 0.05 | 0.27 | <0.001 |
| LN | 3.00 | 6.00 | 5.00 | 4.81 | 0.67 | 0.14 | <0.001 |
| HL | 2.20 | 9.10 | 4.50 | 4.55 | 1.24 | 0.27 | <0.001 |
| SH | 18.7 | 86.7 | 52.3 | 51.0 | 11.3 | 0.22 | <0.001 |
| SM | 164 | 1150 | 663 | 652 | 213 | 0.33 | <0.001 |
| RL-upper | 152 | 1146 | 664 | 662 | 226 | 0.34 | <0.001 |
| RL-lower | 11.5 | 654 | 223 | 236 | 136 | 0.58 | <0.001 |
| RL-fine | 134 | 908 | 448 | 450 | 208 | 0.46 | <0.001 |
| RL-coarse | 175 | 1551 | 849 | 845 | 309 | 0.37 | <0.001 |
| RLR-upper/lower | 1.18 | 16.5 | 3.27 | 3.59 | 2.23 | 0.62 | <0.001 |
| - | RM | SRL | SM | RL- Upper | RL- Lower | RL- Fine | RL- Coarse | RLR-Upper/ Lower |
|---|---|---|---|---|---|---|---|---|
| RL | 0.903 ** | −0.023 ns | 0.818 ** | 0.955 ** | 0.867 ** | 0.593 ** | 0.998 ** | −0.469 ** |
| RM | - | −0.322 ** | 0.803 ** | 0.868 ** | 0.774 ** | 0.505 ** | 0.900 ** | −0.429 ** |
| SRL | - | - | −0.101 ns | −0.055 ns | 0.034 ns | −0.022 ns | −0.033 ns | −0.014 ns |
| SM | - | - | - | 0.812 ** | 0.657 ** | 0.599 ** | 0.820 ** | −0.404 ** |
| RL-upper | - | - | - | - | 0.680 ** | 0.509 ** | 0.951 ** | −0.303 ** |
| RL-lower | - | - | - | - | - | 0.610 ** | 0.869 ** | −0.650 ** |
| RL-fine | - | - | - | - | - | - | 0.608 ** | −0.514 ** |
| RL-coarse | - | - | - | - | - | - | - | −0.474 ** |
| Traits | PC1 | PC2 |
|---|---|---|
| RL | 0.976 | −0.016 |
| RM | 0.917 | −0.309 |
| SRL | −0.083 | 0.883 |
| SM | 0.870 | −0.106 |
| RL-upper | 0.907 | −0.143 |
| RL-lower | 0.889 | 0.199 |
| RL-fine | 0.701 | 0.212 |
| RL-coarse | 0.978 | −0.018 |
| RLR-upper/lower | −0.585 | −0.383 |
| Eigenvalue | 5.96 | 1.14 |
| Variability (%) | 66.2 | 12.6 |
| Cumulative (%) | 66.2 | 78.9 |
| Trait | Unit | 38 DAS | 81 DAS | 160 DAS | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| #054 | #055 | #071 | #054 | #055 | #071 | #054 | #055 | #071 | ||
| RM | g plant−1 | 0.59 b | 1.10 a | 0.89 ab | 1.11 b | 2.32 a | 1.78 ab | 6.70 b | 12.5 a | 2.36 c |
| RSM | - | 0.41 b | 0.46 ab | 0.59 a | 0.23 b | 0.31 a | 0.27 ab | 0.30 ns | 0.30 ns | 0.20 ns |
| HL | cm | 4.25 b | 8.17 a | 5.92 b | 4.05 b | 7.50 a | 7.00 a | 5.00 b | 7.67 a | 6.42 ab |
| SH | cm | 98.0 ab | 107 a | 80.5 b | 100 ab | 114 a | 88.5 b | 108 ab | 121 a | 93.3 b |
| SM | g plant−1 | 1.48 b | 2.38 a | 1.50 b | 4.90 b | 7.47 a | 6.48 ab | 22.1 ab | 41.7 a | 11.6 b |
| RL | m | 36.6 b | 74.0 a | 43.7 b | 70.2 b | 161 a | 75.3 b | - | - | - |
| RD | mm | 0.39 ns | 0.41 ns | 0.40 ns | 0.44 ns | 0.43 ns | 0.42 ns | - | - | - |
| SRL | m g−1 | 6.17 ab | 6.76 a | 4.94 b | 6.34 ab | 6.93 a | 4.24 b | - | - | - |
| RL-upper | m | 36.0 b | 71.0 a | 40.4 b | 62.7 b | 124 a | 60.7 b | - | - | - |
| RL-lower | m | 0.32 b | 3.04 a | 3.31 a | 7.54 b | 37.3 a | 14.6 b | - | - | - |
| RL-fine | m | 30.5 b | 62.7 a | 36.2 b | 57.4 b | 134 a | 62.1 b | - | - | - |
| RL-coarse | m | 5.83 b | 11.2 a | 7.52 ab | 12.8 b | 27.5 a | 13.2 b | - | - | - |
| RLR-upper/lower | m | 116 a | 25.9 b | 12.4 b | 16.5 a | 3.39 b | 5.17 b | - | - | - |
| - | RL | RD | RM | SRL | RSM | HL | SH | SM | RL-Upper | RL-Lower | RL-Fine | RL-Coarse | RLR-Upper/Lower |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RL | - | 0.116 | 0.879 ** | 0.591 | 0.750 * | 0.637 | 0.583 | 0.807 ** | 0.991 ** | 0.958 ** | 0.990 ** | 0.858 ** | −0.385 |
| RD | 0.377 | - | 0.053 | 0.181 | 0.256 | −0.014 | 0.187 | −0.107 | 0.073 | 0.201 | 0.129 | 0.031 | −0.033 |
| RM | 0.865 ** | 0.621 | - | 0.138 | 0.860 ** | 0.885 ** | 0.292 | 0.952 ** | 0.828 ** | 0.935 ** | 0.862 ** | 0.781 * | −0.545 |
| SRL | 0.504 | −0.271 | 0.020 | - | 0.117 | −0.161 | 0.769 * | 0.065 | 0.651 | 0.425 | 0.602 | 0.451 | 0.108 |
| RSM | 0.012 | 0.408 | 0.459 | −0.785 * | - | 0.819 ** | 0.333 | 0.670 * | 0.694 * | 0.827 ** | 0.762 * | 0.574 | −0.552 |
| HL | 0.913 ** | 0.298 | 0.866 ** | 0.265 | 0.191 | - | 0.179 | 0.830 ** | 0.587 | 0.707 * | 0.644 | 0.495 | −0.332 |
| SH | 0.604 | 0.387 | 0.314 | 0.599 | −0.477 | 0.479 | - | 0.203 | 0.595 | 0.522 | 0.649 | 0.247 | −0.072 |
| SM | 0.949 ** | 0.330 | 0.762 * | 0.604 | −0.218 | 0.838 ** | 0.667 * | - | 0.761 * | 0.858 ** | 0.779 * | 0.764 * | −0.509 |
| RL-upper | 0.998 ** | 0.356 | 0.833 ** | 0.554 | −0.046 | 0.893 ** | 0.633 | 0.956 ** | - | 0.912 ** | 0.983 ** | 0.845 ** | −0.286 |
| RL-lower | 0.596 | 0.450 | 0.850 ** | −0.274 | 0.668 * | 0.756 * | 0.011 | 0.457 | 0.538 | - | 0.945 ** | 0.834 ** | −0.575 |
| RL-fine | 0.998 ** | 0.336 | 0.844 ** | 0.528 | −0.014 | 0.917 ** | 0.616 | 0.948 ** | 0.997 ** | 0.581 | - | 0.779 * | −0.380 |
| RL-coarse | 0.953 ** | 0.576 | 0.925 ** | 0.349 | 0.152 | 0.845 ** | 0.509 | 0.902 ** | 0.944 ** | 0.647 | 0.935 ** | - | −0.338 |
| RLR-upper/lower | −0.493 | −0.253 | −0.761 * | 0.365 | −0.770 * | −0.690 * | 0.186 | −0.314 | −0.438 | −0.903 ** | −0.484 | −0.513 | - |
| Traits | (a) 38 DAS | (b) 81 DAS | (c) 160 DAS |
|---|---|---|---|
| RM | 0.591 | 0.665 | 0.901 ** |
| RSM | −0.648 | 0.187 | 0.884 ** |
| HL | 0.690 * | 0.670 * | 0.672 * |
| SH | 0.732 * | 0.801 ** | 0.776 * |
| SM | 0.887 ** | 0.511 | 0.900 ** |
| RL | 0.854 ** | 0.886 ** | - |
| RD | 0.004 | 0.310 | - |
| SRL | −0.397 | −0.563 | - |
| RL-upper | 0.831 ** | 0.851 ** | - |
| RL-lower | 0.299 | 0.866 ** | - |
| RL-fine | 0.179 | 0.202 | - |
| RL-coarse | 0.733 * | 0.664 | - |
| RLR-upper/lower | −0.196 | 0.195 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Begum, N.; An, T.; Zhao, T.; Xu, B.; Zhang, S.; Deng, X.; Lam, H.-M.; Nguyen, H.T.; Siddique, K.H.M.; et al. Characterization of Root System Architecture Traits in Diverse Soybean Genotypes Using a Semi-Hydroponic System. Plants 2021, 10, 2781. https://doi.org/10.3390/plants10122781
Liu S, Begum N, An T, Zhao T, Xu B, Zhang S, Deng X, Lam H-M, Nguyen HT, Siddique KHM, et al. Characterization of Root System Architecture Traits in Diverse Soybean Genotypes Using a Semi-Hydroponic System. Plants. 2021; 10(12):2781. https://doi.org/10.3390/plants10122781
Chicago/Turabian StyleLiu, Shuo, Naheeda Begum, Tingting An, Tuanjie Zhao, Bingcheng Xu, Suiqi Zhang, Xiping Deng, Hon-Ming Lam, Henry T. Nguyen, Kadambot H. M. Siddique, and et al. 2021. "Characterization of Root System Architecture Traits in Diverse Soybean Genotypes Using a Semi-Hydroponic System" Plants 10, no. 12: 2781. https://doi.org/10.3390/plants10122781
APA StyleLiu, S., Begum, N., An, T., Zhao, T., Xu, B., Zhang, S., Deng, X., Lam, H.-M., Nguyen, H. T., Siddique, K. H. M., & Chen, Y. (2021). Characterization of Root System Architecture Traits in Diverse Soybean Genotypes Using a Semi-Hydroponic System. Plants, 10(12), 2781. https://doi.org/10.3390/plants10122781













