Inoculation with Ericoid Mycorrhizal Associations Alleviates Drought Stress in Lowland and Upland Velvetleaf Blueberry (Vaccinium myrtilloides) Seedlings
Abstract
1. Introduction
2. Results
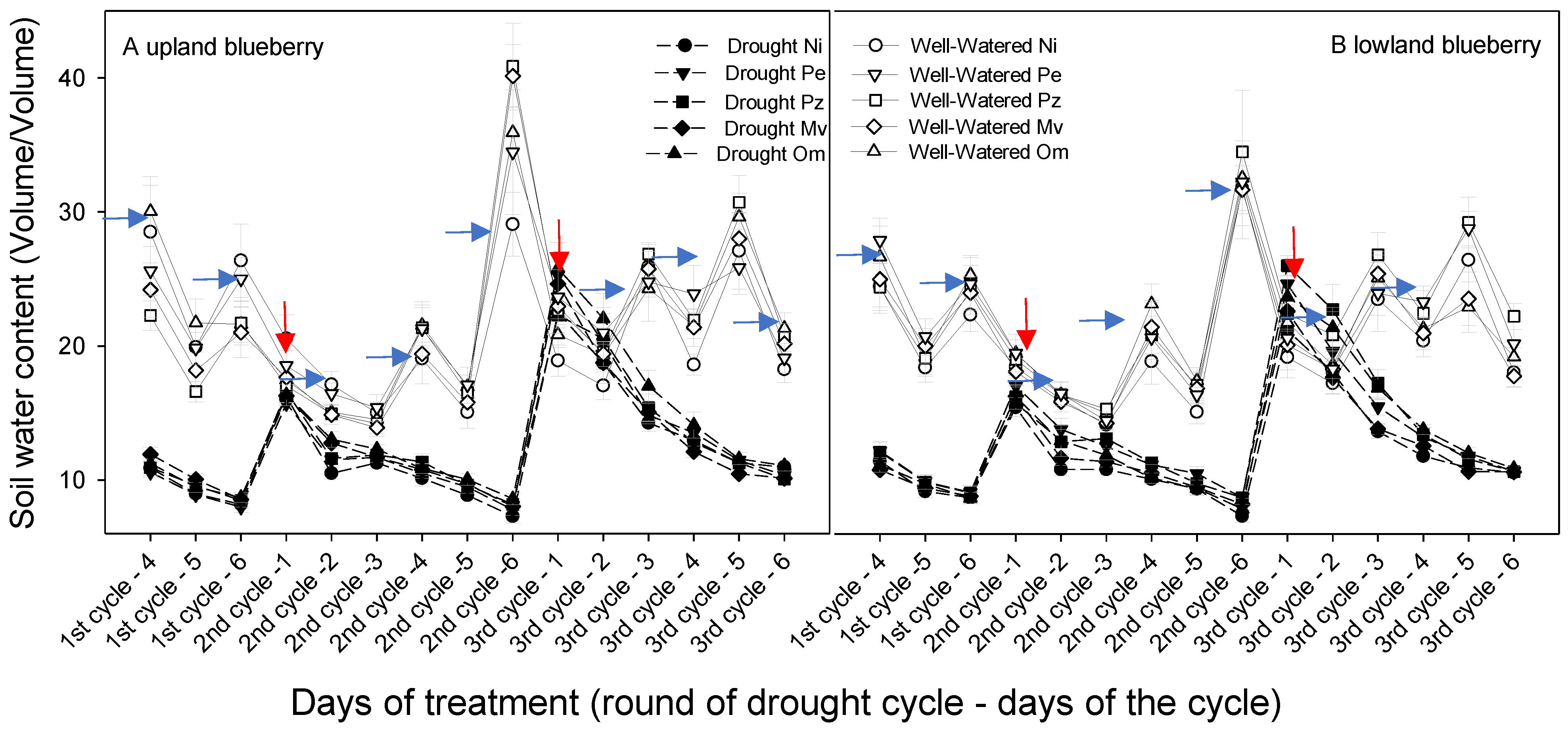
2.1. Volumetric Soil Water Content
2.2. Root Colonization
2.3. Plant Mortality
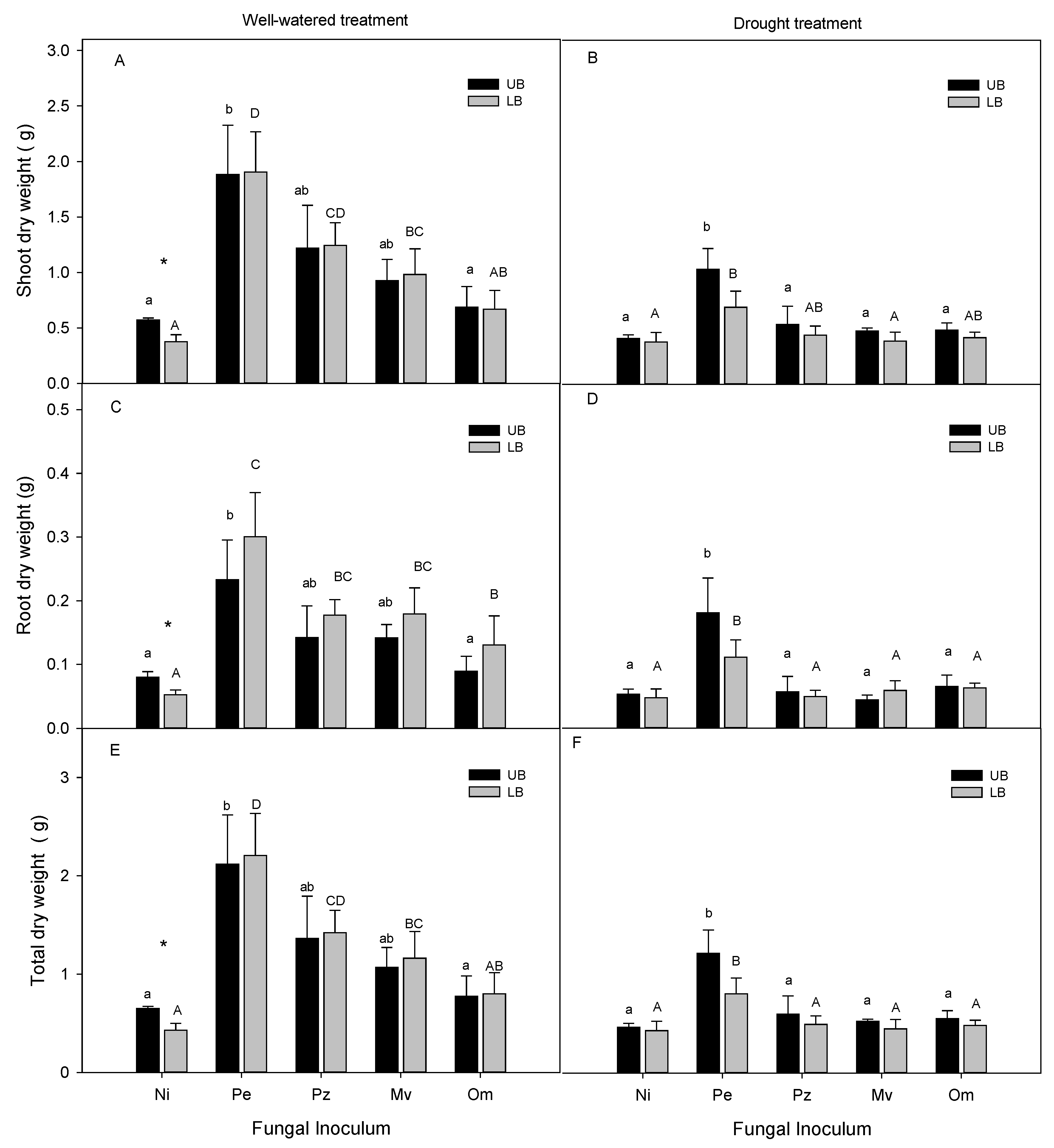
2.4. Dry Weights
2.5. Leaf Chlorophyll Concentrations and Gas Exchange
2.6. Shoot Water Potentials
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Isolation and Identification of ERM Fungi
4.3. Fungal Cultures
4.4. Root Inoculation and Drought Treatment
4.5. Volumetric Soil Water Content
4.6. Root Colonization by ERM Fungi
4.7. Plant Mortality and Dry Weights
4.8. Gas Exchange
4.9. Leaf Chlorophyll Concentrations
4.10. Shoot Water Potentials
4.11. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stevens, P.F.; Luteyn, J.; Oliver, E.G.H.; Bell, T.L.; Brown, E.A.; Crowden, R.K.; George, A.S.; Jordan, G.J.; Ladd, P.; Lemson, K.; et al. Ericaceae. In The Families and Genera of Flowering Plants; Kubitzki, K., Ed.; Springer: Berlin/Heidelberg, Germany, 2004; Volume 6, pp. 145–194. [Google Scholar]
- Read, D.J. The structure and function of the vegetative mycelium of mycorrhizal roots. In The Ecology and Physiology of the Fungal Mycelium; British Mycological Society Symposium 8; Jennings, D.H., Rayner, A.D.M., Eds.; Cambridge University Press: Cambridge, UK, 1984; pp. 215–240. [Google Scholar]
- Perotto, S.; Daghino, S.; Martino, E. Ericoid mycorrhizal fungi and their genomes: Another side to the mycorrhizal symbiosis? New Phytol. 2018, 220, 1141–1147. [Google Scholar] [CrossRef]
- Vohník, M. Ericoid mycorrhizal symbiosis: Theoretical background and methods for its comprehensive investigation. Mycorrhiza 2020, 30, 671–695. [Google Scholar] [CrossRef]
- Leopold, D.R. Ericoid fungal diversity: Challenges and opportunities for mycorrhizal research. Fungal Ecol. 2016, 24, 114–123. [Google Scholar] [CrossRef]
- Van Geel, M.; Jacquemyn, H.; Peeters, G.; Van Acker, K.; Honnay, O.; Ceulemans, T. Diversity and community structure of ericoid mycorrhizal fungi in European bogs and heathlands across a gradient of nitrogen deposition. New Phytol. 2020, 228, 1640–1651. [Google Scholar] [CrossRef]
- Fadaei, S.; Vaziriyeganeh, M.; Young, M.; Sherr, I.; Zwiazek, J.J. Ericoid mycorrhizal fungi enhance salt tolerance in ericaceous plants. Mycorrhiza 2020, 30, 419–429. [Google Scholar] [CrossRef]
- Lehto, T.; Zwiazek, J.J. Ectomycorrhizas and water relations of trees: A review. Mycorrhiza 2011, 21, 71–90. [Google Scholar] [CrossRef]
- Bárzana, G.; Aroca, R.; Bienert, G.P.; Chaumont, F.; Ruiz-Lozano, J.M. New insights into the regulation of aquaporins by the arbuscular mycorrhizal symbiosis in maize plants under drought stress and possible implications for plant performance. Mol. Plant-Microbe Interact. 2014, 27, 349–363. [Google Scholar] [CrossRef]
- Bahadur, A.; Batool, A.; Nasir, F.; Jiang, S.; Mingsen, Q.; Zhang, Q.; Pan, J.; Liu, Y.; Feng, H. Mechanistic insights into arbuscular mycorrhizal fungi-mediated drought stress tolerance in plants. Int. J. Mol. Sci. 2019, 20, 4199. [Google Scholar] [CrossRef]
- Muhsin, T.M.; Zwiazek, J.J. Colonization with Hebeloma crustuliniforme increases water conductance and limits shoot sodium uptake in white spruce (Picea glauca) seedlings. Plant Soil 2002, 238, 217–225. [Google Scholar] [CrossRef]
- Muhsin, T.M.; Zwiazek, J.J. Ectomycorrhizas increase apoplastic water transport and root hydraulic conductivity in Ulmus americana seedlings. New Phytol. 2002, 153, 153–158. [Google Scholar] [CrossRef]
- Marjanović, Ž.; Uehlein, N.; Kaldenhoff, R.; Zwiazek, J.J.; Weiß, M.; Hampp, R.; Nehls, U. Aquaporins in poplar: What a difference a symbiont makes! Planta 2005, 222, 258–268. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Kemppainen, M.; El Kayal, W.; Lee, S.H.; Pardo, A.G.; Cooke, J.E.K.; Zwiazek, J.J. Overexpression of Laccaria bicolor aquaporin JQ585595 alters root water transport properties in ectomycorrhizal white spruce (Picea glauca) seedlings. New Phytol. 2015, 205, 757–770. [Google Scholar] [CrossRef]
- Lee, S.H.; Calvo-Polanco, M.; Chung, G.C.; Zwiazek, J.J. Role of aquaporins in root water transport of ectomycorrhizal jack pine (Pinus banksiana) seedlings exposed to NaCl and fluoride. Plant Cell Environ. 2010, 33, 769–780. [Google Scholar] [CrossRef]
- Siemens, J.A.; Zwiazek, J.J. Hebeloma crustuliniforme modifies root hydraulic responses of trembling aspen (Populus tremuloides) seedlings to changes in external pH. Plant Soil 2011, 345, 247–256. [Google Scholar] [CrossRef]
- Quiroga, G.; Erice, G.; Ding, L.; Chaumont, F.; Aroca, R.; Ruiz-Lozano, J.M. The arbuscular mycorrhizal symbiosis regulates aquaporins activity and improves root cell water permeability in maize plants subjected to water stress. Plant Cell Environ. 2019, 42, 2274–2290. [Google Scholar] [CrossRef]
- Zou, Y.-N.; Wu, H.-H.; Giri, B.; Wu, Q.-S.; Kuča, K. Mycorrhizal symbiosis down-regulates or does not change root aquaporin expression in trifoliate orange under drought stress. Plant Physiol. Biochem. 2019, 144, 292–299. [Google Scholar] [CrossRef]
- Nielsen, S.E.; Dennett, J.M.; Bater, C.W. Predicting occurrence, abundance, and fruiting of a cultural keystone species to inform landscape values and priority sites for habitat enhancements. Forests. 2020, 11, 783. [Google Scholar] [CrossRef]
- Schulz, B.J.E.; Boyle, C.J.C.; Sieber, T.N. Microbial Root Endophytes; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Baba, T.; Hirose, D.; Sasaki, N.; Watanabe, N.; Kobayashi, N.; Kurashige, Y.; Karimi, F.; Ban, T. Mycorrhizal formation and diversity of endophytic fungi in hair roots of Vaccinium oldhamii Miq. in japan. Microbes Environ. 2006, 31, 186–189. [Google Scholar] [CrossRef] [PubMed]
- Vohník, M.; Mrnka, L.; Lukešová, T.; Bruzone, M.C.; Kohout, P.; Fehrer, J. The cultivable endophytic community of Norway spruce ectomycorrhizas from microhabitats lacking ericaceous hosts is dominated by ericoid mycorrhizal Meliniomyces variabilis. Fungal Ecol. 2013, 6, 281–292. [Google Scholar] [CrossRef]
- Baral, H.; Krieglsteiner, L. Hymenoscyphus subcarneus, a little known Bryicolous discomycete found in the Bialowieża National Park. Acta Mycol. 2006, 41, 11–20. [Google Scholar] [CrossRef][Green Version]
- Midgley, D.J.; Greenfield, P.; Bissett, A.; Tran-Dinh, N. First evidence of Pezoloma ericae in Australia: Using the Biomes of Australia Soil Environments (BASE) to explore the Australian phylogeography of known mycorrhizal and root-associated fungi. Mycorrhiza 2017, 27, 587–594. [Google Scholar] [CrossRef]
- Gorzelak, M.A.; Hambleton, S.; Massicotte, H.B. Community structure of ericoid mycorrhizas and root-associated fungi of Vaccinium membranaceum across an elevation gradient in the Canadian Rocky Mountains. Fungal Ecol. 2011, 5, 36–45. [Google Scholar] [CrossRef]
- Kohout, P. Biogeography of ericoid mycorrhiza. In Biogeography of Mycorrhizal Symbiosis. Ecological Studies (Analysis and Synthesis); Tedersoo, L., Ed.; Springer: Cham, Switzerland, 2017; Volume 230, pp. 179–193. [Google Scholar]
- Fadaei, S.; Khan, S.; Young, M.; Sherr, I.; Zwiazek, J.J. Impact of soil stockpiling on ericoid mycorrhizal colonization and growth of velvetleaf blueberry (Vaccinium myrtilloides) and Labrador tea (Ledum groenlandicum). Restor. Ecol. 2021, 29, 13276. [Google Scholar] [CrossRef]
- Hambleton, S.; Nickerson, N.L.; Seifert, K.A. Leohumicola, a new genus of heat-resistant hyphomycetes. Stud. Mycol. 2005, 53, 29–52. [Google Scholar] [CrossRef]
- Yu, L.X.; Nguyen, H.T. Genetic variation detected with RAPD markers among upland and lowland rice cultivars (Oryza sativa L.). Theor. Appl. Genet. 1994, 87, 668–672. [Google Scholar] [CrossRef]
- Luo, Z.; Xiong, J.; Xia, H.; Ma, X.; Gao, M.; Wang, L.; Liu, G.; Yu, X.; Luo, L. Transcriptomic divergence between upland and lowland ecotypes contributes to rice adaptation to a drought-prone agroecosystem. Evol. Appl. 2020, 13, 2484–2496. [Google Scholar] [CrossRef] [PubMed]
- Bernier, J.; Atlin, G.N.; Serraj, R.; Kumar, A.; Spaner, D. Breeding upland rice for drought resistance. J. Sci. Food Agric. 2008, 88, 927–939. [Google Scholar] [CrossRef]
- Oehl, F.; Laczko, E.; Oberholzer, H.-R.; Jansa, J.; Egli, S. Diversity and biogeography of arbuscular mycorrhizal fungi in agricultural soils. Biol. Fertil. Soils 2017, 53, 777–797. [Google Scholar] [CrossRef]
- Kim, S.J.; Eo, J.-K.; Lee, E.-H.; Park, H.; Eom, A.-H. Effects of arbuscular mycorrhizal fungi and soil conditions on crop plant growth. Mycobiology 2017, 45, 20–24. [Google Scholar] [CrossRef]
- Augé, R.M. Water relation, drought and VA mycorrhizal symbiosis. Mycorrhiza 2001, 11, 3–42. [Google Scholar]
- Xu, H.; Zwiazek, J.J. Fungal aquaporins in ectomycorrhizal root water transport. Front. Plant Sci. 2020, 11, 302. [Google Scholar] [CrossRef]
- Bitterlich, M.; Sandmann, M.; Graefe, J. Arbuscular Mycorrhiza alleviates restrictions to substrate water flow and delays transpiration limitation to stronger drought in tomato. Front. Plant Sci. 2018, 9, 154. [Google Scholar] [CrossRef] [PubMed]
- Balestrini, R.; Brunetti, C.; Chitarra, W.; Nerva, L. Photosynthetic traits and nitrogen uptake in crops: Which is the role of arbuscular mycorrhizal fungi? Plants 2020, 9, 1105. [Google Scholar] [CrossRef]
- Mathur, N.; Vyas, A.I. Influence of VA mycorrhizae on net photosynthesis and transpiration of Ziziphus mauritiana. J. Plant Physiol. 1995, 147, 328–330. [Google Scholar] [CrossRef]
- Cordeiro, E.C.N.; De Resende, J.T.V.; Júnior, O.J.S.; Nascimento, D.A.; Zeist, A.R.; Favaro, R.; Córdova, K.R.V.; Gabriel, A. Physiology of the production of strawberries inoculated with arbuscular mycorrhizal fungi. Semin. Ciências Agrárias 2019, 40, 3333–3344. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, H.; Gao, J.; Zhang, Y.; Liu, Y.; Tang, M. Effects of ectomycorrhizal fungi (Suillus variegatus) on the growth, hydraulic function, and non-structural carbohydrates of Pinus tabulaeformis under drought stress. BMC Plant Biol. 2021, 21, 171. [Google Scholar] [CrossRef] [PubMed]
- Quiroga, G.; Erice, G.; Aroca, R.; Chaumont, F.; Ruiz-Lozano, J.M. Contribution of the arbuscular mycorrhizal symbiosis to the regulation of radial root water transport in maize plants under water deficit. Environ. Exp. Bot. 2019, 167, 103821. [Google Scholar] [CrossRef]
- Allen, M.F. Mycorrhizal fungi: Highways for water and nutrients in arid soils. Vadose Zone J. 2007, 6, 291–297. [Google Scholar] [CrossRef]
- Xu, H.; Cooke, J.E.; Zwiazek, J.J. Phylogenetic analysis of fungal aquaporins provides insight into their possible role in water transport of mycorrhizal associations. Botany 2013, 91, 495–504. [Google Scholar] [CrossRef]
- Augé, R.M.; Toler, H.D.; Saxton, A. Mycorrhizal stimulation of leaf gas exchange in relation to root colonization, shoot size, leaf phosphorus and nitrogen: A quantitative analysis of the literature using meta-regression. Front. Plant Sci. 2016, 7, 1084. [Google Scholar] [CrossRef]
- Onwuchekwa, N.E.; Zwiazek, J.J.; Quoreshi, A.; Khasa, D.P. Growth of mycorrhizal jack pine (Pinus banksiana) and white spruce (Picea glauca) seedlings in oil sands reclaimed areas. Mycorrhiza 2014, 24, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Gardes, M.; Bruns, T.D. ITS primers with enhanced specificity for basidiomycetes—Application to identification of mycorhizae and rusts. Mol. Ecol. 1993, 2, 113–118. [Google Scholar] [CrossRef] [PubMed]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Arango-Velez, A.; Zwiazek, J.J.; Thomas, B.R.; Tyree, M.T. Xylem cavitation and leaf water relations of poplar clones with different drought resistance strategies. Physiol. Plant. 2011, 143, 154–165. [Google Scholar] [CrossRef] [PubMed]
- Trouvelot, A.; Kough, J.; Gianinazzi-Pearson, V. Evaluation of VA infection levels in root systems. Research for estimation methods having a functional significance. In Physiological and Genetical Aspects of Mycorrhizae; Gianinazzi-Pearson, V., Gianinazzi, S., Eds.; INRA Press: Paris, France, 1986; pp. 217–221. [Google Scholar]
- Sestak, Z.; Catský, J.; Jarvis, P.G. Plant Photosynthetic Production: Manual of Methods; W. Junk Publishers: The Hague, The Netherlands, 1971. [Google Scholar]


| Inoculation | Treatment | Population | M% ± SE | |
|---|---|---|---|---|
| Non-inoculated | Drought | Upland | 20.7 ± 6.6 | a |
| Lowland | 17.4 ± 3.8 | |||
| Well-watered | Upland | 17.5 ± 3.8 | ||
| Lowland | 14.7 ± 4.9 | |||
| Pezicula ericae | Drought | Upland | 55.8 ± 3.2 | c |
| Lowland | 63.8 ± 5.0 | |||
| Well-watered | Upland | 69.3 ± 4.5 | ||
| Lowland | 71.1 ± 4.4 | |||
| Pezoloma ericae | Drought | Upland | 67.3 ± 4.0 | b |
| Lowland | 51.3 ± 4.2 | |||
| Well-watered | Upland | 56.9 ± 3.8 | ||
| Lowland | 53.3 ± 7.0 | |||
| Meliniomyces variabilis | Drought | Upland | 55.2 ± 4.1 | bc |
| Lowland | 51.8 ± 3.4 | |||
| Well-watered | Upland | 62.5 ± 6.3 | ||
| Lowland | 78.2 ± 4.2 | |||
| Oidiodendron maius | Drought | Upland | 66.0 ± 4.1 | d |
| Lowland | 78.7 ± 3.8 | |||
| Well-watered | Upland | 77.4 ± 2.7 | ||
| Lowland | 78.3 ± 3.3 |
| Plant Group | NI | Pezicula ericae | Pezoloma ericae | Meliniomyces variabilis | Oidiodendron maius |
|---|---|---|---|---|---|
| LB | 33.3 | 5.6 | 5.6 | 16.7 | 11.1 |
| UB | 22.2 | 11.1 | 11.1 | 5.6 | 11.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mu, D.; Du, N.; Zwiazek, J.J. Inoculation with Ericoid Mycorrhizal Associations Alleviates Drought Stress in Lowland and Upland Velvetleaf Blueberry (Vaccinium myrtilloides) Seedlings. Plants 2021, 10, 2786. https://doi.org/10.3390/plants10122786
Mu D, Du N, Zwiazek JJ. Inoculation with Ericoid Mycorrhizal Associations Alleviates Drought Stress in Lowland and Upland Velvetleaf Blueberry (Vaccinium myrtilloides) Seedlings. Plants. 2021; 10(12):2786. https://doi.org/10.3390/plants10122786
Chicago/Turabian StyleMu, Deyu, Ning Du, and Janusz J. Zwiazek. 2021. "Inoculation with Ericoid Mycorrhizal Associations Alleviates Drought Stress in Lowland and Upland Velvetleaf Blueberry (Vaccinium myrtilloides) Seedlings" Plants 10, no. 12: 2786. https://doi.org/10.3390/plants10122786
APA StyleMu, D., Du, N., & Zwiazek, J. J. (2021). Inoculation with Ericoid Mycorrhizal Associations Alleviates Drought Stress in Lowland and Upland Velvetleaf Blueberry (Vaccinium myrtilloides) Seedlings. Plants, 10(12), 2786. https://doi.org/10.3390/plants10122786







