Phenolic Compounds and Oxidative Enzymes Involved in Female Fertility in Banana Plants of the Cavendish Subgroup
Abstract
1. Introduction
2. Results and Discussion
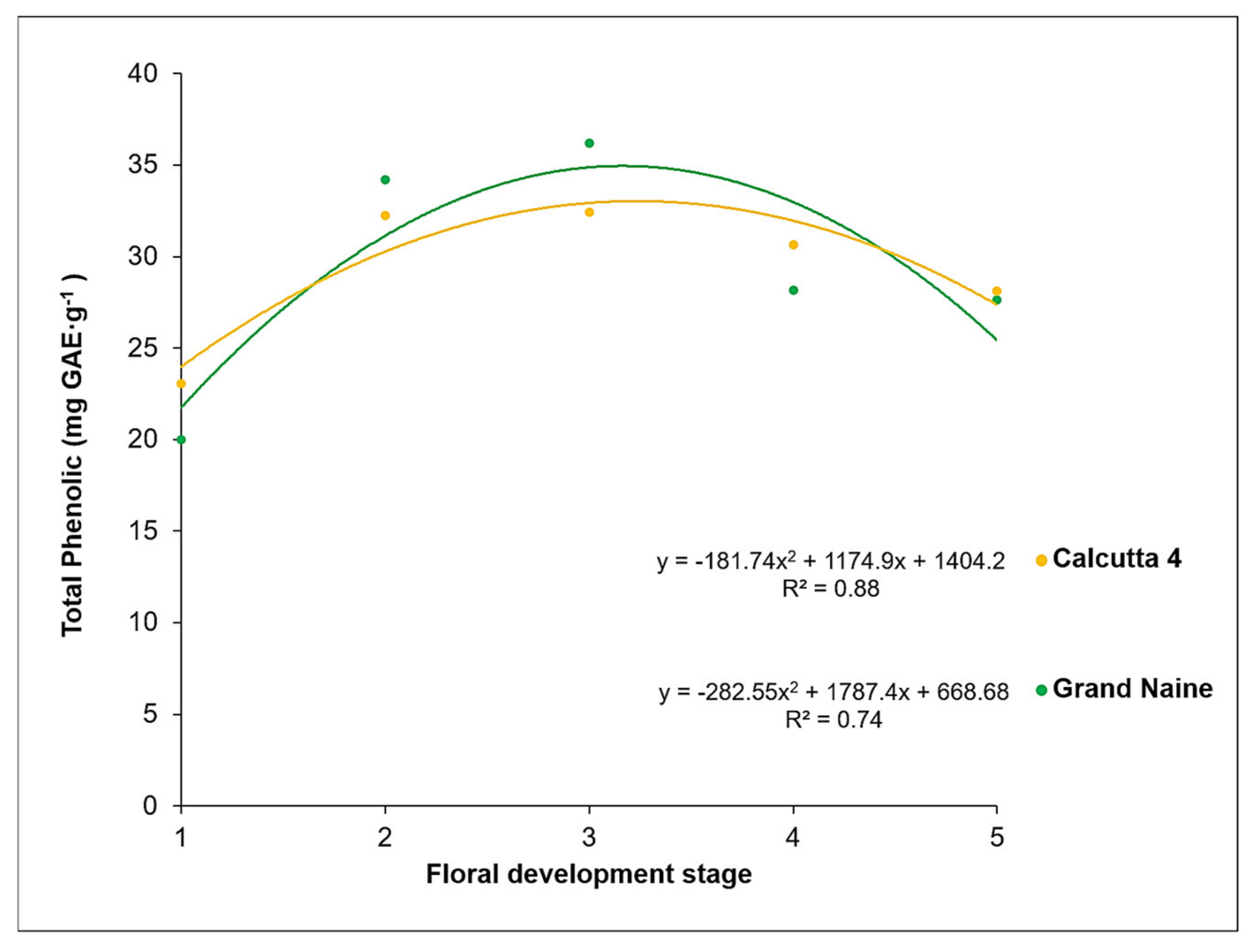
2.1. Total Phenolic Compounds Content
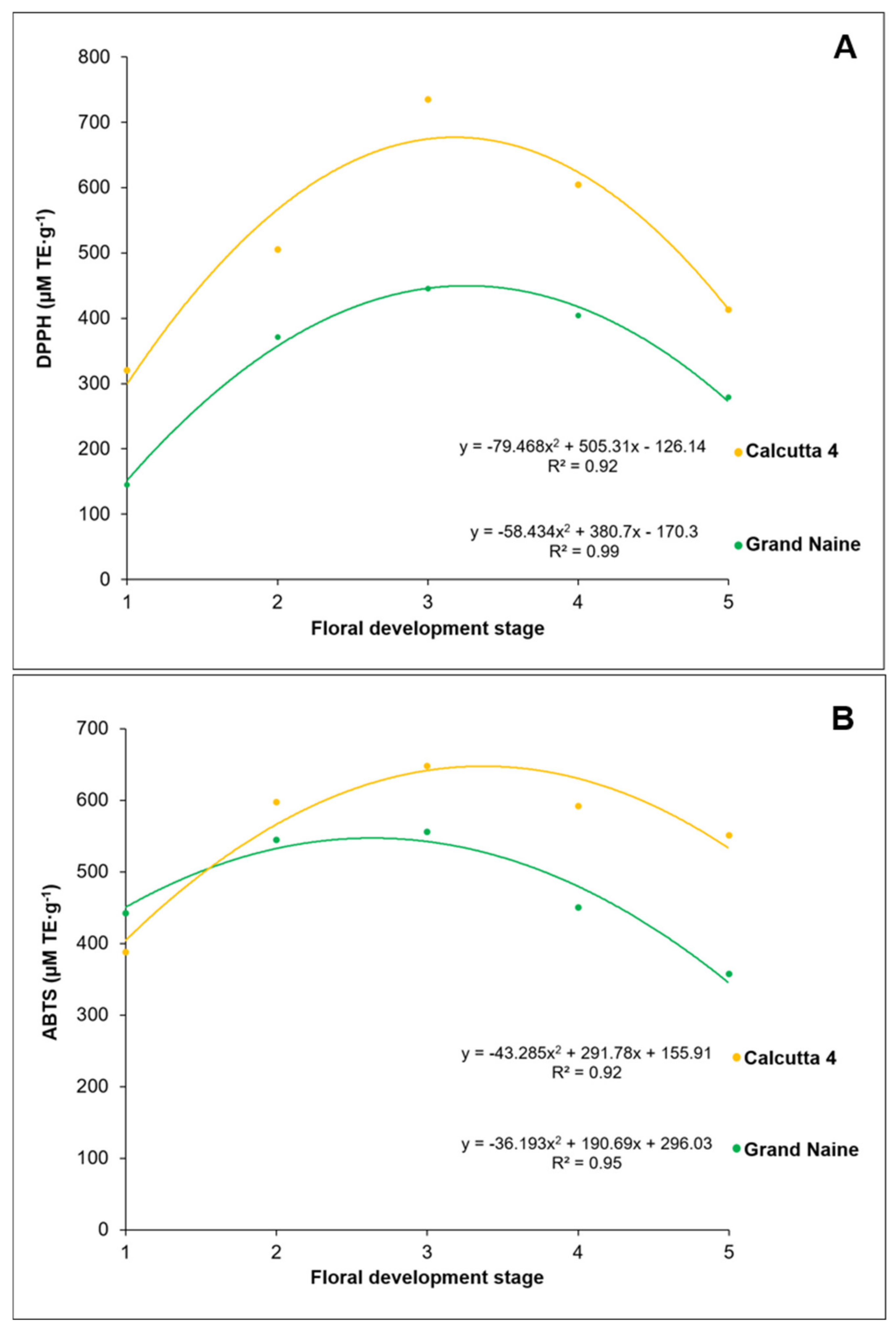
2.2. Antioxidant Activity
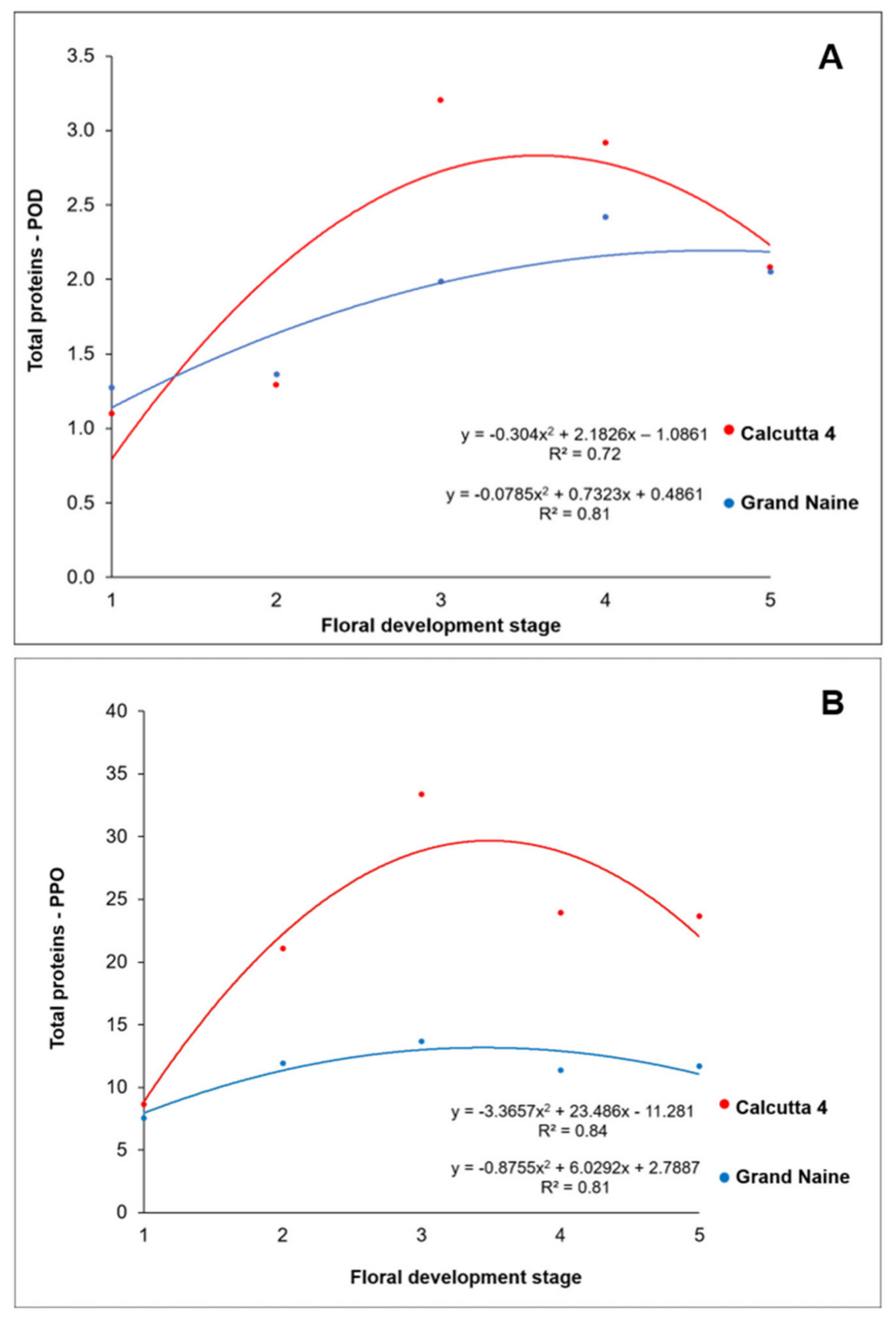
2.3. Enzyme Activity
3. Materials and Methods
3.1. Plant Material
3.2. Floral Development Stages
3.3. Sample Preparation
3.4. Analysis of Total Phenolic Compounds
3.4.1. Extract Preparation
3.4.2. Determination of Total Phenolic Compounds Content
3.5. Determination of Antioxidant Activity
3.5.1. DPPH Radical Scavenging Assay
3.5.2. ABTS Radical Scavenging Assay
3.6. Determination of Enzyme Activity
3.6.1. POD Activity
3.6.2. PPO Activity
3.7. Determination of Total Proteins
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations Statistics. Available online: http://www.fao.org/faostat/en/ (accessed on 13 July 2021).
- Uwimana, B.; Zorrilla-Fontanesi, Y.; van Wesemael, J.; Mduma, H.; Brown, A.; Carpentier, S.; Swennen, R. Effect of Seasonal Drought on the Agronomic Performance of Four Banana Genotypes (Musa spp.) in the East African Highlands. Agronomy 2021, 11, 4. [Google Scholar] [CrossRef]
- Cordeiro, Z.J.M.; Matos, A.P.; Haddad, F. Doenças fúngicas e bacterianas. In O Agronegócio da Banana; Ferreira, C.F., Silva, S.O., Amorim, E.P., Santos-Serejo, J.A., Eds.; Embrapa: Brasília, Brazil, 2016; pp. 545–576. [Google Scholar]
- Silva, S.O.; Amorim, E.P.; Santos-Serejo, J.A.; Borges, A.L. Cultivares. In O Agronegócio da Banana; Ferreira, C.F., Silva, S.O., Amorim, E.P., Santos-Serejo, J.A., Eds.; Embrapa: Brasília, Brazil, 2016; pp. 135–168. [Google Scholar]
- Alakonya, A.E.; Kimunye, J.; Mahuku, G.; Amah, D.; Uwimana, B.; Brown, A.; Swennen, R. Progress in understanding Pseudocercospora banana pathogens and the development of resistant Musa germplasm. Plant Pathol. 2018, 67, 759–770. [Google Scholar] [CrossRef]
- Nascimento, F.D.S.; Souza, Y.M.; Rocha, A.J.; Ferreira, C.F.; Haddad, F.; Amorim, E.P. Sources of black Sigatoka resistance in wild banana diploids. Rev. Bras. Frutic. 2020, 42, 1–10. [Google Scholar] [CrossRef]
- Thangavelu, R.; Edwin Raj, E.; Pushpakanth, P.; Loganathan, M.; Uma, S. Draft genome of Fusarium oxysporum f. sp. cubense strain Tropical Race-4 infecting Cavendish (AAA) groupof banana in India. Plant Dis. 2020, 105, 481–483. [Google Scholar] [CrossRef]
- Rebouças, T.A.; Rocha, A.J.; Cerqueira, T.S.; Adorno, P.R.; Barreto, R.Q.; Ferreira, M.S.; Lino, L.S.M.; Amorim, V.B.; Santos-Serejo, J.A.; Haddad, F.; et al. Pre-selectionof banana somaclones resistantto Fusarium oxysporum f. sp. cubense, subtropical race 4. Crop Prot. 2021, 147, 1056–1092. [Google Scholar] [CrossRef]
- Ploetz, R.C. Fusarium wilt of banana. Phytopathology 2015, 105, 1512–1521. [Google Scholar] [CrossRef]
- Rocha, A.D.J.; Soares, J.M.D.S.; Nascimento, F.D.S.; Santos, A.S.; Amorim, V.B.D.O.; Ferreira, C.F.; Haddad, F.; Santos-Serejo, J.A.D.; Amorim, E.P. Improvements in the Resistance of the Banana Species to Fusarium Wilt: A Systematic Review of Methods and Perspectives. J. Fungi 2021, 7, 249. [Google Scholar] [CrossRef]
- Scheerer, L.; Pemsl, D.; Dita, M.; Perez Vicente, L.; Staver, C. A quantified approach to projecting losses caused by Fusarium wilt tropical race 4. Acta Hortic. 2018, 1196, 211–218. [Google Scholar] [CrossRef]
- Staver, C.; Pemsl, D.E.; Scheerer, L.; Perez Vicente, L.; Dita, M. Ex Ante Assessment of Returns on Research Investments to Address the Impact of Fusarium Wilt Tropical Race 4 on Global Banana Production. Front. Plant Sci. 2020, 11, 844. [Google Scholar] [CrossRef]
- Molinari, H.B.C.; Vieira, L.R.; Silva, N.V.E.; Prado, G.S.; Lopes Filho, J.H. Tecnologia CRISPR na Edição Genômica de Plantas: Biotecnologia Aplicada à Agricultura; Embrapa: Brasília, Brazil, 2020; p. 207. [Google Scholar]
- Angelotti-Mendonça, J.; Oliveira, F.F.; Silva, N.V. Genome editing: Propelling the next generation of crop improvement. Colloq. Agrar. 2021, 17, 83–101. [Google Scholar] [CrossRef]
- Fortescue, J.A.; Turner, D.W. Reproductive Biology. In Banana Breeding: Progress and Challenges; Pillay, M., Tenkouano, A., Eds.; CRC Press: Boca Raton, FL, USA, 2011; Volume 1, pp. 145–179. [Google Scholar]
- Amorim, E.P.; Amorim, V.B.O.; Silva, M.S.; Haddad, F.; Ferreira, C.F.; Santos-Serejo, J.A. Developing Hybrid Banana Varieties with Improved Properties. In Achieving Sustainable Cultivation of Bananas: Germplasm and Genetic Improvement; Burleigh Dodds Science Publishing Limited: Cambridge, UK, 2020; Volume 2, pp. 323–338. [Google Scholar] [CrossRef]
- Waniale, A.; Swennen, R.; Mukasa, S.B.; Tugume, A.K.; Kubiriba, J.; Tushemereirwe, W.K.; Batte, M.; Brown, A.; Tumuhimbise, R. Seed Set Patterns in East African Highland Cooking Bananas Show Asymmetric Distribution in Bunches and Fruits. Agronomy 2021, 11, 763. [Google Scholar] [CrossRef]
- Amah, D.; Turner, D.W.; Gibbs, D.J.; Wanilae, A.; Gram, G.; Swennen, R. Overcoming the fertility crisis in bananas (Musa spp.). In Achieving Sustainable Cultivation of Bananas: Germplasm and Genetic Improvement; Kema, G.H.J., Drenth, A., Eds.; Burleigh Dodds Science Publishing Limited: Cambridge, UK, 2021; Volume 2, pp. 257–306. [Google Scholar] [CrossRef]
- Aguilar-Morán, J.F. Improvement of Cavendish banana cultivars through conventional breeding. Acta Hortic. 2013, 986, 205–208. [Google Scholar] [CrossRef]
- Soares, T.L.; Souza, E.H.; Costa, M.A.P.C.; Silva, S.O.; Santos-Serejo, J.A. In vivo fertilization of banana. Ciênc. Rural 2014, 44, 37–42. [Google Scholar] [CrossRef]
- Barbosa, A.O. Aspectos Reprodutivos e Fertilidade em Bananeiras Diploides e Triploides. Master’s Thesis, Federal University of Recôncavo da Bahia, Cruz das Almas, Brasil, 2015. [Google Scholar]
- Veitch, N.C. Horseradish peroxidase: A modern view of a classic enzyme. Phytochemistry 2004, 65, 249–259. [Google Scholar] [CrossRef]
- Siqueira, J.O.; Safir, G.R.; Nair, M.G. Stimulation of vesicular-arbuscular mycorrhiza formation and growth of white clover by flavonoid compounds. New Phytol. 1991, 118, 87–93. [Google Scholar] [CrossRef]
- Rocha, M.M. Extratos Florais de Bananeira e Sua Influência na Germinação In Vitro de Grãos de Pólen. Master’s Thesis, Federal University of Recôncavo da Bahia, Cruz das Almas, Brazil, 2014. [Google Scholar]
- Silva, S.O.; Amorim, E.P.; Santos-Serejo, J.A.; Ferreira, C.F.; Rodriguez, M.A.D. Melhoramento genético da bananeira: Estratégias e tecnologias disponíveis. Rev. Bras. Frutic. 2013, 35, 919–931. [Google Scholar] [CrossRef][Green Version]
- Fahn, A.; Benouaiche, P. Ultrastructure, Development and Secretion in the Nectary of Banana Flowers. Ann. Bot. 1979, 44, 85–93. [Google Scholar] [CrossRef]
- Possobom, C.C.F.; Machado, S.R. Elaiophores: Their taxonomic distribution, morphology and functions. Acta Bot. Bras. 2017, 31, 503–524. [Google Scholar] [CrossRef]
- Talcott, S.T.; Percival, S.S.; Pittet-Moore, J.; Celoria, C. Phytochemical composition and antioxidant stability of fortified yellow passion fruit (Passiflora edulis). J. Agric. Food Chem. 2003, 51, 935–941. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997, 2, 152–159. [Google Scholar] [CrossRef]
- Kuskoski, E.M.; Asuero, A.G.; Troncoso, A.M.; Mancini-Filho, J.; Fett, R. Aplicación de diversos método químico para determinar actividad antioxidante em pulpa de fruto. Ciênc. Tecnol. Aliment. 2005, 25, 726–732. [Google Scholar] [CrossRef]
- Pérez-Jiménez, J.; Arranz, S.; Tabernero, M.; Díaz-Rubio, M.E.; Serrano, J.; Goni, I.; Saura-Calixto, F. Updated methodology to determine antioxidant capacity in plant foods, oils and beverages: Extraction, measurement and expression of results. Food Res. 2008, 41, 274–285. [Google Scholar] [CrossRef]
- Sakihama, Y.; Yamasaki, H. Lipid peroxidation induced by phenolics in conjunction with aluminum ions. Biol. Plant. 2002, 45, 249–254. [Google Scholar] [CrossRef]
- Giada, M.L.R.; Mancini Filho, J. Importância dos compostos fenólicos da dieta na promoção da saúde humana. Ciênc. Biol. Saúde 2016, 12, 7–15. [Google Scholar] [CrossRef]
- Shepherd, K. Seed fertility of edible bananas. J. Hortic. Sci. 1960, 35, 6–20. [Google Scholar] [CrossRef]
- Swennen, R.; Vuylsteke, D. Breeding black sigatoka resistant plantains with a wild banana. Trop. Agric. 1993, 70, 74–77. [Google Scholar]
- Soares, T.L.; Souza, E.H.; Costa, M.A.P.C.; Silva, S.O.; Santos-Serejo, J.A. Viability of pollen grains of tetraploid banana. Bragantia 2016, 75, 145–151. [Google Scholar] [CrossRef]
- Andersen, W.C.A.A. revised medium for shoot multiplication of Rhododendron. J. Am. Soc. Hortic. Sci. 1986, 109, 343–347. [Google Scholar]
- Kears, C.A.; Inouye, D.W. Techniques for Pollination Biologists; University Press of Colorado: Niwot, CO, USA, 1993; p. 586. [Google Scholar]
- Kim, Y.H.; Kwak, S.S. The role of antioxidant enzymes during leaf development. In Reactive Oxygen Species and Antioxidants in Higher Plants; Gupta, S.D., Ed.; CRC Press: Boca Raton, FL, USA, 2010; pp. 129–150. [Google Scholar]
- Locato, V.; Pinto, M.C.; Paradiso, A.; Gara, L. Reactive Oxygen Species and Ascorbate-Glutathione Interplay in Signaling and Stress Responses; Science Publishers: Riad, Saudi Arabia, 2010; pp. 45–64. [Google Scholar]
- Jimenez, M.; García-Carmona, F. The effect of sodium dodecyl sulphate on polyphenoloxidase. Phytochemistry 1996, 42, 1503–1509. [Google Scholar] [CrossRef]
- Vilas Boas, E.V.B. Frutas Minimamente Processadas: Banana. In Proceedings of the III Encontro Sobre Processamento Mínimo de Frutas e Hortaliças: Palestras, Resumos e Oficinas, Viçosa, Brazil, 25–28 May 2004; pp. 111–121. [Google Scholar]
- Shepherd, K.; Dantas, J.L.L.; Alves, E.J. Banana breeding in Brazil. In Banana and Plantain Breeding Strategies; Persley, G.J., De Langhe, E.A., Eds.; Australian Center for International Agricultural Research: Camberra, Australia, 1986; pp. 78–83. [Google Scholar]
- Silva, T.S.M.; Coelho Filho, M.A.; Coelho, E.F. Boletim Meteorológico da Estação Convencional de Cruz das Almas, BA: Variabilidade e Tendências Climáticas; Documentos 216; Embrapa Mandioca e Fruticultura: Cruz das Almas, Brazil, 2016; 79p. [Google Scholar]
- Menezes, A.J.E.A.; Galvão, E.U.P. Bananeira: Recomendações de Cultivo; Comunicado Técnico; Embrapa: Cruz das Almas, Brazil, 2004; Volume 113, pp. 1–4. [Google Scholar]
- Fortescue, J.A.; Turner, D.W. The association between low temperatures and anatomical changes in preanthetic ovules of Musa (Musaceae). Sci. Hortic. 2005, 104, 433–444. [Google Scholar] [CrossRef]
- Larrauri, J.A.; Rupérez, P.; Sauracalixto, F. Effect of drying temperature on the stability of polyphenols and antioxidant activity of red grape pomace peels. J. Agric. Food Chem. 1997, 45, 1390–1393. [Google Scholar] [CrossRef]
- Rufino, M.S.M.; Alves, R.E.; Brito, E.S.; Morais, S.M.; Sampaio, C.G.; Pérez-Jiménez, J.; Saura-Calixto, F.D. Metodologia Científica: Determinação da Atividade Antioxidante Total em Frutas Pela Captura do Radical Livre DPPH; Comunicado Técnico; Embrapa: Cruz das Almas, Brazil, 2007; pp. 1–4. [Google Scholar]
- Allain, C.C.; Poon, L.S.; Chan, C.S.G.; Richmond, W.; Fu, P.C. Enzymatic Determination of Total Serum Cholesterol. Clin. Chem. 1974, 20, 470–475. [Google Scholar] [CrossRef] [PubMed]
- Lima, G.P.P. Efeito do Cálcio Sobre o Teor de Poliaminas e Atividade da Peroxidase e Redutase do Nitrato em Calos de Arroz (Oriza sativa L. cv. IAA 4440). Ph.D. Thesis, State University of São Paulo, São Paulo, Brazil, 1994. [Google Scholar]
- Cano, P.M.; Ancos, B.; Matallana, M.C.; Camara, M.; Reglero, G.; Tabera, J. Differences among spanish and layin-american banana cultivars: Morphological, chemical and sensory characteristics. Food Chem. 1997, 59, 411–419. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- R Foundation for Statistical Computing. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: http://www.r-project.org/index.html (accessed on 6 January 2021).


| Acronym | Stage | Description | |
|---|---|---|---|
| S1 | Stage 1 | Partial emission of the pseudostem inflorescence—vertical position (without viewing the base of the inflorescence) |  |
| S2 | Stage 2 | Total emission of the pseudostem inflorescence—vertical position (base of visible inflorescence) |  |
| S3 | Stage 3 | Total inflorescence emission—horizontal position to the ground | 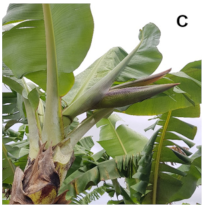 |
| S4 | Stage 4 | Inflorescence in pendular position to the pseudostem with closed flowers (Pre-anthesis) |  |
| S5 | Stage 5 | Inflorescence in pendular position to the pseudostem with open flowers (Anthesis) |  |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
dos Santos Silva, M.; da Hora Góes, N.; dos Santos-Serejo, J.A.; Ferreira, C.F.; Amorim, E.P. Phenolic Compounds and Oxidative Enzymes Involved in Female Fertility in Banana Plants of the Cavendish Subgroup. Plants 2021, 10, 2790. https://doi.org/10.3390/plants10122790
dos Santos Silva M, da Hora Góes N, dos Santos-Serejo JA, Ferreira CF, Amorim EP. Phenolic Compounds and Oxidative Enzymes Involved in Female Fertility in Banana Plants of the Cavendish Subgroup. Plants. 2021; 10(12):2790. https://doi.org/10.3390/plants10122790
Chicago/Turabian Styledos Santos Silva, Manassés, Naiala da Hora Góes, Janay Almeida dos Santos-Serejo, Claudia Fortes Ferreira, and Edson Perito Amorim. 2021. "Phenolic Compounds and Oxidative Enzymes Involved in Female Fertility in Banana Plants of the Cavendish Subgroup" Plants 10, no. 12: 2790. https://doi.org/10.3390/plants10122790
APA Styledos Santos Silva, M., da Hora Góes, N., dos Santos-Serejo, J. A., Ferreira, C. F., & Amorim, E. P. (2021). Phenolic Compounds and Oxidative Enzymes Involved in Female Fertility in Banana Plants of the Cavendish Subgroup. Plants, 10(12), 2790. https://doi.org/10.3390/plants10122790







