Abstract
Kidney diseases are regarded as one of the major public health issues in the world. The objectives of this study were: (i) to investigate the causative factors involved in kidney disease and the therapeutic aspects of Moringa oleifera, as well as (ii) the effectiveness of M. oleifera in the anti-inflammation and antioxidant processes of the kidney while minimizing all potential side effects. In addition, we proposed a hypothesis to improve M. oleifera based drug development. This study was updated by searching the key words M. oleifera on kidney diseases and M. oleifera on oxidative stress, inflammation, and fibrosis in online research databases such as PubMed and Google Scholar. The following validation checking and scrutiny analysis of the recently published articles were used to explore this study. The recent existing research has found that M. oleifera has a plethora of health benefits. Individual medicinal properties of M. oleifera leaf extract, seed powder, stem extract, and the whole extract (ethanol/methanol) can up-increase the activity of antioxidant enzymes like superoxide dismutase (SOD), catalase (CAT), and glutathione (GSH), while decreasing the activity of inflammatory cytokines such as TNF-α, IL-1β, IL-6, and COX-2. In our study, we have investigated the properties of this plant against kidney diseases based on existing knowledge with an updated review of literature. Considering the effectiveness of M. oleifera, this study would be useful for further research into the pharmacological potential and therapeutic insights of M. oleifera, as well as prospects of Moringa-based effective medicine development for human benefits.
1. Introduction
Kidney diseases are considered among the major health problems worldwide. Acute kidney injury (AKI) is closely connected with chronic kidney diseases (CKD). Since 1990, CKD has been included in the list of non-communicable conditions investigated by the global burden of disease study. As the disease’s growth rate accelerates, it has become a global concern. The majority of incidents occur in low and lower-middle income countries [1,2,3]. The kidneys gradually lose their ability to function in CKD patients, and the glomerular filtration rate (GFR) falls below 60 mL/min per 1.73 m2 [1,2]. Mainly people who have been already suffering from diabetes, heart disease, or high blood pressure are at a high risk of developing CKD. Few drugs, such as prolyl hydroxylase domain inhibitors against anemia in CKD [3], can be used to treat CKD complications. The main pathologies involved in kidney complications are inflammation, oxidative stress, apoptosis, and fibrosis [4]. Unfortunately, no potential drug for treating kidney diseases exists at this time. Therefore, the search for a potential drug with fewer side effects to combat this disease is becoming increasingly important. M. oleifera Lam., also known as drumstick tree, is a Moringaceae family member that grows in the Indian subcontinent. This plant’s various parts have medicinal applications, such as antifungal, antiviral, anti-inflammatory, etc. [5,6,7,8]. Moringa leaves also have a low calorific value and can be included in the diet of obese individuals [9]. Furthermore, it contains numerous bioactive phytochemicals such as flavonoids, saponin, vanillin, omega fatty acids, carotenoids, ascorbates, tocopherols, beta-sitosterol, moringine, kaempferol, and quercetin that have been reported in its flowers, roots, fruits, and seeds, and can play a variety of roles in medicine [10,11,12,13]. In general, the choice of the most suitable bioactive substance for therapeutic purposes necessarily depends on the chemical formula of that specific compound, its structure giving its unique properties, and implicitly its mode of action [14]. Kaempferol has been shown to promote cancer cell apoptosis, such as MCF-7 and A549 cells [15]. Due to its anti-inflammatory and antioxidant properties, quercetin has the potential to be hepatoprotective, hypocholesterolemic, hypolipidemic, and anti-atherosclerotic [16]. Moringa has an anti-hyperglycemic effect, according to researchers who studied it in vivo on mice models [17].
Previous studies indicate that the juice of the super food M. oleifera enhances antimicrobial defense [18] and regulates insulin level, as well as glucose uptake in muscles [19,20]. Interestingly, M. oleifera showed a significant reduction of hyperglycemia, low-density lipoprotein (LDL) cholesterol, total cholesterol, fatty substances, FPG, and VLDL-cholesterol [21]. M. oleifera is also beneficial for skin, hair, liver, eye, blood pressure, treating anemia, kidney disease, and diabetes [22]. Several recent studies have documented the beneficial impacts of M. oleifera in alleviating renal diseases in animal model. Nafiu et al. [23] marked that gentamicin-induced impairment and oxidative stress significantly reduced by ethanolic extract of Moringa oleifera seeds in plasma, urine and kidney homogenate of rats. Akinrinde et al. [24] observed that M. oleifera extract attenuates the deleterious effects of renal ischemia-reperfusion through alleviation of oxidative stress. Soliman et al. [25] explored the ameliorative effects of M. oleifera against oxidative stress and methotrexate-induced hepato–renal dysfunction. Recently, Abu-Zeid et al. [26] discovered that the ecofriendly selenium nanoparticle using M. oleifera and/or M. oleifera ethanolic leaf extract reduces melamine-induced nephrotoxicity by alleviating of renal function impairments, oxidative stress, and apoptosis in rat kidney. Despite the great progress of M. oleifera in this field in recent years, less attention has been given to the effectiveness of M. oleifera, particularly against kidney related diseases. Therefore, there are still some issues which need further exploration, such as the protective effects of M. oleifera in kidney related disease difficulties and its prospects in drug development for human benefits.
This review updates the existing knowledge concerning the causative factors involved in kidney disease, as well as the therapeutic aspects of M. oleifera. Furthermore, this study provides a hypothesis on how M. oleifera would be effective in the anti-inflammation and antioxidant processes of the kidney, with the least amount of side effects.
2. Methods
This systematic review was carried out following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [27]. Databases such as Scopus, PubMed, and Google Scholar were accessed to retrieve information using the keywords ‘MeSH terms’, on ‘kidney diseases’ and ‘oxidative stress’ and ‘inflammation’, and ‘fibrosis’ and ‘Moringa oleifera’. The information was retrieved from 2011 to 15 June 2021. Automatic search tools were used to exclude some of the articles, while others were screened manually. Articles published in languages other than English were excluded. Reviews, book chapters, expert opinions, conference papers, and letters to editors were also excluded from this review. A total of 151 research articles were retrieved from the databases and discussed in this study (Figure 1). All information compiled in the table was obtained from these research articles.
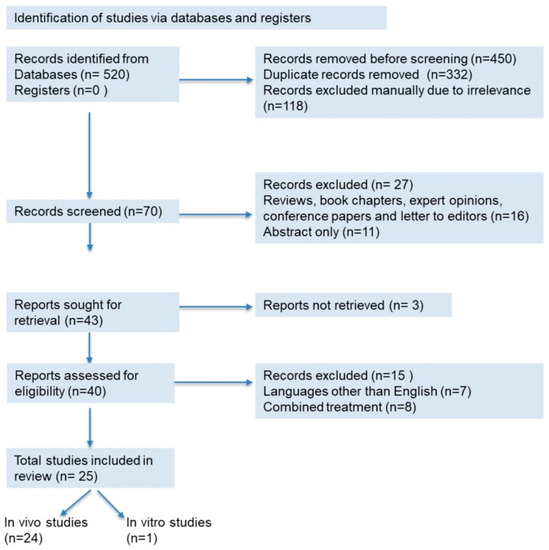
Figure 1.
PRISMA 2020 flow diagram for the systematic review.
3. Phytochemical Content and Pharmacological Potential of M. oleifera on Kidney Diseases
M. oleifera contains several bioactive phytochemicals including flavonoids and isothiocyanates [10]; polyphenols, carotenoids, alkaloids, and terpenoids [11]; and triterpenoids, moringyne, monopalmitic, di-oleic triglyceride, campesterol, stigmasterol, β-sitosterol, avenasterol, and vitamin A [12]. These bioactive phytochemicals are found in M. oleifera roots, fruits, and seeds. These phytochemicals have medicinal properties which have been shown to be effective antioxidant, antimicrobial, inflammatory, and anti-carcinogenic agents [28]. More studies are required to explore the role of bioactive phytochemicals specially in kidney diseases.
M. oleifera also possesses a variety of pharmacological properties, which are closely associated with the presence of its bioactive compounds. Therefore, in the following section we highlighted the pharmacological potential of M. oleifera. M. oleifera showed pharmacological potential against some plausible factors such as oxidative stress, inflammation, fibrosis, and other pathologies responsible for kidney diseases. The potential effects of M. oleifera against risk factors associated with kidney disease in the following sections as shown in Figure 2 and Figure 3.
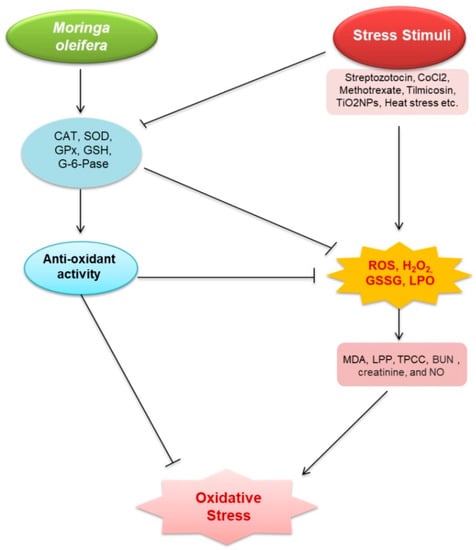
Figure 2.
Renoprotective effects of M. oleifera against oxidative stress. Stress stimuli (streptozotocin, CoCl2, methotrexate, tilmicosin, TiO2NPs, acetaminophen (APAP), glycerol, and Salmonella) increased malondialdehyde (MDA), lipid peroxidation products (LPP), total protein carbonyl content (TPCC), blood urea nitrogen (BUN), creatinine, and nitric oxide (NO) production via triggering reactive oxygen species (ROS), H2O2, glutathione disulfide (GSSG), and lactoperoxidase (LPO). Oxidative stress emerged as a result of these events. MO—induced models, on the other hand, increased the expression of catalase (CAT); superoxide dismutase (SOD); glutathione peroxidase (GPx); glutathione (GSH), total antioxidant capacity (TAC); delta-amino levulinic acid dehydratase (ALAD), and G-6-Pase, which then activates glutathione (GSH). These stressors inhibit the expression of oxidative stress suppressive factors. ROS, H2O2, GSSG, and LPO, all related to oxidative stress, were decreased by GSH. GSH is also capable of reducing oxidative stress.
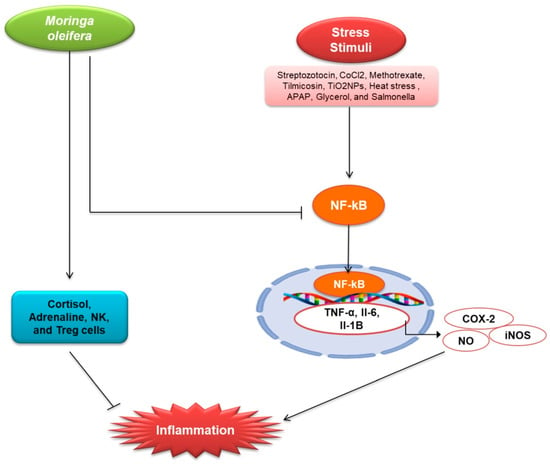
Figure 3.
Renoprotective effects of M. oleifera against inflammation. The expression of C-reactive protein (CRP), which activates NF-kB in the cytosol, is linked to stress factors. TNF-, Il-6, Il-1B, iNOS, and COX-2 are all activated when NF-kB enters the nucleus and binds to DNA. All of these elements have been linked to the development of inflammation. NO is activated even more by iNOS. NO is thought to be a pro-inflammatory mediator that causes inflammation. In the cytosol, M. oleifera suppressed the expression of CRP and NF-kB. It also boosted cortisol, adrenaline, NK, and Treg cells, which helped reduce inflammation. Anti-inflammatory hormones Cortisol and Adrenaline Both NK cells and Treg cells are anti-inflammatory regulators.
3.1. Oxidative Stress
Oxidative stress is caused by an imbalance between the excessive free radical generation and insufficient antioxidant defense [29,30]. It is frequently observed in CKD [31,32,33], and has become a diagnostic factor [34]. A number of studies documented that M. oleifera has antioxidative properties to protect and/or alleviate cellular damage (Table 1 and Figure 2). M. oleifera extracts and compounds, particularly quercetin, kaempferol, isothiocyanates, rutin, myricetin, ascorbic acid, and β-carotene, showed antioxidant potentials either via direct scavenging of free radicals [35].

Table 1.
Summary on the protective effects of M. oleifera against kidney diseases.
Methanol extract of M. oleifera reduced the oxidative stress in STZ induced male rats by lowering the production of MDA, ROS, LDL, and CHOL, which increase the risk of CKD [36,54]. Methanol extract also lowered the generation of MDA, AOPP, NO, H2O2, GPx, and GST, all of which induce oxidative stress in ischemia-induced Wistar rats [29]. Another study showed that metabolic extract reduced the levels of BUN and creatinine, and total protein is increased in CKD patients [42]. Ethanolic extract of M. oleifera inhibits oxidative stress and atherosclerosis in CKD by lowering LDL [20]. 8-OHdG causes oxidative stress to DNA and promotes cancer [56], ameliorated by the ethanolic extract of M. oleifera [56]. Ethanol extracts decrease the plasma creatinine level by enhancing the process of creatinine clearance [30]. Plasma sodium and potassium levels were raised after treating nickel-induced Wistar rats with ethanolic extract of M. oleifera [34]. Ethanolic extract detoxified plasma by reducing the bilirubin levels (indirect/direct), urea levels, etc., in ML-induced male Sprague Dawley rats [48]. HO-1 and Nrf2 expression were stimulated by leaf extract of M. oleifera at dosages of 300 and 400 mg/kg body weight, respectively [25,41]. Leaf extracts up-regulated the level of total thiol TiO2NPs induced male albino rats, which play an important role in antioxidant protection [41]. Leaf extract of M. oleifera also downregulated the oxidative stress generating mediators in sodium fluoride (NaF)-induced Oreochromis niloticus, gentamicin-induced rabbit, and APAP-treated mice [23,42,57].
M. oleifera alcoholic extract reduced oxidative stress by lowering the lipid peroxidation, and ROS in iodide injected rabbits [51]. Furthermore, fermented leaf extract of M. oleifera boosts the antioxidant activity in bacteria-induced mice [53]. M. oleifera extract reduced the manifestation of MDA, indicating that the free radicle overproduction was reduced in both Tilmicosin and Hg induced rats. Abarikwu et al. showed that SOD level was increased after treatment with M. oleifera in tilmicosin induced rats [40]. Hydroalcoholic root extract raised blood sugar, antioxidant enzyme activities, and G-6-phase activities, which protect the kidney from nephropathy in Beryllium-induced rats [45]. Seed powder reduced free radical species, TPCC, metal content, and increased ALAD activity in lead-treated rats [57]. In arsenic-treated rats, seed powder of M. oleifera considerably increased antioxidant function including GSH, CAT, and ALAD [46].
3.2. Inflammation
The kidney is responsible for maintaining whole-body homeostasis. Kidney disease is characterized by inflammation as a major pathology [58,59,60]. Acute or chronic disease such as ischemia, toxins, or inflammation affects kidney tubules, causing kidney fibrosis that is associated with reduction of GFR in kidneys [61]. Kidney injury is linked to the production of cytokines levels, which prolongs the acute phase of kidney disease [62]. Moreover, chronic inflammation is regarded as a comorbid condition in CKD [63]. Many plants have an anti-inflammatory action through active substances such as hesperidin, diosmin, withaferin, fucoidan, thymoquinone, etc. [64,65,66,67]. Here, the anti-inflammatory effects of M. oleifera has been discussed. M. oleifera has been reported to exhibit strong inflammatory activity (Table 1 and Figure 3). Methanolic extract of M. oleifera reduced inflammation in STZ induced male Wister rats by down-regulating the tumor necrosis factor (TNF-α), IL-6, and MCP-1, an important chemokine [36,54]. Tang et al. investigated the effects of ethanolic extract of M. oleifera in metformin-induced mice and observed that the M. oleifera declines the production of inflammatory markers and the expression of cyclooxygenase-2 (COX-2) and nitric oxide synthase (iNOS) by reducing the phosphorylation of mitogen-activated protein kinase (MAPK) pathway [20]. Ethanolic extract of M. oleifera down-regulates the inflammatory cytokines in CoCl2-induced rats, including NO, which is involved in the pathogenesis of inflammation [37]. Leaf extract of M. oleifera inhibits inflammatory cytokines production and regulates the inflammation by inhibiting NF-kB [25]. It was also observed that inflammation in Tilmicosin (Til) induced rats was reduced by M. oleifera extracts [39]. M. oleifera leaf extract protects against interstitial kidney inflammation with fibrosis by down-regulating KIM-1 in TiO2NPs induced male albino rats [41]. M. oleifera extract increases the secretion of cortisol, adrenaline, Treg cells, NK, and leptin, promoting anti-inflammatory cytokines and regulating the immune system [47]. M. oleifera treatment reduced the expression of KIM-1, TIMP-1, and TNF-α in ML-induced male Sprague Dawley rats [48]. TNF-α, an inflammatory cytokine that stimulates IL-1; IL-6, downregulated by M. oleifera in Seabream (Sparus aurata); and activated TGF-β, elicits anti-inflammatory effects [49]. M. oleifera also reduced the inflammatory cytokines in APAP-treated mice, where APAP induces AKI [50]. Fermented extract of leaves also reduces the Nrf2 in Salmonella-induced mice [53].
Moringa seed’s phytochemicals can reduce the production of nitric oxide (NO) and the gene expression of LPS-inducible iNOS and interleukins 1β and 6 (IL-1β and IL-6) compared to curcumin [68]. Flavonoids have been shown to be effective inhibitors of nitric oxide synthase type 2 (NOS-2) actions, and it also inhibits protein tyrosine kinase action that is involved in the NOS-2 expression at the molecular level [69,70,71]. Flower extract can cause the activation of pro-inflammatory proteins such as toll-like receptors. In the flowers, quercetin and kaempferol can inhibit the signal transducer and activator of transcription 1 (STAT-1) and the NF-κB pathways [72,73]. M. oleifera flowers contain 80% hydroethanolic, a potent agent of anti-inflammation in the NF-κB signaling pathway [74]. Scientists discovered that phenolic glycosides suppress inducible iNOS expression and NO production in mouse macrophage cells, as well as COX-2 and iNOS proteins [75,76]. Moringa extracts eventually down-regulate the inflammatory mediators because its seeds and flowers contain many bioactive compounds. Each of these compounds has its individual effects.
3.3. Fibrosis
Kidney fibrosis is defined as a radical harmful connective tissue deposition on the kidney parenchyma, which leads to renal dysfunction. Epithelial to mesenchymal transition (EMT) is the main mechanism of kidney fibrosis, and the TGFβ-1-SMAD pathway and hypoxia are known as the main modulator of EMT [32,77]. TGF-β-induced expression of fibronectin, type I collagen, and PAI-1 rat kidney fibroblast cells is reduced by M. oleifera extract [55]. Furthermore, moringa root extract selectively inhibited TGF-β-induced phosphorylation of SMAD4 and ERK expression. These results suggest that moringa root extract may reduce renal fibrosis by a mechanism related to its antifibrotic activity in rat kidney fibroblast cells. Oral administration of M. oleifera seed extract reduced CCl4-induced liver fibrosis in rats [78].
3.4. Other Pathologies Those Are Associated with Kidney Diseases
Autophagy has a critical role in kidney physiology and homeostasis [79], and, thus, its regulation is an important determinant of kidney diseases [61]. AKI or CKD causes mitochondrial damage, but damaged mitochondria begin to accumulate in response to these types of stimuli. Autophagy protects the kidney through the removal of ROS-producing mitochondria [80,81,82]. Apoptosis is a type of programmed cell death in which cells are killed by a controlled system. It is an energy-dependent complex process [83]. It contributes to develop AKI, even organ failure [84]. Ischemia/reperfusion (I/R) induces apoptosis or necrosis in the kidney and loss of tubular cells, leading to decreased GFR [85,86]. Renal tubular cells express cell surface ‘death receptors’ of TNF-α which is responsible for inducing apoptosis [87]. Also, ROS production in kidney disease is responsible for promoting apoptosis [86].
TNF-α inducer of apoptosis, also increased the expression of apoptosis-related molecules which was down-regulated by ethanol extract of M. oleifera in CoCl2-treated rats [37,88]. Leaf extract at a dose of 300 mg/kg body weight reduced the expression of caspase-9, the precursor of caspase-3, leading to apoptosis [25,89]. Bcl-2 inhibited apoptosis by blocking cytochrome c release and preventing caspase activation [90] while it was up-regulated by ethanol extract of M. oleifera in ML-induced rats. M. oleifera also reduced the expression of TIMP-1, which is involved in renal fibrosis and apoptosis [48].
4. Prospects for M. oleifera in Drug Development
Researchers are targeting the development of drugs from natural sources instead of the synthetic drug because natural sources have fewer side effects than synthetic sources. Nigerian scientists proved that M. oleifera is a beneficial herb and causes no harm to the body and kidneys [91]. Another study reported that higher doses of M. oleifera created toxicity in rats, but a moderate level dose of M. oleifera is safe [92]. M. oleifera has been shown to alleviate diabetic nephropathy in alloxan-induced rats [93]. Acetaminophen causes hepato-renal toxicity, which can be cured by M. oleifera treatment at the dosage of 500 mg/kg [94]. M. oleifera reduced necrosis, dilatation of renal tubules in Cd-induced rats, where Saleh et al. suggested that M. oleifera could be used as an herbal drug [95]. M. oleifera leaf extracts reduced oxidative stress, kidney, and liver damage [96]. A randomized placebo-controlled study suggested that M. oleifera leaf capsules can be used to control blood sugar level and blood pressure level [97]. Moreover, aqueous extracts of M. oleifera can reduce metal (As (III), Cd, Ni and Pb) toxicity and showed the protective effects in Saccharomyces cerevisiae [98].
The rich phytochemical profile and advances in biotechnological techniques have made this tree indispensable for opening a new era in medical science. An in vitro propagation technique provides new insights into developing more effective, eco-friendly, and biodegradable products using mass multiplication and production techniques. Though efficiency in in vitro propagation techniques for M. oleifera has been established, there are still gaps in the production of metabolites and those specific metabolites in the human body. The use of biotechnological approaches will help in the commercialization of important plant products. There is no doubt that biotechnological protocols will allow great research to make M. oleifera one of the essential solutions for various health issues including kidney diseases.
5. Conclusions
Kidney function declines with age, and aging-related kidney complications proportionately increase. Their side effects limit the effectiveness of existing drugs for treating kidney diseases and, therefore, natural compounds with fewer side effects are being evaluated. The literature discussed in this review suggests that M. oleifera alleviates several pathological factors associated with kidney diseases, including inflammation and oxidative stress. However, a mechanism associated with protective potential of M. oleifera against kidney diseases has been provided in this study (Figure 4).
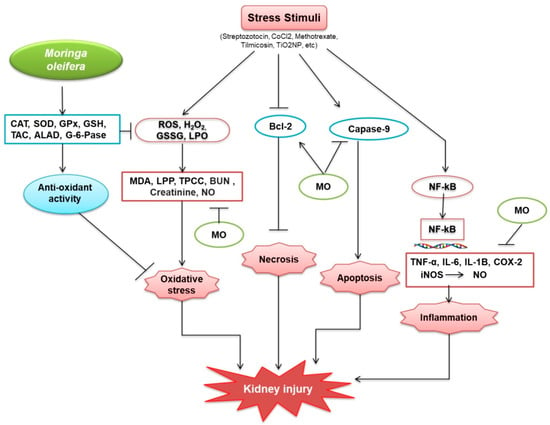
Figure 4.
Protective mechanisms of M. oleifera against kidney injury. M. oleifera increased the production of catalase (CAT); superoxide dismutase (SOD); glutathione peroxidase (GPx); glutathione (GSH); total antioxidant capacity (TAC); delta-amino levulinic acid dehydratase (ALAD); and G-6-Pase, which facilitated oxidative stress reduction by activating glutathione (GSH), a non-protein thiol that suppresses free radicals. GSH suppresses the oxidative stress situation. M. oleifera also suppressed oxidative stressors caused by ROS, H2O2, GSSG, and LPO by inhibiting MDA, LPP, TPCC, BUN, Creatinine, and NO. Bcl-2 was similarly produced by stress stimuli and was linked to the suppression of necrosis, induced by M. oleifera. M. oleifera inhibited the expression of Caspase-9, a protein involved in the formation of caspases. Following NF-kB, stress stimuli also increased CRP expression. NF-kB then moved from the cytosol to the nucleus, bound to DNA, and activated inflammation-related proteins. M. oleifera inhibited the mechanism by which inflammation factors were produced, hence, reducing inflammation. M. oleifera has been linked to a reduction in the progression of kidney disease.
This study discusses the insights of M. oleifera against kidney diseases including AKI and CKD, which have not been reported previously. In addition, further studies are needed to confirm the effects of the bioactive phytochemicals (vitamins, alkaloids, polyphenols, isothiocyonates, glucosinolates, tannins, and saponins) of M. oleifera against kidney diseases. We anticipate that the points raised in this review will provide a future research direction for understanding how pharmacological interventions based on natural products could modulate kidney disease. In contrast, it would shed light on how M. oleifera-based drugs would potentially be a kidney protective agent in treating aging-associated kidney abnormalities. Considering the harmful effects of synthetic resources and their non-renewable nature, the use of natural resources as a source of medicine has received a lot of attention in recent years. M. oleifera based medicine would be an excellent protective agent against several risk factors associated with kidney diseases.
Author Contributions
Conceptualization, M.J.U., A.M. and M.A.R.; data curation, T.A. and M.A.I.A.; funding acquisition, M.J.U.; methodology, T.A., M.A.I.A. and A.F.; initial draft, T.A. and M.A.R.; supervision, M.J.U.; writing—review and editing, T.A., M.A.R., M.A.H. and M.J.U. All authors have read and agreed to the published version of the manuscript.
Funding
This study received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This work acknowledges National Research Foundation (No. 2020R1I1A1A01072879 and 2015H1D3A1062189), and Brain Pool program funded by the Ministry of Science, and ICT through the National Research Foundation (No. 2020H1D3A2A02110924), Korea.
Conflicts of Interest
There are no conflicts of interest regarding this work.
References
- Romagnani, P.; Remuzzi, G.; Glassock, R.; Levin, A.; Jager, K.J.; Tonelli, M.; Massy, Z.; Wanner, C.; Anders, H.J. Chronic kidney disease. Nat. Rev. Dis. Primers 2017, 3, 17088. [Google Scholar] [CrossRef]
- Webster, A.C.; Nagler, E.V.; Morton, R.L.; Masson, P. Chronic kidney disease. Lancet 2017, 389, 1238–1252. [Google Scholar] [CrossRef]
- Sugahara, M.; Tanaka, T.; Nangaku, M. Prolyl hydroxylase domain inhibitors as a novel therapeutic approach against anemia in chronic kidney disease. Kidney Int. 2017, 92, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.J.; Dorotea, D.; Pak, E.S.; Ha, H. Fyn kinase: A potential therapeutic target in acute kidney injury. Biomol. Ther. 2020, 28. [Google Scholar] [CrossRef]
- Ahmed, S.R.; Romi, I.J.; Ahmed, J.; Hasan, M.; Roy, R.; Khan, M.M.H. Phytochemical profiling and antioxidant potentiality of medicinal plants along with their antibacterial efficacy. JABET 2019, 2, 140–145. [Google Scholar] [CrossRef]
- Aktar, S.; Das, P.K.; Asha, S.Y.; Siddika, M.A.; Islam, F.; Khanam, J.A.; Rakib, M.A. Moringa oleifera leaves methanolic extract inhibits angiotensin converting enzyme activity in vitro which ameliorates hypertension. JABET 2019, 2, 73–77. [Google Scholar] [CrossRef]
- Das, P.K.; Asha, S.Y.; Siddika, M.A.; Siddika, A.; M Tareq, A.R.; Islam, F.; Khanam, J.A.; Rakib, M.A. Methanolic extract of Moringa oleifera leaves mediates anticancer activities through inhibiting NF-ĸB and enhancing ROS in ehrlich ascites carcinoma cells in mice. JABET 2021, 4, 161–170. [Google Scholar]
- Islam, A.; Mandal, C.; Habib, A. Antibacterial potential of synthesized silver nanoparticles from leaf extract of Moringa oleifera. JABET 2021, 4, 67–73. [Google Scholar] [CrossRef]
- Gopalakrishnan, L.; Doriya, K.; Kumar, D.S. Moringa oleifera: A review on nutritive importance and its medicinal application. Food Sci. Hum. Wellness. 2016, 5, 49–56. [Google Scholar] [CrossRef] [Green Version]
- Kou, X.; Li, B.; Olayanju, J.B.; Drake, J.M.; Chen, N. Nutraceutical or pharmacological potential of Moringa oleifera Lam. Nutrients 2018, 10, 343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Z.F.; Ahmad, J.; Zhang, H.; Khan, I.; Muhammad, S. Evaluation of phytochemical and medicinal properties of moringa (Moringa oleifera) as a potential functional food. S. Afr. J. Bot. 2020, 129, 40–46. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Tiwari, P.; Sahu, P.K.; Kumar, S. A review of the phytochemical and pharmacological characteristics of Moringa oleifera. J. Pharm. Bioallied. Sci. 2018, 10, 181–191. [Google Scholar] [PubMed]
- Valdez-Solana, M.A.; Mejía-García, V.Y.; Téllez-Valencia, A.; García-Arenas, G.; Salas-Pacheco, J.; Alba-Romero, J.J.; Sierra-Campos, E. Nutritional content and elemental and phytochemical analyses of Moringa oleifera grown in Mexico. J.Chem. 2015, 2015, 860381. [Google Scholar] [CrossRef] [Green Version]
- Glevitzky, I.; Dumitrel, G.-A.; Mirel, G.; Pasca, M.B.; Otřísal, P.; Bungau, S.; Cioca, G.; Carmen, P.; Maria, P. Statistical analysis of the relationship between antioxidant activity and the structure of flavonoid compounds. Rev. Chim. 2019, 70. [Google Scholar] [CrossRef]
- Kashyap, D.; Sharma, A.; Tuli, H.S.; Sak, K.; Punia, S.; Mukherjee, T.K. Kaempferol—A dietary anticancer molecule with multiple mechanisms of action: Recent trends and advancements. J. Funct. Foods 2017, 30, 203–219. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Zhang, J.; Chen, X. Bioactive flavonoids in Moringa oleifera and their health-promoting properties. J. Funct. Foods 2018, 47, 469–479. [Google Scholar] [CrossRef]
- Vargas-Sánchez, K.; Garay-Jaramillo, E.; González-Reyes, R.E. Effects of Moringa oleifera on glycaemia and insulin levels: A review of animal and human studies. Nutrients 2019, 11, 2907. [Google Scholar] [CrossRef] [Green Version]
- Rahman, M.M.; Sheikh, M.M.I.; Sharmin, S.A.; Islam, M.S.; Rahman, M.A.; Rahman, M.M.; Alam, M.J.C.J.N.S. Antibacterial activity of leaf juice and extracts of Moringa oleifera Lam. against some human pathogenic bacteria. CMU J. Nat. Sci. 2009, 8, 219–227. [Google Scholar]
- Khan, W.; Parveen, R.; Chester, K.; Parveen, S.; Ahmad, S. Hypoglycemic potential of aqueous extract of Moringa oleifera leaf and In Vivo GC-MS metabolomics. Front. Pharmacol. 2017, 8, 577. [Google Scholar] [CrossRef] [Green Version]
- Tang, Y.; Choi, E.J.; Han, W.C.; Oh, M.; Kim, J.; Hwang, J.Y.; Park, P.J.; Moon, S.H.; Kim, Y.S.; Kim, E.K. Moringa oleifera from cambodia ameliorates oxidative stress, hyperglycemia, and kidney dysfunction in type 2 diabetic mice. J. Med. Food 2017, 20, 502–510. [Google Scholar] [CrossRef]
- Pedraza-Chaverri, J.; Sánchez-Lozada, L.G.; Osorio-Alonso, H.; Tapia, E.; Scholze, A. New pathogenic concepts and therapeutic approaches to oxidative stress in chronic kidney disease. Oxid. Med. Cell Longev. 2016, 2016, 6043601. [Google Scholar] [CrossRef] [Green Version]
- Meireles, D.; Gomes, J.; Lopes, L.; Hinzmann, M.; Machado, J. A review of properties, nutritional and pharmaceutical applications of Moringa oleifera: Integrative approach on conventional and traditional Asian medicine. ADTM 2020, 1–21. [Google Scholar] [CrossRef]
- Nafiu, A.O.; Akomolafe, R.O.; Alabi, Q.K.; Idowu, C.O.; Odujoko, O.O. Effect of fatty acids from ethanol extract of Moringa oleifera seeds on kidney function impairment and oxidative stress induced by gentamicin in rats. Biomed. Pharmacother. 2019, 117, 109154. [Google Scholar] [CrossRef]
- Akinrinde, A.S.; Oduwole, O.; Akinrinmade, F.J.; Bolaji-Alabi, F.B. Nephroprotective effect of methanol extract of Moringa oleifera leaves on acute kidney injury induced by ischemia-reperfusion in rats. Afr. Health Sci. 2020, 20, 1382–1396. [Google Scholar] [CrossRef]
- Soliman, M.M.; Aldhahrani, A.; Alkhedaide, A.; Nassan, M.A.; Althobaiti, F.; Mohamed, W.A. The ameliorative impacts of Moringa oleifera leaf extract against oxidative stress and methotrexate-induced hepato-renal dysfunction. Biomed. Pharmacother. 2020, 128, 110259. [Google Scholar] [CrossRef]
- Abu-Zeid, E.H.; Abdel Fattah, D.M.; Arisha, A.H.; Ismail, T.A.; Alsadek, D.M.; Metwally, M.M.M.; El-Sayed, A.A.; Khalil, A.T. Protective prospects of eco-friendly synthesized selenium nanoparticles using Moringa oleifera or Moringa oleifera leaf extract against melamine induced nephrotoxicity in male rats. Ecotoxicol. Environ. Saf. 2021, 221, 112424. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Vergara-Jimenez, M.; Almatrafi, M.M.; Fernandez, M.L. Bioactive components in Moringa oleifera leaves protect against chronic disease. Antioxidants 2017, 6, 91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daenen, K.; Andries, A.; Mekahli, D.; Van Schepdael, A.; Jouret, F.; Bammens, B. Oxidative stress in chronic kidney disease. Pediatr. Nephrol. 2019, 34, 975–991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ling, X.C.; Kuo, K.-L. Oxidative stress in chronic kidney disease. Renal. Replace. Ther. 2018, 4, 53. [Google Scholar] [CrossRef] [Green Version]
- Uddin, M.J.; Kim, E.H.; Hannan, M.A.; Ha, H. Pharmacotherapy against oxidative stress in chronic kidney disease: Promising small molecule natural products targeting nrf2-ho-1 signaling. Antioxidants 2021, 10, 258. [Google Scholar] [CrossRef]
- Sohn, M.; Kim, K.; Uddin, M.J.; Lee, G.; Hwang, I.; Kang, H.; Kim, H.; Lee, J.H.; Ha, H. Delayed treatment with fenofibrate protects against high-fat diet-induced kidney injury in mice: The possible role of ampk autophagy. Am. J. Physiol. Renal. Physiol. 2017, 312, F323–F334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, I.; Uddin, M.J.; Lee, G.; Jiang, S.; Pak, E.S.; Ha, H. Peroxiredoxin 3 deficiency accelerates chronic kidney injury in mice through interactions between macrophages and tubular epithelial cells. Free Radic. Biol. Med. 2019, 131, 162–172. [Google Scholar] [CrossRef]
- Rapa, S.F.; Di Iorio, B.R.; Campiglia, P.; Heidland, A.; Marzocco, S. Inflammation and oxidative stress in chronic kidney disease-potential therapeutic role of minerals, vitamins and plant-derived metabolites. Int. J. Mol. Sci. 2019, 21, 263. [Google Scholar] [CrossRef] [Green Version]
- Pakade, V.; Cukrowska, E.; Chimuka, L. Comparison of antioxidant activity of Moringa oleifera and selected vegetables in South Africa. S. Af. J. Sci. 2013, 109. [Google Scholar] [CrossRef]
- Omodanisi, E.I.; Aboua, Y.G.; Oguntibeju, O.O. Assessment of the anti-hyperglycaemic, anti-inflammatory and antioxidant activities of the methanol extract of Moringa oleifera in diabetes-induced nephrotoxic male Wistar rats. Molecules 2017, 22, 439. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Daim, M.M.; Khalil, S.R.; Awad, A.; Abu Zeid, E.H.; El-Aziz, R.A.; El-Serehy, H.A. Ethanolic extract of Moringa oleifera leaves influences NF-κB signaling pathway to restore kidney tissue from cobalt-mediated oxidative injury and inflammation in rats. Nutrients 2020, 12, 1031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adeyemi, O.S.; Elebiyo, T.C. Moringa oleifera supplemented diets prevented nickel-induced nephrotoxicity in Wistar Rats. J. Nutr. Metab. 2014, 2014, 958621. [Google Scholar] [CrossRef] [Green Version]
- Abou-Zeid, S.M.; Ahmed, A.I.; Awad, A.; Mohammed, W.A.; Metwally, M.M.M.; Almeer, R.; Abdel-Daim, M.M.; Khalil, S.R. Moringa oleifera ethanolic extract attenuates tilmicosin-induced renal damage in male rats via suppression of oxidative stress, inflammatory injury, and intermediate filament proteins mRNA expression. Biomed. Pharmacother. 2021, 133, 110997. [Google Scholar] [CrossRef]
- Abarikwu, S.O.; Benjamin, S.; Ebah, S.G.; Obilor, G.; Agbam, G. Protective effect of Moringa oleifera oil against HgCl2-induced hepato- and nephro-toxicity in rats. J. Basic Clin. Physiol. Pharmacol. 2017, 28, 337–345. [Google Scholar] [CrossRef]
- Abdou, K.H.; Moselhy, W.A.; Mohamed, H.M.; El-Nahass, E.S.; Khalifa, A.G. Moringa oleifera leaves extract protects titanium dioxide nanoparticles-induced nephrotoxicity via Nrf2/HO-1 signaling and amelioration of oxidative stress. Biol. Trace. Elem. Res. 2019, 187, 181–191. [Google Scholar] [CrossRef]
- Ahmed, N.F.; Sadek, K.M.; Soliman, M.K.; Khalil, R.H.; Khafaga, A.F.; Ajarem, J.S.; Maodaa, S.N.; Allam, A.A. Moringa oleifera leaf extract repairs the oxidative misbalance following Sub-chronic exposure to sodium fluoride in Nile tilapia Oreochromis niloticus. Animals 2020, 10, 626. [Google Scholar] [CrossRef] [Green Version]
- Ouédraogo, M.; Lamien-Sanou, A.; Ramdé, N.; Ouédraogo, A.S.; Ouédraogo, M.; Zongo, S.P.; Goumbri, O.; Duez, P.; Guissou, P.I. Protective effect of Moringa oleifera leaves against gentamicin-induced nephrotoxicity in rabbits. Exp. Toxicol. Pathol. 2013, 65, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Velaga, M.K.; Daughtry, L.K.; Jones, A.C.; Yallapragada, P.R.; Rajanna, S.; Rajanna, B. Attenuation of lead-induced oxidative stress in rat brain, liver, kidney and blood of male Wistar rats by Moringa oleifera seed powder. J. Environ. Pathol. Toxicol. Oncol. 2014, 33, 323–337. [Google Scholar] [CrossRef]
- Agrawal, N.D.; Nirala, S.K.; Shukla, S.; Mathur, R. Co-administration of adjuvants along with Moringa oleifera attenuates beryllium-induced oxidative stress and histopathological alterations in rats. Pharm. Biol. 2015, 53, 1465–1473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, R.; Kannan, G.M.; Sharma, M.; SJ, S.F. Therapeutic effects of Moringa oleifera on arsenic-induced toxicity in rats. Environ. Toxicol. Pharmacol. 2005, 20, 456–464. [Google Scholar] [CrossRef]
- Abdel-Latif, M.; Sakran, T.; Badawi, Y.K.; Abdel-Hady, D.S. Influence of Moringa oleifera extract, vitamin C, and sodium bicarbonate on heat stress-induced HSP70 expression and cellular immune response in rabbits. Cell Stress Chaperones 2018, 23, 975–984. [Google Scholar] [CrossRef] [PubMed]
- Abd-Elhakim, Y.M.; Mohamed, W.A.M.; El Bohi, K.M.; Ali, H.A.; Mahmoud, F.A.; Saber, T.M. Prevention of melamine-induced hepatorenal impairment by an ethanolic extract of Moringa oleifera: changes in KIM-1, TIMP-1, oxidative stress, apoptosis, and inflammation-related genes. Gene 2021, 764, 145083. [Google Scholar] [CrossRef]
- Mansour, A.T.; Miao, L.; Espinosa, C.; García-Beltrán, J.M.; Ceballos Francisco, D.C.; Esteban, M. Effects of dietary inclusion of Moringa oleifera leaves on growth and some systemic and mucosal immune parameters of seabream. Fish. Physiol. Biochem. 2018, 44, 1223–1240. [Google Scholar] [CrossRef]
- Karthivashan, G.; Kura, A.U.; Arulselvan, P.; Md Isa, N.; Fakurazi, S. The modulatory effect of Moringa oleifera leaf extract on endogenous antioxidant systems and inflammatory markers in an acetaminophen-induced nephrotoxic mice model. Peer J. 2016, 4, e2127. [Google Scholar] [CrossRef] [Green Version]
- Altaee, R.A.; Fadheel, Q.J. The nephroprotective effects of moringa oleifera extract against contrast induced nephrotoxicity. J. Pharm. Res. Int. 2021, 33, 63–70. [Google Scholar] [CrossRef]
- Adedapo, A.; Ue, E.; Falayi, O.; Ogunpolu, B.; Omobowale, T.; Oyagbemi, A.; Oguntibeju, O. Methanol stem extract of Moringa oleifera mitigates glycerol-induced acute kidney damage in rats through modulation of KIM-1 and NF-kB signaling pathways. Sci. Afr. 2020, 9, e00493. [Google Scholar] [CrossRef]
- Widodo, N.; Widjajanto, E.; Jatmiko, Y.; Rifa’i, M. Red Moringa oleifera leaf fermentation extract protecting Hepatotoxicity in Balb/C mice injected with Salmonella typhi through Nrf-2, HO-1, and SOD-2 signaling pathways. R. J. Pharm. Technol. 2020, 13, 5947–5952. [Google Scholar]
- Omodanisi, E.I.; Aboua, Y.G.; Chegou, N.N.; Oguntibeju, O.O. Hepatoprotective, antihyperlipidemic, and anti-inflammatory Activity of Moringa oleifera in diabetic-induced damage in male Wistar Rats. Pharmacogn. Res. 2017, 9, 182–187. [Google Scholar]
- Park, S.-H.; Chang, Y.-C. Anti-fibrotic effects by Moringa root extract in rat kidney fibroblast. J. Life Sci. 2012, 22, 1371–1377. [Google Scholar] [CrossRef] [Green Version]
- Valavanidis, A.; Vlachogianni, T.; Fiotakis, C. 8-hydroxy-2′-deoxyguanosine (8-OHdG): A critical biomarker of oxidative stress and carcinogenesis. J. Environ. Sci. Health C. Environ. Carcinog. Ecotoxicol. Rev. 2009, 27, 120–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oyagbemi, A.A.; Omobowale, T.O.; Azeez, I.O.; Abiola, J.O.; Adedokun, R.A.; Nottidge, H.O. Toxicological evaluations of methanolic extract of Moringa oleifera leaves in liver and kidney of male Wistar rats. J. Basic Clin. Physiol. Pharmacol. 2013, 24, 307–312. [Google Scholar] [CrossRef]
- Anders, H.J.; Schaefer, L. Beyond tissue injury-damage-associated molecular patterns, toll-like receptors, and inflammasomes also drive regeneration and fibrosis. J. Am. Soc. Nephrol. 2014, 25, 1387–1400. [Google Scholar] [CrossRef]
- Uddin, M.J.; Pak, E.S.; Ha, H. Carbon monoxide releasing molecule-2 protects mice against acute kidney injury through inhibition of ER stress. Korean J. Physiol. Pharmacol. 2018, 22, 567–575. [Google Scholar] [CrossRef] [Green Version]
- Uddin, M.J.; Jeong, J.; Pak, E.S.; Ha, H. Co-releasing molecule-2 prevents acute kidney Injury through suppression of ROS-Fyn-ER stress signaling in mouse model. Oxid. Med. Cell. Longev. 2021, 2021, 9947772. [Google Scholar] [CrossRef]
- Mackensen-Haen, S.; Bader, R.; Grund, K.E.; Bohle, A. Correlations between renal cortical interstitial fibrosis, atrophy of the proximal tubules and impairment of the glomerular filtration rate. Clin. Nephrol. 1981, 15, 167–171. [Google Scholar] [PubMed]
- Akcay, A.; Nguyen, Q.; Edelstein, C.L. Mediators of inflammation in acute kidney injury. Mediators Inflamm. 2009, 2009, 137072. [Google Scholar] [CrossRef]
- Akchurin, O.M.; Kaskel, F. Update on inflammation in chronic kidney disease. Blood Purif. 2015, 39, 84–92. [Google Scholar] [CrossRef]
- Elhelaly, A.E.; AlBasher, G.; Alfarraj, S.; Almeer, R.; Bahbah, E.I.; Fouda, M.M.A.; Bungău, S.G.; Aleya, L.; Abdel-Daim, M.M. Protective effects of hesperidin and diosmin against acrylamide-induced liver, kidney, and brain oxidative damage in rats. Environ. Sci. Pollut. Res. Int. 2019, 26, 35151–35162. [Google Scholar] [CrossRef]
- Behl, T.; Sharma, A.; Sharma, L.; Sehgal, A.; Zengin, G.; Brata, R.; Fratila, O.; Bungau, S. Exploring the multifaceted therapeutic potential of withaferin a and its derivatives. Biomedicines 2020, 8, 571. [Google Scholar] [CrossRef]
- Abdel-Daim, M.M.; Abushouk, A.I.; Bahbah, E.I.; Bungău, S.G.; Alyousif, M.S.; Aleya, L.; Alkahtani, S. Fucoidan protects against subacute diazinon-induced oxidative damage in cardiac, hepatic, and renal tissues. Environ. Sci. Pollut. Res. 2020, 27, 11554–11564. [Google Scholar] [CrossRef]
- Abdel-Daim, M.M.; Abo El-Ela, F.I.; Alshahrani, F.K.; Bin-Jumah, M.; Al-Zharani, M.; Almutairi, B.; Alyousif, M.S.; Bungau, S.; Aleya, L.; Alkahtani, S. Protective effects of thymoquinone against acrylamide-induced liver, kidney and brain oxidative damage in rats. Environ. Sci. Pollut. Res. 2020, 27, 37709–37717. [Google Scholar] [CrossRef] [PubMed]
- Jaja-Chimedza, A.; Graf, B.L.; Simmler, C.; Kim, Y.; Kuhn, P.; Pauli, G.F.; Raskin, I. Biochemical characterization and anti-inflammatory properties of an isothiocyanate-enriched moringa (Moringa oleifera) seed extract. PLoS ONE 2017, 12, e0182658. [Google Scholar] [CrossRef] [Green Version]
- Oblak, M.; Randic, M.; Solmajer, T. Quantitative structure-activity relationship of flavonoid analogues. 3. Inhibition of p56lck protein tyrosine kinase. J. Chem. Inf. Comput. Sci. 2000, 40, 994–1001. [Google Scholar] [CrossRef] [PubMed]
- Olszanecki, R.; Gebska, A.; Kozlovski, V.I.; Gryglewski, R.J. Flavonoids and nitric oxide synthase. J. Physiol. Pharmacol. 2002, 53, 571–584. [Google Scholar]
- Sulaiman, M.R.; Zakaria, Z.A.; Bujarimin, A.S.; Somchit, M.N.; Israf, D.A.; Moin, S. Evaluation of Moringa oleifera aqueous extract for antinociceptive and anti-inflammatory activities in animal models. Pharm. Biol. 2008, 46, 838–845. [Google Scholar] [CrossRef] [Green Version]
- García-Mediavilla, V.; Crespo, I.; Collado, P.S.; Esteller, A.; Sánchez-Campos, S.; Tuñón, M.J.; González-Gallego, J. The anti-inflammatory flavones quercetin and kaempferol cause inhibition of inducible nitric oxide synthase, cyclooxygenase-2 and reactive C-protein, and down-regulation of the nuclear factor kappaB pathway in Chang Liver cells. Eur. J. Pharmacol. 2007, 557, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Hämäläinen, M.; Nieminen, R.; Vuorela, P.; Heinonen, M.; Moilanen, E. Anti-inflammatory effects of flavonoids: Genistein, kaempferol, quercetin, and daidzein inhibit STAT-1 and NF-kappaB activations, whereas flavone, isorhamnetin, naringenin, and pelargonidin inhibit only NF-kappaB activation along with their inhibitory effect on iNOS expression and NO production in activated macrophages. Mediators. Inflamm. 2007, 2007, 45673. [Google Scholar] [PubMed] [Green Version]
- Tan, W.S.; Arulselvan, P.; Karthivashan, G.; Fakurazi, S. Moringa oleifera flower extract suppresses the activation of inflammatory mediators in lipopolysaccharide-stimulated raw 264.7 macrophages via NF-κB Pathway. Mediators. Inflamm. 2015, 2015, 720171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, E.J.; Cheenpracha, S.; Chang, L.C.; Kondratyuk, T.P.; Pezzuto, J.M. Inhibition of lipopolysaccharide-induced cyclooxygenase-2 and inducible nitric oxide synthase expression by 4-[(2′-O-acetyl-α-L-rhamnosyloxy)benzyl] isothiocyanate from Moringa oleifera. Nutr. Cancer 2011, 63, 971–982. [Google Scholar] [CrossRef] [Green Version]
- Fard, M.T.; Arulselvan, P.; Karthivashan, G.; Adam, S.K.; Fakurazi, S. Bioactive extract from Moringa oleifera inhibits the pro-inflammatory mediators in lipopolysaccharide stimulated macrophages. Pharmacogn. Mag. 2015, 11, S556. [Google Scholar] [CrossRef] [PubMed]
- Efstratiadis, G.; Divani, M.; Katsioulis, E.; Vergoulas, G. Renal fibrosis. Hippokratia 2009, 13, 224–229. [Google Scholar]
- Hamza, A.A. Ameliorative effects of Moringa oleifera Lam seed extract on liver fibrosis in rats. Food Chem. Toxicol. 2010, 48, 345–355. [Google Scholar] [CrossRef]
- Lin, T.A.; Wu, V.C.; Wang, C.Y. Autophagy in chronic kidney diseases. Cells 2019, 8, 61. [Google Scholar] [CrossRef] [Green Version]
- Kimura, T.; Takabatake, Y.; Takahashi, A.; Kaimori, J.Y.; Matsui, I.; Namba, T.; Kitamura, H.; Niimura, F.; Matsusaka, T.; Soga, T.; et al. Autophagy protects the proximal tubule from degeneration and acute ischemic injury. J. Am. Soc. Nephrol. 2011, 22, 902–913. [Google Scholar] [CrossRef]
- Takahashi, A.; Kimura, T.; Takabatake, Y.; Namba, T.; Kaimori, J.; Kitamura, H.; Matsui, I.; Niimura, F.; Matsusaka, T.; Fujita, N.; et al. Autophagy guards against cisplatin-induced acute kidney injury. Am. J. Pathol. 2012, 180, 517–525. [Google Scholar] [CrossRef]
- Liu, S.; Hartleben, B.; Kretz, O.; Wiech, T.; Igarashi, P.; Mizushima, N.; Walz, G.; Huber, T.B. Autophagy plays a critical role in kidney tubule maintenance, aging and ischemia-reperfusion injury. Autophagy 2012, 8, 826–837. [Google Scholar] [CrossRef] [Green Version]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Bonegio, R.; Lieberthal, W. Role of apoptosis in the pathogenesis of acute renal failure. Curr. Opin. Nephrol. Hypertens. 2002, 11, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Molitoris, B.A. Acute renal failure. Drugs Today (Barc) 1999, 35, 659–666. [Google Scholar] [CrossRef]
- Havasi, A.; Borkan, S.C. Apoptosis and acute kidney injury. Kidney Int. 2011, 80, 29–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feldenberg, L.R.; Thevananther, S.; del Rio, M.; de Leon, M.; Devarajan, P. Partial ATP depletion induces Fas- and caspase-mediated apoptosis in MDCK cells. Am. J. Physiol. 1999, 276, F837–F846. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; de Jong, P.E.; Coresh, J.; El Nahas, M.; Astor, B.C.; Matsushita, K.; Gansevoort, R.T.; Kasiske, B.L.; Eckardt, K.U. The definition, classification, and prognosis of chronic kidney disease: A KDIGO controversies conference report. Kidney Int. 2011, 80, 17–28. [Google Scholar] [CrossRef] [Green Version]
- Mace, P.D.; Riedl, S.J. Molecular cell death platforms and assemblies. Curr. Opin. Cell Biol. 2010, 22, 828–836. [Google Scholar] [CrossRef] [Green Version]
- Tzifi, F.; Economopoulou, C.; Gourgiotis, D.; Ardavanis, A.; Papageorgiou, S.; Scorilas, A. The role of BCL2 family of apoptosis regulator proteins in acute and chronic leukemias. Adv. Hematol. 2012, 2012, 524308. [Google Scholar] [CrossRef] [Green Version]
- Adebola, A.O.; Oluwatoyin, O.; Toyin, A.I.; Nnena Linda, N. Histological variances of Moringa olifera on the kidney of adult Wistar rats. Innov.J. Med. Sci. 2021, 9, 36–38. [Google Scholar] [CrossRef]
- Hassan, I.M.; Saidu, B.; Afaru, J.; Ishaq, A.; Dahiru, A.; Abdulazeez, N.; Yusuf, H.; Karofi, D.; Pilau, N.; Abubakar, A.A.; et al. Effects of Moringa oleifera biochemical constituents on kidney, liver and brain of Wister rats. SJAC 2020, 8, 128–132. [Google Scholar] [CrossRef]
- Akpan, H.; Akande, A.; Ojewale, A.; Oladipupo, F.; Akinpelu, O.F.; Jimoh, S. Moringa oleifera ameliorates nephropathic changes in alloxaninduced diabetic adult Wistar rats. J. Afr. Assoc. Physiol. Sci. 2018, 6, 110–118. [Google Scholar]
- Abaekwume, C.O.; Kagbo, H.D. Hepato-renal-curative effect of the herbal supplement of Aloe vera Linn Gel versus Moringa oleifera on acetaminophen-induced damage on the liver and Kidney of Wistar rats (Rattus novergicus). JAMPS 2021, 23, 12–23. [Google Scholar] [CrossRef]
- Saleh, A. Evaluation of hepatorenal protective activity of Moringa oleifera on histological and biochemical parameters in cadmium intoxicated rats. Toxin Rev. 2018, 38, 1–8. [Google Scholar] [CrossRef]
- Arafat, N.; Awadin, W.; ElShafei, R.; El-Metwalley, V.; Saleh, R. Protective Role of Moringa oleifera Leaves Extract Against Gentamicin-induced Nephro- and Hepato- Toxicity in Chickens. Alex. J. Vet. Sci. 2018, 58, 173. [Google Scholar] [CrossRef]
- Taweerutchana, R.; Lumlerdkij, N.; Vannasaeng, S.; Akarasereenont, P.; Sriwijitkamol, A. Effect of Moringa oleifera leaf capsules on glycemic control in therapy-naïve type 2 diabetes patients: A randomized placebo controlled study. Evid. Based. Complement. Alternat. Med. 2017, 2017, 6581390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kerdsomboon, K.; Chumsawat, W.; Auesukaree, C. Effects of Moringa oleifera leaf extracts and its bioactive compound gallic acid on reducing toxicities of heavy metals and metalloid in Saccharomyces cerevisiae. Chemosphere 2021, 270, 128659. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).