Secretion-Based Modes of Action of Biocontrol Agents with a Focus on Pseudozyma aphidis
Abstract
:1. Introduction
2. Antibiosis: Fungal Metabolites as a Source for New Pesticides
3. Effectors and Hyperbiotrophy-Dependent Inhibition
4. Induced Resistance by Fungal Metabolites
5. Mycoparasitisim and Cell Wall-Degrading Enzymes
6. Competition for Space and Nutrients
7. Conclusions
Funding
Conflicts of Interest
References
- The State of Food Insecurity in the World 2009: Economic Crisis–Impact and Lesson Learned; Food and Agriculture Organization of the United Nations: Rome, Italy, 2009.
- Oerke, E.C.; Dehne, H.W. Safeguarding production—Losses in major crops and the role of crop protection. Crop Prot. 2004, 23, 275–285. [Google Scholar] [CrossRef]
- Bebber, D.P.; Gurr, S.J. Crop-destroying fungal and oomycete pathogens challenge food security. Fungal Genet. Biol. 2015, 74, 62–64. [Google Scholar] [CrossRef] [PubMed]
- Oerke, E.C. Crop losses to pests. J. Agric. Sci. 2006, 144, 31–43. [Google Scholar] [CrossRef]
- Lee, N.; D’Souza, C.A.; Kronstad, J.W. Of smuts, blasts, mildews, and blights: cAMP Signaling in Phytopathogenic Fungi. Annu. Rev. Phytopathol. 2003, 41, 399–427. [Google Scholar] [CrossRef] [PubMed]
- Agrios, G. (Ed.) Plant Pathology, 5th ed.; Elsevier Academic Press: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Kennedy, B.W.; Alcorn, S.M. Estimates of U.S. Crop Losses to Procaryote Plant Pathogens. Plant Dis. 1980, 64, 674. [Google Scholar] [CrossRef]
- Vidhyasekaran, P. Bacterial Disease Resistance in Plants: Molecular Biology and Biotechnological Applications; CRC Press: Boca, Florida, USA, 2002; ISBN 9781560229254. [Google Scholar]
- Denholm, I.; Rowland, M.W. Tactics for Managing Pesticide Resistance in Arthropods: Theory and Practice. Annu. Rev. Entomol. 1992, 37, 91–112. [Google Scholar] [CrossRef] [PubMed]
- Leroux, P.; Fritz, R.; Debieu, D.; Albertini, C.; Lanen, C.; Bach, J.; Gredt, M.; Chapeland, F. Mechanisms of resistance to fungicides in field strains of Botrytis cinerea. Pest Manag. Sci. 2002, 58, 876–888. [Google Scholar] [CrossRef] [PubMed]
- Vela-Corcía, D.; Aditya Srivastava, D.; Dafa-Berger, A.; Rotem, N.; Barda, O.; Levy, M. MFS transporter from Botrytis cinerea provides tolerance to glucosinolate-breakdown products and is required for pathogenicity. Nat. Commun. 2019, 10, 2886. [Google Scholar] [CrossRef] [Green Version]
- Leroux, P.; Walker, A.S. Activity of fungicides and modulators of membrane drug transporters in field strains of Botrytis cinerea displaying multidrug resistance. Eur. J. Plant Pathol. 2013, 135, 683–693. [Google Scholar] [CrossRef]
- Arendrup, M.C.; Patterson, T.F. Multidrug-Resistant Candida: Epidemiology, Molecular Mechanisms, and Treatment. J. Infect. Dis. 2017, 216, S445–S451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rupp, S.; Weber, R.W.S.; Rieger, D.; Detzel, P.; Hahn, M. Spread of Botrytis cinerea strains with multiple fungicide resistance in German horticulture. Front. Microbiol. 2017, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leroch, M.; Plesken, C.; Weber, R.W.S.; Kauff, F.; Scalliet, G.; Hahn, M. Gray mold populations in German strawberry fields are resistant to multiple fungicides and dominated by a novel clade closely related to Botrytis cinerea. Appl. Environ. Microbiol. 2013, 79, 159–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mernke, D.; Dahm, S.; Walker, A.S.; Lalève, A.; Fillinger, S.; Leroch, M.; Hahn, M. Two promoter rearrangements in a drug efflux transporter gene are responsible for the appearance and spread of multidrug resistance phenotype MDR2 in Botrytis cinerea isolates in French and German vineyards. Phytopathology 2011, 101, 1176–1183. [Google Scholar] [CrossRef] [Green Version]
- Russ, D.; Kishony, R. Additivity of inhibitory effects in multidrug combinations. Nat. Microbiol. 2018, 3, 1339–1345. [Google Scholar] [CrossRef] [PubMed]
- Dayan, F.; Romagni, J.; Tellez, M.; Romando, A.; Duke, S. Managing weeds with natural products. Pestic. Outlook 1999, 10, 185–188. [Google Scholar]
- Dayan, F.E.; Cantrell, C.L.; Duke, S.O. Natural products in crop protection. Bioorganic Med. Chem. 2009, 17, 4022–4034. [Google Scholar] [CrossRef] [PubMed]
- Copping, L.G.; Duke, S.O. Natural products that have been used commercially as crop protection agents. Pest Manag. Sci. 2007, 63, 524–554. [Google Scholar] [CrossRef]
- Isman, M.B. Repellents in modern agriculture and an increasingly regulated world. Annu. Rev. Entomol. 2006, 51, 45–66. [Google Scholar] [CrossRef] [Green Version]
- Wilson, R.A.; Jenkinson, J.M.; Gibson, R.P.; Littlechild, J.A.; Wang, Z.Y.; Talbot, N.J. Tps1 regulates the pentose phosphate pathway, nitrogen metabolism and fungal virulence. EMBO J. 2007, 26, 3673–3685. [Google Scholar] [CrossRef] [Green Version]
- Elad, Y.; Freeman, S. Biological Control of Fungal Plant Pathogens. In Agricultural Applications; Springer: Berlin/Heidelberg, Germany, 2002; pp. 93–109. [Google Scholar]
- Butt, M.T.; Jackson, C.; Magan, N. (Eds.) Fungi as Biocontrol Agents: Progress, Problems and Potential, 1st ed.; CABI: Wallingford, UK, 2001. [Google Scholar]
- Raaijmakers, J.M.; Vlami, M.; de Souza, J.T. Antibiotic production by bacterial biocontrol agents. Antonie Van Leeuwenhoek 2002, 81, 537–547. [Google Scholar] [CrossRef]
- Kim, B.S.; Hwang, B.K. Microbial Fungicides in the Control of Plant Diseases. J. Phytopathol. 2007, 155, 641–653. [Google Scholar] [CrossRef]
- Porter, N. Physicochemical biophysical panel symposium biologically active secondary metabolites. Pestic. Sci. 1985, 16, 422–427. [Google Scholar]
- Früh, T.; Chemla, P.; Ehrler, J.; Farooq, S. Natural products as pesticides: Two examples of stereoselective synthesis. Pestic. Sci. 1996, 46, 37–47. [Google Scholar] [CrossRef]
- Zhang, X.; Harvey, P.R.; Stummer, B.E.; Warren, R.A.; Zhang, G.; Guo, K.; Li, J.; Yang, H. Antibiosis functions during interactions of Trichoderma afroharzianum and Trichoderma gamsii with plant pathogenic Rhizoctonia and Pythium. Funct. Integr. Genom. 2015, 15, 599–610. [Google Scholar] [CrossRef]
- Howell, C.R. Mechanisms employed by Trichoderma species in the biological control of plant diseases: The history and evolution of current concepts. Plant Dis. 2003, 87, 4–10. [Google Scholar] [CrossRef] [Green Version]
- Reino, J.L.; Guerrero, R.F.; Hernández-Galán, R.; Collado, I.G. Secondary metabolites from species of the biocontrol agent Trichoderma. Phytochem. Rev. 2008, 7, 89–123. [Google Scholar] [CrossRef]
- Sood, M.; Kapoor, D.; Kumar, V.; Sheteiwy, M.S.; Ramakrishnan, M.; Landi, M.; Araniti, F.; Sharma, A. Trichoderma: The “Secrets” of a Multitalented Biocontrol Agent. Plants 2020, 9, 762. [Google Scholar] [CrossRef]
- Krause, C.; Kirschbaum, J.; Jung, G.; Brückner, H. Sequence diversity of the peptaibol antibiotic suzukacillin-A from the mold Trichoderma viride. J. Pept. Sci. 2006, 12, 321–327. [Google Scholar] [CrossRef]
- Szekeres, A.; Leitgeb, B.; Kredics, L.; Antal, Z.; Hatvani, L.; Manczinger, L.; Vágvölgyi, C. Peptaibols and related peptaibiotics of Trichoderma: A review. Acta Microbiol. Immunol. Hung. 2005, 52, 137–168. [Google Scholar] [CrossRef]
- Shi, M.; Chen, L.; Wang, X.W.; Zhang, T.; Zhao, P.B.; Song, X.Y.; Sun, C.Y.; Chen, X.L.; Zhou, B.C.; Zhang, Y.Z. Antimicrobial peptaibols from Trichoderma pseudokoningii induce programmed cell death in plant fungal pathogens. Microbiology 2012, 158, 166–175. [Google Scholar] [CrossRef] [Green Version]
- Schirmbock, M.; Lorito, M.; Wang, Y.L.; Hayes, C.K.; Arisan-Atac, I.; Scala, F.; Harman, G.E.; Kubicek, C.P. Parallel formation and synergism of hydrolytic enzymes and peptaibol antibiotics, molecular mechanisms involved in the antagonistic action of Trichoderma harzianum against phytopathogenic fungi. Appl. Environ. Microbiol. 1994, 60, 4364–4370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hajlaoui, M.R.; Bélanger, R.R. Comparative effects of temperature and humidity on the activity of three potential antagonists of rose powdery mildew. Neth. J. Plant Pathol. 1991, 97, 203–208. [Google Scholar] [CrossRef]
- Hajlaoui, M.R.; Traquair, J.A.; Jarvis, W.R.; Belanger, B. Antifungal Activity of Extracellular Metabolites Produced by Sporothrix flocculosa. Biocontrol Sci. Technol. 1994, 4, 229–237. [Google Scholar] [CrossRef]
- Jarvis, W.R.; Shaw, L.A.; Traquair, J.A. Factors affecting antagonism of cucumber powdery mildew by Stephanoascus flocculosus and S. rugulosus. Mycol. Res. 1989, 92, 162–165. [Google Scholar] [CrossRef]
- Hajlaoui, M.R.; Belanger, R.R. Antagonism of the Yeast-like Phylloplane Fungus Sporothrix flocculosa against Erysiphe graminis var tritici. Biocontrol Sci. Technol. 1993, 3, 427–434. [Google Scholar] [CrossRef]
- Dik, A.J.; Verhaar, M.A.; Bélanger, R.R. Comparison of three biological control agents against cucumber powdery mildew (Sphaerotheca fuliginea) in semi-commercial-scale glasshouse trials. Eur. J. Plant Pathol. 1998, 104, 413–423. [Google Scholar] [CrossRef]
- Hammami, W.; Castro, C.Q.; Rémus-Borel, W.; Labbé, C.; Bélanger, R.R. Ecological basis of the interaction between Pseudozyma flocculosa and powdery mildew fungi. Appl. Environ. Microbiol. 2011, 77, 926–933. [Google Scholar] [CrossRef] [Green Version]
- Buxdorf, K.; Rahat, I.; Gafni, A.; Levy, M. The epiphytic fungus Pseudozyma aphidis induces jasmonic acid- and salicylic acid/nonexpressor of PR1-independent local and systemic resistance. Plant Physiol. 2013, 161, 2014–2022. [Google Scholar] [CrossRef] [Green Version]
- Gafni, A.; Calderon, C.E.; Harris, R.; Buxdorf, K.; Dafa-Berger, A.; Zeilinger-Reichert, E.; Levy, M. Biological control of the cucurbit powdery mildew pathogen Podosphaera xanthii by means of the epiphytic fungus Pseudozyma aphidis and parasitism as a mode of action. Front. Plant Sci. 2015, 6, 132. [Google Scholar] [CrossRef] [Green Version]
- Avis, T.J.; Boulanger, R.R.; Bélanger, R.R. Synthesis and biological characterization of (Z)-9-heptadecenoic and (Z)-6-methyl-9-heptadecenoic acids: Fatty acids with antibiotic activity produced by Pseudozyma flocculosa. J. Chem. Ecol. 2000, 26, 987–1000. [Google Scholar] [CrossRef]
- Benyagoub, M.; Bel Rhlid, R.; Bélanger, R.R. Purification and characterization of new fatty acids with antibiotic activity produced by Sporothrix flocculosa. J. Chem. Ecol. 1996, 22, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, S.R.; Traquair, J.A.; Jarvis, W.R. 4-Methyl-7,11-heptadecadienal and 4-methyl-7,11-heptadecadienoic acid: New antibiotics from Sporothrix flocculosa and Sporothrix rugulosa. J. Nat. Prod. 1994, 57, 700–704. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, S.R.; Traquair, J.A.; Jarvis, W.R. New extracellular fatty acids in culture filtrates of Sporothrix flocculosa and S. rugulosa. Can. J. Chem. 1995, 73, 84–87. [Google Scholar] [CrossRef]
- Hajlaoui, M.R. Cytochemical Study of the Antagonistic Activity of Sporothrix flocculosa on Rose Powdery Mildew, Sphaerotheca pannosa var. rosae. Phytopathology 1992, 82, 583. [Google Scholar] [CrossRef]
- Avis, T.J.; Belanger, R.R. Specificity and mode of action of the antifungal fatty acid cis-9-heptadecenoic acid produced by Pseudozyma flocculosa. Appl. Environ. Microbiol. 2001, 67, 956–960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Golubev, W.I.; Pfeiffer, I.; Golubeva, E.W. Mycocin production in Pseudozyma tsukubaensis. Mycopathologia 2006, 162, 313–316. [Google Scholar] [CrossRef]
- Golubev, W.I. Mycocinogeny in smut yeast-like fungi of the genus Pseudozyma. Microbiology 2007, 76, 719–722. [Google Scholar] [CrossRef]
- Cheng, Y.; McNally, D.J.; Labbé, C.; Voyer, N.; Belzile, F.; Bélanger, R.R. Insertional mutagenesis of a fungal biocontrol agent led to discovery of a rare cellobiose lipid with antifungal activity. Appl. Environ. Microbiol. 2003, 69, 2595–2602. [Google Scholar] [CrossRef] [Green Version]
- Mimee, B.; Labbé, C.; Pelletier, R.; Bélanger, R.R. Antifungal activity of flocculosin, a novel glycolipid isolated from Pseudozyma flocculosa. Antimicrob. Agents Chemother. 2005, 49, 1597–1599. [Google Scholar] [CrossRef] [Green Version]
- Mimee, B.; Pelletier, R.; Bélanger, R.R. In vitro antibacterial activity and antifungal mode of action of flocculosin, a membrane-active cellobiose lipid. J. Appl. Microbiol. 2009, 107, 989–996. [Google Scholar] [CrossRef]
- Kulakovskaya, T.V.; Shashkov, A.S.; Kulakovskaya, E.V.; Golubev, W.I. Ustilagic acid secretion by Pseudozyma fusiformata strains. FEMS Yeast Res. 2005, 5, 919–923. [Google Scholar] [CrossRef] [Green Version]
- Golubev, W.I.; Kulakovskaya, T.V.; Shashkov, A.S.; Kulakovskaya, E.V.; Golubev, N. V Antifungal cellobiose lipid secreted by the epiphytic yeast Pseudozyma graminicola. Microbiology 2008, 77, 171–175. [Google Scholar] [CrossRef]
- Haskins, R.H.; Thorn, J.A. Biochemistry of the Ustilaginales: VII. Antibiotic activity of Ustilagic acid. Can. J. Bot. 1951, 29, 585–592. [Google Scholar] [CrossRef]
- Lefebvre, F.; Joly, D.L.; Labbé, C.; Teichmann, B.; Linning, R.; Belzile, F.; Bakkeren, G.; Bélanger, R.R. The transition from a phytopathogenic smut ancestor to an anamorphic biocontrol agent deciphered by comparative whole-genome analysis. Plant Cell 2013, 25, 1946–1959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bélanger, R.R.; Labbé, C.; Lefebvre, F.; Teichmann, B. Mode of action of biocontrol agents: All that glitters is not gold. Can. J. Plant Pathol. 2012, 34, 469–478. [Google Scholar] [CrossRef]
- Barda, O.; Shalev, O.; Alster, S.; Buxdorf, K.; Gafni, A.; Levy, M. Pseudozyma aphidis induces salicylic-acid-independent resistance to Clavibacter michiganensis in tomato plants. Plant Dis. 2015, 99, 621–626. [Google Scholar] [CrossRef]
- Calderón, C.E.; Rotem, N.; Harris, R.; Vela-Corcía, D.; Levy, M. Pseudozyma aphidis activates reactive oxygen species production, programmed cell death and morphological alterations in the necrotrophic fungus Botrytis cinerea. Mol. Plant Pathol. 2019, 20, 562–574. [Google Scholar] [CrossRef] [Green Version]
- Nyfeler, R.; Ackermann, P. Phenylpyrroles, a New Class of Agricultural Fungicides Related to the Natural Antibiotic Pyrrolnitrin. In Synthesis and Chemistry of Agrochemicals, 3rd ed.; ACS Publications: Washington, DC, USA, 1992; pp. 395–404. [Google Scholar]
- Anke, T.; Oberwinkler, F.; Steglich, W.; Schramm, G. The strobilurins—New antifungal antibiotics from the basidiomycete Strobilurus tenacellus. J. Antibiot. 1977, 30, 806–810. [Google Scholar] [CrossRef]
- Bartlett, D.W.; Clough, J.M.; Godwin, J.R.; Hall, A.A.; Hamer, M.; Parr-Dobrzanski, B. The strobilurin fungicides. Pest Manag. Sci. 2002, 58, 649–662. [Google Scholar] [CrossRef]
- Balba, H. Review of strobilurin fungicide chemicals. J. Environ. Sci. Health B 2007, 42, 441–451. [Google Scholar] [CrossRef]
- Takahashi, M.; Koyama, K.; Natori, S. Four New Azaphilones from Chaetomium globosum var. flavo-viridae. Chem. Pharm. Bull. 1990, 38, 625–628. [Google Scholar] [CrossRef] [Green Version]
- Park, J.H.; Gyung, J.C.; Kyoung, S.J.; He, K.L.; Heung, T.K.; Kwang, Y.C.; Kim, J.C. Antifungal activity against plant pathogenic fungi of chaetoviridins isolated from Chaetomium globosum. FEMS Microbiol. Lett. 2005, 252, 309–313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altomare, C.; Pengue, R.; Favilla, M.; Evidente, A.; Visconti, A. structure-activity relationships of derivatives of Fusapyrone, an antifungal metabolite of Fusarlum semitectum. J. Agric. Food Chem. 2004, 52, 2997–3001. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.C.; Choi, G.J.; Kim, H.J.; Kim, H.T.; Ahn, J.W.; Cho, K.Y. Verlamelin, an antifungal compound produced by a mycoparasite, Acremonium strictum. Plant Pathol. J. 2002, 18, 102–105. [Google Scholar] [CrossRef]
- Lee, D.W.; Kim, B.S. Antimicrobial cyclic peptides for plant disease control. Plant Pathol. J. 2015, 31, 1–11. [Google Scholar] [CrossRef]
- Kämper, J.; Kahmann, R.; Bölker, M.; Ma, L.J.; Brefort, T.; Saville, B.J.; Banuett, F.; Kronstad, J.W.; Gold, S.E.; Müller, O.; et al. Insights from the genome of the biotrophic fungal plant pathogen Ustilago maydis. Nature 2006, 444, 97–101. [Google Scholar] [CrossRef]
- Win, J.; Chaparro-Garcia, A.; Belhaj, K.; Saunders, D.G.O.; Yoshida, K.; Dong, S.; Schornack, S.; Zipfel, C.; Robatzek, S.; Hogenhout, S.A.; et al. Effector biology of plant-associated organisms: Concepts and perspectives. Cold Spring Harb. Symp. Quant. Biol. 2012, 77, 235–247. [Google Scholar] [CrossRef]
- Doehlemann, G.; van der Linde, K.; Assmann, D.; Schwammbach, D.; Hof, A.; Mohanty, A.; Jackson, D.; Kahmann, R. Pep1, a secreted effector protein of Ustilago maydis, is required for successful invasion of plant cells. PLoS Pathog. 2009, 5, e1000290. [Google Scholar] [CrossRef] [Green Version]
- Doehlemann, G.; Reissmann, S.; Aßmann, D.; Fleckenstein, M.; Kahmann, R. Two linked genes encoding a secreted effector and a membrane protein are essential for Ustilago maydis-induced tumour formation. Mol. Microbiol. 2011, 81, 751–766. [Google Scholar] [CrossRef]
- Sharma, R.; Ökmen, B.; Doehlemann, G.; Thines, M. Saprotrophic yeasts formerly classified as Pseudozyma have retained a large effector arsenal, including functional Pep1 orthologs. Mycol. Prog. 2019, 18, 763–768. [Google Scholar] [CrossRef]
- Laur, J.; Ramakrishnan, G.B.; Labbé, C.; Lefebvre, F.; Spanu, P.D.; Bélanger, R.R. Effectors involved in fungal-fungal interaction lead to a rare phenomenon of hyperbiotrophy in the tritrophic system biocontrol agent-powdery mildew-plant. New Phytol. 2018, 217, 713–725. [Google Scholar] [CrossRef] [Green Version]
- Guzmán-Guzmán, P.; Alemán-Duarte, M.I.; Delaye, L.; Herrera-Estrella, A.; Olmedo-Monfil, V. Identification of effector-like proteins in Trichoderma spp. and role of a hydrophobin in the plant-fungus interaction and mycoparasitism. BMC Genet. 2017, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramírez-Valdespino, C.A.; Casas-Flores, S.; Olmedo-Monfil, V. Trichoderma as a model to study effector-like molecules. Front. Microbiol. 2019, 10, 1030. [Google Scholar] [CrossRef] [PubMed]
- Djonović, S.; Pozo, M.J.; Dangott, L.J.; Howell, C.R.; Kenerley, C.M. Sm1, a proteinaceous elicitor secreted by the biocontrol fungus Trichoderma virens induces plant defense responses and systemic resistance. Mol. Plant Microbe Interact. 2006, 19, 838–853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seidl, V.; Marchetti, M.; Schandl, R.; Allmaier, G.; Kubicek, C.P. Epl1, the major secreted protein of Hypocrea atroviridis on glucose, is a member of a strongly conserved protein family comprising plant defense response elicitors. FEBS J. 2006, 273, 4346–4359. [Google Scholar] [CrossRef] [PubMed]
- Salas-Marina, M.A.; Isordia-Jasso, M.I.; Islas-Osuna, M.A.; Delgado-Sánchez, P.; Jiménez-Bremont, J.F.; Rodríguez-Kessler, M.; Rosales-Saavedra, M.T.; Herrera-Estrella, A.; Casas-Flores, S. The Epl1 and Sm1 proteins from Trichoderma atroviride and Trichoderma virens differentially modulate systemic disease resistance against different life style pathogens in Solanum lycopersicum. Front. Plant Sci. 2015, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crutcher, F.K.; Moran-Diez, M.E.; Ding, S.; Liu, J.; Horwitz, B.A.; Mukherjee, P.K.; Kenerley, C.M. A paralog of the proteinaceous elicitor SM1 is involved in colonization of maize roots by Trichoderma virens. Fungal Biol. 2015, 119, 476–486. [Google Scholar] [CrossRef] [PubMed]
- Gaderer, R.; Lamdan, N.L.; Frischmann, A.; Sulyok, M.; Krska, R.; Horwitz, B.A.; Seidl-Seiboth, V. Sm2, a paralog of the Trichoderma cerato-platanin elicitor Sm1, is also highly important for plant protection conferred by the fungal-root interaction of Trichoderma with maize. BMC Microbiol. 2015, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shcherbakova, L.A.; Odintsova, T.I.; Stakheev, A.A.; Fravel, D.R.; Zavriev, S.K. Identification of a novel small cysteine-rich protein in the fraction from the biocontrol Fusarium oxysporum strain CS-20 that mitigates fusarium wilt symptoms and triggers defense responses in tomato. Front. Plant Sci. 2016, 6. [Google Scholar] [CrossRef] [Green Version]
- Schuster, M.; Schweizer, G.; Kahmann, R. Comparative analyses of secreted proteins in plant pathogenic smut fungi and related basidiomycetes. Fungal Genet. Biol. 2018, 112, 21–30. [Google Scholar] [CrossRef]
- Hemetsberger, C.; Mueller, A.N.; Matei, A.; Herrberger, C.; Hensel, G.; Kumlehn, J.; Mishra, B.; Sharma, R.; Thines, M.; Hückelhoven, R.; et al. The fungal core effector Pep1 is conserved across smuts of dicots and monocots. New Phytol. 2015, 206, 1116–1126. [Google Scholar] [CrossRef] [PubMed]
- Malinovsky, F.G.; Fangel, J.U.; Willats, W.G.T. The role of the cell wall in plant immunity. Front. Plant Sci. 2014, 5, 178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saijo, Y.; Loo, E.P.I.; Yasuda, S. Pattern recognition receptors and signaling in plant–microbe interactions. Plant J. 2018, 93, 592–613. [Google Scholar] [CrossRef] [PubMed]
- Grant, M.; Lamb, C. Systemic immunity. Curr. Opin. Plant Biol. 2006, 9, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Glazebrook, J. Contrasting Mechanisms of Defense against Biotrophic and Necrotrophic Pathogens. Annu. Rev. Phytopathol. 2005, 43, 205–227. [Google Scholar] [CrossRef] [PubMed]
- Somssich, I.E. Closing another gap in the plant SAR puzzle. Cell 2003, 113, 815–816. [Google Scholar] [CrossRef] [Green Version]
- Haas, D.; Défago, G. Biological control of soil-borne pathogens by fluorescent pseudomonads. Nat. Rev. Microbiol. 2005, 3, 307–319. [Google Scholar] [CrossRef]
- Heil, M. Ecological costs of induced resistance. Curr. Opin. Plant Biol. 2002, 5, 345–350. [Google Scholar] [CrossRef]
- Llorens, E.; García-Agustín, P.; Lapeña, L. Advances in induced resistance by natural compounds: Towards new options for woody crop protection. Sci. Agric. 2017, 74, 90–100. [Google Scholar] [CrossRef]
- Pieterse, C.M.J.; Van Loon, L.C. Salicylic acid-independent plant defence pathways. Trends Plant Sci. 1999, 4, 52–58. [Google Scholar] [CrossRef] [Green Version]
- Penninckx, I.A.M.A.; Thomma, B.P.H.J.; Buchala, A.; Métraux, J.P.; Broekaert, W.F. Concomitant activation of jasmonate and ethylene response pathways is required for induction of a plant defensin gene in Arabidopsis. Plant Cell 1998, 10, 2103–2113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomma, B.P.H.J.; Eggermont, K.; Penninckx, I.A.M.A.; Mauch-Mani, B.; Vogelsang, R.; Cammue, B.P.A.; Broekaert, W.F. Separate jasmonate-dependent and salicylate-dependent defense-response pathways in Arabidopsis are essential for resistance to distinct microbial pathogens. Proc. Natl. Acad. Sci. USA 1998, 95, 15107–15111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Penninckx, I.A.; Eggermont, K.; Terras, F.R.; Thomma, B.P.; De Samblanx, G.W.; Buchala, A.; Métraux, J.P.; Manners, J.M.; Broekaert, W.F. Pathogen-induced systemic activation of a plant defensin gene in Arabidopsis follows a salicylic acid-independent pathway. Plant Cell 1996, 8, 2309–2323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pieterse, C.M.J.; Zamioudis, C.; Berendsen, R.L.; Weller, D.M.; Van Wees, S.C.M.; Bakker, P.A.H.M. Induced systemic resistance by beneficial microbes. Annu. Rev. Phytopathol. 2014, 52, 347–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buxdorf, K.; Rahat, I.; Levy, M. Pseudozyma aphidis induces ethylene-independent resistance in plants. Plant Signal. Behav. 2013, 8, e26273. [Google Scholar] [CrossRef]
- Agrawal, A.A. Induced responses to herbivory and increased plant performance. Science 1998, 279, 1201–1202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baldwin, I.T. Jasmonate-induced responses are costly but benefit plants under attack in native populations. Proc. Natl. Acad. Sci. USA 1998, 95, 8113–8118. [Google Scholar] [CrossRef] [Green Version]
- Conrath, U.; Beckers, G.J.M.; Flors, V.; García-Agustín, P.; Jakab, G.; Mauch, F.; Newman, M.A.; Pieterse, C.M.J.; Poinssot, B.; Pozo, M.J.; et al. Priming: Getting ready for battle. Mol. Plant Microbe Interact. 2006, 19, 1062–1071. [Google Scholar] [CrossRef] [Green Version]
- Perazzolli, M.; Roatti, B.; Bozza, E.; Pertot, I. Trichoderma harzianum T39 induces resistance against downy mildew by priming for defense without costs for grapevine. Biol. Control 2011, 58, 74–82. [Google Scholar] [CrossRef]
- Yoshioka, Y.; Ichikawa, H.; Naznin, H.A.; Kogure, A.; Hyakumachi, M. Systemic resistance induced in Arabidopsis thaliana by Trichoderma asperellum SKT-1, a microbial pesticide of seedborne diseases of rice. Pest Manag. Sci. 2012, 68, 60–66. [Google Scholar] [CrossRef]
- Shoresh, M.; Yedidia, I.; Chet, I. Involvement of jasmonic acid/ethylene signaling pathway in the systemic resistance induced in cucumber by Trichoderma asperellum T203. Phytopathology 2005, 95, 76–84. [Google Scholar] [CrossRef] [Green Version]
- Molitor, A.; Zajic, D.; Voll, L.M.; Pons-Kühnemann, J.; Samans, B.; Kogel, K.H.; Waller, F. Barley leaf transcriptome and metabolite analysis reveals new aspects of compatibility and Piriformospora indica-mediated systemic induced resistance to powdery mildew. Mol. Plant Microbe Interact. 2011, 24, 1427–1439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aime, S.; Alabouvette, C.; Steinberg, C.; Olivain, C. The endophytic strain Fusarium oxysporum Fo47: A good candidate for priming the defense responses in tomato roots. Mol. Plant Microbe Interact. 2013, 26, 918–926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, G.; Lee, S.-H.; Kim, K.M.; Ryu, C.-M. Foliar application of the leaf-colonizing yeast Pseudozyma churashimaensis elicits systemic defense of pepper against bacterial and viral pathogens. Sci. Rep. 2017, 7, 39432. [Google Scholar] [CrossRef] [PubMed]
- Oostendorp, M.; Kunz, W.; Dietrich, B.; Staub, T. Induced disease resistance in plants by chemicals. Eur. J. Plant Pathol. 2001, 107, 19–28. [Google Scholar] [CrossRef]
- Friedrich, L.; Lawton, K.; Ruess, W.; Masner, P.; Specker, N.; Rella, M.G.; Meier, B.; Dincher, S.; Staub, T.; Uknes, S.; et al. A benzothiadiazole derivative induces systemic acquired resistance in tobacco. Plant J. 1996, 10, 61–70. [Google Scholar] [CrossRef]
- Walters, D.R.; Ratsep, J.; Havis, N.D. Controlling crop diseases using induced resistance: Challenges for the future. J. Exp. Bot. 2013, 64, 1263–1280. [Google Scholar] [CrossRef]
- Ton, J.; Jakab, G.; Toquin, V.; Flors, V.; Iavicoli, A.; Maeder, M.N.; Métraux, J.P.; Mauch-Mani, B. Dissecting the β-aminobutyric acid-induced priming phenomenon in Arabidopsis. Plant Cell 2005, 17, 987–999. [Google Scholar] [CrossRef] [Green Version]
- Aranega-Bou, P.; de la O Leyva, M.; Finiti, I.; Garcfa-Agustfn, P.; Gonzalez-Bosch, C. Priming of plant resistance by natural compounds. Hexanoic acid as a model. Front. Plant Sci. 2014, 5, 488. [Google Scholar] [CrossRef] [Green Version]
- Iriti, M.; Castorina, G.; Vitalini, S.; Mignani, I.; Soave, C.; Fico, G.; Faoro, F. Chitosan-induced ethylene-independent resistance does not reduce crop yield in bean. Biol. Control 2010, 54, 241–247. [Google Scholar] [CrossRef]
- Ahn, I.P.; Kim, S.; Lee, Y.H.; Suh, S.C. Vitamin B1-induced priming is dependent on hydrogen peroxide and the NPR1 gene in arabidopsis. Plant Physiol. 2007, 143, 838–848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noutoshi, Y.; Okazaki, M.; Kida, T.; Nishina, Y.; Morishita, Y.; Ogawa, T.; Suzuki, H.; Shibata, D.; Jikumaru, Y.; Hanada, A.; et al. Novel plant immune-priming compounds identified via high-throughput chemical screening target salicylic acid glucosyltransferases in Arabidopsis. Plant Cell 2012, 24, 3795–3804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soufleros, E.H.; Mygdalia, S.A.; Natskoulis, P. Production process and characterization of the traditional Greek fruit distillate “Koumaro” by aromatic and mineral composition. J. Food Compos. Anal. 2005, 18, 699–716. [Google Scholar] [CrossRef]
- Hossain, M.M.; Sultana, F.; Kubota, M.; Koyama, H.; Hyakumachi, M. The Plant Growth-Promoting Fungus Penicillium simplicissimum GP17-2 Induces Resistance in Arabidopsis thaliana by Activation of Multiple Defense Signals. Plant Cell Physiol. 2007, 48, 1724–1736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naznin, H.A.; Kiyohara, D.; Kimura, M.; Miyazawa, M.; Shimizu, M.; Hyakumachi, M. Systemic resistance induced by volatile organic compounds emitted by plant growth-promoting fungi in Arabidopsis thaliana. PLoS ONE 2014, 9, e86882. [Google Scholar] [CrossRef] [Green Version]
- Viterbo, A.; Wiest, A.; Brotman, Y.; Chet, I.; Kenerley, C. The 18mer peptaibols from Trichoderma virens elicit plant defence responses. Mol. Plant Pathol. 2007, 8, 737–746. [Google Scholar] [CrossRef]
- Vinale, F.; Sivasithamparam, K.; Ghisalberti, E.L.; Marra, R.; Barbetti, M.J.; Li, H.; Woo, S.L.; Lorito, M. A novel role for Trichoderma secondary metabolites in the interactions with plants. Physiol. Mol. Plant Pathol. 2008, 72, 80–86. [Google Scholar] [CrossRef]
- Weindling R Trichoderma lignorum as a parasite of other soil fungi. Phytopathology 1932, 22, 837–845.
- Lorito, M.; Harman, G.; Hayes, C.; Broadway, R.; Tronsmo, A.; Woo, S.; Di Pietro, A. Chitinolytic enzymes produced by Trichoderma harzianum: Antifungal activity of purified Endochitinase and Chitobiosidase. Phytopathology 1993, 83, 302. [Google Scholar] [CrossRef]
- Viterbo, A.; Haran, S.; Friesem, D.; Ramot, O.; Chet, I. Antifungal activity of a novel endochitinase gene (chit36) from Trichoderma harzianum Rifai TM. FEMS Microbiol. Lett. 2001, 200, 169–174. [Google Scholar] [CrossRef]
- Elad, Y. Biological control of foliar pathogens by means of Trichoderma harzianum and potential modes of action. Crop Prot. 2000, 19, 709–714. [Google Scholar] [CrossRef]
- Boyce, K.J.; Andrianopoulos, A. Fungal dimorphism: The switch from hyphae to yeast is a specialized morphogenetic adaptation allowing colonization of a host. FEMS Microbiol. Rev. 2015, 39, 797–811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murad, A.M.A.; D’Enfert, C.; Gaillardin, C.; Tournu, H.; Tekaia, F.; Talibi, D.; Marechal, D.; Marchais, V.; Cottin, J.; Brown, A.J.P. Transcript profiling in Candida albicans reveals new cellular functions for the transcriptional repressors CaTup1, CaMig1 and CaNrg1. Mol. Microbiol. 2001, 42, 981–993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lo, H.J.; Köhler, J.R.; Didomenico, B.; Loebenberg, D.; Cacciapuoti, A.; Fink, G.R. Nonfilamentous C. albicans mutants are avirulent. Cell 1997, 90, 939–949. [Google Scholar] [CrossRef] [Green Version]
- Berrocal, A.; Oviedo, C.; Nickerson, K.W.; Navarrete, J. Quorum sensing activity and control of yeast-mycelium dimorphism in Ophiostoma floccosum. Biotechnol. Lett. 2014, 36, 1503–1513. [Google Scholar] [CrossRef]
- Jacobsen, I.D.; Wilson, D.; Wächtler, B.; Brunke, S.; Naglik, J.R.; Hube, B. Candida albicans dimorphism as a therapeutic target. Expert Rev. Anti. Infect. Ther. 2012, 10, 85–93. [Google Scholar] [CrossRef] [Green Version]
- Vanittanakom, N.; Cooper, C.R.; Fisher, M.C.; Sirisanthana, T. Penicillium marneffei infection and recent advances in the epidemiology and molecular biology aspects. Clin. Microbiol. Rev. 2006, 19, 95–110. [Google Scholar] [CrossRef] [Green Version]
- Gullino, M.L. Control of Botrytis Rot of Grapes and Vegetables with Trichoderma Spp. In Biological Control of Plant Diseases; Springer: New York, NY, USA, 1992; pp. 125–132. [Google Scholar]
- Vargas, W.A.; Mandawe, J.C.; Kenerley, C.M. Plant-derived sucrose is a key element in the symbiotic association between Trichoderma virens and maize plants. Plant Physiol. 2009, 151, 792–808. [Google Scholar] [CrossRef] [Green Version]
- Delgado-Jarana, J.; Moreno-Mateos, M.Á.; Benítez, T. Glucose uptake in Trichoderma harzianum: Role of gtt1. Eukaryot. Cell 2003, 2, 708–717. [Google Scholar] [CrossRef] [Green Version]
- Benítez, T.; Rincón, A.M.; Limón, M.C.; Codón, A.C. Biocontrol mechanisms of Trichoderma strains. Int. Microbiol. 2004, 7, 249–260. [Google Scholar]
- Ghosh, S.K.; Banerjee, S.; Sengupta, C. Siderophore production by antagonistic fungi. J. Biopest. 2017, 10, 105–112. [Google Scholar]
- Liu, P.; Luo, L.; Long, C. An Characterization of competition for nutrients in the biocontrol of Penicillium italicum by Kloeckera apiculata. Biol. Control 2013, 67, 157–162. [Google Scholar] [CrossRef]
- Zhao, Y.; Tu, K.; Shao, X.; Jing, W.; Su, Z. Effects of the yeast Pichia guilliermondii against Rhizopus nigricans on tomato fruit. Postharvest Biol. Technol. 2008, 49, 113–120. [Google Scholar] [CrossRef]
- Ippolito, A.; El Ghaouth, A.; Wilson, C.L.; Wisniewski, M. Control of postharvest decay of apple fruit by Aureobasidium pullulans and induction of defense responses. Postharvest Biol. Technol. 2000, 19, 265–272. [Google Scholar] [CrossRef]
- Kunitake, E.; Tanaka, T.; Ueda, H.; Endo, A.; Yarimizu, T.; Katoh, E.; Kitamoto, H. CRISPR/Cas9-mediated gene replacement in the basidiomycetous yeast Pseudozyma antarctica. Fungal Genet. Biol. 2019, 130, 82–90. [Google Scholar] [CrossRef]
- Massart, S.; Perazzolli, M.; Höfte, M.; Pertot, I.; Jijakli, M.H. Impact of the omic technologies for understanding the modes of action of biological control agents against plant pathogens. BioControl 2015, 60, 725–746. [Google Scholar] [CrossRef]
- Wang, Q.-M.; Begerow, D.; Groenewald, M.; Liu, X.-Z.; Theelen, B.; Bai, F.-Y.; Boekhout, T. Multigene phylogeny and taxonomic revision of yeasts and related fungi in the Ustilaginomycotina. Stud. Mycol. 2015, 81, 55–83. [Google Scholar] [CrossRef] [Green Version]
- Piątek, M.; Lutz, M.; Yorou, N.S. A molecular phylogenetic framework for Anthracocystis (Ustilaginales), including five new combinations (inter alia for the asexual Pseudozyma flocculosa), and description of Anthracocystis grodzinskae sp. nov. Mycol. Prog. 2015, 14, 1–15. [Google Scholar] [CrossRef] [Green Version]
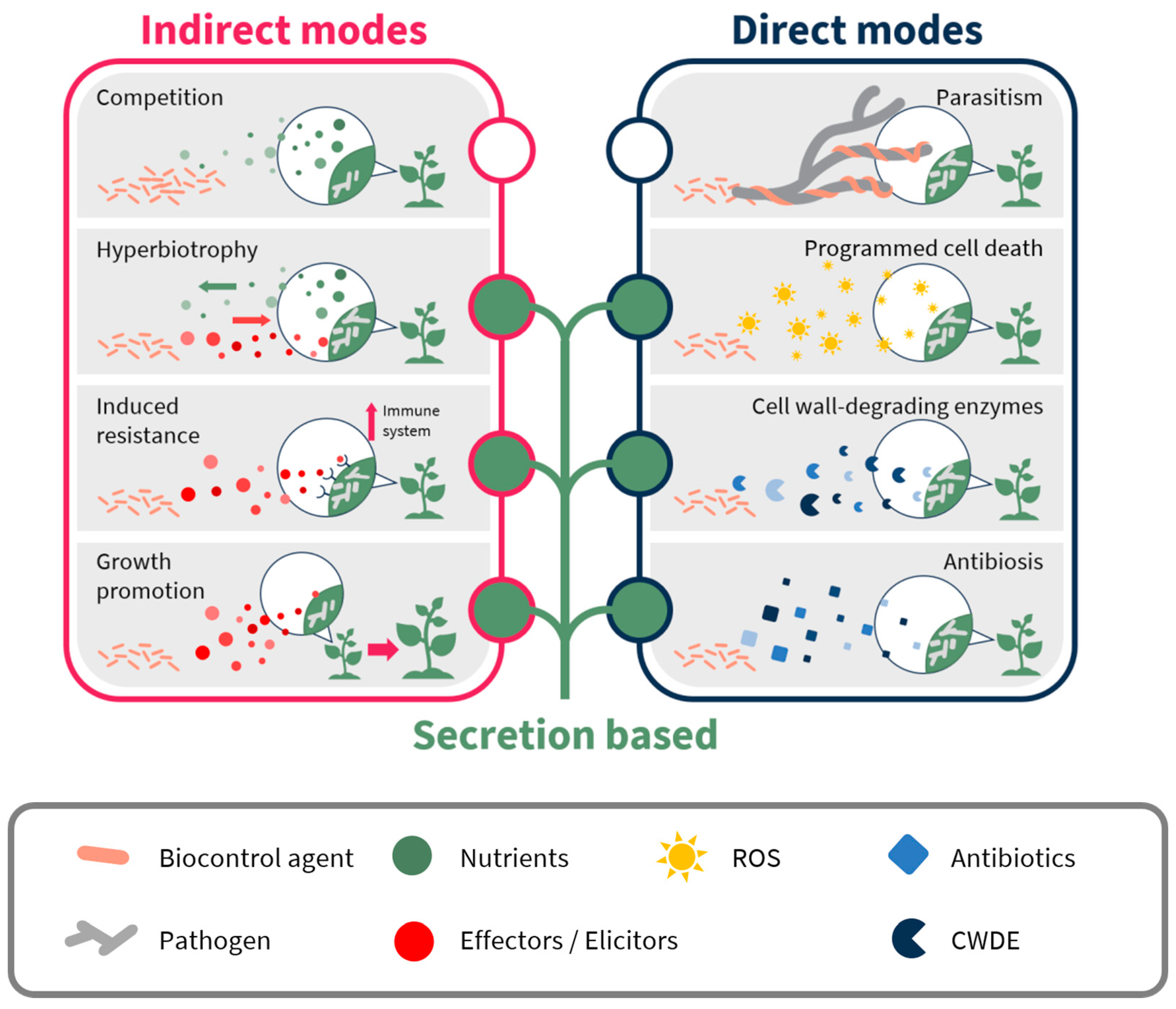
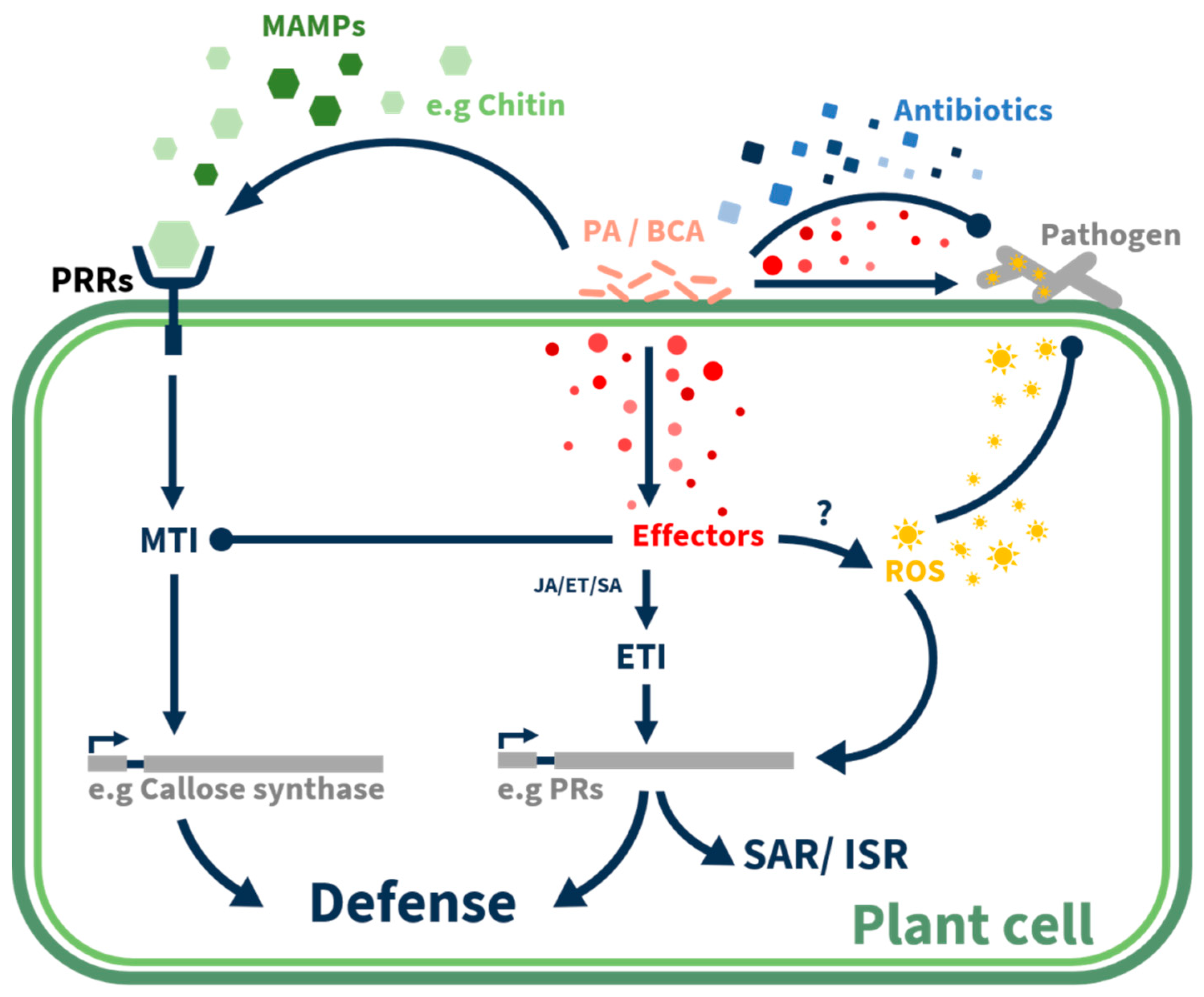
| Biocontrol Agent | Compound/Protein/Gene | Mechanism/Activity | Pathogen | Ref. |
|---|---|---|---|---|
| Antibiosis/ ROS/ PCD | ||||
| Acremonium strictum | Verlamelin | Unknown | Erysiphe graminis f. sp. hordei Puccnia recondita Botrytis cinerea | [5,70] |
| Trichoderma virens Trichoderma spp. | Peptaibols | Formation of pores in bilayer lipid membrane. | B. cinerea Mucor mucedo | [3,6,31,33,122] |
| Trichoderma pseudokoningii | Peptaibols | Induces metacaspase-independent apoptotic cell death. | Fusarium oxysporum | [35] |
| T. virens | Peptaibols | Induced resistance. | Pseudomonas syringae | [3,12,122] |
| Pseudozyma aphidis | Mostly lipophilic compounds. | ROS/PCD | Podosphaera xanthii B. cinerea Clavibacter michiganensis | [44,61,62,101] |
| Biocontrol Agent | Compound/Protein/Gene | Mechanism/Activity | Pathogen | Ref. |
| Psudozyma rugulosa Pseudozyma flocculosa (syn.Sporothrix flocculosa) | (Z)-9-heptadecenoic Z)-6-methyl-9-hepta decenoic acids Cis-9-Heptadecenoic acid (CHDA) | Disturbance in fluidity of the cell membrane, leakage of electrolytes and proteins. | E. graminis var. tritici, powdery mildews | [45,46,47,48,49,50] |
| P. flocculosa | Flocculosin | Leakage of cell membrane. | Candida albicans, Trichosporon asahii, powdery mildews | [53,54,55] |
| Pseudozyma tsukubaensis Pseudozyma prolifica | Mycocins | Membrane disruption. | Ustilaginomycetes | [51,52] |
| Pseudozyma graminicola Pseudozyma fusiformata | Ustilagic acid | Disruption of the cytoplasmic membrane permeability. | ~300 tested species of yeastlike and mycelial fungi | [56,57] |
| Chaetomium globosum | Chaetoviridins A and B | Antibiosis | Puccinia recondita, Magnaporthe grisea | [67,68] |
| Fusarium semitectum | Fusapyrone and deoxyfusapyrone | Antibiosis | B.cinerea, Aspergillus parasiticus, and Penicillium brevi | [69] |
| Gliocladium virens p | Gliovirin and heptelidic acid | Suppressing TNF-alpha synthesis. | Pythium ultimum | [30] |
| G. irens q | Gliotoxin and dimethylgliotoxin | Oxidative stress | Rhizoctonia solani | [30] |
| Effectors | ||||
| P. flocculosa | pf02826, pf00303 and pf02382 | Dissemination and sequestration of nutrient. | Blumeria graminis | [77] |
| Pseudozyma sp. | Pep1, Cmu1, Cwh41 and Hum3 | BCA -Plant interactions | [76,87] | |
| T. virens | tvlysm1 | Colonization of BCA and defense against the pathogen. | Rhizoctonia solani | [78] |
| T. virens | Cerato-platanin protein Sm1 | Induces ROS and PR genes expression in cotton. | Cochliobolus heterostrophus | [80] |
| T. virens | Sm2 and Epl2 | Induced resistance | C. heterostrophus | [80] |
| Fusarium oxysporum strain CS-20 | CS20EP | Elicits defense responses and ion exchange in tomato plants. | F. oxysporum | [85] |
| Hydrolytic Enzymes | ||||
| Trichoderma harzianum | Endo-chitinase, chitobiosidase | B.cinerea; F. oxysporum Sclerotium rolfsii | [125,126,127] | |
| P. aphidis | Chitinase, protease lipase, cellulase | P. xanthii, B. cinerea | [44,62] | |
| Parasitism | ||||
| Trichoderma lignorum | Mycoparasitism, haustoria formation. | R. solani | [30,124] | |
| P. flocculosa | Mycoparasitism, Hyperbiotrophy. | P. xanthii B. graminis | [55,77] | |
| P. aphidis | Ecto-parasitism | P. xanthii | [44] | |
| Biocontrol Agent | Compound/Protein/Gene | Mechanism/Activity | Pathogen | Ref. |
| Induced resistance (IR) | ||||
| T. harzianum T39 | Through jasmonic acid(JA) and ethylene(ET) signals. | Induced resistance in grapevine. | Plasmopara viticola | [105] |
| Trichoderma asperellum SKT-1 | Through salicylic acid (SA), JA and ET signaling pathways. | Induced resistance in Arabidopsis thaliana. | P. syringae | [106] |
| T. asperellum T203 | Through JA and ET signals. | Induced resistance in cucumber. | P. syringae | [107] |
| T. virens | Sm1 and 18 mer peptaibols. | Induced resistance in cucumber. | P. syringae | [80,122] |
| Piriformospora indica | ISR, upregulation of PR genes and heat-shock proteins. | Induced resistance in barley. | B. graminis | [108] |
| F. oxysporum Fo47 | Root colonization upregulation of GLUA and PR-1a. | Induced resistance in tomato. | Fusarium wilt | [109] |
| Penicillium simplicissimum GP17-2 | Through SA, JA and ET signaling pathways. | Induced resistance in A. thaliana. | P. syringae | [111] |
| Ampelomyces sp. and Cladosporium sp | Volatiles; m-cresol and methyl benzoate induce ISR. | Induced resistance in A. thaliana. | P.syringae pv. tomato DC3000 | [121] |
| P. aphidis | Induce ISR and SAR through JA/ET and SA pathways. | Induce resistance in A. thaliana, tomato and cucumber. | B. cinerea C. michiganensis | [43,61,101] |
| Competition | ||||
| T. harzianum | Competition for space. | B. cinerea | [134] | |
| P. aphidis | Competition for space and nutrients. | P. xanthii, B. cinerea | [43,44,62] | |
| Pichia guilliermondii | Competition for space and nutrients. | Rhizopus nigricans | [140] | |
| Kloeckera apiculate | Competition for nutrients | Penicillium italicum | [139] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Srivastava, D.A.; Harris, R.; Breuer, G.; Levy, M. Secretion-Based Modes of Action of Biocontrol Agents with a Focus on Pseudozyma aphidis. Plants 2021, 10, 210. https://doi.org/10.3390/plants10020210
Srivastava DA, Harris R, Breuer G, Levy M. Secretion-Based Modes of Action of Biocontrol Agents with a Focus on Pseudozyma aphidis. Plants. 2021; 10(2):210. https://doi.org/10.3390/plants10020210
Chicago/Turabian StyleSrivastava, Dhruv Aditya, Raviv Harris, Gilli Breuer, and Maggie Levy. 2021. "Secretion-Based Modes of Action of Biocontrol Agents with a Focus on Pseudozyma aphidis" Plants 10, no. 2: 210. https://doi.org/10.3390/plants10020210
APA StyleSrivastava, D. A., Harris, R., Breuer, G., & Levy, M. (2021). Secretion-Based Modes of Action of Biocontrol Agents with a Focus on Pseudozyma aphidis. Plants, 10(2), 210. https://doi.org/10.3390/plants10020210





