Global Plant Virus Disease Pandemics and Epidemics
Abstract
:1. Introduction
2. Definitions and Concepts
2.1. Definitions
2.2. Concepts
3. Cereals
3.1. Maize
Maize Lethal Necrosis Disease
3.2. Wheat
3.2.1. Yellow Dwarf Disease
3.2.2. Wheat Streak Mosaic Disease
3.3. Rice
Rice Tungro Disease
4. Root and Tuber Crops
4.1. Potato
Potato Tuber Necrotic Ringspot Disease
4.2. Sweet Potato
Sweet Potato Virus Disease
5. Plantation and Orchard Crops
5.1. Banana
Banana Bunchy Top Disease
5.2. Citrus Fruit
Citrus Tristeza Disease
5.3. Stone Fruit
Plum Pox Disease
6. Grain Legumes
Faba Bean Necrotic Yellows Disease
7. Annual Horticultural Crops
7.1. Tomato
7.1.1. Tomato Brown Rugose Fruit Disease
7.1.2. Pepino Mosaic Disease
7.2. Cucurbits
Cucumber Green Mottle Mosaic Disease
8. Management
9. Conclusions
- (i)
- Introduction of vulnerable higher-yielding crop cultivars with MLND, WYDD, RTD, SPVD, BBTD, CTD, PPD and FBNYD;
- (ii)
- Agricultural intensification to increase crop yields with MLND, WSMD, RTD, SPVD, BBTD, CTD, FBNYD, PepMD, TBRFD and CGMMD;
- (iii)
- Virus recombination events resulting in more virulent virus variants with WYDD, RTD, PTNRD and TBRFD; and
- (iv)
- Increased, or more efficient, vector populations with MLND, WSMD, BBTD and CTD.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Klinkowski, M. Catastrophic plant diseases. Annu. Rev. Phytopathol. 1970, 8, 37–60. [Google Scholar] [CrossRef]
- Thurston, U.D. Threatening plant diseases. Annu. Rev. Phytopathol. 1973, 11, 27–52. [Google Scholar] [CrossRef]
- Bos, L. Crop losses caused by viruses. Crop Prot. 1982, 1, 263–282. [Google Scholar] [CrossRef]
- Thresh, J.M. The origins and epidemiology of some important plant virus diseases. Appl. Biol. 1980, 5, 1–65. [Google Scholar]
- Anderson, P.K.; Cunningham, A.A.; Patel, N.G.; Morales, F.J.; Epstein, P.R.; Daszak, P. Emerging infectious diseases of plants: Pathogen pollution, climate change and agrotechnology drivers. Trends Ecol. Evol. 2004, 19, 535–544. [Google Scholar] [CrossRef]
- Thresh, J.M. Crop viruses and virus diseases: A global perspective. In Virus Diseases and Crop Biosecurity; Cooper, J.I., Kuehne, T., Polischuk, V.P., Eds.; Springer: Dordrecht, The Netherlands, 2006; pp. 9–32. [Google Scholar]
- Jones, R.A.C. Plant virus emergence and evolution: Origins, new encounter scenarios, factors driving emergence, effects of changing world conditions, and prospects for control. Virus Res. 2009, 141, 113–130.5. [Google Scholar] [CrossRef]
- Hull, R. Mathews’ Plant Virology, 5th ed.; Academic Press: London, UK, 2014. [Google Scholar]
- Jones, R.A.C.; Naidu, R.A. Global dimensions of plant virus diseases: Current status and future perspectives. Annu. Rev. Virol. 2019, 6, 387–409. [Google Scholar] [CrossRef]
- Brunt, A.; Crabtree, K.; Gibbs, A.J. Viruses of Tropical Plants: Descriptions and Lists from the VIDE Database; CAB International: Wallingford, UK, 1990. [Google Scholar]
- Bos, L. New plant virus problems in developing countries: A corollary of agricultural modernization. Adv. Virus Res. 1992, 41, 349–407. [Google Scholar]
- Thresh, J.M. Control of plant virus diseases in sub-Saharan Africa: The possibility and feasibility of an integrated approach. Afr. Crop Sci. J. 2003, 11, 199–223. [Google Scholar] [CrossRef] [Green Version]
- Thresh, J.M. Control of tropical plant virus diseases. Adv. Virus Res. 2006, 67, 245–295. [Google Scholar]
- Thresh, J.M. Plant virus epidemiology: The concept of host genetic vulnerability. Adv. Virus Res. 2006, 67, 89–125. [Google Scholar] [PubMed]
- Loebenstein, G.; Thottappilly, G. Virus and Virus-like Diseases of Major Crops in Developing Countries; Springer: Dordrecht, The Netherlands, 2013. [Google Scholar]
- Jones, R.A.C. Plant virus ecology and epidemiology: Historical perspectives, recent progress and future prospects. Ann. Appl. Biol. 2014, 164, 320–347. [Google Scholar] [CrossRef]
- Sastry, S.K.; Zitter, T.A. Management of virus and viroid diseases of crops in the tropics. In Plant Virus and Viroid Diseases in the Tropics, Epidemiology and Management; Sastry, S.K., Zitter, T.A., Eds.; Springer: Dordrecht, The Netherlands, 2014; Volume 2, pp. 149–480. [Google Scholar]
- Thresh, J.M. Cropping practices and virus spread. Annu. Rev. Phytopathol. 1982, 20, 193–218. [Google Scholar] [CrossRef]
- Rodoni, B. The role of plant biosecurity in preventing and controlling emerging plant virus disease epidemics. Virus Res. 2009, 141, 150–157. [Google Scholar] [CrossRef]
- IMF. Globalization: Threat or Opportunity? International Monetary Fund: Washington, DC, USA, 2000. [Google Scholar]
- James, P.; Steger, M.B. A genealogy of ‘globalization’: The career of a concept. Globalizations 2014, 11, 417–434. [Google Scholar] [CrossRef]
- Thresh, J.M. An ecological approach to the epidemiology of plant virus diseases. In Comparative Epidemiology: A Tool for Better Disease Management; Palti, J., Kranz, J., Eds.; Centre for Agricultural Publishing and Documentation: Wageningen, The Netherlands, 1980; pp. 57–70. [Google Scholar]
- Thresh, J.M. Pests, Pathogens and Vegetation; Pitman: London, UK, 1981. [Google Scholar]
- Jones, R.A.C. Plant and insect viruses in managed and natural environments: Novel and neglected transmission pathways. Adv. Virus Res. 2018, 101, 149–187. [Google Scholar]
- Jones, R.A.C. Disease pandemics and major epidemics arising from new encounters between indigenous viruses and introduced crops. Viruses 2020, 12, 1388. [Google Scholar] [CrossRef]
- Canto, T.; Aranda, M.A.; Fereres, A. Climate change effects on physiology and population processes of hosts and vectors that influence the spread of hemipteran-borne plant viruses. Global Change Biol. 2009, 15, 1884–1894. [Google Scholar] [CrossRef] [Green Version]
- Jones, R.A.C.; Barbetti, M.J. Influence of climate change on plant disease infections and epidemics caused by viruses and bacteria. Plant Sci. Rev. 2012, 22, 1–31. [Google Scholar] [CrossRef]
- Jones, R.A.C. Future scenarios for plant virus pathogens as climate change progresses. Adv. Virus. Res. 2016, 95, 87–147. [Google Scholar]
- Trebicki, P. Climate change and plant virus epidemiology. Virus Res. 2020, 286, 198059. [Google Scholar] [CrossRef] [PubMed]
- Dombrovsky, A.; Smith, E. Seed transmission of Tobamoviruses: Aspects of global disease distribution. In Advances in Seed Biology; Jimenez-Lopez, J.C., Ed.; IntechOpen: London, UK, 2017; pp. 233–260. [Google Scholar]
- Constable, F.; Daly, A.; Terras, M.A.; Penrose, L.; Dall, D. Detection in Australia of cucumber green mottle mosaic virus in seed lots of cucurbit crops. Aust. Plant Dis. Notes 2018, 13, 18. [Google Scholar] [CrossRef] [Green Version]
- Irwin, M.E.; Fereres, A. John Michael Thresh, founding father of plant virus epidemiology: A tribute. Virus Res. 2017, 241, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Thresh, J.M. Insect-borne viruses of rice and the Green Revolution. Int. J. Pest Manag. 1989, 35, 264–272. [Google Scholar] [CrossRef]
- Thresh, J.M. Plant virus epidemiology: The battle of the genes. In Recognition and Response in Plant-Virus Interactions; [NATO ASI Series H: Cell Biology]; Springer: Berlin/Heidelberg, Germany, 1990; Volume 41, pp. 93–121. [Google Scholar]
- Thresh, J.M. The impact of plant virus diseases in developing countries. In Virus and Virus-like Diseases of Major Crops in Developing Countries; Loebenstein, G., Thottappilly, G., Eds.; Springer: Dordrecht, The Netherlands, 2004; pp. 1–30. [Google Scholar]
- Jeger, M.J.; Thresh, J.M. Modelling reinfection of replanted cocoa by swollen shoot virus in pandemically diseased areas. J. Appl. Ecol. 1993, 30, 187–196. [Google Scholar] [CrossRef]
- Otim-Nape, G.W.; Thresh, J.M. The current pandemic of cassava mosaic virus disease in Uganda. In The Epidemiology of Plant Diseases; Jones, G., Ed.; Kluwer: Dordrecht, The Netherlands, 1998; pp. 423–443. [Google Scholar]
- Rybicki, E.P.; Pietersen, G. Plant virus disease problems in the developing world. Adv. Virus Res. 1999, 53, 127–175. [Google Scholar]
- Dombrovsky, A.; Tran-Nguyen, L.T.T.; Jones, R.A.C. Cucumber green mottle mosaic virus: Rapidly increasing global distribution, etiology, epidemiology, and management. Annu. Rev. Phytopathol. 2017, 55, 231–256. [Google Scholar] [CrossRef]
- Karasev, A.V.; Gray, S.M. Continuous and emerging challenges of Potato virus Y in potato. Annu. Rev. Phytopathol. 2013, 51, 571–586. [Google Scholar] [CrossRef]
- Rey, C.; Vanderschuren, H. Cassava mosaic and brown streak diseases: Current perspectives and beyond. Annu. Rev. Virol. 2017, 4, 429–452. [Google Scholar] [CrossRef]
- Redinbaugh, M.G.; Stewart, L.R. Maize lethal necrosis: An emerging, synergistic viral disease. Annu. Rev. Virol. 2018, 5, 301–322. [Google Scholar] [CrossRef]
- Tomlinson, K.R.; Bailey, A.M.; Alicai, T.; Seal, S.; Foster, G.D. Cassava brown streak disease: Historical timeline, current knowledge and future prospects. Mol. Plant Pathol. 2018, 19, 1282–1294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moriones, E.; Praveen, S.; Chakraborty, S. Tomato leaf curl New Delhi virus: An emerging virus complex threatening vegetable and fibre crops. Viruses 2017, 9, 264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Legg, J.P.; Jeremiah, S.C.; Obiero, H.M.; Maruthi, M.N.; Ndyetabula, I.; Okao-Okuja, G.; Bouwmeester, H.; Bigirimana, S.; Tata-Hangy, W.; Gashaka, G.; et al. Comparing the regional epidemiology of the cassava mosaic and cassava brown streak virus pandemics in Africa. Virus Res. 2011, 159, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Waggoner, P.E.; Aylor, D.E. Epidemiology: A science of patterns. Annu. Rev. Phytopathol. 2000, 38, 71–94. [Google Scholar] [CrossRef] [PubMed]
- Robert, Y. Epidemiology of Plant Virus Diseases; John Wiley & Sons Ltd.: Bognor Regis, UK, 2001. [Google Scholar]
- Wilson, C.R. Applied Plant Virology; CABI Press: Wallingford, UK, 2014. [Google Scholar]
- Jones, R.A.C. Using epidemiological information to develop effective integrated virus disease management strategies. Virus Res. 2004, 100, 5–30. [Google Scholar] [CrossRef]
- Jones, R.A.C. Control of plant virus diseases. Adv. Virus Res. 2006, 67, 205–244. [Google Scholar]
- Cooper, J.I.; Jones, A.T. Responses of plants to viruses: Proposals for the use of terms. Phytopathology 1983, 73, 127–128. [Google Scholar] [CrossRef] [Green Version]
- Harlan, J.R. Agricultural origins: Centres and non-centres. Science 1971, 174, 468–474. [Google Scholar] [CrossRef]
- Harlan, J.R. Ecological settings for the emergence of agriculture. In Pests, Pathogens and Vegetation; Thresh, J.M., Ed.; Pitman Press: London, UK, 1981; pp. 3–22. [Google Scholar]
- Fargette, D.; Konate, G.; Fauquet, C.; Muller, E.; Peterscmitt, M.; Thresh, J.M. Molecular ecology and emergence of tropical plant viruses. Annu. Rev. Phytopathol. 2006, 44, 235–260. [Google Scholar] [CrossRef]
- García-Arenal, F.; Zerbini, F.M. Life on the edge: Geminiviruses at the interface between crops and wild plant hosts. Annu. Rev. Virol. 2019, 6, 411–433. [Google Scholar] [CrossRef] [Green Version]
- Spetz, C.; Taboada, A.M.; Darwich, S.; Ramsell, J.; Salazar, L.F.; Valkonen, J.P.T. Molecular resolution of a complex of potyviruses infecting solanaceous crops at the centre of origin in Peru. J. Gen. Virol. 2003, 84, 2565–2578. [Google Scholar] [CrossRef]
- Santillan, F.W.; Fribourg, C.E.; Adams, I.P.; Gibbs, A.J.; Boonham, N.; Kehoe, M.A.; Maina, S.; Jones, R.A.C. The biology and phylogenetics of Potato virus S isolates from the Andean region of South America. Plant Dis. 2018, 102, 869–885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuentes, S.; Jones, R.A.C.; Matsuoka, H.; Ohshima, K.; Kreuze, J.; Gibbs, A.J. Potato virus Y; the Andean connection. Virus Evol. 2019, 5, vez037. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, S.; Gibbs, A.J.; Adams, I.; Wilson, C.R.; Botermans, M.; Fox, A.; Kreuze, J.; Boonham, N.; Kehoe, M.A.; Jones, R.A.C. Potato virus A isolates from three continents: Their biological properties, phylogenetics and prehistory. Phytopathology 2021. [Google Scholar] [CrossRef]
- Hajizadeh, M.; Gibbs, A.J.; Amirnia, F.; Glasa, M. The global phylogeny of Plum pox virus is emerging. J. Gen. Virol. 2011, 100, 1457–1468. [Google Scholar] [CrossRef]
- Banerjee, A.; Roy, S.; Tarafdar, J. Phylogenetic analysis of Rice tungro bacilliform virus ORFs revealed strong correlation between evolution and geographical distribution. Virus Genes 2011, 43, 398–408. [Google Scholar] [CrossRef]
- Mathur, S.; Dasgupta, I. Further support of genetic conservation in Indian isolates of Rice tungro bacilliformvirus by sequence analysis of an isolate from North–Western India. Virus Genes 2013, 46, 387–391. [Google Scholar] [CrossRef]
- Kumar, P.L.; Hanna, R.; Alabi, O.J.; Soko, M.M.; Oben, T.T.; Vangu, G.H.P.; Naidu, R.A. Banana bunchy top virus in sub-Saharan Africa: Investigations on virus distribution and diversity. Virus Res. 2011, 159, 171–182. [Google Scholar] [CrossRef]
- Kumar, P.L.; Selvarajan, R.; Iskra-Caruana, M.L.; Chabannes, M.; Hanna, R. Biology, etiology, and control of virus diseases of banana and plantain. Adv. Virus Res. 2015, 91, 229–269. [Google Scholar]
- Shiferaw, B.; Prasanna, B.M.; Hellin, J.; Bänziger, M. Crops that feed the world 6. Past successes and future challenges to the role played by maize in global food security. Food Secur. 2011, 3, 307–327. [Google Scholar] [CrossRef] [Green Version]
- FAOSTAT. Food and Agricultural Organization Statistical Databases; United Nations: Rome, Italy, 2020. [Google Scholar]
- Doebley, J.F. The genetics of maize evolution. Annu. Rev. Genet. 2004, 38, 37–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bedoya, C.A.; Dreisigacker, S.; Hearne, S.; Franco, J.; Mir, C.; Prasanna, B.M.; Taba, S.; Charcosset, A.; Warburton, M.L. Genetic diversity and population structure of native maize populations in Latin America and the Caribbean. PLoS ONE 2017, 12, e0173488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCann, J. Maize and grace: History, corn, and Africa’s new landscapes, 1500–1999. Comp. Stud. Soc. Hist. 2001, 43, 246–272. [Google Scholar] [CrossRef] [Green Version]
- Munkvold, G.P.; White, D.G. Compendium of Corn Diseases; APS Press: St. Paul, MN, USA, 2016. [Google Scholar]
- Niblett, C.L.; Claflin, L.E. Corn lethal necrosis—A new virus disease of corn in Kansas. Plant Dis. Report. 1978, 62, 5–19. [Google Scholar]
- CABI. Compendium Datasheets; CABI: Wallingford, UK, 2020. [Google Scholar]
- Boddupalli, P.; Suresh, L.M.; Mwatuni, F.; Beyene, Y.; Makumbi, D.; Gowda, M.; Olsen, M.; Hodson, D.; Worku, M.; Mezzalama, M.; et al. Maize lethal necrosis (MLN): Efforts toward containing the spread and impact of a devastating transboundary disease in sub-Saharan Africa. Virus Res. 2020, 282, 197943. [Google Scholar] [CrossRef]
- Braidwood, L.; Quito-Avila, D.F.; Cabanas, D.; Bressan, A.; Wangai, A.; Baulcombe, D.C. Maize chlorotic mottle virus exhibits low divergence between differentiated regional sub-populations. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Irwin, M.E.; Thresh, J.M. Epidemiology of barley yellow dwarf: A study in ecological complexity. Annu. Rev. Phytopathol. 1990, 28, 393–424. [Google Scholar] [CrossRef]
- D’Arcy, C.J.; Burnett, P.A. Barley Yellow Dwarf: Forty Years of Progress; APS Press: St. Paul, MN, USA, 1995. [Google Scholar]
- Miller, W.A.; Rasochova, L. Barley yellow dwarf viruses. Annu. Rev. Phytopathol. 1997, 35, 167–190. [Google Scholar] [CrossRef] [Green Version]
- Plumb, R.T. Barley yellow dwarf virus: A global problem. In Plant Virus Epidemiology. The Spread and Control of Insect-Borne Viruses; Plumb, R.T., Thresh, J.M., Eds.; Blackwell Scientific Publications: Oxford, UK, 1983; pp. 185–198. [Google Scholar]
- Bockus, W.W.; Bowden, R.L.; Hunger, R.M.; Morrill, W.L.; Murray, T.D.; Smiley, R.W. Compendium of Wheat Diseases and Insects; APS Press: St Paul, MN, USA, 2009. [Google Scholar]
- Byamukama, E.; Wegulo, S.N.; Tatineni, S.; Hein, G.L.; Graybosch, R.A.; Baenziger, P.S.; French, R. Quantification of yield loss caused by triticum mosaic virus and wheat streak mosaic virus in winter wheat under field conditions. Plant Dis. 2014, 98, 127–133. [Google Scholar] [CrossRef] [Green Version]
- Coutts, B.A.; Strickland, G.R.; Kehoe, M.; Severtson, D.L.; Jones, R.A.C. The epidemiology of Wheat streak mosaic virus in Australia: Case histories, gradients, mite vectors and alternative hosts. Aust. J. Agric. Res. 2008, 59, 844–853. [Google Scholar] [CrossRef]
- Coutts, B.A.; Banovic, M.; Kehoe, M.A.; Severtson, D.L.; Jones, R.A.C. Epidemiology of Wheat streak mosaic virus in wheat in a Mediterranean-type environment. Eur. J. Plant Pathol. 2014, 140, 797–813. [Google Scholar] [CrossRef]
- Singh, K.; Wegulo, S.N.; Skoracka, A.; Kundu, J.K. Wheat streak mosaic virus: A century old virus with rising importance worldwide. Mol. Plant Pathol. 2018, 19, 2193–2206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hibino, H. Biology and epidemiology of rice viruses. Annu. Rev. Phytopathol. 1996, 34, 249–274. [Google Scholar] [CrossRef] [PubMed]
- Bunawan, H.; Dusik, L.; Bunawan, S.N.; Amin, N.M. Rice tungro disease: From identification to disease control. World Appl. Sci. J. 2014, 31, 1221–1226. [Google Scholar]
- Mangrauthia, S.K.; Malathi, P.; Agarwal, S.; Ramkumar, G.; Krishnaveni, D.; Neeraja, C.N.; Madhav, M.S.; Ladhalakshmi, D.; Balachandran, S.M.; Viraktamath, B.C. Genetic variation of coat protein gene among the isolates of Rice tungro spherical virus from tungro-endemic states of the India. Virus Genes 2012, 44, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Rabindran, R.; Robin, S.; Dasgupta, I. Analysis of the complete DNA sequence of rice tungro bacilliform virus from southern India indicates it to be a product of recombination. Arch. Virol. 2011, 156, 2257–2262. [Google Scholar] [CrossRef] [PubMed]
- Sailaja, B.; Anjum, N.; Patil, Y.K.; Agarwal, S.; Malathi, P.; Krishnaveni, D.; Balachandran, S.M.; Viraktamath, B.C.; Mangrauthia, S.K. The complete genome sequence of a south Indian isolate of Rice tungro spherical virus reveals evidence of genetic recombination between distinct isolates. Virus Genes 2013, 47, 515–523. [Google Scholar] [CrossRef]
- Jones, R.A.C. Virus disease problems facing potato industries worldwide: Viruses found, climate change implications, rationalizing virus strain nomenclature, and addressing the Potato virus Y issue. In The Potato: Botany, Production and Uses; Navarre, R., Pavek, M.J., Eds.; CABI: Wallingford, UK, 2014; pp. 202–224. [Google Scholar]
- Kreuze, J.F.; Souza-Dias, J.A.C.; Jeevalatha, A.; Figueira, A.R.; Valkonen, J.P.T.; Jones, R.A.C. Viral diseases in potato. In The Potato Crop; Campos, H., Ortiz, O., Eds.; Springer: New York, NY, USA, 2020; pp. 389–430. [Google Scholar]
- Gray, S.; De Boer, S.; Lorenzen, J.; Karasev, A.; Whitworth, J.; Nolte, P.; Singh, R.; Boucher, A.; Xu, H. Potato virus Y: An evolving concern for potato crops in the United States and Canada. Plant Dis. 2010, 94, 1384–1397. [Google Scholar] [CrossRef] [Green Version]
- Gibbs, A.J.; Ohshima, K.; Yasaka, R.; Mohammadi, M.; Gibbs, M.J.; Jones, R.A.C. The phylogenetics of the global population of potato virus Y and its necrogenic recombinants. Virus Evol. 2017, 3, vex002. [Google Scholar] [CrossRef] [Green Version]
- Torrance, L.; Talianksy, M.E. Potato Virus Y emergence and evolution from the Andes of South America to become a major destructive pathogen of potato and other solanaceous crops worldwide. Viruses 2020, 12, 1430. [Google Scholar] [CrossRef]
- Tairo, F.; Mukasa, S.B.; Jones, R.A.C.; Kullaya, A.; Rubaihayo, P.R.; Valkonen, J.P. Unravelling the genetic diversity of the three main viruses involved in sweet potato virus disease (SPVD), and its practical implications. Mol. Plant Pathol. 2005, 6, 199–211. [Google Scholar] [CrossRef] [PubMed]
- Clark, C.A.; Davis, J.A.; Abad, J.A.; Cuellar, W.J.; Fuentes, S.; Kreuze, J.F.; Gibson, R.W.; Mukasa, S.B.; Tugume, A.K.; Tairo, F.D.; et al. Sweetpotato viruses: 15 years of progress on understanding and managing complex diseases. Plant Dis. 2012, 96, 168–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibson, R.W.; Mpembe, I.; Alicai, T.; Carey, E.E.; Mwanga, R.O.M.; Seal, S.E.; Vetten, H.J. Symptoms, aetiology and serological analysis of sweet potato virus disease in Uganda. Plant Pathol. 1998, 47, 95–102. [Google Scholar] [CrossRef]
- Karyeija, R.F.; Gibson, R.W.; Valkonen, J.P.T. The significance of sweet potato feathery mottle virus in subsistence sweet potato production in Africa. Plant Dis. 1998, 82, 4–15. [Google Scholar] [CrossRef]
- Cuellar, W.J.; Galvez, M.; Fuentes, S.; Tugume, J.; Kreuze, J. Synergistic interactions of begomoviruses with Sweet potato chlorotic stunt virus (genus Crinivirus) in sweet potato (Ipomoea batatas L.). Mol. Plant Pathol. 2015, 16, 459–471. [Google Scholar] [CrossRef]
- Loebenstein, G. Control of sweet potato virus diseases. Adv. Virus Res. 2015, 91, 33–45. [Google Scholar]
- Zhang, Z.C.; Qiao, Q.; Qin, Y.H.; Zhang, D.S.; Tian, Y.T. First evidence for occurrence of Sweet potato virus disease (SPVD) caused by dual infection of Sweet potato feathery mottle virus and Sweet potato chlorotic stunt virus in China. Acta Phytopathol. Sin. 2012, 3. Available online: http://en.cnki.com.cn/Article_en/CJFDTOTAL-ZWBL201203017.htm (accessed on 8 December 2020).
- Qazi, J. Banana bunchy top virus and the bunchy top disease. J. Gen. Plant Pathol. 2016, 82, 2–11. [Google Scholar] [CrossRef]
- Bar-Joseph, M.; Marcus, R.; Lee, R.F. The continuous challenge of Citrus tristeza virus control. Annu. Rev. Phytopathol. 1989, 27, 291–316. [Google Scholar] [CrossRef]
- Bar-Joseph, M.; Batuman, O.; Roistacher, C. The history of Citrus tristeza virus revisited. In Citrus Tristeza Virus Complex and Tristeza Diseases; Karasev, A.V., Hilf, M.E., Eds.; APS Press: St Paul, MN, USA, 2010; pp. 3–26. [Google Scholar]
- Bar-Joseph, M.; Dawson, W.O. Citrus tristeza virus. In Encyclopedia of Virology; Mahy, B.W.J., van Regenmortel, M.H.V., Eds.; Elsevier Ltd.: Amsterdam, The Netherlands, 2008; pp. 520–525. [Google Scholar]
- Moreno, P.; Garnsey, S.M. Citrus tristeza diseases. A worldwide perspective. In Citrus Tristeza Virus Complex and Tristeza Diseases; Karasev, A.V., Hilf, M.E., Eds.; APS Press: St Paul, MN, USA, 2010; pp. 27–49. [Google Scholar]
- Moreno, P.; Ambrós, S.; Albiach-Martí, M.R.; Guerri, J.; Peña, L. Citrus tristeza virus: A pathogen that changed the course of the citrus industry. Mol. Plant Pathol. 2008, 9, 251–268. [Google Scholar] [CrossRef]
- Yokomi, R.K.; Lastra, R.; Stoetzel, M.B.; Damsteegt, V.D.; Lee, R.F.; Garnsey, S.M.; Gottwald, T.R.; Rocha-Peña, M.A.; Niblett, C.L. Establishment of the brown citrus aphid (Homoptera: Aphididae) in Central America and the Caribbean Basin and transmission of citrus tristeza virus. J. Econ. Entomol. 1994, 87, 1078–1085. [Google Scholar] [CrossRef]
- Dawson, W.O.; Garnsey, S.M.; Tatineni, S.; Folimonova, S.Y.; Harper, S.J.; Gowda, S. Citrus tristeza virus-host interactions. Front. Microbiol. 2013, 4, 88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dawson, W.O.; Bar-Joseph, M.; Garnsey, S.M.; Moreno, P. Citrus tristeza virus: Making an ally from an enemy. Annu. Rev. Phytopathol. 2015, 53, 137–155. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.F. Control of virus diseases of citrus. Adv. Virus Res. 2015, 92, 143–173. [Google Scholar]
- Németh, M. History and importance of plum pox virus in stone-fruit production. EPPO Bull. 1994, 24, 525–536. [Google Scholar] [CrossRef]
- Dunez, J.; Ravelonandro, M.; Candresse, T. Plum pox: Advances in research on the disease and its causal agent, and possible means of control. EPPO Bull. 1994, 24, 537–542. [Google Scholar] [CrossRef]
- Cambra, M.; Capote, N.; Myrta, A.; Llácer, G. Plum pox virus and the estimated costs associated with sharka disease. EPPO Bull. 2006, 36, 202–204. [Google Scholar] [CrossRef]
- García, J.A.; Cambra, M. Plum pox virus and sharka disease. Plant Viruses 2007, 1, 69–79. [Google Scholar]
- Barba, M.; Hadidi, A.; Candresse, T.; Cambra, M. Plum pox virus. In Virus and Virus-Like Diseases of Pome and Stone Fruits; Hadidi, A., Barba, M., Candresse, T., Jelkmann, W., Eds.; APS Press: St Paul, MN, USA, 2011; pp. 185–198. [Google Scholar]
- Rimbaud, L.; Sylvie Dallot, S.; Gottwald, T.; Decroocq, V.; Jacquot, E.; Soubeyrand, S.; Thébaud, G. Sharka. Epidemiology and worldwide management strategies: Learning lessons to optimize disease control in perennial plants. Annu. Rev. Phytopathol. 2015, 53, 357–378. [Google Scholar] [CrossRef] [Green Version]
- Makkouk, K.M. Plant pathogens which threaten food security: Viruses of chickpea and other cool season legumes in West Asia and North Africa. Food Secur. 2020, 12, 495–502. [Google Scholar] [CrossRef]
- Makkouk, K.M.; Kumari, S.G.; van Leur, J.A.G.; Jones, R.A.C. Control of plant virus diseases in cool-season grain legume crops. Adv. Virus Res. 2014, 90, 207–253. [Google Scholar] [PubMed]
- Kumari, S.G.; Makkouk, K.M. Virus diseases of faba bean (Vicia faba L.) in Asia and Africa. Plant Viruses 2007, 1, 93–105. [Google Scholar]
- Makkouk, K.M.; Bos, L.; Azzam, O.I.; Koumari, S.; Rizkallah, A. Survey of viruses affecting faba bean in six Arab countries. Arab J. Plant Prot. 1988, 6, 53–61. [Google Scholar]
- EPPO. EPPO Global Database; European and Mediterranean Plant Protection Organization (EPPO): Paris, France, 2018. [Google Scholar]
- Oladokun, J.O.; Halabi, M.H.; Barua, P.; Nath, P.D. Tomato brown rugose fruit disease: Current distribution, knowledge and future prospects. Plant Pathol. 2019, 68, 1579–1586. [Google Scholar] [CrossRef] [Green Version]
- Davino, S.; Caruso, A.G.; Bertacca, S.; Barone, S.; Panno, S. Tomato brown rugose fruit virus: Seed transmission rate and efficacy of different seed disinfection treatments. Plants 2020, 9, 1615. [Google Scholar] [CrossRef]
- Klap, C.; Luria, N.; Smith, E.; Bakelman, E.; Belausov, E.; Laskar, O.; Lachman, O.; Gal-On, A.; Dombrovsky, A. The potential risk of plant-virus disease initiation by infected tomatoes. Plants 2020, 9, 623. [Google Scholar] [CrossRef]
- Jones, R.A.C.; Koenig, R.; Lesseman, D.E. Pepino mosaic virus, a new potexvirus from pepino (Solanum muricatum). Ann. Appl. Biol. 1980, 94, 61–68. [Google Scholar] [CrossRef]
- Shipp, J.L.; Buitenhuis, R.; Stobbs, L.; Wang, K.; Kim, W.S.; Ferguson, G. Vectoring of Pepino mosaic virus by bumble-bees in tomato greenhouses. Ann. Appl. Biol. 2008, 53, 149–155. [Google Scholar] [CrossRef]
- Hanssen, I.M.; Lapidot, M.; Thomma, B.P.H.J. Emerging viral diseases of tomato crops. Mol. Plant-Microbe Interact. 2010, 23, 539–548. [Google Scholar] [CrossRef] [Green Version]
- Hanssen, I.M.; Thomma, B.P.H.J. Pepino mosaic virus: A successful pathogen that rapidly evolved from emerging to endemic in tomato crops. Mol. Plant Pathol. 2010, 11, 179–189. [Google Scholar] [CrossRef]
- Agüero, J.; Gómez-Aix, C.; Sempere, R.N.; García-Villalba, J.; García-Núñez, J.; Hernando, Y.; Aranda, M.A. Stable and broad spectrum cross-protection against pepino mosaic virus attained by mixed infection. Front. Plant Sci. 2018, 9, 1810. [Google Scholar] [CrossRef] [PubMed]
- Hollings, M.; Komuro, Y.; Tochihara, H. Cucumber green mottle mosaic virus. In CMI/AAB Descriptions of Plant Viruses; No. 154; CABI: Wellesbourne, UK, 1975. [Google Scholar]
- Varveri, C.; Vassilakos, N.; Bem, F. Characterization and detection of cucumber green mottle mosaic virus in Greece. Phytoparasitica 2002, 30, 493–501. [Google Scholar] [CrossRef]
- Reingold, V.; Lachman, O.; Koren, A.; Dombrovsky, A. First report of Cucumber green mottle mosaic virus (CGMMV) symptoms in watermelon used for the discrimination of non-marketable fruits in Israeli commercial fields. Plant Pathol. 2013, 28, 11. [Google Scholar]
- Reingold, V.; Lachman, O.; Belausov, E.; Koren, A.; Mor, N.; Dombrovsky, A. Epidemiological study of Cucumber green mottle mosaic virus in greenhouses enables reduction of disease damage in cucurbit production. Ann. Appl. Biol. 2016, 168, 29–40. [Google Scholar] [CrossRef]
- Li, J.X.; Liu, S.S.; Gu, Q.S. Transmission efficiency of Cucumber green mottle mosaic virus via seeds, soil, pruning and irrigation water. J. Phytopathol. 2015, 5, 300–309. [Google Scholar]
- Darzi, E.; Smith, E.; Shargil, D.; Lachman, O.; Ganot, L.; Dombrovsky, A. The honeybee Apis mellifera contributes to Cucumber green mottle mosaic virus spread via pollination. Plant Pathol. 2018, 67, 244–251. [Google Scholar] [CrossRef]
- Shargil, D.; Smith, E.; Lachman, O.; Reingold, V.; Darzi, E.; Tam, Y.; Dombrovsky, A. New weed hosts for Cucumber green mottle mosaic virus in wild Mediterranean vegetation. Eur. J. Plant Pathol. 2017, 148, 473–480. [Google Scholar] [CrossRef]
- Castillo, J.; Hebert, T.T. Nueva enfermedad virosa afectando al maiz en el Peru. Fitopatologia 1974, 9, 79–84. [Google Scholar]
- Heun, M.; Schäfer-Pregl, R.; Klawan, D.; Castagna, R.; Accerbi, M.; Borghi, B.; Salamini, F. Site of einkorn wheat domestication identified by DNA fingerprinting. Science 1997, 278, 1312–1314. [Google Scholar] [CrossRef] [Green Version]
- Shiferaw, B.; Smale, M.; Braun, H.-J.; Duveiller, E.; Reynolds, M.; Muricho, G. Crops that feed the world 10. Past successes and future challenges to the role played by wheat in global food security. Food Secur. 2013, 5, 291–317. [Google Scholar] [CrossRef] [Green Version]
- Trębicki, P.; Nancarrow, N.; Cole, E.; Bosque-Pérez, N.A.; Constable, F.E.; Freeman, A.J.; Rodoni, B.; Yen, A.L.; Luck, J.E.; Fitzgerald, G.J. Virus disease in wheat predicted to increase with a changing climate. Global Change Biol. 2015, 21, 3511–3519. [Google Scholar] [CrossRef] [PubMed]
- Oswald, J.W.; Houston, B.E. The yellow-dwarf virus disease of cereal crops. Phytopathology 1953, 43, 128–136. [Google Scholar]
- Strange, R.N.; Scott, P.R. Plant disease: A threat to global food security. Annu. Rev. Phytopathol. 2005, 43, 83–116. [Google Scholar] [CrossRef] [PubMed]
- Thackray, D.J.; Ward, L.T.; Thomas-Carroll, M.L.; Jones, R.A.C. Role of winter-active aphids spreading Barley yellow dwarf virus in decreasing wheat yields in a Mediterranean-type environment. Aust. J. Agric. Res. 2005, 56, 1089–1099. [Google Scholar] [CrossRef]
- McKirdy, S.J.; Jones, R.A.C. Use of imidacloprid and newer generation synthetic pyrethroids to control the spread of barley yellow dwarf luteovirus in cereals. Plant Dis. 1996, 80, 895–901. [Google Scholar] [CrossRef]
- McKirdy, S.J.; Jones, R.A.C.; Nutter, F.W., Jr. Quantification of yield losses caused by Barley yellow dwarf virus in wheat and oats. Plant Dis. 2002, 86, 769–773. [Google Scholar] [CrossRef] [Green Version]
- Wu, B.; Blanchard-Letort, A.; Liu, Y.; Zhou, G.; Wang, X.; Elena, S.F. Dynamics of molecular evolution and phylogeography of barley yellow dwarf virus-PAV. PLoS ONE 2011, 6, e16896. [Google Scholar] [CrossRef] [Green Version]
- Thackray, D.J.; Diggle, A.J.; Jones, R.A.C. BYDV PREDICTOR: A simulation model to predict aphid arrival, epidemics of Barley yellow dwarf virus and yield losses in wheat crops in a Mediterranean-type environment. Plant Pathol. 2009, 58, 186–202. [Google Scholar] [CrossRef]
- Gourmet, C.; Kolb, F.L.; Smyth, C.A.; Pedersen, W.L. Use of imidacloprid as a seed-treatment insecticide to control barley yellow dwarf virus (BYDV) in oat and wheat. Plant Dis. 1996, 80, 136–141. [Google Scholar] [CrossRef]
- Woodcock, B.A.; Ridding, L.; Freeman, S.N.; Pereira, M.G.; Sleep, D.; Redhead, J.; Aston, D.; Carreck, N.L.; Shore, R.F.; Bullock, J.M.; et al. Neonicotinoid residues in UK honey despite European Union moratorium. PLoS ONE 2018, 13, e0189681. [Google Scholar] [CrossRef]
- Velandia, M.; Rejesus, R.M.; Jones, D.C.; Price, J.A.; Workneh, F.; Rush, C.M. Economic impact of Wheat streak mosaic virus in the Texas High Plains. Crop Prot. 2010, 29, 699–703. [Google Scholar] [CrossRef]
- Jones, R.A.C.; Coutts, B.A.; Mackie, A.E.; Dwyer, G.I. Seed transmission of Wheat streak mosaic virus shown unequivocally in wheat. Plant Dis. 2005, 89, 1048–1050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dwyer, G.I.; Gibbs, M.J.; Gibbs, A.J.; Jones, R.A.C. Wheat streak mosaic virus in Australia: Relationship to isolates from the Pacific Northwest of the USA and its dispersion via seed transmission. Plant Dis. 2007, 91, 164–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hadi, B.A.R.; Langham, M.A.C.; Osborne, L.; Tilmon, K.J. Wheat streak mosaic virus on wheat: Biology and management. J. Integ. Pest Manag. 2011, 1, J1–J5. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Sánchez, H.; Henry, M.; Cárdenas-Soriano, E.; Alvizo-Villasana, H.F. Identification of Wheat streak mosaic virus and its vector Aceria tosichella in Mexico. Plant Dis. 2001, 85, 13–17. [Google Scholar] [CrossRef] [Green Version]
- Seck, P.A.; Diagne, A.; Mohanty, S.; Wopereis, M.C. Crops that feed the world 7: Rice. Food Secur. 2012, 4, 7–24. [Google Scholar] [CrossRef]
- Sweeney, M.; McCouch, S. The complex history of the domestication of rice. Ann. Bot. 2007, 100, 951–957. [Google Scholar] [CrossRef] [Green Version]
- Azzam, O.; Chancellor, T.C. The biology, epidemiology, and management of rice tungro disease in Asia. Plant Dis. 2002, 86, 88–100. [Google Scholar] [CrossRef] [Green Version]
- Chancellor, T.C.B.; Thresh, J.M. Epidemiology and Management of Rice Tungro Disease; Natural Resources Institute: Chatham, UK, 1997. [Google Scholar]
- Hawkes, J.G. The Potato: Evolution, Biodiversity and Genetic Resources; Belhaven Press: London, UK, 1990. [Google Scholar]
- Nunn, N.; Qian, N. The Columbian Exchange: A history of disease, food, and ideas. J. Econ. Perspect. 2010, 24, 163–188. [Google Scholar] [CrossRef] [Green Version]
- Glenndinning, D.R. Potato introductions and breeding up to the 20th century. New Phytol. 1983, 94, 479–505. [Google Scholar] [CrossRef]
- Birch, P.R.; Bryan, G.; Fenton, B.; Gilroy, E.M.; Hein, I.; Jones, J.T.; Prashar, A.; Taylor, M.A.; Torrance, L.; Toth, I.K. Crops that feed the world 8: Potato: Are the trends of increased global production sustainable? Food Secur. 2012, 4, 477–508. [Google Scholar] [CrossRef]
- Scott, G.J. A review of root, tuber and banana crops in developing countries: Past, present and future. Int. J. Food Sci. Technol. 2020, 1–22. [Google Scholar] [CrossRef]
- Stevenson, W.R.; Loria, R.; Franc, G.D.; Weingartner, D.P. Compendium of Potato Diseases, 2nd ed.; APS Press: St. Paul, MN, USA, 2001. [Google Scholar]
- Salaman, R.N. Degeneration of the potato—An urgent problem. J. Nat. Inst. Agric. Bot. 1925, 1, 39–51. [Google Scholar]
- De Bokx, J.A.; van der Want, J.P.H. Viruses of Potatoes and Seed-Potato Production, 2nd ed.; PUDOC: Wageningen, The Netherlands, 1987. [Google Scholar]
- Jones, R.A.C. Strain group specific and virus specific hypersensitive reactions to infection with potyviruses in potato cultivars. Ann. Appl. Biol. 1990, 117, 93–105. [Google Scholar] [CrossRef]
- Kerlan, C. Potato virus Y. In Descriptions of Plant Viruses; No. 414; Association of Applied Biologists: Wellesbourne, UK, 2006; p. 42. [Google Scholar]
- Coutts, B.A.; Jones, R.A.C. Potato virus Y: Contact transmission, stability, inactivation, and infection sources. Plant Dis. 2015, 99, 387–394. [Google Scholar] [CrossRef] [Green Version]
- Jones, R.A.C. The ecology of viruses infecting wild and cultivated potatoes in the Andean Region of South America. In Pests, Pathogens and Vegetation; Thresh, J.M., Ed.; Pitman: London, UK, 1981; pp. 89–107. [Google Scholar]
- Hawkes, J.G. History of the potato. In The Potato Crop; Harris, P.M., Ed.; Springer: New York, NY, USA, 1978; pp. 1–14. [Google Scholar]
- Cockerham, G. Genetical studies on resistance to potato viruses X and Y. Heredity 1970, 25, 309–348. [Google Scholar] [CrossRef]
- Green, K.; Quintero-Ferrer, A.; Chikh-Ali, M.; Jones, R.A.C.; Karasev, A.V. Genetic diversity of nine new non-recombinant potato virus Y isolates from three biological strain groups: Historical and geographical insights. Plant Dis. 2020, 104, 2317–3222. [Google Scholar] [CrossRef]
- Funke, C.N.; Nikolaeva, O.V.; Green, K.J.; Tran, L.T.; Chikh-Ali, M.; Quintero-Ferrer, A.; Cating, R.A.; Frost, K.E.; Hamm, P.B.; Olsen, N.; et al. Strain-specific resistance to Potato virus Y (PVY) in potato and its effect on the relative abundance of PVY strains in commercial potato fields. Plant Dis. 2017, 101, 20–28. [Google Scholar] [CrossRef] [Green Version]
- Roullier, C.; Benoit, L.; McKey, D.B.; Lebot, V. Historical collections reveal patterns of diffusion of sweet potato in Oceania obscured by modern plant movements and recombination. Proc. Natl. Acad. Sci. USA 2013, 110, 2205–2210. [Google Scholar] [CrossRef] [Green Version]
- Roullier, C.; Duputié, A.; Wennekes, P.; Benoit, L.; Bringas, V.M.F.; Rossel, G.; Tay, D.; McKey, D.; Lebot, V. Disentangling the origins of cultivated sweet potato (Ipomoea batatas (L.) Lam.). PLoS ONE 2013, 8, e62707. [Google Scholar] [CrossRef]
- Low, J.; Lynam, J.; Lemaga, B.; Crissman, C.; Barker, I.; Thiele, G.; Namanda, S.; Wheatley, C.; Andrade, M. Sweetpotato in Sub-Saharan Africa. In The Sweetpotato; Loebenstein, G., Thottappilly, G., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 359–390. [Google Scholar]
- Mukhopadhyay, S.K.; Chattopadhyay, A.; Chakraborty, I.; Bhattacharya, I. Crops that feed the world 5. Sweetpotato. Sweetpotatoes for income and food security. Food Secur. 2011, 3, 283–305. [Google Scholar] [CrossRef]
- CIP. Sweetpotato Facts and Figures; CIP: Lima, Peru, 2020; Available online: https://cipotato.org/sweetpotato/sweetpotato-facts-and-figures (accessed on 3 November 2020).
- Wasswa, P.; Mukasa, S.B.; Gibson, R.W. Identification of a ‘mild’ strain of sweet potato chlorotic stunt virus and impact on titres of co-infecting SPFMV. Afr. Crop Sci. J. 2018, 26, 349–363. [Google Scholar] [CrossRef]
- Loebenstein, G.; Fuentes, S.; Cohen, J.; Salazar, L.F. Sweetpotato. In Viruses and Virus-Like Diseases of Major Crops in Developing Countries; Loebenstein, G., Thottapilly, G., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2003; pp. 223–248. [Google Scholar]
- Kokkinos, C.D.; Clark, C.A. Interactions among Sweet potato chlorotic stunt virus and different potyviruses and potyvirus strains infecting sweetpotato in the United States. Plant Dis. 2006, 90, 1347–1352. [Google Scholar] [CrossRef] [PubMed]
- Untiveros, M.; Fuentes, S.; Salazar, L.F. Synergistic interaction of Sweet potato chlorotic stunt virus (Crinivirus) with carla-, cucumo-, ipomo-, and potyviruses infecting sweet potato. Plant Dis. 2007, 91, 669–676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kashif, M.; Pietilä, S.; Artola, K.; Jones, R.A.C.; Tugume, A.K.; Mäkinen, V.; Valkonen, J.P.T. Detection of viruses in sweetpotato from Honduras and Guatemala augmented by deep-sequencing of small-RNAs. Plant Dis. 2012, 96, 1430–1437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tugume, A.K.; Mukasa, S.B.; Kalkkinen, N.; Valkonen, J.P. Recombination and selection pressure in the ipomovirus Sweet potato mild mottle virus (Potyviridae) in wild species and cultivated sweetpotato in the centre of evolution in East Africa. J. Gen. Virol. 2010, 91, 1092–1108. [Google Scholar] [CrossRef]
- Tugume, A.K.; Amayo, R.; Weinheimer, I.; Mukasa, S.B.; Rubaihayo, P.R.; Valkonen, J.P. Genetic variability and evolutionary implications of RNA silencing suppressor genes in RNA1 of Sweet potato chlorotic stunt virus isolates infecting sweetpotato and related wild species. PLoS ONE 2013, 8, e81479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibson, R.W.; Aritua, V. The perspective of sweetpotato chlorotic stunt virus in sweetpotato production in Africa: A review. Afr. Crop Sci. J. 2002, 10, 281–310. [Google Scholar] [CrossRef]
- Tugume, A.K.; Cuellar, W.J.; Mukasa, S.B.; Valkonen, J.P. Molecular genetic analysis of virus isolates from wild and cultivated plants demonstrates that East Africa is a hotspot for the evolution and diversification of Sweet potato feathery mottle virus. Mol. Ecol. 2010, 19, 3139–3156. [Google Scholar] [CrossRef]
- Tugume, A.K.; Mukasa, S.B.; Valkonen, J.P.T. Natural wild hosts of Sweet potato feathery mottle virus show spatial differences in virus incidence and virus-like diseases in Uganda. Phytopathology 2008, 98, 640–652. [Google Scholar] [CrossRef] [Green Version]
- Simmonds, N.W. The Evolution of the Bananas; Longmans Green: London, UK, 1962. [Google Scholar]
- Heslop-Harrison, J.S.; Schwarzacher, T. Domestication, genomics and the future for banana. Ann. Bot. 2007, 100, 1073–1084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dale, J.L. Banana bunchy top: An economically important tropical plant virus disease. Adv. Virus Res. 1987, 33, 301–325. [Google Scholar] [PubMed]
- Wu, G.A.; Terol, J.; Ibanez, V.; López-García, A.; Pérez-Román, E.; Borredá, C.; Domingo, C.; Tadeo, F.R.; Carbonell-Caballero, J.; Alonso, R.; et al. Genomics of the origin and evolution of Citrus. Nature 2018, 554, 311–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ollitrault, P.; Navarro, L. Citrus. In Fruit Breeding. Handbook of Plant Breeding; Badenes, M.L., Byrne, D.H., Eds.; Springer: Boston, MA, USA, 2012; Volume 8, pp. 623–662. [Google Scholar]
- Roistacher, C.N. Diagnosis and management of virus and virus like diseases of citrus. In Diseases of Fruits and Vegetables; Naqvi, S.A.M.H., Ed.; Springer: Dordrecht, The Netherlands, 2004; Volume 1, pp. 109–189. [Google Scholar]
- Rocha-Peña, M.A.; Lee, R.F.; Lastra, R.; Niblett, C.L.; Ochoa-Corona, F.M.; Garnsey, S.M.; Yokomi, R.K. Citrus tristeza virus and its aphid vector Toxoptera citricida: Threats to citrus production in the Caribbean and Central and North America. Plant Dis. 1995, 79, 437–445. [Google Scholar] [CrossRef]
- Das, B.; Ahmed, N.; Singh, P. Prunus diversity-early and present development: A review. Int. J. Biodiv. Conserv. 2011, 3, 721–734. [Google Scholar]
- Hadidi, A.; Barba, M.; Candresse, T.; Jelkmann, W. Virus and Virus-like Diseases of Pome and Stone Fruits; APS Press: St Paul, MN, USA, 2011. [Google Scholar]
- Foyer, C.H.; Lam, H.M.; Nguyen, H.T.; Siddique, K.H.; Varshney, R.K.; Colmer, T.D.; Cowling, W.; Bramley, H.; Mori, T.A.; Hodgson, J.M.; et al. Neglecting legumes has compromised human health and sustainable food production. Nat. Plants 2016, 2, 1–10. [Google Scholar] [CrossRef]
- Bos, L. Research on Viruses of Legume Crops and the International Working Group on Legume Viruses—Historical Facts and Personal Reminiscences; ICARDA: Aleppo, Syria, 1996; 151p. [Google Scholar]
- Rachie, K.O.; Roberts, L.M. Grain legumes of the lowland tropics. Adv. Agron. 1974, 26, 1–132. [Google Scholar]
- Caracuta, V.; Barzilai, O.; Khalaily, H.; Milevski, I.; Paz, Y.; Vardi, J.; Regev, L.; Boaretto, E. The onset of faba bean farming in the Southern Levant. Sci. Rep. 2015, 5, 14370. [Google Scholar] [CrossRef] [Green Version]
- Albrechtsen, S.E. Testing Methods for Seed-Transmitted Viruses: Principles and Protocols; CABI: Press, Wallingford, UK, 2006. [Google Scholar]
- Sastry, K.S. Seed-Borne Plant Virus Diseases; Springer: Dordrecht, The Netherlands, 2013. [Google Scholar]
- Klee, H.J.; Resende, M.F., Jr. Plant domestication: Reconstructing the route to modern tomatoes. Curr. Biol. 2020, 30, R359–R361. [Google Scholar] [CrossRef]
- Razifard, H.; Ramos, A.; Della Valle, A.L.; Bodary, C.; Goetz, E.; Manser, E.J.; Li, X.; Zhang, L.; Visa, S.; Tieman, D.; et al. Genomic evidence for complex domestication history of the cultivated tomato in Latin America. Mol. Biol. Evol. 2020, 37, 1118–1132. [Google Scholar] [CrossRef]
- Salem, N.; Mansour, A.; Ciuo, M.; Falk, B.; Turina, M. A new tobamovirus infecting tomato crops in Jordan. Arch. Virol. 2016, 161, 503–506. [Google Scholar] [CrossRef] [PubMed]
- Luria, N.; Smith, E.; Reingold, V.; Bekelman, I.; Lapidot, M.; Levin, I.; Elad, N.; Tam, Y.; Sela, N.; Abu-Ras, A.; et al. A new Israeli Tobamovirus isolate infects tomato plants harboring Tm-22 resistance genes. PLoS ONE 2017, 12, e0170429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maayan, Y.; Pandaranayaka, E.P.J.; Srivastava, D.A.; Lapidot, M.; Levin, I.; Dombrovsky, A.; Harel, A. Using genomic analysis to identify tomato Tm-2 resistance-breaking mutations and their underlying evolutionary path in a new and emerging tobamovirus. Arch Virol. 2018, 163, 1863–1875. [Google Scholar] [CrossRef]
- Panno, S.; Caruso, A.G.; Blanco, G.; Davino, S. First report of Tomato brown rugose fruit virus infecting sweet pepper in Italy. New Dis. Rep. 2020, 41, 20. [Google Scholar] [CrossRef] [Green Version]
- Levitzky, N.; Smith, E.; Lachman, O.; Luria, N.; Mizrahi, Y.; Bakelman, H.; Sela, N.; Laskar, O.; Milrot, E.; Dombrovsky, A. The bumblebee Bombus terrestris carries a primary inoculum of Tomato brown rugose fruit virus contributing to disease spread in tomatoes. PLoS ONE 2019, 14, e0210871. [Google Scholar] [CrossRef]
- Van der Vlugt, R.A.A.; Stijger, C.C.M.M.; Verhoeven, J.T.J.; Lesemann, D.E. First report of Pepino mosaic virus on tomato. Plant Dis. 2000, 84, 103. [Google Scholar] [CrossRef]
- Soler, S.; Prohens, J.; Díez, M.J.; Nuez, F. Natural occurrence of Pepino mosaic virus in Lycopersicon species in central and southern Peru. J. Phytopathol. 2002, 150, 49–53. [Google Scholar] [CrossRef]
- Córdoba, M.C.; Martínez-Priego, L.; Jordá, C. New natural hosts of Pepino mosaic virus in Spain. Plant Dis. 2004, 88, 90. [Google Scholar] [CrossRef]
- Endl, J.; Achigan-Dako, E.G.; Pandey, A.K.; Monforte, A.J.; Pico, B.; Schaefer, H. Repeated domestication of melon (Cucumis melo) in Africa and Asia and a new close relative from India. Amer. J. Bot. 2018, 105, 1662–1671. [Google Scholar] [CrossRef] [Green Version]
- Chomicki, G.; Schaefer, H.; Renner, S.S. Origin and domestication of Cucurbitaceae crops: Insights from phylogenies, genomics and archaeology. New Phytol. 2020, 226, 1240–1255. [Google Scholar] [CrossRef] [Green Version]
- Ainsworth, G.C. Mosaic disease of cucumber. Ann. Appl. Biol. 1935, 22, 55–67. [Google Scholar] [CrossRef]
- Shim, C.K.; Han, K.S.; Lee, J.H.; Bae, D.W.; Kim, D.K.; Kim, H.K. Isolation and characterization of watermelon isolate of Cucumber green mottle mosaic virus (CGMMV-HY1) from watermelon plants with severe mottle mosaic symptoms. Plant Pathol. J. 2005, 21, 167–171. [Google Scholar] [CrossRef] [Green Version]
- Fletcher, J.T. Cucumber green mottle mosaic virus, its effect on yield and its control in the Lea Valley, England. Plant Pathol. 1969, 18, 16–22. [Google Scholar] [CrossRef]
- Antignus, Y.; Lachman, O.; Pearlsman, A.; Koren, A. Containment of Cucumber fruit mottle mosaic virus (CFMMV) infection through roots by planting into a virus-free intermediating medium. Phytoparasitica 2005, 33, 85–87. [Google Scholar] [CrossRef]
- Vani, S.; Varma, A. Properties of cucumber green mottle mosaic virus isolated from water of river Jamuna. Ind. Phytopathol. 1993, 46, 118–122. [Google Scholar]
- Desbiez, C.; Lecoq, H. Zucchini yellow mosaic virus. Plant Pathol. 1997, 46, 809–829. [Google Scholar] [CrossRef]
- Lecoq, H.; Katis, N. Control of cucurbit viruses. Adv. Virus Res. 2014, 90, 255–296. [Google Scholar]
- Clarke, R.; Webster, C.G.; Kehoe, M.A.; Coutts, B.A.; Broughton, S.; Warmington, M.; Jones, R.A.C. Epidemiology of Zucchini yellow mosaic virus in cucurbit crops in a remote tropical environment. Virus Res. 2020, 281, 197897. [Google Scholar] [CrossRef]
- Clarke, R.; Kehoe, M.A.; Broughton, S.; Jones, R.A.C. Host plant affiliations of aphid vector species found in a remote tropical environment. Virus Res. 2020, 281, 197934. [Google Scholar] [CrossRef]
- Rojas, M.R.; Macedo, M.A.; Maliano, M.R.; Soto-Aguilar, M.; Souza, J.O.; Briddon, R.W.; Kenyon, L.; Rivera-Bustamante, R.F.; Zerbini, F.M.; Adkins, S.; et al. World management of geminiviruses. Annu. Rev. Phytopathol. 2018, 56, 637–677. [Google Scholar] [CrossRef]
- Jones, R.A.C. Trends in plant virus epidemiology: Opportunities from new or improved technologies. Virus Res. 2014, 186, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.A.C.; Salam, M.U.; Maling, T.J.; Diggle, A.J.; Thackray, D.J. Principles of predicting plant virus disease epidemics. Annu. Rev. Phytopathol. 2010, 48, 179–203. [Google Scholar] [CrossRef] [PubMed]
- Jeger, M.J.; Madden, L.V.; Van Den Bosch, F. Plant virus epidemiology: Applications and prospects for mathematical modeling and analysis to improve understanding and disease control. Plant Dis. 2018, 102, 837–854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeger, M.J. The epidemiology of plant virus disease: Towards a new synthesis. Plants 2020, 9, 1768. [Google Scholar] [CrossRef] [PubMed]
- Adams, I.P.; Glover, R.H.; Monger, W.A.; Mumford, R.; Jackeviciene, E.; Navalinskiene, M.; Samuitiene, M.; Boonham, N. Next-generation sequencing and metagenomic analysis: A universal diagnostic tool in plant virology. Mol. Plant Pathol. 2009, 10, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Kreuze, J.F.; Perez, A.; Untiveros, M.; Quispe, D.; Fuentes, S.; Barker, I.; Simon, R. Complete viral genome sequence and discovery of novel viruses by deep sequencing of small RNAs: A generic method for diagnosis, discovery and sequencing of viruses. Virology 2009, 388, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roossinck, M.J.; Martin, D.P.; Roumagnac, P. Plant virus metagenomics: Advances in virus discovery. Phytopathology 2015, 105, 716–727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, R.A.C.; Boonham, N.; Adams, I.P.; Fox, A. Historical virus isolate collections: An invaluable resource connecting plant virology’s pre-sequencing and post-sequencing eras. Plant Pathol. 2021, 70, 235–248. [Google Scholar] [CrossRef]
- Legg, J.; Somado, E.A.; Barker, I.; Beach, L.; Ceballos, H.; Cuellar, W.; Elkhoury, W.; Gerling, D.; Helsen, J.; Hershey, C.; et al. A global alliance declaring war on cassava viruses in Africa. Food Secur. 2014, 6, 231–248. [Google Scholar] [CrossRef] [Green Version]





| Disease (Pandemic or Major Epidemic) | Continents or Regions Affected | Causal Agent(s) | Virus Genus | Vector(s) | Crop Diseased | Crop Domestication Center | Disease Impact | Virus(es) Origin(s) | Causes(s) of Appearance | Alternative Hosts | Factors Favoring Increased Importance/Distribution | Key Citations |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Maize lethal necrosis (pandemic) | East and Central Africa, East Asia, Southeast Asia, North and South America, Europe | Virus complex: Maize chlorotic mottle virus (MCMV), plus sugarcane mosaic virus (SCMV), maize dwarf mosaic virus (MDMV) or wheat streak mosaic virus (WSMV) | Machlomovirus(MCMV), plus Potyvirus (SCMV, MDMV)) or Trtimovirus (WSMV) | Several beetle and thrips species (including the thrips Frankliniella williamsi) (MCMV). Several aphid species (SCMV, MDMV). Eriophyid mite (Aceria tosichella) (WSMV) | Maize (Zea mays) | North America (Mexico) | Widespread devastating yield losses. Food shortages (especially in East and Central Africa) | Probably all coevolved with the principal crop they each infect within each of its domestication centers: Mexico (MCMV, MDMV), Indian subcontinent and Southeast Asia (SCMV) Middle East (WSMV) | Ancestral viruses probably first spread by respective vectors to maize from wild maize ancestors (MCMV, MDMV), sugar cane ancestors (SCMV), or wheat ancestors (WSMV) | Sugar cane, Sorghum, millet, wheat, barley, and several pasture and weed grasses (MCMV). Several cereals and wild grasses (MDMV, SCMV, WSMV) | Seed-borne MCMV spread in contaminated maize seed resulting in mixed infections with locally occurring MDMV, SCMV or WSMV. Exacerbated by growing vulnerable maize cultivars, agricultural intensification to increase production and widespread occurrence of MCMVs Frankliniella williamsi vector | [42,71,72,73,74] |
| Wheat yellow dwarf disease (major epidemic) | Europe, Middle East, South, Central and East Asia, Southeast Asia, Oceania, North, Central and South America, North Africa, sub-Saharan Africa | Barley yellow dwarf virus (BYDV), and cereal yellow dwarf virus (CYDV). Distinct BYDV strains are PAV, MAV, RMV and SGV | Luteovirus (BYDV), Polerovirus (CYDV) | Aphids, especially Rhopalosiphum padi (PAV, CYDV), R. maidis (RMV), Sitobion avenae (MAV) and Schizaphis graminium (SGV) | Wheat (Triticum aestivum) | Middle East (Fertile crescent region) | Sporadic epidemics. Widespread yield losses | BYDV and CYDV both probably first originated in wheat’s Middle East domestication center. May have re-emerged separately in other world regions | Ancestral viruses probably first spread by aphid vectors from wild grasses to wheat May have done this several times | Barley, oats, rye, triticale, maize, rice and several pasture and weed grasses | Growing vulnerable wheat cultivars. Virus recombination generating virulent new variants | [4,72,75,76,77,78,79] |
| Wheat streak mosaic disease (major epidemic) | Europe, Middle East, Central and East Asia, Australasia, North and South America, North Africa, sub-Saharan Africa | Wheat streak mosaic virus | Tritimovirus | Leaf curl mite (Aceria tosichella) | Wheat (Triticum aestivum) | Middle East (Fertile crescent region) | Sporadic epidemics, severe yield losses, especially in Great Plains of North America. | Probably coevolved with wheat in its Middle East domestication center. May have re-emerged separately in other world regions | Ancestral virus probably first spread from wild wheat or grasses to wheat by Aceria tosichella vector. Likely occurred several times. | Barley, maize, oats, rye, sorghum, and some mostly annual grasses | Spread as seed-borne WSMV to other continents in contaminated wheat seed. Extended cropping periods and growing wheat under warm conditions that favor its mite vector | [4,72,79,80,81,82,83] |
| Rice tungro disease (pandemic) | Southeast Asia, East Asia (China) and the Indian subcontinent | Virus complex: rice tungro bacilliform virus (RTBV), and rice tungro spherical virus (RTSV) | Tungrovirus (RTBV)) and Waikavirus (RTSV) | Several leafhopper vector species transmit both viruses, Nephotettix virescens most efficient vector species | Asian rice (Oryza sativa) | East Asia (China) | Devastating yield losses, famine. Major deterrent to rice cultivation | Both viruses have the major phylogroups, Indian and Southeast Asian (RTBV), or Indian and Southeast Asian /East Asian (RTSV). Probably coevolved with rice separately within different parts of its domestication center | Ancestral RTBV and RTSV likely first spread by leafhopper vectors from infected wild rice or grasses to rice plantings | Wild rice and grass weeds often associated with rice paddies are alternative hosts of both viruses | Favored by agricultural intensification to increase production, growing vulnerable cultivars, and virus recombination generating more virulent variants | [4,13,14,61,62,84,85,86,87,88] |
| Potato necrotic ringspot disease (major epidemic) | All continents except Antarctic | Potato virus Y (PVY) necrogenic R2 variants | Potyvirus | Several aphid species, Myzus persicae most efficient vector species | Potato (Solanum tuberosum) | South America (Andean region (Peru, Ecuador) | R2-affected tubers unsaleable. Inability to manage effectively in many heathy seed potato schemes | PVY itself originally coevolved with potato in its Andean region domestication center | PVY ancestor spread by aphid vectors to potato from wild potato ancestors | Pepper, tomato, tobacco and many wild Solanaceae species | R2 arose in Europe by recombination between strains PVYO and PVYN. It caused PTNRD and subtle or no foliage symptoms, and was more readily aphid transmissible. R2 spread globally in infected seed potato tubers. Largely unmanageable in many healthy seed tuber schemes | [40,58,89,90,91,92,93] |
| Sweet potato virus disease (pandemic) | Sub-Saharan Africa, North Africa, Middle East, Southeast Asia, East Asia, and North, Central and South America | Virus complex: Sweet potato chlorotic stunt virus (SPCSV) plus sweet potato feathery mottle virus (SPFMV), or sweet potato mild mottle virus (SPMMV) | Crinivirus (SPCSV), plus Potyvirus (SPFMV) or Ipomovirus (SPMMV) | Whiteflies Bemisia tabaci (SPCSV, SPMMV) and Trialeurodes abutilonea (SPCSV). Aphid species Myzus persicae and Aphis gossypii (SPFMV) | Sweet potato (Ipomoea batatas) | Occurred twice, separately in Central and South America | Devastating yield losses. Major deterrent to sweet potato cultivation | SPCSV and SPFMV probably coevolved with sweet potato in one of its two domestication centers in Central or South America. SPMMV spread to sweet potato from wild Convolulaceae hosts in East Africa | Ancestral viruses likely first spread by their respective vectors to sweet potato from its wild ancestors (SPCSV, SPFMV) or other wild alternative hosts (SPMMV) | SPCSV, SPFMV and SPMMV all infect wild Convolvulaceae: Ipomoea spp. (several species), Hewittia sublobata and Lepistemon owariensis | SPCSV and SPFMV spread globally in infected tuberous roots. Displacement of local sweet potato land races by vulnerable high-yielding cultivars. Spread favored by agricultural intensification to increase production | [72,94,95,96,97,98,99,100] |
| Banana bunchy top disease (pandemic) | Sub-Saharan and North Africa, Middle East (Iran), Indian subcontinent, Southeast Asia, East Asia, Oceania | Banana bunchy top virus | Babuvirus | Aphid (Pentalonia nigronervosa) | Banana, including plantain (Musa spp.) | Southeast Asia (especially Malaysia), Polynesia, Indian subcontinent | Devastating yield losses. Major deterrent to banana cultivation | Within banana’s wider domestication center, two major phylogroups diverged, the Pacific-Indian Oceans and Southeast Asian | Ancestral virus likely first spread by its aphid vector to banana from its wild ancestors or alternative hosts | Musa paradisiaca M. textilis, and Ensete ventriculosum | Wide-scale transportation of infected planting material of vulnerable cultivars to new geographic locations. Frequent new introductions of its Pentalonia nigronervosa vector. Agricultural intensification to increase production. | [38,63,64,72,101] |
| Citrus tristeza disease (pandemic) | South, Central and North America, Sub-Saharan and North Africa, Europe, Middle East, Indian subcontinent, East Asia, Southeast Asia, Oceania | Citrus tristeza virus | Closterovirus | Aphids. Toxoptera citricida most efficient vector. Less efficiently vectored by A. gossypii, T. aurantii and A. spiraecola | Citrus sp. Especially orange, lemon, mandarin, grapefruit, lime | Southeast Asia mainly, but also in the Indian subcontinent, East Asia and Melanesia | Devastating yield losses. Plants killed. Plantations abandoned | Co-evolved with Citrus species within the broader citrus domestication center (Southeast Asia, Indian subcontinent, East Asia and Melanesia) | Ancestral virus first spread by its aphid vectors to citrus from its wild citrus ancestors or related genera | Wild Citrus species and species in related genera, such as Fortunella and Poncirus. | Wide-scale transportation of CTV-infected and Toxoptera citricida-infested planting material to new geographic locations. Widespread growing of citrus trees derived from CTV-vulnerable cultivar scions grafted onto CTV-susceptible sour orange rootstocks. | [4,72,102,103,104,105,106,107,108,109,110] |
| Plum pox disease (pandemic) | Europe, Middle East, Indian subcontinent, East Asia, North Africa, South and North America | Plum pox virus | Potyvirus | Aphids. Myzus persicae most efficient vector | Prunus spp. Especially plum, peach, apricot, nectarine | China (peaches, nectarines). Europe, Asia, North America (plum, cherry) | Devastating losses in fruit quality, premature fruit drop and yield. Diminished orchard lifespan | Probably co-evolved with plum and cherry within its wider domestication center (central and eastern Europe and the Levant) | Ancestral virus spread by aphid vectors and grafting to plum and cherry from wild Prunus ancestors. Spread to peach and nectarine from infected Prunus after introduction to Europe and the Levant | Ornamental Prunus trees. Wild Prunus species | Spread by wide-scale transportation of PPV-infected Prunus planting material and germplasm to new geographic locations. Local spread by aphid vectors. Widespread adoption of vulnerable stone fruit cultivars | [4,14,60,72,111,112,113,114,115,116] |
| Faba bean necrotic yellows disease (major epidemic) | Europe (Spain), North Africa, Horn of Africa, Middle East and Arabia, Indian subcontinent (Pakistan) | Faba bean necrotic yellows virus | Nanovirus | Aphids. Aphis craccivora, A. fabae and Acyrthosiphon pisum. | Faba bean (Vicia faba) | Middle East | Sporadic epidemics. Devastating yield losses. Major deterrent to faba bean cultivation | Probably coevolved with faba bean (and other cultivated legumes) within faba bean’s domestication center | Ancestral virus probably spread by its aphid vectors to faba bean crops from other crop or wild legumes | Common bean, cowpea, chickpea, lentil and several wild and pasture legumes, and Amaranthus spp. | Growing vulnerable faba bean cultivars combined with agricultural intensification to increase production | [72,117,118,119,120] |
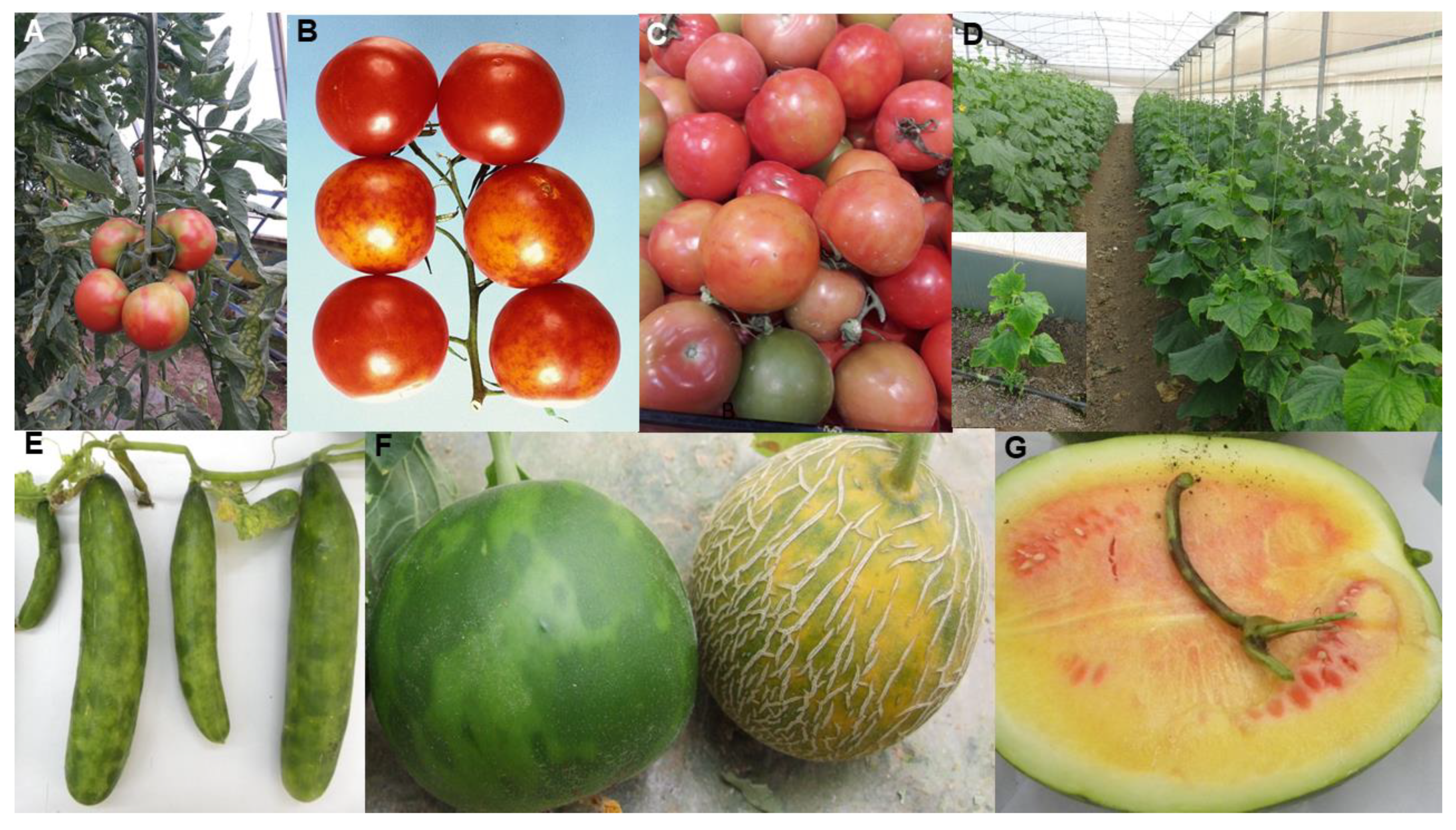
| Tomato brown rugose fruit disease (major epidemic) | Middle East, Europe, East Asia (China), North Africa (Egypt, Sudan), North (Mexico, USA) and South (Chile) America | Tomato brown rugose fruit virus | Tobamovirus | Contact transmission and by bee pollinators | Tomato (Solanum lycopersicum) | Middle East | Unmarketable fruit cause major losses. Recently spreading rapidly, especially in protected cropping | Uncertain. Possibly infected tomato in new encounter with indigenous virus in Middle East, or virus already coevolved with tomato beforehand but remained unnoticed until recently | Uncertain. Ancestral virus spread by contact to tomato from unknown host, or the virus itself was already present infecting tomatoes worldwide | Infects pepper. Possible alternative hosts include eggplant, petunia and the weed Solanum nigrum (natural infection yet to be confirmed) | Seed-borne international spread in contaminated tomato seed, and to a lesser extent in infected seedlings and fruit. Mutation or recombination event that broke resistance gene Tm-22. Spread favored by intensive protected cropping procedures | [121,122,123,124] |
| Pepino mosaic disease (major epidemic) | South and North America, Europe, Middle East, Africa (Morocco, South Africa), East Asia (China) | Pepino mosaic virus | Potexvirus | Contact transmission and by bee pollinators | Tomato (Solanum lycopersicum) | Andean region of South America | Unmarketable fruit cause major losses. | Probably coevolved with, pepino, pepper and semi-domesticated tomato in the Andean region of South America | Ancestral virus spread by contact from wild tomato to pepino, pepper and tomato crops in the Andean region | Infects pepper and pepino crops, wild tomato species; and 18 weed species from seven different non-Solanaceae families in Spain | Seed-borne international spread in contaminated tomato seed, and to a lesser extent in infected seedlings and fruit. Spread favored by intensive protected cropping procedures | [7,72,124,125,126,127,128,129] |
| Cucumber green mottle mosaic disease (major epidemic) | All continents except Antarctic | Cucumber green mottle mosaic virus | Tobamovirus | Contact transmission and by bee pollinators | Fruit and vegetable cucurbits | Americas (squash, zucchini, pumpkin). Indian subcontinent (cucumber, melon). Africa (watermelon, melon, gherkin) | Diminished marketable yields or fruit unmarketable due to poor quality. Substantial gross yield losses. | Likely coevolved with cucumber and melon in Indian subcontinent domestication center. Spread from these two crops to squash, zucchini, gherkin watermelon, pumpkin | Ancestral virus probably spread by contact from wild cucumber or melon ancestor to cucumber and/or melon in Indian subcontinent | Infects cultivated gourds and wild species in nine families including Amarthaceae, Cucurbitaceae, Euphorbiaceae, Lamiaceae and Solanacaeae | Seed-borne international spread in contaminated cucurbit seed, and to a lesser extent in infected cucurbit seedlings and fruit. Spread favored by intensive protected cropping procedures | [39,72,130,131,132,133,134,135,136] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jones, R.A.C. Global Plant Virus Disease Pandemics and Epidemics. Plants 2021, 10, 233. https://doi.org/10.3390/plants10020233
Jones RAC. Global Plant Virus Disease Pandemics and Epidemics. Plants. 2021; 10(2):233. https://doi.org/10.3390/plants10020233
Chicago/Turabian StyleJones, Roger A. C. 2021. "Global Plant Virus Disease Pandemics and Epidemics" Plants 10, no. 2: 233. https://doi.org/10.3390/plants10020233
APA StyleJones, R. A. C. (2021). Global Plant Virus Disease Pandemics and Epidemics. Plants, 10(2), 233. https://doi.org/10.3390/plants10020233






