Complete Chloroplast Genome of Abutilon fruticosum: Genome Structure, Comparative and Phylogenetic Analysis
Abstract
1. Introduction
2. Results and Discussion
2.1. Characteristics of A. fruticosum Chloroplast Genome
2.2. Repeat Analyses
2.2.1. Long Repeats
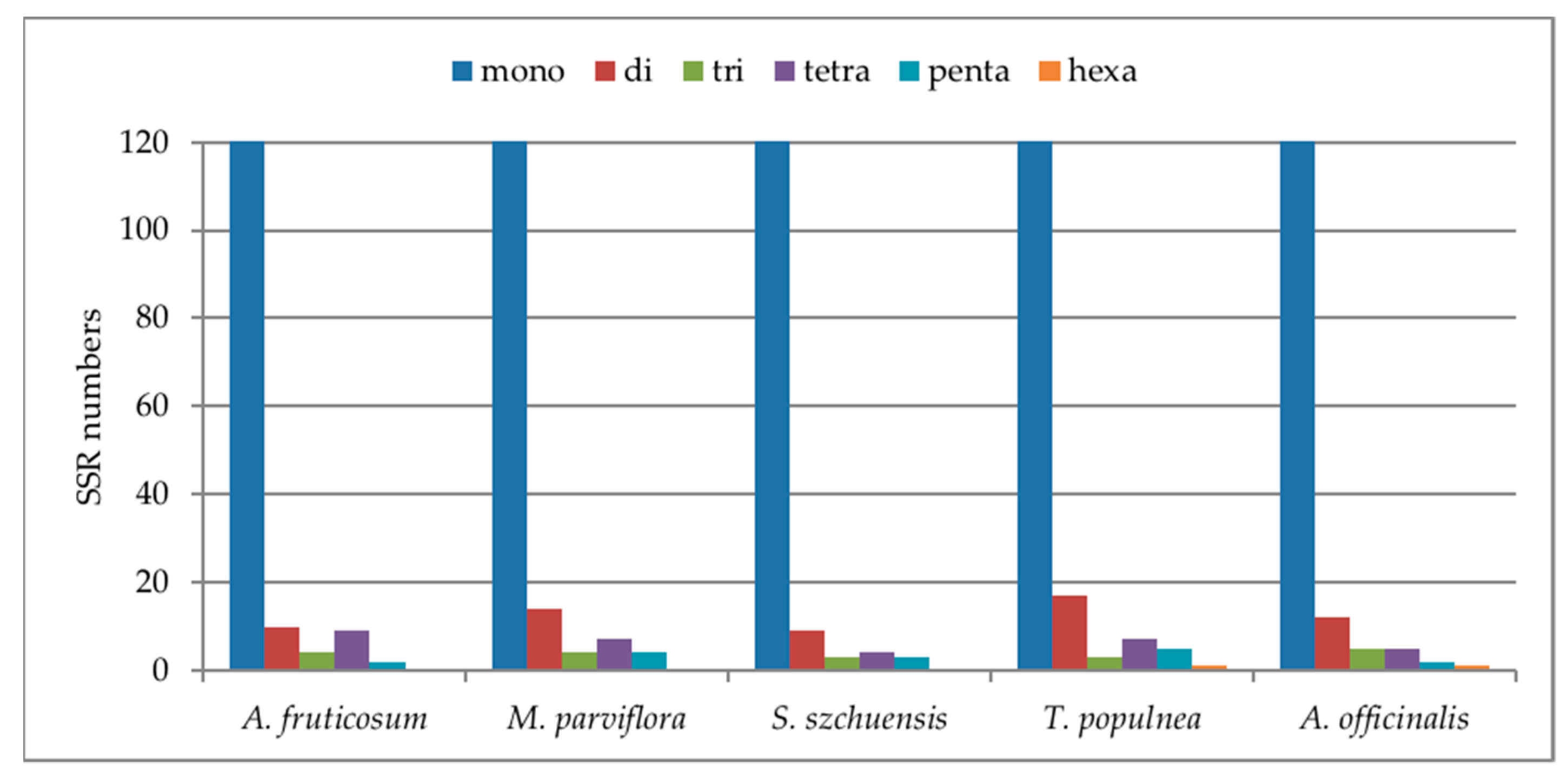
2.2.2. Simple Sequence Repeats (SSRs)
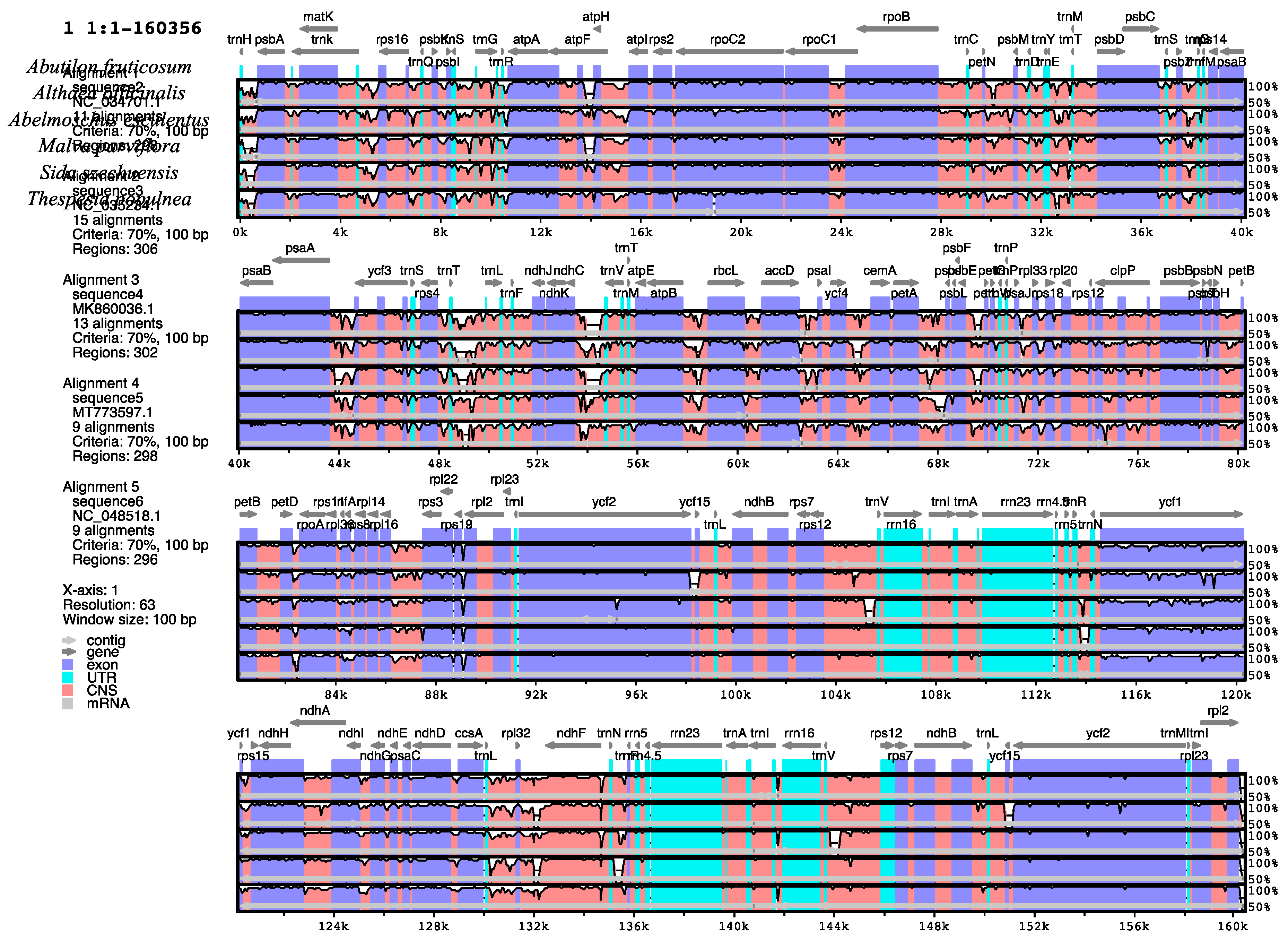
2.3. Comparative Analysis of Plastomes of Malvaceae Species
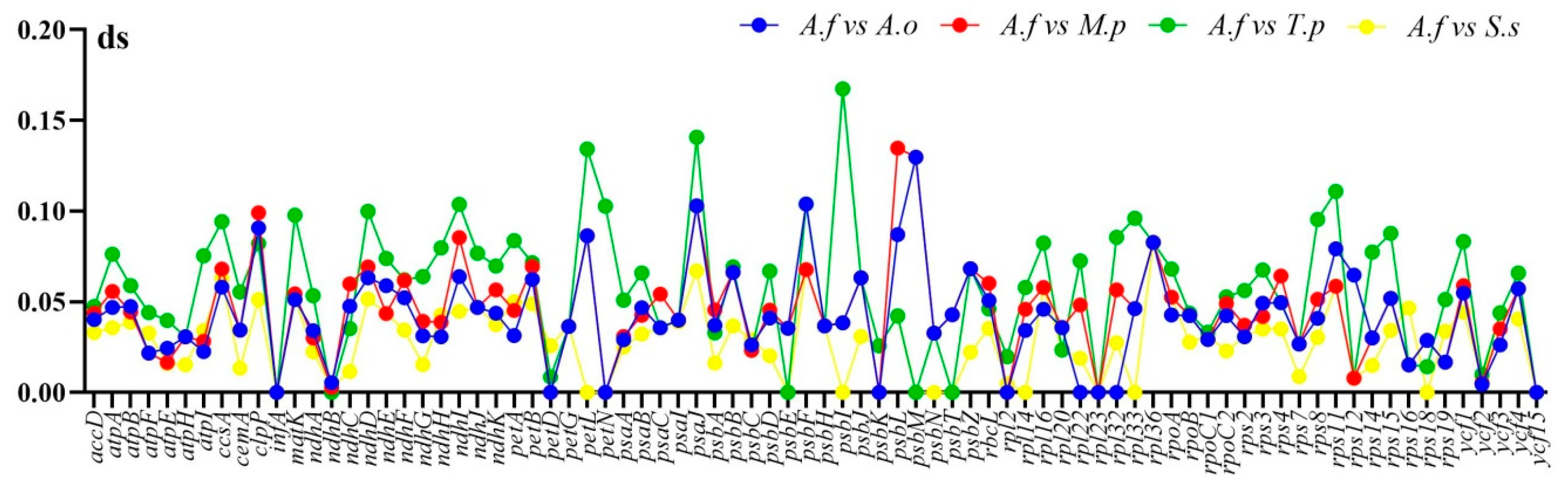
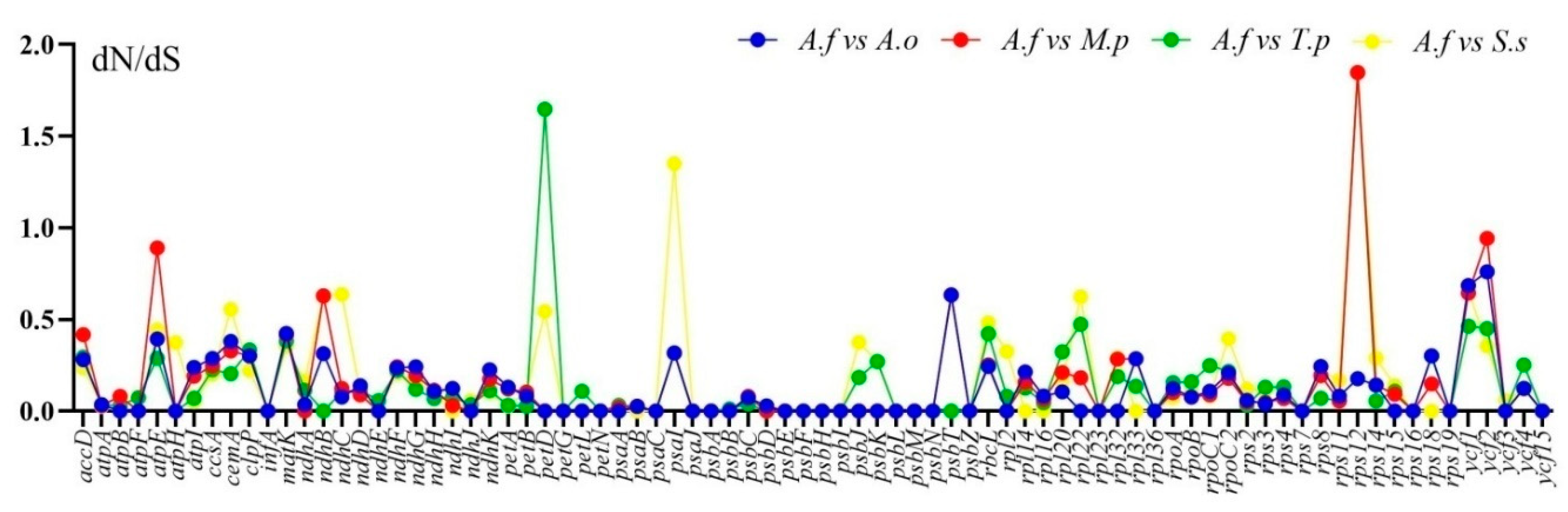
2.4. Divergence of Protein-Coding Gene Sequences
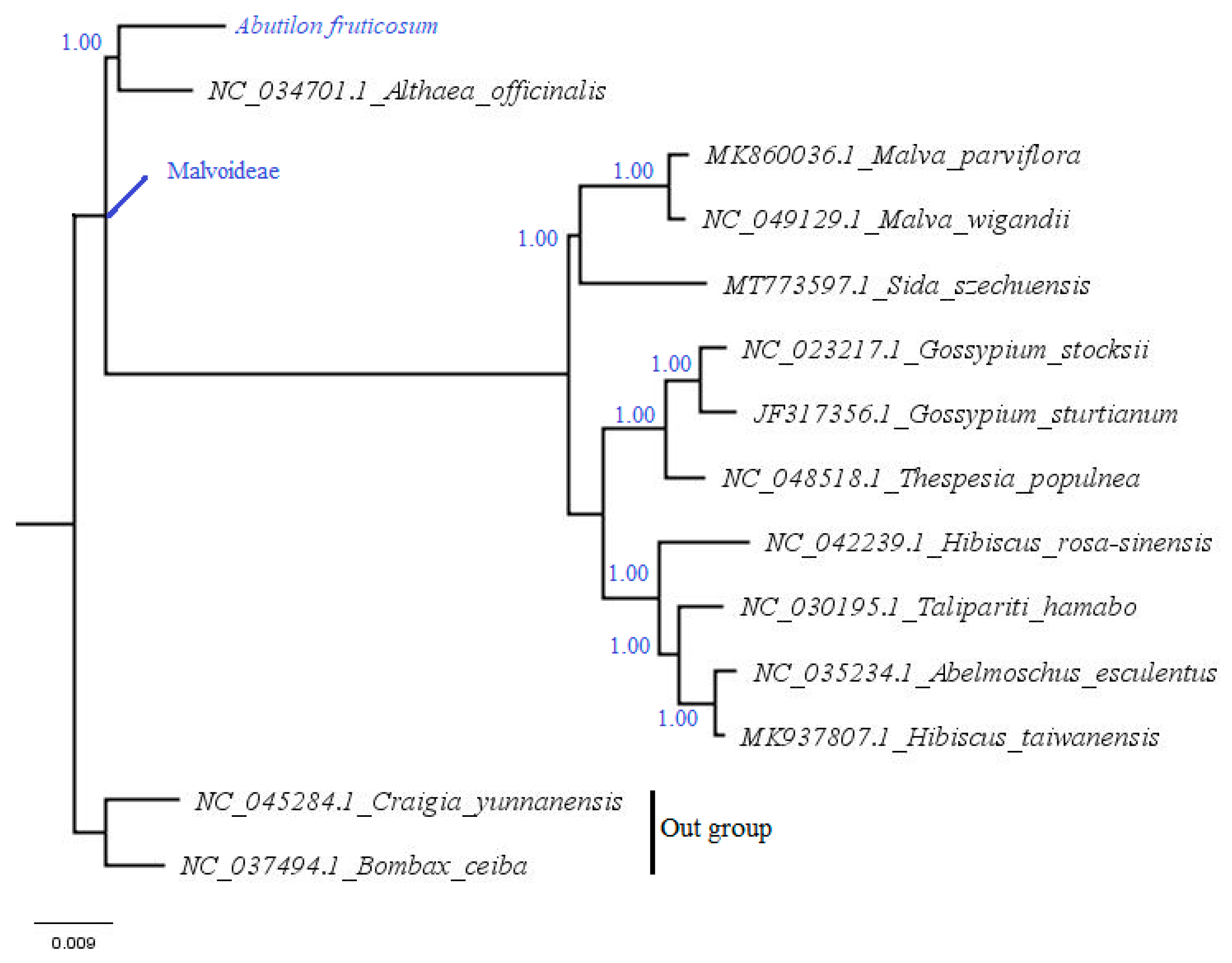
2.5. Phylogenetic Analysis
3. Materials and Methods
3.1. Plant Material and DNA Extraction
3.2. Library Construction, Sequencing and Assembly of the Chloroplast Genome
3.3. Gene Annotation
3.4. Sequence Analysis
3.5. Repeat Analysis
3.6. Genome Comparison
3.7. Characterization of Substitution Rate
3.8. Phylogenetic Analysis
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Miller, P. The Gardeners Dictionary 3:23; John and James Rivington: London, UK, 1731. [Google Scholar] [CrossRef][Green Version]
- Sweet, R. Hortus Britannicus, 1st ed.; J. Ridgway: London, UK, 1826. [Google Scholar]
- Sivarajan, V.V.; Pradeep, A.K. Malvaceae of Southern Peninsular India: A Taxonomic Monograph; Daya Publ. House: Delhi, India, 1996; pp. 201–204. [Google Scholar]
- Kearney, T.H. A tentative key to the South American species of Abutilon. Leafl. West. Bot. 1958, 8, 201–216. [Google Scholar]
- Fryxell, P.A. The American genera of Malvaceae-II. Brittonia 1997, 49, 204–269. [Google Scholar] [CrossRef]
- Fryxell, P.A. An Abutilon nomenclator (Malvaceae). Lundellia 2002, 5, 79–118. [Google Scholar] [CrossRef]
- Esteves, G.L.; Krapovickas, A. New species of Abutilon (Malvaceae) from Sao Paulo State, Brazil. Kew Bull. 2002, 57, 479–482. [Google Scholar] [CrossRef]
- Husain, S.A.; Baquar, S.R. Biosystematic studies in the genus Abutilon from Pakistan. Phyton 1974, 15, 219–234. [Google Scholar]
- Patel, M.K.; Rajput, A.P. Therapeutic significance of Abutilon indicum: An overview. Am. J. Pharm. Tech. Res. 2013, 4, 20–35. [Google Scholar]
- Khadabadi, S.S.; Bhajipale, N.S. A review on some important medicinal plants of Abutilon spp. Res. J. Pharm. Biol. Chem. Sci. 2010, 1, 718–729. [Google Scholar]
- Pingale, S.S.; Virkar, P.S. Evaluation of acute toxicity for Abutilon indicum. Pharm. Lett. 2011, 3, 37–42. [Google Scholar]
- Bano, I.; Deora, G.S. Studies on micro morphological taxonomic variations in Abutilon species of Indian Thar Desert. IOSR J. Pharm. Biol. Sci. 2017, 12, 60–68. [Google Scholar]
- Baquar, S.R. Medicinal and Poisonous Plants of Pakistan; Printas: Karachi, Pakistan, 1989. [Google Scholar]
- Ramar, K.; Ayyadurai, V. The present investigation deals with in-vitro Callus induction and plant regeneration of Abutilon indicum (L.). J. Pharmacogn. Phytochem. 2015, 3, 248–251. [Google Scholar]
- Christenhusz, M.J.M.; Byng, J.W. The number of known plants species in the world and its annual increase. Phytotaxa 2016, 261, 201–217. [Google Scholar] [CrossRef]
- Xu, Z.; Deng, M. Malvaceae, Identification and Control of Common Weeds, 1st ed.; Springer: Dordrecht, The Netherlands, 2017; pp. 717–735. [Google Scholar]
- Grevich, J.J.; Daniell, H. Chloroplast Genetic Engineering: Recent Advances and Future Perspectives. Crit. Rev. Plant Sci. 2005, 24, 83–107. [Google Scholar] [CrossRef]
- Neuhaus, H.; Emes, M. Nonphotosynthetic metabolism in plastids. Ann. Rev. Plant Biol. 2000, 51, 111–140. [Google Scholar] [CrossRef] [PubMed]
- Wicke, S.; Schneeweiss, G.M.; Depamphilis, C.W.; Kai, F.M.; Quandt, D. The evolution of the plastid chromosome in land plants: Gene content, gene order, gene function. Plant Mol. Biol. 2011, 76, 273–297. [Google Scholar] [CrossRef]
- Oldenburg, D.J.; Bendich, A.J. The linear plastid chromosomes of maize: Terminal sequences, structures and implications for DNA replication. Curr. Genet. 2016, 62, 431–442. [Google Scholar] [CrossRef]
- Shaw, J.; Shafer, H.L.; Leonard, O.R.; Kovach, M.J.; Schorr, M.; Morris, A.B. Chloroplast DNA sequence utility for the lowest phylogenetic and phylogeographic inferences in angiosperms: Thetortoise and the hare IV. Am. J. Bot. 2014, 101, 1987–2004. [Google Scholar] [CrossRef]
- Guisinger, M.M.; Chumley, T.W.; Kuehl, J.V.; Boore, J.L.; Jansen, R.K. Implications of the plastid genome sequence of Typha (Typhaceae, poales) for understanding genome evolution in poaceae. J. Mol. Evol. 2010, 70, 149–166. [Google Scholar] [CrossRef]
- Yang, J.B.; Tang, M.; Li, H.T.; Zhang, Z.R.; Li, D.Z. Complete chloroplast genome of the genus Cymbidium: Lights into the species identification, phylogenetic implications and population genetic analysis. BMC Evol. Biol. 2013, 13. [Google Scholar] [CrossRef]
- Chen, H.; Shao, J.; Zhang, H.; Jiang, M.; Huang, L.; Zhang, Z.; Yang, D.; He, M.; Ronaghi, M.; Luo, X.; et al. Sequencing and analysis of Strobilanthes cusia (Nees) Kuntze chloroplast Genome revealed the rare simultaneous contraction and expansion of the inverted repeat region in Angiosperm. Front. Plant Sci. 2018, 9, 324. [Google Scholar] [CrossRef]
- Chang, C.C.; Lin, H.C.; Lin, I.P.; Chow, T.Y.; Chen, H.H.; Chen, W.H.; Cheng, C.H.; Lin, C.Y.; Liu, S.M.; Chang, C.C.; et al. The chloroplast genome of Phalaenopsis Aphrodite (Orchidaceae): Comparative analysis of evolutionary rate with that of grasses and its phylogenetic implications. Mol. Biol. Evol. 2006, 23, 279–291. [Google Scholar] [CrossRef]
- Raman, G.; Park, S. The complete chloroplast genome sequence of Ampelopsis: Gene organization, comparative analysis, and phylogenetic relationships to other angiosperms. Front. Plant Sci. 2016, 341, 7. [Google Scholar] [CrossRef] [PubMed]
- Park, I.; Kim, W.J.; Yeo, S.-M.; Choi, G.; Kang, Y.-M.; Piao, R.; Moon, B.C. The complete chloroplast genome sequences of Fritillaria ussuriensis maxim. In addition, Fritillaria cirrhosa D. Don, and comparative analysis with other Fritillaria species. Molecules. 2017, 282, 22. [Google Scholar]
- Li, B.; Lin, F.; Huang, P.; Guo, W.; Zheng, Y. Complete chloroplast genome sequence of Decaisnea insignis: Genome organization, genomic resources and comparative analysis. Sci. Rep. 2017, 7, 10073. [Google Scholar] [CrossRef] [PubMed]
- Campbell, W.H.; Gowri, G. Codon usage in higher plants, green algae, and cyanobacteria. Plant Physiol. 1990, 92, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Dou, S.; Ji, Z.; Xue, Q. Synonymous codon usage and gene function are strongly related in Oryza sativa. Biosystems 2005, 80, 123–131. [Google Scholar] [CrossRef]
- Srivastava, D.; Shanker, A. Identification of simple sequence repeats in chloroplast genomes of Magnoliids through bioinformatics approach. Interdiscip. Sci. Comput. Life Sci. 2016, 8, 327–336. [Google Scholar] [CrossRef]
- Zhou, J.; Chen, X.; Cui, Y.; Sun, W.; Li, Y.; Wang, Y. Molecular structure and phylogenetic analyses of complete chloroplast genomes of two Aristolochia medicinal species. Int. J. Mol. Sci. 2017, 18, 1839. [Google Scholar] [CrossRef]
- Jiang, D.; Zhao, Z.; Zhang, T.; Zhong, W.; Liu, C.; Yuan, Q.; Huang, L. The chloroplast genome sequence of Scutellaria baicalensis provides insight into intraspecific and interspecific chloroplast genome diversity in Scutellaria. Gene 2017, 8, 227. [Google Scholar] [CrossRef]
- Zhou, J.; Cui, Y.; Chen, X.; Li, Y.; Xu, Z.; Duan, B.; Li, Y.; Song, J.; Yao, H. Complete chloroplast genomes of Papaver rhoeas and Papaver orientale: Molecular structures, comparative analysis, and phylogenetic analysis. Molecules 2018, 23, 437. [Google Scholar] [CrossRef]
- Mower, J.P. The PREP suite: Predictive RNA editors for plant mitochondrial genes, chloroplast genes and user-defined alignments. Nucleic Acids Res. 2009, 37, 253–259. [Google Scholar] [CrossRef]
- Bundschuh, R.; Altmuller, J.; Becker, C.; Nurnberg, P.; Gott, J.M. Complete characterization of the edited transcriptome of the mitochondrion of Physarum polycephalum using deep sequencing of RNA. Nucleic Acids Res. 2011, 39, 6044–6055. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.H.; Liao, S.C.; Chang, C.C. Identification of RNA editing sites in chloroplast transcripts of Phalaenopsis Aphrodite and comparative analysis with those of other seed plants. Plant Cell Physiol. 2007, 48, 362–368. [Google Scholar] [CrossRef][Green Version]
- Wang, W.; Yu, H.; Wang, J.; Lei, W.; Gao, J.; Qiu, X.; Wang, J. The complete chloroplast genome sequences of the medicinal plant Forsythia suspense (oleaceae). Int. J. Mol. Sci. 2017, 18, 2288. [Google Scholar] [CrossRef] [PubMed]
- Kumbhar, F.; Nie, X.; Xing, G.; Zhao, X.; Lin, Y.; Wang, S.; Weining, S. Identification and characterisation of RNA editing sites in chloroplast transcripts of einkorn wheat (Triticum monococcum). Ann. Appl. Biol. 2018, 172, 197–207. [Google Scholar] [CrossRef]
- Park, M.; Park, H.; Lee, H.; Lee, B.-H.; Lee, J. The complete plastome sequence of an antarctic Bryophyte Sanionia uncinata (Hedw.) loeske. Int. J. Mol. Sci. 2018, 19, 709. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.G.; Xu, W.Q.; Zou, W.T.; Jiang, D.Y.; Liu, X.H. Complete chloroplast genome sequences of two endangered Phoebe (Lauraceae) species. Bot. Stud. 2017, 58, 37. [Google Scholar] [CrossRef]
- Greiner, S.; Wang, X.; Rauwolf, U.; Silber, M.V.; Mayer, K.; Meurer, J.; Haberer, G.; Herrmann, R.G. The complete nucleotide sequences of the five genetically distinct plastid genomes of Oenothera, subsection Oenothera: I. sequence evaluation and plastome evolution. Nucleic Acids Res. 2008, 36, 2366–2378. [Google Scholar] [CrossRef]
- Song, Y.; Wang, S.; Ding, Y.; Xu, J.; Li, M.F.; Zhu, S.; Chen, N. Chloroplast Genomic Resource of Paris for Species Discrimination. Sci. Rep. 2017, 7, 3427. [Google Scholar] [CrossRef]
- Bryan, G.J.; McNicol, J.W.; Meyer, R.C.; Ramsay, G.; De Jong, W.S. Polymorphic simple sequence repeat markers in chloroplast genomes of Solanaceous plants. Theor. Appl. Genet. 1999, 99, 859–867. [Google Scholar] [CrossRef]
- Provan, J. Novel chloroplast microsatellites reveal cytoplasmic variation in Arabidopsis thaliana. Mol. Ecol. 2000, 9, 2183–2185. [Google Scholar] [CrossRef]
- Ebert, D.; Peakall, R. Chloroplast simple sequence repeats (cpSSRs): Technical resources and recommendations for expanding cpSSR discovery and applications to a wide array of plant species. Mol. Ecol. Resour. 2009, 9, 673–690. [Google Scholar] [CrossRef] [PubMed]
- Jacinta, N.M.; Xiang, D.; Jia-Xin, Y.; Elijah, M.M.; Vincent, O.W.; Millicent, A.O.; Josphat, K.S.; Paulm, M.M.; Guang-Wan, H. Complete chloroplast genome of Chlorophytum comosum and Chlorophytum gallabatense: Geome structures, comparative and phylogenetic analysis. Plants 2020, 9, 296. [Google Scholar]
- Dhafer, A.A.; Samaila, S.Y.; Enas, J.A.; Abidina, A. Complete chloroplast genome sequence of Barleria prionitis, Comparative chloroplast genomics and phylogenetic relationships among Acanthoideae. BMC Genom. 2020. [Google Scholar] [CrossRef]
- Philippe, H.; Delsuc, F.; Brinkmann, H.; Lartillot, N. Phylogenomics, Annual Review of Ecology. Evol. Syst. 2005, 36, 541–562. [Google Scholar] [CrossRef]
- Raubeson, L.A.; Peery, R.; Chumley, T.W.; Dziubek, C.; Fourcade, H.M.; Boorem, J.L.; Jansen, R.K. Comparative chloroplast genomics: Analyses including new sequences from the angiosperms Nupharadvena and Ranunculus macranthus. BMC Genom. 2007, 8, 174–201. [Google Scholar] [CrossRef] [PubMed]
- Borsch, T.; Quandt, D. Mutational dynamics and phylogenetic utility of noncoding chloroplast DNA. Plant Syst. Evol. 2009, 282, 169–199. [Google Scholar] [CrossRef]
- Dong, W.P.; Liu, J.; Yu, J.; Wang, L.; Zhou, S.L. Highly variable chloroplast markers for evaluating plant phylogeny at low taxonomic levels and for DNA barcoding. PLoS ONE 2012, 7, e35071. [Google Scholar] [CrossRef]
- Tong, W.; Kim, T.S.; Park, Y.J. Rice chloroplast genome variation architecture and phylogenetic dissection in diverse Oryza species assessed by whole-genome resequencing. Rice 2016, 9, 57. [Google Scholar] [CrossRef]
- Dong, W.-P.; Liu, H.; Xu, C.; Zuo, Y.; Chen, Z.; Zhou, S. A chloroplast genomic strategy for designing taxon specific DNA mini-barcodes: A case study on ginsengs. BMC Genet. 2014, 15, 138. [Google Scholar] [CrossRef]
- Du, Y.-P.; Bi, Y.; Yang, F.-P.; Zhang, M.-F.; Chen, X.-Q.; Xue, J.; Zhang, X.-H. Complete chloroplast genome sequences of Lilium: Insights into evolutionary dynamics and phylogenetic analyses. Sci. Rep. 2017, 7, 5751. [Google Scholar] [CrossRef]
- Judd, W.S.; Manchester, S.R. Circumscription of Malvaceae (Malvales) as determined by a preliminary cladistic analysis of morphological, anatomical, palynological, and chemical characters. Brittonia 1997, 49, 384–405. [Google Scholar] [CrossRef]
- Alverson, W.S.; Whitlock, B.A.; Nyffeler, R.; Bayer, C.; Baum, D.A. Phylogeny of the core Malvales: Evidence from ndhF sequence data. Am. J. Bot. 1999, 86, 1474–1486. [Google Scholar] [CrossRef] [PubMed]
- Bayer, C.; Fay, M.F.; Bruijn, A.Y.D.; Savolainen, V.; Morton, C.M.; Kubitzki, K.; Alverson, W.S.; Chase, M.W. Support for an expanded family concept of Malvaceae within a recircumscribed order Malvales: A combined analysis of plastid atpB and rbcL DNA sequences. Bot. J. Linn. Soc. 1999, 129, 267–303. [Google Scholar] [CrossRef]
- Jennifer, A.T.; Javier, F.A.; Steven, J.W.; John, C.L.; Tracey, A.B.D.; Beryl, B.S. Phylogenetic relationships within the tribe Malveae (Malvaceae, subfamily Malvoideae) as inferred from ITS sequence data. Am. J. Bot. 2005, 92, 602. [Google Scholar]
- La Duke, J.C.; Doebley, J. A chloroplast DNA based phylogeny of the Malvaceae. Syst. Bot. 1995, 20, 259–271. [Google Scholar] [CrossRef]
- Bayer, C.; Kubitzki, K. Malvaceae. In Flowering Plants, Dicotyledons: Malvales, Capparales, and Nonbetalain Caryophyllales; Kubitzki, K., Bayer, C., Eds.; Springer: Berlin, Germany, 2003; pp. 225–311. [Google Scholar]
- Bentham, G.; Hooker, J.D. Malvaceae. In Genera Plantarum; Bentham, G., Hooker, J.D., Eds.; Reeve & Co.: London, UK, 1862; pp. 195–213. [Google Scholar]
- Schumann, K. Malvaceae. In Die naturlichen Pflanzenfamilien; Wilhelm Engelmann: Leipzig, Germany, 1890; pp. 30–53. [Google Scholar]
- Hutchinson, J. The Genera of Flowering Plants; Clarendon Press: Oxford, UK, 1967. [Google Scholar]
- Schmieder, R.; Edwards, R. Quality control and preprocessingof metagenomic datasets. Bioinformatics 2011, 27, 863–864. [Google Scholar] [CrossRef]
- Dierckxsens, N.; Mardulyn, P.; Smits, G. NOVOPlasty: De novo assembly of organelle genomes from whole genome data. Nucleic Acids Res. 2016, 45, e18. [Google Scholar]
- Wyman, S.K.; Jansen, R.K.; Boore, J.L. Automatic annotation of organellar genomes with DOGMA. Bioinformatics 2004, 20, 3252–3255. [Google Scholar] [CrossRef]
- Schattner, P.; Brooks, A.N.; Lowe, T.M. The tRNA scan-SE, snoscan and snoGPS web servers for the detection of tRNAs and snoRNAs. Nucleic Acids Res. 2005, 33, 686–689. [Google Scholar] [CrossRef]
- Lohse, M.; Drechsel, O.; Bock, R. OrganellarGenomeDRAW (OGDRAW): A tool for the easy generation of high-quality custom graphical maps of plastid and mitochondrial genomes. Curr. Genet. 2007, 52, 267–274. [Google Scholar] [CrossRef]
- Thiel, T.; Michalek, W.; Varshney, R.; Graner, A. Exploiting EST databases for the development and characterization of gene-derived SSR-markers in barley (Hordeum vulgare L.). Theor. Appl. Genet. 2003, 106, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, S.; Choudhuri, J.V.; Ohlebusch, E.; Schleiermacher, C.; Stoye, J.; Giegerich, R. Reputer: The manifold applications of repeat analysis on a genomic scale. Nucleic Acids Res. 2001, 29, 4633–4642. [Google Scholar] [CrossRef] [PubMed]
- Mayor, C.; Brudno, M.; Schwartz, J.R.; Poliakov, A.; Rubin, E.M.; Frazer, K.A.; Pachter, L.S.; Dubchak, I. VISTA: Visualizing global DNA sequence alignments of arbitrary length. Bioinformatics 2000, 16, 1046–1047. [Google Scholar] [CrossRef] [PubMed]
- Frazer, K.A.; Pachter, L.; Poliakov, A.; Rubin, E.M.; Dubchak, I. VISTA: Computational tools for comparative genomics. Nucleic Acids Res. 2004, 32, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Librado, P.; Rozas, J. DnaSP v5: A software for comprehensive analysis of DNA polymorphism data. Bioinformatics 2009, 25, 1451–1452. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Fredrik, R.; Maxim, T.; Paul, V.M.; Daniel, L.A.; Aaron, D.; Sebastian, H.; Bret, L.; Liang, L.; Mar, A.S.; John, P.H. MrBayes 3.2: Efficient Bayesian Phylogenetic Inference and Model Choice Across a Large Model Space. Syst. Biol. 2012, 61, 539–542. [Google Scholar]
- Posada, D. jModelTest: Phylogenetic model averaging. Mol. Biol. Evol. 2008, 25, 1253–1259. [Google Scholar] [CrossRef]
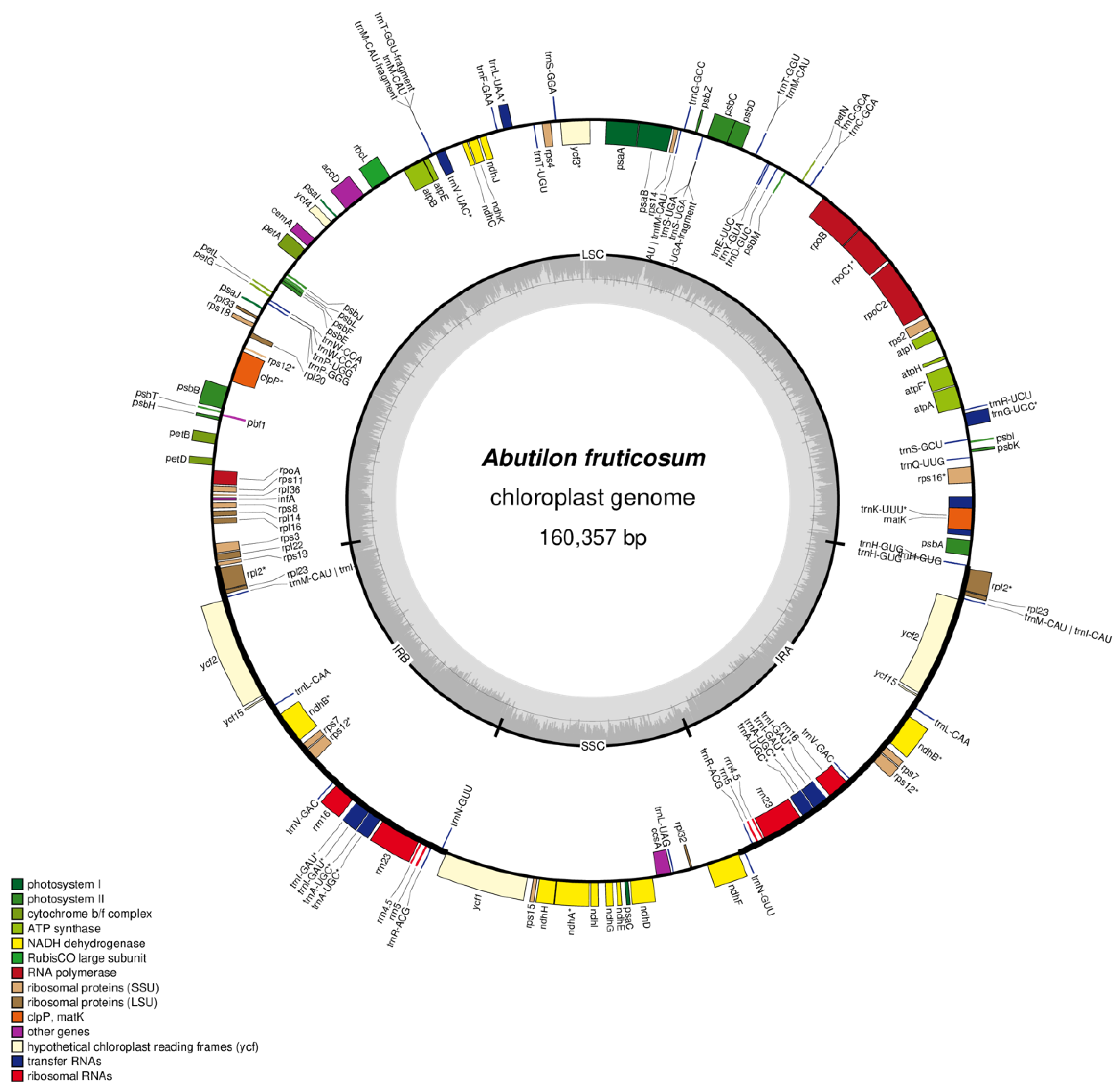
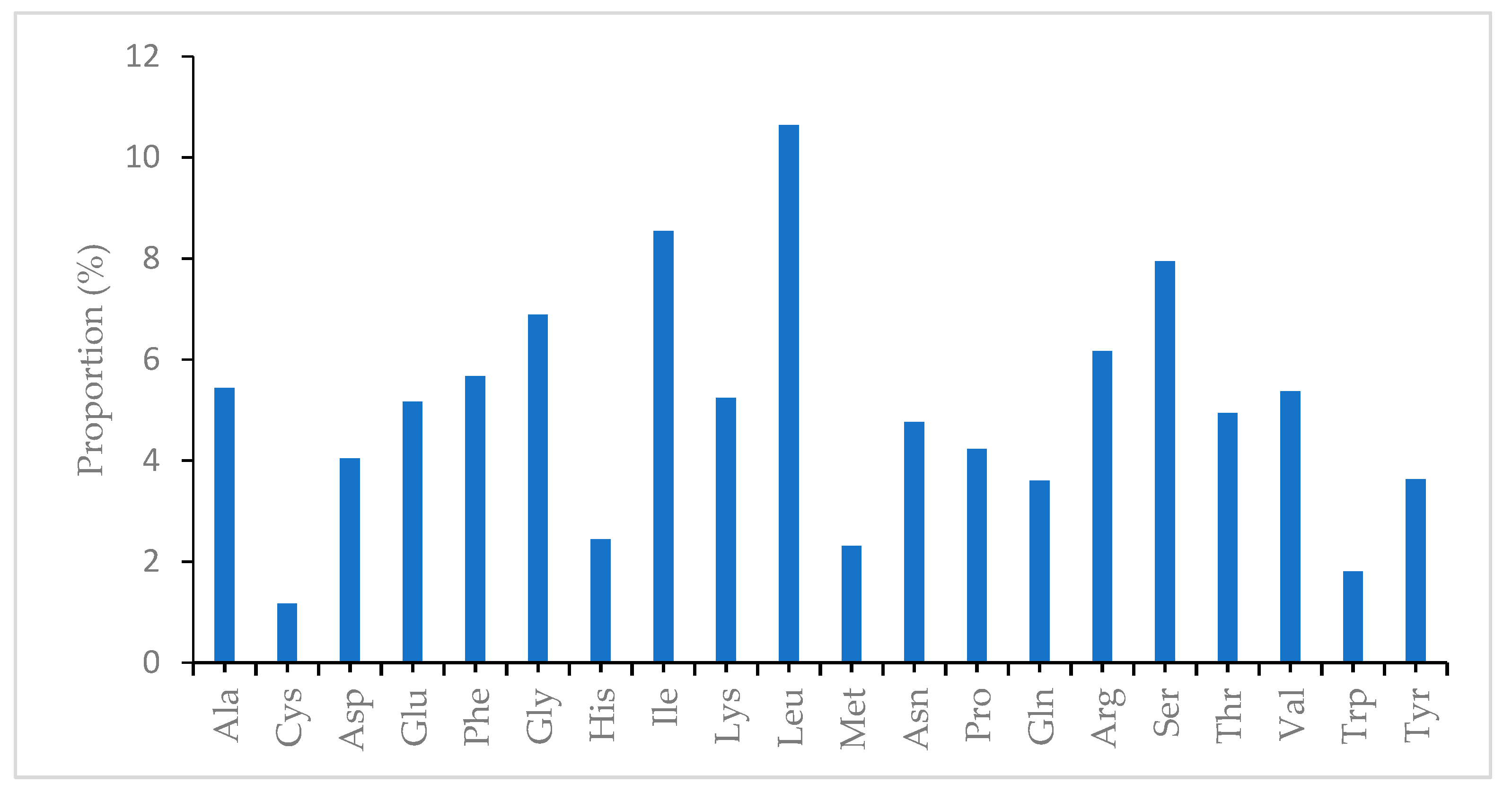

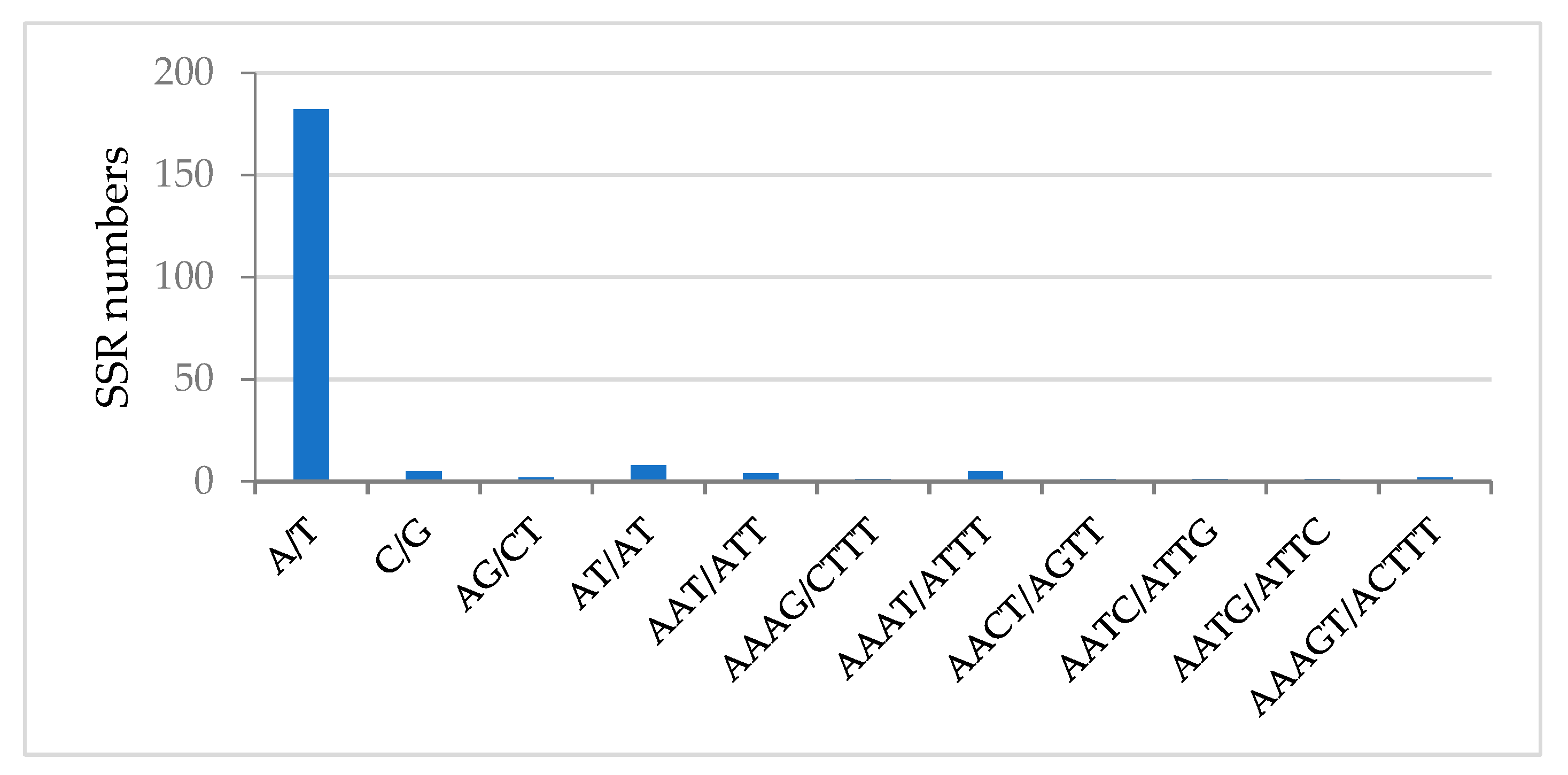
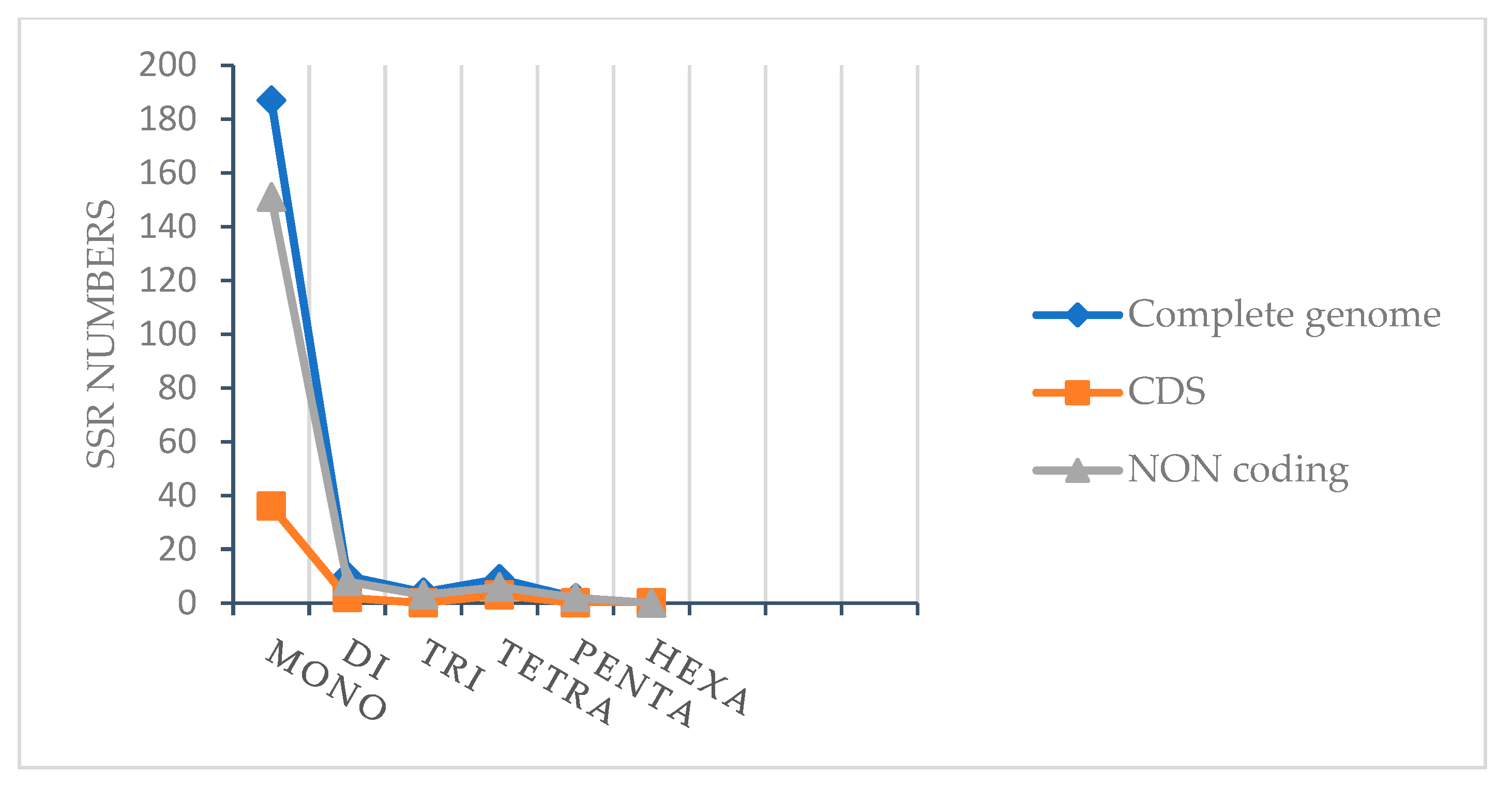

| Region | T(U) (%) | C (%) | A (%) | G (%) | Total (bp) | |
|---|---|---|---|---|---|---|
| cp genome | 31.13 | 18.26 | 31.9 | 18.69 | 160,357 | |
| LSC | 33.38 | 17.94 | 31.84 | 16.82 | 89,032 | |
| SSC | 33.88 | 15.03 | 34.57 | 16.94 | 20,031 | |
| IRA | 28.51 | 22.26 | 28.59 | 20.63 | 25,646 | |
| IRB | 28.58 | 20.61 | 28.53 | 22.26 | 25,646 | |
| 1st Position | 31.24 | 18.34 | 31.81 | 18.59 | 53,453 | |
| 2nd Position | 31.07 | 18.25 | 32.02 | 18.64 | 53,452 | |
| 3rd Position | 31.09 | 18.18 | 31.86 | 18.85 | 53,452 |
| Category | Group of Genes | Name of Genes |
|---|---|---|
| RNA genes | Ribosomal RNA genes (rRNA) | rrn5, rrn4.5, rrn16, rrn23 |
| Transfer RNA genes (tRNA) | trnH-GUG, trnK-UUU +, trnQ-UUG, trnS-GCU, trnG-UCC, trnR-UCU, trnC-GCA, trnD-GUC, trnY-GUA, trnE-UUC, trnM-CAU, trnT-GGU, trnS-UGA, trnG-GCC, trnfM-CAU, trnS-GGA, trnT-UGU, trnL-UAA +, trnF-GAA, trnV-UAC +, trnW-CCA, trnP-UGG, trnP-GGG, trnL-CAA a, trnV-GAC a, trnI-GAU +,a, trnA-UGC +,a, trnR-ACG a, trnN-GUU a, trnL-UAG. | |
| Ribosomal proteins | Small subunit of ribosome | rps2, rps3, rps4, rps7 a, rps8, rps11, rps12 a, rps14, rps15, rps16 +, rps18, rps19 |
| Transcriptiongenes | Large subunit of ribosome | rpl2 +,a, rpl14, rpl16, rpl20, rpl22, rpl23a, rpl32, rpl33, rpl36. |
| DNA-dependent RNA polymerase | rpoA, rpoB, rpoC1 +, rpoC2 | |
| Protein genes | Photosystem I | psaA, psaB, psaC, psaI, psaJ, ycf3 ++ |
| Photosystem II | psbA, psbB, psbC, psbD, psbE, psbF, psbH, psbI, psbJ, psbK, psbL, psbM, psbN, psbT, psbZ | |
| Subunit of cytochrome | petA, petB, petD, petG, petL, petN | |
| Subunit of synthase | atpA, atpB, atpE, atpF +, atpH, atpI | |
| Large subunit of rubisco | rbcL | |
| NADH dehydrogenase | ndhA +, ndhB +,a, ndhC, ndhD, ndhE, ndhF, ndhG, ndhH, ndhI, ndhJ, ndhK | |
| ATP-dependent protease subunit P | clpP ++ | |
| Chloroplast envelope membrane protein | cemA | |
| Other genes | Maturase | matK |
| Subunit acetyl-coA carboxylase | accD | |
| C-type cytochrome synthesis | ccsA | |
| Translational initiation factor | infA | |
| Hypothetical proteins | ycf2 a, ycf4, ycf15 a | |
| Component of TIC complex | ycf1 a |
| Gene | Location | Exon I (bp) | Intron I (bp) | Exon II (bp) | Intron II (bp) | Exon III (bp) |
|---|---|---|---|---|---|---|
| rps16 | LSC | 224 | 889 | 35 | ||
| atpF | LSC | 410 | 790 | 158 | ||
| rpoC1 | LSC | 1634 | 759 | 434 | ||
| ycf3 | LSC | 152 | 773 | 227 | 763 | 125 |
| ndhK | LSC | 716 | 7 | 155 | ||
| accD | LSC | 44 | 6 | 1487 | ||
| clpP | LSC | 227 | 640 | 293 | 914 | 70 |
| rpI2 | IR | 434 | 682 | 392 | ||
| ycf15 | IR | 131 | 24 | 125 | ||
| ndhB | IR | 755 | 684 | 776 | ||
| ndhA | SSC | 551 | 1124 | 539 | ||
| trnK-UUU | LSC | 34 | 2574 | 36 | ||
| trnG-UCC | LSC | 31 | 789 | 60 | ||
| trnL-UAA | LSC | 36 | 560 | 49 | ||
| trnV-UAC | LSC | 36 | 590 | 37 | ||
| trnI-GAU | IR | 41 | 958 | 34 | ||
| trnA-UGC | IR | 37 | 800 | 34 |
| Codon | Amino Acid | RSCU | tRNA | Codon | Amino Acid | RSCU | tRNA |
|---|---|---|---|---|---|---|---|
| UUU | Phe | 1.27 | trnF-GAA | UAU | Tyr | 1.57 | trnY-GUA |
| UUC | Phe | 0.73 | UAC | Tyr | 0.43 | ||
| UUA | Leu | 1.85 | trnL-UAA | UAA | Stop | 1.35 | |
| UUG | Leu | 1.24 | trnL-CAA | UAG | Stop | 1.06 | |
| CUU | Leu | 1.22 | trnL-UAG | CAU | His | 1.49 | trnH-GUG |
| CUC | Leu | 0.46 | CAC | His | 0.51 | ||
| CUA | Leu | 0.83 | CAA | Gln | 1.5 | trnQ-UUG | |
| CUG | Leu | 0.41 | CAG | Gln | 0.5 | ||
| AUU | Ile | 1.46 | trnI-GAU | AAU | Asn | 1.52 | trnN-GUU |
| AUC | Ile | 0.62 | AAC | Asn | 0.48 | ||
| AUA | Ile | 0.93 | trnI-CAU | AAA | Lys | 1.48 | trnK-UUU |
| AUG | Met | 1 | trnM-CAU | AAG | Lys | 0.52 | |
| GUU | Val | 1.45 | trnV-GAC | GAU | Asp | 1.61 | trnD-GUC |
| GUC | Val | 0.53 | GAC | Asp | 0.39 | ||
| GUA | Val | 1.46 | GAA | Glu | 1.45 | trnE-UUC | |
| GUG | Val | 0.56 | trnV-UAC | GAG | Glu | 0.55 | |
| UCU | Ser | 1.65 | trnS-GGA | UGU | Cys | 1.5 | trnC-GCA |
| UCC | Ser | 0.98 | UGC | Cys | 0.5 | ||
| UCA | Ser | 1.22 | UGA | Stop | 0.59 | ||
| UCG | Ser | 0.57 | trnS-UGA | UGG | Trp | 1 | trnW-CCA |
| CCU | Pro | 1.47 | trnP-UGG | CGU | Arg | 1.2 | trnR-ACG |
| CCC | Pro | 0.77 | CGC | Arg | 0.49 | trnR-UCU | |
| CCA | Pro | 1.15 | CGA | Arg | 1.35 | ||
| CCG | Pro | 0.62 | CGG | Arg | 0.52 | ||
| ACU | Thr | 1.57 | AGA | Arg | 1.71 | ||
| ACC | Thr | 0.78 | AGG | Arg | 0.73 | ||
| ACA | Thr | 1.16 | trnT-GGU | AGU | Ser | 1.14 | trnS-GCU |
| ACG | Thr | 0.49 | trnT-UGU | AGC | Ser | 0.43 | |
| GCU | Ala | 1.76 | trnA-UGC | GGU | Gly | 1.28 | |
| GCC | Ala | 0.7 | GGC | Gly | 0.46 | ||
| GCA | Ala | 1.03 | GGA | Gly | 1.51 | ||
| GCG | Ala | 0.52 | GGG | Gly | 0.75 | trnG-UCC |
| Gene | Nucleotide Position | Amino Acid Position | Codon | Amino Acid | Score |
|---|---|---|---|---|---|
| accD | 854 | 285 | TCG => TTG | S => L | 0.8 |
| 1463 | 488 | CCT => CTT | P => L | 1 | |
| atpA | 914 | 305 | TCA => TTA | S => L | 1 |
| 1148 | 383 | TCA => TTAA | S => L | 1 | |
| atpF | 92 | 31 | CCA => CTA | P => L | 0.86 |
| atpI | 629 | 210 | TCA => TTA | S => L | 1 |
| ccsA | 662 | 221 | ACT => ATT | T => I | 0.86 |
| clpP | 559 | 187 | CAT => TAT | H => Y | 1 |
| matK | 457 | 153 | CAT => TAT | H => Y | 1 |
| 634 | 212 | CAT => TAT | H => Y | 1 | |
| 1237 | 413 | CAC => TAC | H => Y | 1 | |
| ndhA | 341 | 114 | TCG => TTG | S => L | 1 |
| 566 | 189 | TCA => TTA | S => L | 1 | |
| ndhB | 149 | 50 | TCA => TTA | S => L | 1 |
| 467 | 156 | CCA => CTA | P => L | 1 | |
| 542 | 181 | ACG => ATG | T => M | 1 | |
| 586 | 196 | CAT => TAT | H => Y | 1 | |
| 611 | 204 | TCA => TTA | S => L | 0.8 | |
| 737 | 246 | CCA => CTA | P => L | 1 | |
| 746 | 249 | TCT => TTT | S => F | 1 | |
| 830 | 277 | TCAG => TTG | S => L | 1 | |
| 836 | 279 | TCA => TTA | S => L | 1 | |
| 1255 | 419 | CAT => TAT | H => Y | 1 | |
| 1291 | 431 | CTC => CTA | L => F | 1 | |
| 1481 | 494 | CCA => CTA | P => L | 1 | |
| ndhD | 2 | 1 | ACG => ATG | T => M | 1 |
| 26 | 9 | ACA => ATA | T => I | 1 | |
| 47 | 16 | TCT => TTT | S => F | 0.8 | |
| 383 | 128 | TCA => TTA | S => L | 1 | |
| 568 | 190 | CCT => TCT | P => S | 1 | |
| 674 | 225 | TCG => TTG | S => L | 1 | |
| 878 | 293 | TCA => TTA | S => L | 1 | |
| 1298 | 433 | TCA => TTA | S => L | 0.8 | |
| ndhF | 290 | 97 | TCA => TTA | S => L | 1 |
| 1549 | 517 | CTT => TTT | L => F | 1 | |
| 1826 | 609 | ACA => ATA | T => I | 0.8 | |
| 1892 | 631 | GCG => GTG | A => V | 0.8 | |
| ndhG | 166 | 56 | CAT => TAT | H => Y | 0.8 |
| 314 | 105 | ACA => ATA | T => I | 0.8 | |
| petB | 425 | 142 | CGG => TGG | R => W | 1 |
| psbF | 77 | 26 | TCT => TTT | S => F | 1 |
| rpl20 | 308 | 103 | TCA => TTA | S => L | 0.86 |
| rpoA | 329 | 110 | GCC => GTC | A => V | 0.86 |
| 830 | 277 | TCA => TTA | S => L | 1 | |
| rpoB | 338 | 113 | TCT => TTT | S => F | 1 |
| 551 | 184 | TCA => TTA | S => L | 1 | |
| 566 | 189 | TCG => TTG | S => L | 1 | |
| 2426 | 809 | TCA => TTA | S => L | 0.86 | |
| rpoC1 | 41 | 14 | TCA => TTA | S => L | 1 |
| 1273 | 425 | CCG => TCG | P => S | 0.86 | |
| rpoC2 | 2296 | 766 | CGG => TGG | R => W | 1 |
| 3188 | 1063 | CCC => CTC | P => L | 0.86 | |
| rps2 | 248 | 83 | TCG => TTG | S => L | 1 |
| 325 | 109 | CCC => TCC | P => S | 1 | |
| rps8 | 217 | 73 | CAT => TAT | H => Y | 1 |
| rps14 | 149 | 50 | TCA => TTA | S => L | 1 |
| SN | Repeat Size | Repeat Position 1 | Repeat Type | Repeat Location | Repeat Position 2 | Repeat Location 2 | E-Value |
|---|---|---|---|---|---|---|---|
| 1 | 58 | 33,156 | F | IGS | 33,213 | IGS | 8.71 × 10−26 |
| 2 | 50 | 0 | P | IGS | 89,084 | IGS | 5.71 × 10−21 |
| 3 | 36 | 44,187 | F | IGS | 44,205 | IGS | 1.53 × 10−12 |
| 4 | 36 | 103,838 | P | IGS | 123,909 | ndhA-Intron | 1.53 × 10−12 |
| 5 | 36 | 123,909 | F | ndhA-Intron | 145,617 | IGS | 1.53 × 10−12 |
| 6 | 30 | 64,915 | F | IGS | 64,930 | IGS | 6.27 × 10−9 |
| 7 | 29 | 8535 | P | trnS-GCU | 47,062 | IGS | 2.51 × 10−8 |
| 8 | 26 | 6039 | P | IGS | 6039 | IGS | 1.61 × 10−6 |
| 9 | 26 | 10,662 | P | IGS | 10,662 | IGS | 1.61 × 10−6 |
| 10 | 26 | 96,437 | F | ycf2 | 96,455 | ycf2 | 1.61 × 10−6 |
| 11 | 26 | 96,437 | P | ycf2 | 153,010 | ycf2 | 1.61 × 10−6 |
| 12 | 26 | 96,455 | P | ycf2 | 153,028 | ycf2 | 1.61 × 10−6 |
| 13 | 26 | 153,010 | F | ycf2 | 153,028 | ycf2 | 1.61 × 10−6 |
| 14 | 25 | 53,987 | P | IGS | 58,381 | IGS | 6.42 × 10−6 |
| 15 | 25 | 54,230 | R | IGS | 54,230 | IGS | 6.42 × 10−6 |
| 16 | 24 | 19,010 | F | rpoC2 | 19,034 | rpoC2 | 2.57 × 10−6 |
| 17 | 24 | 40,578 | F | psaB | 42,802 | psaA | 2.57 × 10−5 |
| 18 | 24 | 48,652 | F | IGS | 48,672 | IGS | 2.57 × 10−5 |
| 19 | 23 | 48,902 | R | IGS | 48,902 | IGS | 1.03 × 10−4 |
| 20 | 23 | 88,769 | F | rps19 | 88,792 | rps19 | 1.03 × 10−4 |
| 21 | 23 | 112,986 | F | IGS | 113,018 | IGS | 1.03 × 10−4 |
| 22 | 23 | 112,986 | P | IGS | 136,450 | IGS | 1.03 × 10−4 |
| 23 | 23 | 113,018 | P | IGS | 136,482 | IGS | 1.03 × 10−4 |
| 24 | 23 | 136,450 | F | IGS | 136,482 | IGS | 1.03 × 10−4 |
| 25 | 22 | 10,362 | P | IGS | 10,388 | IGS | 4.11 × 10−4 |
| 26 | 22 | 38,111 | P | IGS | 38,111 | IGS | 4.11 × 10−4 |
| 27 | 22 | 113,764 | F | IGS | 113,785 | IGS | 4.11 × 10−4 |
| 28 | 22 | 113,764 | P | IGS | 135,684 | IGS | 4.11 × 10−4 |
| 29 | 22 | 113,785 | P | IGS | 135,705 | IGS | 4.11 × 10−4 |
| 30 | 22 | 135,684 | F | IGS | 135,705 | IGS | 4.11 × 10−4 |
| 31 | 21 | 8540 | F | trnS-GCU | 37,036 | trnS-UGA | 1.64 × 10−3 |
| 32 | 21 | 9201 | F | IGS | 9220 | IGS | 1.64 × 10−3 |
| 33 | 21 | 10,136 | F | IGS | 10,157 | IGS | 1.64 × 10−3 |
| 34 | 21 | 10,491 | R | trnR-UCU | 10,491 | trnR-UCU | 1.64 × 10−3 |
| 35 | 21 | 37,036 | P | trnS-UGA | 47,065 | IGS | 1.64 × 10−3 |
| 36 | 21 | 43,822 | R | IGS | 43,822 | IGS | 1.64 × 10−3 |
| 37 | 21 | 78,782 | P | psbN | 78,810 | psbN | 1.64 × 10−3 |
| 38 | 21 | 96,428 | F | ycf2 | 96,482 | ycf2 | 1.64 × 10−3 |
| 39 | 21 | 96,428 | P | ycf2 | 152,988 | ycf2 | 1.64 × 10−3 |
| 40 | 21 | 96,482 | P | ycf2 | 153,042 | ycf2 | 1.64 × 10−3 |
| 41 | 21 | 152,988 | F | ycf2 | 153,042 | ycf2 | 1.64 × 10−3 |
| 42 | 20 | 402 | P | IGS | 402 | IGS | 6.58 × 10−3 |
| 43 | 20 | 5288 | F | IGS | 5307 | IGS | 6.58 × 10−3 |
| 44 | 20 | 10,128 | C | IGS | 82,502 | IGS | 6.58 × 10−3 |
| 45 | 20 | 14,151 | P | IGS | 54,042 | IGS | 6.58 × 10−3 |
| 46 | 20 | 50,997 | P | trnF-GAA | 55,404 | IGS | 6.58 × 10−3 |
| 47 | 20 | 53,309 | R | ndhC | 53,309 | ndhC | 6.58 × 10−3 |
| 48 | 20 | 55,405 | P | IGS | 108,890 | ycf2 | 6.58 × 10−3 |
| 49 | 20 | 55,405 | F | IGS | 140,581 | ycf2 | 6.58 × 10−3 |
| Repeat | Length (bp) | Number | Start Position |
|---|---|---|---|
| A | 8 | 40 | 4686; 5481; 5865; 6926; 7897; 12,271; 13,473; 13,702; 15,318; 19,663; 22,147; 23,354; 30,009; 30,421; 31,419; 49,433; 50,206; 51,576; 54,400; 69,630; 74,881; 76,238; 82,296; 84,962; 85,726; 115,568; 117,338; 118,072; 120,375; 120,736; 128,085; 130,563;131,446; 131,580; 135,564; 135,895; 140,843; 141,163; 145,401; 148,617 |
| 9 | 12 | 4151; 8835; 16,318; 23,595; 67,970; 80,923; 87,139; 94,543; 117,759; 119,376; 125,042; 125,285 | |
| 10 | 11 | 5166; 7193; 28,188; 37,725; 48,400; 74,710; 75,051; 76,083; 115,690; 128,759; 131,209 | |
| 11 | 5 | 52,345; 82,469; 116,213; 117,460; 118,643 | |
| 12 | 3 | 320; 2221; 65,189 | |
| 14 | 1 | 14,115 | |
| 15 | 1 | 145,189 | |
| C | 8 | 1 | 27,432 |
| 10 | 1 | 14,953 | |
| 11 | 1 | 30,215 | |
| T | 8 | 28 | 116; 4444; 9679; 28,812; 32,051; 36,848; 37,359; 58,437; 61,319; 63,814; 67,383; 67,842; 68,540; 71,227; 79,674; 81,661; 88,975; 100,748; 103,964; 108,202; 108,522; 113,470; 113,801; 115,834; 126,161; 127,095; 130,350; 131,860 |
| 9 | 13 | 2643; 6292; 13,241; 20,064; 31,032; 43,959; 51,110; 54,805; 71,700; 82,737; 87,273; 123,499; 154,821 | |
| 10 | 15 | 5982; 9102; 10,369; 10,642; 12,750; 14,567; 17,590; 30,779; 34,153; 49,053; 58,034; 64,821; 66,148; 81,282; 84,685 | |
| 11 | 6 | 19,520; 21,952; 29,839; 70,361; 75,959; 85,189 | |
| 12 | 4 | 27,254; 54,171; 57,834; 63,361 | |
| 15 | 1 | 104,169 | |
| 16 | 1 | 8658 | |
| AT | 5 | 3 | 20,902; 53,741; 63,067 |
| 6 | 1 | 28,039 | |
| TA | 5 | 2 | 10,115; 33,119 |
| TC | 5 | 1 | 65,402 |
| CT | 5 | 1 | 17,231 |
| AAT | 4 | 1 | 13,837 |
| TTA | 4 | 1 | 160,252 |
| AATA | 3 | 1 | 13,014 |
| AGAA | 3 | 1 | 115,404 |
| ATCA | 3 | 1 | 126,521 |
| GAAT | 3 | 1 | 118,455 |
| TAGT | 3 | 1 | 62,483 |
| TTTA | 3 | 1 | 72,098 |
| ACTTT | 3 | 1 | 139,519 |
| TAAAG | 3 | 1 | 109,838 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alzahrani, D.A. Complete Chloroplast Genome of Abutilon fruticosum: Genome Structure, Comparative and Phylogenetic Analysis. Plants 2021, 10, 270. https://doi.org/10.3390/plants10020270
Alzahrani DA. Complete Chloroplast Genome of Abutilon fruticosum: Genome Structure, Comparative and Phylogenetic Analysis. Plants. 2021; 10(2):270. https://doi.org/10.3390/plants10020270
Chicago/Turabian StyleAlzahrani, Dhafer A. 2021. "Complete Chloroplast Genome of Abutilon fruticosum: Genome Structure, Comparative and Phylogenetic Analysis" Plants 10, no. 2: 270. https://doi.org/10.3390/plants10020270
APA StyleAlzahrani, D. A. (2021). Complete Chloroplast Genome of Abutilon fruticosum: Genome Structure, Comparative and Phylogenetic Analysis. Plants, 10(2), 270. https://doi.org/10.3390/plants10020270





