Phytosanitary Interventions for Safe Global Germplasm Exchange and the Prevention of Transboundary Pest Spread: The Role of CGIAR Germplasm Health Units
Abstract
1. Introduction
1.1. International Germplasm Transfers for Food Security and Biodiversity Conservation
1.2. Pathogen and Pest Threats to International Germplasm Transfers
1.3. Transboundary Pest Risk to Germplasm Distribution and Premises for the Establishment of CGIAR Germplasm Health Programs
2. Historical Evolution of GHU and Its Core Functions
2.1. Development of Institutional Capacity for the Prevention of Transboundary Pest Spread through Germplasm
2.2. GHUs as CGIAR Gateway for Safe Germplasm Exchange
3. Procedures for Germplasm Health Testing and Safe International Transfers
3.1. Multistage Phytosanitary Controls for Pest Prevention
3.2. Criteria for Pest Monitoring
4. Germplasm Health Testing and Pest Elimination
4.1. True Seed Crops
4.1.1. Cereals
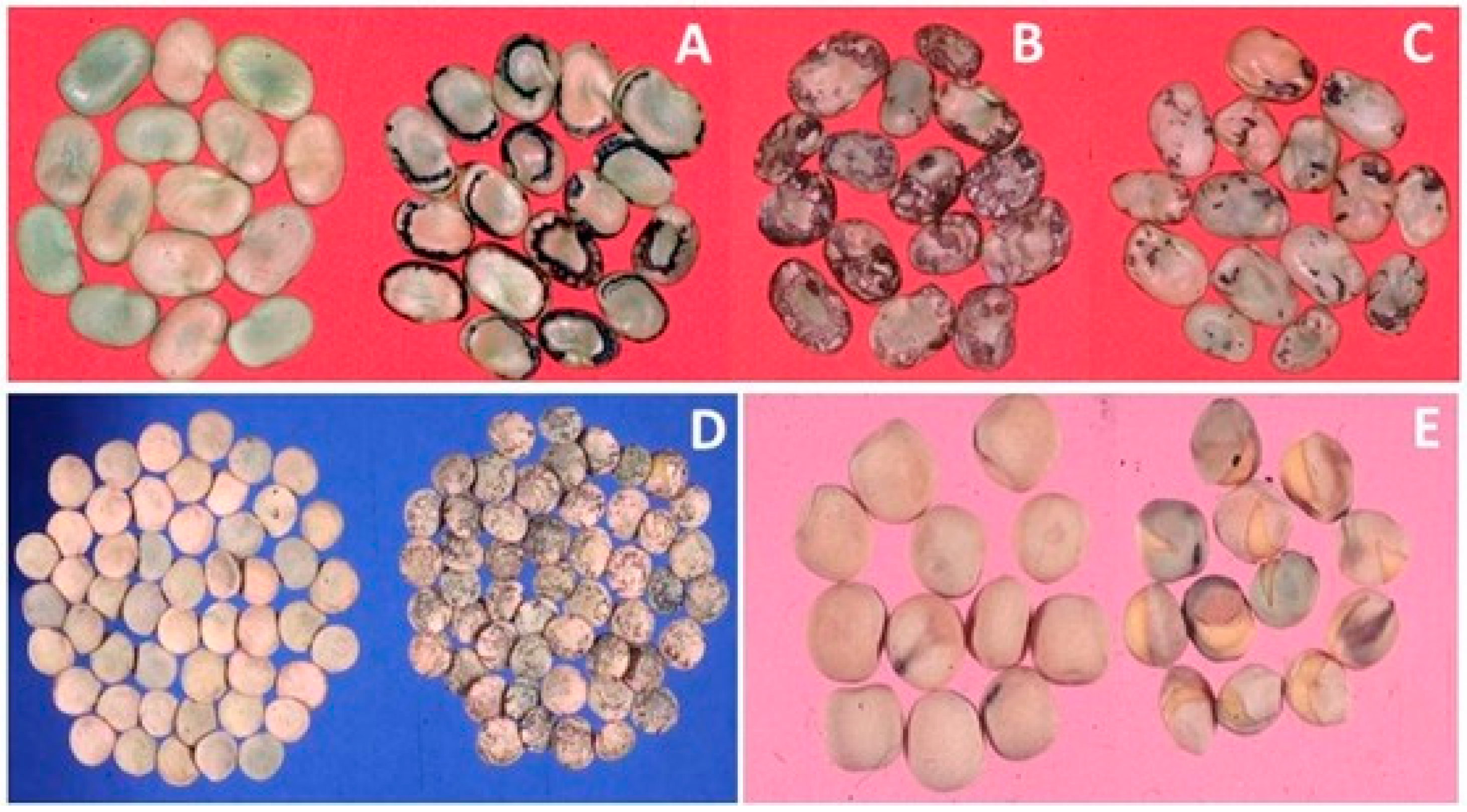
4.1.2. Grain and Oil Seed Legumes
4.2. Vegetatively Propagated Crops
4.3. Trees
4.4. Forages
5. GHU Support for CGIAR Programs
5.1. Enabling Safe Germplasm Transfers
5.2. Partnerships Enabling GHU Functions
5.3. GHUs in Capacity Development
6. Challenges and Opportunities
6.1. Evaluation and Reevaluation of Germplasm Health
6.2. Variable Standards and Different Phytosanitary Demands
6.3. Changes in Pest Dynamics
6.4. Keeping up with Evolving Technologies
6.5. Insufficient Phytosanitary Standards for Germplasm Transfers from Genebanks and Breeding Programs
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khoury, C.K.; Achicanoy, H.A.; Bjorkman, A.D.; Navarro-Racines, C.; Guarino, L.; Flores-Palacios, X.; Engels, J.M.M.; Wiersema, J.H.; Dempewolf, H.; Sotelo, S.; et al. Origins of food crops connect countries worldwide. Proc. R. Soc. B Boil. Sci. 2016, 283, 20160792. [Google Scholar] [CrossRef]
- Galluzz, G.; Halewood, M.; Noriega, I.L.; Vernooy, R. Twenty-five years of international exchanges of plant genetic re-sources facilitated by the CGIAR genebanks: A case study on global interdependence. Biodivers Conserv. 2016, 25, 1421–1446. [Google Scholar] [CrossRef]
- Noriega, I.L.; Halewood, M.; Abberton, M.; Amri, A.; Angarawai, I.I.; Anglin, N.; Blümmel, M.; Bouman, B.; Campos, H.; Costich, D.; et al. CGIAR Operations under the Plant Treaty Framework. Crop. Sci. 2019, 59, 819–832. [Google Scholar] [CrossRef]
- Alston, J.M.; Pardey, P.G.; Rao, X. The Payoff to Investing in CGIAR Research; SoAR Foundation: Arlington, VA, USA, October 2020; Available online: https://www.instepp.umn.edu/products/payoff-investing-cgiar-research (accessed on 15 November 2020).
- GBP Ann Rep. CGIAR Genebank Platform Annual Report 2019. 27p. Available online: https://www.genebanks.org/resources/annual-reports/ (accessed on 28 June 2020).
- Byerlee, D.; Dubin, H.J. Crop improvement in the CGIAR as a global success story of open access and international col-laboration. Int. J. Commons. 2010, 4, 453–480. [Google Scholar]
- Halewood, M.; Jamora, N.; Noriega, I.L.; Anglin, N.L.; Wenzl, P.; Payne, T.; Ndjiondjop, M.-N.; Guarino, L.; Kumar, P.L.; Yazbek, M.; et al. Germplasm Acquisition and Distribution by CGIAR Genebanks. Plants 2020, 9, 1296. [Google Scholar] [CrossRef] [PubMed]
- CGIAR. Report from CGIAR Consortium to the FAO Commission on Genetic Resources for Food and Agriculture—2018. CGRFA/WG-PGR-9/18/Inf.15. Available online: http://www.fao.org/3/CA0105EN/ca0105en.pdf (accessed on 24 May 2020).
- CtEH. Initiative on “Crops to End Hunger” Strategy and Options for CGIAR Support to Plant Breeding. Available online: https://storage.googleapis.com/cgiarorg/2018/11/SC7-B_Breeding-Initiative-1.pdf (accessed on 25 August 2020).
- Elmer, W.H. Seeds as vehicles for pathogen introduction. Biol. Invasions 2001, 3, 263–271. [Google Scholar] [CrossRef]
- Klinkowski, M. Catastrophic Plant Diseases. Annu. Rev. Phytopathol. 1970, 8, 37–60. [Google Scholar] [CrossRef]
- Miew, T.W.; Misra, K.K. (Eds.) Rice Seed Health Testing; IRRI: Los Banos, Philippines, 1994; 122p. [Google Scholar]
- Bebber, D.P.; Holmes, T.; Gurr, S.J. The global spread of crop pests and pathogens. Glob. Ecol. Biogeogr. 2014, 23, 1398–1407. [Google Scholar] [CrossRef]
- Simler, A.B.; Williamson, M.A.; Schwartz, M.W.; Rizzo, D.M. Amplifying plant disease risk through assisted migration. Conserv. Lett. 2019, 12, 12605. [Google Scholar] [CrossRef]
- Kumar, P.L.; Legg, J.P.; Ayodele, M.; Mahuku, G.; Ortega-Beltran, A.; Bandyopadhyay, R. Pest and disease surveillance, diagnostics and germplasm health in crop protection in sub-Saharan Africa. In Critical Issues in Plant Health: 50 Years of Research in African Agriculture; Neuenschwander, P., Tamò, M., Eds.; Burleigh Dodds Science Publishing Limited: Cambridge, UK, 2019; pp. 41–73. [Google Scholar]
- Mahuku, G.; Lockhart, B.E.; Wanjala, B.; Jones, M.W.; Kimunye, J.N.; Stewart, L.R.; Cassone, B.J.; Sevgan, S.; Nyasani, J.O.; Kusia, E.; et al. Maize Lethal Necrosis (MLN), an Emerging Threat to Maize-Based Food Security in Sub-Saharan Africa. Phytopathology 2015, 105, 956–965. [Google Scholar] [CrossRef] [PubMed]
- Goergen, G.; Kumar, P.L.; Sankung, S.B.; Togola, A.; Tamò, M. First Report of Outbreaks of the Fall Armyworm Spodoptera frugiperda (J E Smith) (Lepidoptera, Noctuidae), a New Alien Invasive Pest in West and Central Africa. PLoS ONE 2016, 11, e0165632. [Google Scholar] [CrossRef] [PubMed]
- Minato, N.; Sok, S.; Chen, S.; Delaquis, E.; Phirun, I.; Le, V.X.; Burra, D.D.; Newby, J.C.; Wyckhuys, K.A.G.; De Haan, S. Surveillance for Sri Lankan cassava mosaic virus (SLCMV) in Cambodia and Vietnam one year after its initial detection in a single plantation in 2015. PLoS ONE 2019, 14, e0212780. [Google Scholar] [CrossRef]
- Kumar, P.L.; Selvarajan, R.; Iskra-Caruana, M.-L.; Chabannes, M.; Hanna, R. Biology, Etiology, and Control of Virus Diseases of Banana and Plantain. Adv. Virus Res. 2015, 91, 229–269. [Google Scholar] [CrossRef] [PubMed]
- Maymon, M.; Sela, N.; Shpatz, U.; Galpaz, N.; Freeman, S. The origin and current situation of Fusarium oxysporum f. sp. cubense tropical race 4 in Israel and the Middle East. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Mburu, H.; Cortada, L.; Haukeland, S.; Ronno, W.; Nyongesa, M.; Kinyua, Z.; Bargul, J.L.; Coyne, D. Potato Cyst Nematodes: A New Threat to Potato Production in East Africa. Front. Plant Sci. 2020, 11, 670. [Google Scholar] [CrossRef]
- Islam, T.; Croll, D.; Gladieux, P.; Soanes, D.M.; Persoons, A.; Bhattacharjee, P.; Hossain, M.S.; Gupta, D.R.; Rahman, M.; Mahboob, M.G.; et al. Emergence of wheat blast in Bangladesh was caused by a South American lineage of Magnaporthe oryzae. BMC Biol. 2016, 14. [Google Scholar] [CrossRef] [PubMed]
- Tembo, B.; Mulenga, R.M.; Sichilima, S.; M’Siska, K.K.; Mwale, M.; Chikoti, P.C.; Singh, P.K.; He, X.; Pedley, K.F.; Peterson, G.L.; et al. Detection and characterization of fungus (Magnaporthe oryzae pathotype Triticum) causing wheat blast disease on rain-fed grown wheat (Triticum aestivum L.) in Zambia. PLoS ONE 2020, 15, e0238724. [Google Scholar] [CrossRef]
- Caicedo, J.D.; Simbaña, L.L.; Calderón, D.A.; Lalangui, K.P.; Rivera-Vargas, L.I. First report of ‘Candidatus Liberibacter solanacearum’ in Ecuador and in South America. Australas. Plant Dis. Notes 2020, 15, 6. [Google Scholar] [CrossRef]
- McCullough, D.G.; Work, T.T.; Cavey, J.F.; Liebhold, A.M.; Marshall, D. Interceptions of Nonindigenous Plant Pests at US Ports of Entry and Border Crossings Over a 17-year Period. Biol. Invasions 2006, 8, 611–630. [Google Scholar] [CrossRef]
- Chalam, C.; Parakh, D.B.; Maurya, A.K. Role of viral diagnostics in quarantine for plant genetic resources and prepared-ness. Indian J. Plant Genet. Resour. 2017, 30, 271–285. [Google Scholar] [CrossRef]
- Santini, A.; Liebhold, A.; Migliorini, D.; Woodward, S. Tracing the role of human civilization in the globalization of plant pathogens. ISME J. 2018, 12, 647–652. [Google Scholar] [CrossRef]
- Paini, D.R.; Shappard, A.W.; Cook, A.W.; De Barro, P.J.; Woomer, S.P.; Thomas, M.B. Global threat to agriculture from invasive species. Proc. Natl. Acad. Sci. USA 2016, 113, 7575–7579. [Google Scholar] [CrossRef]
- Plant Health—State of Research. In the State of the World Plants. 2017. Available online: https://stateoftheworldsplants.com/2017/plant-health.html (accessed on 18 November 2020).
- Early, R.; Bradley, B.A.; Dukes, J.S.; Lawler, J.L.; Olden, J.D.; Blumenthal, D.M.; Gonzalez, P.; Grosholz, E.D.; Ibañez, I.; Miller, L.P.; et al. Global threats from invasive alien species in the twenty-first century and national response capacities. Nat. Commun. 2016, 7, 12485. [Google Scholar] [CrossRef] [PubMed]
- Thormann, I.; Engels, J.M.M. Genetic Diversity and Erosion—A Global Perspective. In Genetic Diversity and Erosion in Plants. Sustainable Development and Biodiversity; Ahuja, M., Jain, S., Eds.; Springer: Cham, Switzerland, 2015; Volume 7. [Google Scholar]
- Stace-Smith, R. Role of plant breeders in disease dissemination of virus diseases. HortScience 1985, 20, 834–837. [Google Scholar]
- Khetarpal, R.K.; Singh, S.; Parakh, D.B.; Maurya, A.K.; Chalam, V.C. Viruses intercepted in exotic germplasm during 1991–2000 in quarantine. Indian J. Plant Genet. Resour. 2001, 14, 127–129. [Google Scholar]
- MacLeod, A.; Pautasso, M.; Jeger, M.J.; Haines-Young, R. Evolution of the international regulation of plant pests and challenges for future plant health. Food Secur. 2010, 2, 49–70. [Google Scholar] [CrossRef]
- FAO. Genebank Standards for Plant Genetic Resources for Food and Agriculture; FAO: Rome, Italy, 2013; 181p. [Google Scholar]
- Frison, E.A.; Putter, C.A.J. FAO/IBPGR Technical Guidelines for the Safe Movement of Germplasm. In Proceedings of the 5th International Congress of Plant Pathology, Kyoto, Japan, 20–27 August 1988; p. 279. [Google Scholar]
- Plucknett, D.L.; Smith, N.J.H. Plant Quarantine and the International Transfer of Germplasm; CGIAR Study Paper Number 25; The World Bank: Washington, DC, USA, 1988; 65p. [Google Scholar]
- Chiarappa, L. Plant protection at FAO-40 years of service international plant pest management. FAO Plant Prot. Bull. 1985, 33, 131–138. [Google Scholar]
- ITC. The International Transit Center (ITC). Available online: https://www.promusa.org/ITC (accessed on 23 November 2020).
- Hewitt, W.B.; Chiarappa, L. Plant Health and Quarantine in International Transfer of Genetic Resources; CRC Press: Boca Raton, FL, USA, 1977; 378p. [Google Scholar]
- Frison, E.A. Proceedings of the Inter-Centre Meeting on Germplasm Health and Movement; IBPGR: Rome, Italy, 1991. [Google Scholar]
- Mezzalama, M.; Das, B.; Prasanna, B.M. MLN Pathogen Diagnosis, MLN-Free Seed Production and Safe Exchange to Non-Endemic Countries; CIMMYT brochure: Mexico City, Mexico, 2015; 10p. [Google Scholar]
- IITA. Cassava In Vitro Processing and Gene Banking; IITA: Ibadan, Nigeria, 2009; 38p. [Google Scholar]
- Boonham, N.; Kreuze, J.; Winter, S.; van der Vlugt, R.; Bergervoet, J.; Tomlinson, J.; Mumford, R. Methods in virus diagnostics: From ELISA to next generation sequencing. Virus Res. 2014, 186, 20–31. [Google Scholar] [CrossRef]
- CABI. Crop Protection Compendium. 2020. Available online: https://www.cabi.org/cpc/ (accessed on 5 October 2020).
- FAO. Glossary of Phytosanitary Terms. In International Standard for Phytosanitary Measures No. 5; FAO on behalf of the Secretariat of the International Plant Protection Convention (IPPC): Rome, Italy, 2019; 35p. [Google Scholar]
- FAO. Guidelines on List of Regulated Pests. In International Standard for Phytosanitary Measures No. 19; FAO on behalf of the Secretariat of the International Plant Protection Convention (IPPC): Rome, Italy, 2005; 61p. [Google Scholar]
- FAO. Pest Risk Analysis for Quarantine Pests. In International Standard for Phytosanitary Measures No. 11; FAO on behalf of the Secretariat of the International Plant Protection Convention (IPPC): Rome, Italy, 2019; 40p. [Google Scholar]
- Picard, C.; Afonso, T.; Benko-Beloglavec, A.; Karadjova, O.; Matthews-Berry, S.; Paunovic, S.A.; Pietsch, M.; Reed, P.; Van Der Gaag, D.J.; Ward, M. Recommended regulated non-quarantine pests (RNQP s), associated thresholds and risk management measures in the European and Mediterranean region. EPPO Bull. 2018, 48, 552–568. [Google Scholar] [CrossRef]
- Rao, K.N.; Hanson, J.; Dulloo, E.M.; Ghosh, K.; Nowell, D.; Larinde, M. Handbooks for Gene Banks No. 8 Manual of Seed Handling in Genebanks; Biodiversity International: Rome, Italy, 2006; pp. 50–82. [Google Scholar]
- Aveling, T.A.S. Global standards in seed health testing. In Global Perspectives on the Health of Seeds and Plant Propagation Material; Gullino, M.L., Munkvold, G., Eds.; Springer: London, UK, 2014; pp. 17–28. [Google Scholar]
- Albrechtsen, S.E. Testing Methods for Seed-Transmitted Viruses: Principles and Protocols; CABI Publication: Wallingford, UK, 2006; 268p. [Google Scholar]
- International Seeds Testing Association (ISTA). International Rules for Seed Testing; Secretariat, Zürichstrasse 50; CH-8303 Bassersdorf: Luzern, Switzerland, 2009. [Google Scholar]
- Thakur, R.P.; Gunjotikar, G.A.; Rao, V.P. Safe Movement of ICRISAT’s Seed Crops Germplasm; Information Bulletin no. 81; Patancheru 502 324; International Crops Research Institute for the Semi-Arid Tropics: Andhra Pradesh, India, 2010; 244p, ISBN 978-92-9066-524-3. [Google Scholar]
- Panis, B.; Nagel, M.; Houwe, I.V.D. Challenges and Prospects for the Conservation of Crop Genetic Resources in Field Genebanks, in In Vitro Collections and/or in Liquid Nitrogen. Plants 2020, 9, 1634. [Google Scholar] [CrossRef]
- De Clerck, C.; Crew, K.; Houwe, I.V.D.; McMichael, L.; Berhal, C.; Lassois, L.; Jijakli, M.H.; Roux, N.; Thomas, J.E.; Sebastien, M. Lessons learned from the virus indexing of Musa germplasm: Insights from a multiyear collaboration. Ann. Appl. Biol. 2017, 171, 15–27. [Google Scholar] [CrossRef]
- Legg, J.P.; Iita, T.; Kumar, P.L.; Mahuku, G.; Wosula, E.; Stavolone, L.; Terry, E.; Bosque-Pérez, N.; Iita, N.; Lab, U.N.M. Viruses Affecting African Crops and Their Vectors; Burleigh Dodds Science Publishing: Cambridge, UK, 2019; pp. 95–135. [Google Scholar]
- Thomas, J.E.; Sharman, M.; Lassois, L.; Declercq, C.; Iskara-Caruana, M.-L. Technical Guidelines for the Safe Exchange of Musa germplasm; Bioversity International: Montpellier, France, 2015; 65p. [Google Scholar]
- Legg, J.P.; Kumar, P.L.; Makeshkumar, T.; Tripathi, L.; Ferguson, M.; Kanju, E.; Ntawuruhunga, P.; Cuellar, W. Cassava virus diseases: Biology, epidemiology, and management. Adv. Virus Res. 2015, 91, 85–142. [Google Scholar] [PubMed]
- Carvajal-Yepes, M.; Olaya, C.; Lozano, I.; Cuervo, M.; Castaño, M.; Cuellar, W.J. Unraveling complex viral infections in cassava (Manihot esculenta Crantz) from Colombia. Virus Res. 2014, 186, 76–86. [Google Scholar] [CrossRef]
- Kreuze, J.; Souza-Dias, J.A.C.; Jeevalatha, A.; Figueira, A.R.; Valkonen, J.; Jones, R.A.C. Viral Diseases in Potato. In The Potato Crop; Springer: Cham, Switzerland, 2019; pp. 389–430. [Google Scholar]
- Loebenstein, G.; Fuentes, S.; Cohen, J.; Salazar, L.F. Sweetpotato. In Virus and Virus-Like Diseases of Major Crops in Developing Countries; Loebenstein, G., Thottappilly, G., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2003; pp. 223–248. [Google Scholar]
- Kreuze, J.; Fuentes, S. Sweetpotato viruses. In Encyclopedia of Virology, 3rd ed.; Mahy, B.W.J., Van Regenmortel, M.H.V., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; pp. 659–669. [Google Scholar]
- Mahalakshmi, V.; Ng, Q.; Atalobhor, J.; Ogunsola, D.; Lawson, M.; Ortiz, R. Development of a West African yam Dioscorea spp. core collection. Genet. Resour. Crop. Evol. 2007, 54, 1817–1825. [Google Scholar] [CrossRef]
- Mignouna, B.D.; Kumar, P.L.; Coyne, D.; Bandyopadhyay, R.; Ortega-Beltran, A.; Bhattacharjee, R.; De Koeyer, D. Identifying and Managing Plant Health Risks for Key African Crops: Yam, Taro and Cocoyam; Burleigh Dodds Science Publishing Limited: Cambridgeshire, UK, 2019; pp. 213–228. [Google Scholar]
- Balogun, M.; Maroya, N.; Augusto, J.; Ajayi, A.; Kumar, L.; Aighewi, B.; Asiedu, R. Relative efficiency of positive selection and tissue culture for generating pathogen-free planting materials of yam (Dioscorea spp.). Czech J. Genet. Plant Breed. 2017, 53, 9–16. [Google Scholar] [CrossRef]
- Loo, J.; Souvannavong, O.; Dawson, I.K. Seeing the trees as well as the forest: The importance of managing forest genetic resources. For. Ecol. Manag. 2014, 333, 1–8. [Google Scholar] [CrossRef]
- Graziosi, I.; Tembo, M.; Kuate, J.; Muchugi, A. Pests and diseases of trees in Africa: A growing continental emergency. PLANTS PEOPLE Planet 2019, 2, 14–28. [Google Scholar] [CrossRef]
- Cherotich, S.; Njuguna, J.; Muchugi, A.; Mutamia, J.; Otaye, D.; Graziosi, I.; Kinyanjui, Z. Botryosphaeriaceae associated with baobab (Adansonia digitata L.) and marula (Sclerocarya birrea A. Rich.) in agroforestry systems in Kenya. Afr. J. Plant Sci. 2020, 14, 411–419. [Google Scholar]
- Germplasm Health Units. Available online: https://www.genebanks.org/the-platform/germplasm-health/ (accessed on 25 November 2020).
- Montuori, M. Recognising the importance of plant health in today’s world. South Afr. J. Sci. 2020, 116, 11–12. [Google Scholar] [CrossRef]
- Eschen, R.; O’Hanlon, R.; Santini, A.; Vannini, A.; Roques, A.; Kirichenko, N.; Kenis, M. Safeguarding global plant health: The rise of sentinels. J. Pest Sci. 2018, 92, 29–36. [Google Scholar] [CrossRef]
- Kreuze, J.F.; Perez, A.; Untiveros, M.; Quispe, D.; Fuentes, S.; Barker, I.; Simon, R. Complete viral genome sequence and dis-covery of novel viruses by deep sequencing of small RNAs: A generic method for diagnosis, discovery and sequencing of viruses. Virology 2009, 388, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Massart, S.; Candresse, T.; Gil, J.; Lacomme, C.; Predajna, L.; Ravnikar, M.; Reynard, J.-S.; Rumbou, A.; Saldarelli, P.; Škorić, D.; et al. A Framework for the Evaluation of Biosecurity, Commercial, Regulatory, and Scientific Impacts of Plant Viruses and Viroids Identified by NGS Technologies. Front. Microbiol. 2017, 8, 45. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Zhu, S.; Liu, F.; He, Y.; Bao, Y.; Zhang, A.C. Hyperspectral imaging for seed quality and safety inspection: A review. Plant Methods 2019, 15, 1–25. [Google Scholar] [CrossRef]
- FAO. Integrated Measures for Plants for Planting. In International Standard for Phytosanitary Measures No. 36; FAO on behalf of the Secretariat of the International Plant Protection Convention (IPPC): Rome, Italy, 2019; 22p. [Google Scholar]
- FAO. International Movement of Seeds. In International Standard for Phytosanitary Measures No. 38; FAO on behalf of the Secretariat of the International Plant Protection Convention (IPPC): Rome, Italy, 2019; 22p. [Google Scholar]
- GreenPass Puts Germplasm Distribution in the Fast Lane. Available online: https://www.genebanks.org/news-activities/news/greenpass/ (accessed on 19 November 2020).
- Saving the World. Nat. Plants 2017, 3, 17069. [CrossRef] [PubMed][Green Version]
- Pingali, P.L. Green Revolution: Impacts, limits, and the path ahead. Proc. Natl. Acad. Sci. USA 2012, 109, 12302–12308. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, M.P.; Braun, H.J.; Cavalieri, A.J.; Chapotin, S.; Davies, W.J.; Ellul, P.; Feuillet, C.; Govaerts, B.; Kropff, M.J.; Lucas, H.; et al. Improving global integration of crop research. Science 2017, 357, 359–360. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Nopsa, J.F.H.; Andersen, K.F.; Andrade-Piedra, J.L.; Beed, F.D.; Blomme, G.; Carvajal-Yepes, M.; Coyne, D.L.; Cuellar, W.J.; Forbes, G.A.; et al. Global Cropland Connectivity: A Risk Factor for Invasion and Saturation by Emerging Pathogens and Pests. Bioscience 2020, 70, 744–758. [Google Scholar] [CrossRef] [PubMed]
- Anderson, P.K.; Cunningham, A.A.; Patel, N.G.; Morales, F.J.; Epstein, P.R.; Daszak, P. Emerging infectious diseases of plants: Pathogen pollution, climate change and agrotechnology drivers. Trends Ecol. Evol. 2004, 19, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Turbelin, A.J.; Malamud, B.D.; Francis, R.A. Mapping the global state of invasive alien species: Patterns of invasion and policy responses. Glob. Ecol. Biogeogr. 2016, 26, 78–92. [Google Scholar] [CrossRef]
- OneCGIAR. Available online: https://www.cgiar.org/food-security-impact/one-cgiar/ (accessed on 25 November 2020).




| Disease | Pest | Hosts | First Detection and Spread in SSA | Spread Pathway |
|---|---|---|---|---|
| Asian soybean rust | Phakopsora pachyrhizi (fungus) | Soybean, >150 legume species | First detected in Zambia in the 1980s; spread to most of sub-Saharan Africa | Possibly spread through air-borne urediniospores, which blew from Western India to East Africa |
| Banana bunchy top | Banana bunchy top virus (virus) | Banana/plantain | First reported in Egypt in 1902; and then in the Democratic Republic of Congo; spread to most of Central Africa, and adjoining countries in Southern and Western sub-Saharan Africa | Introduced from South-Pacific or Asia through infected suckers; further spread through exchange of infected planting material and aphid (Pentalonia nigronervosa) vectors |
| Banana bacterial wilt | Xanthomonas campestris pv. Musacearum (bacterium) | Banana/plantain | Burundi, Rwanda, Democratic Republic of Congo, Uganda, Kenya, Tanzania | Possibly introduced from Ethiopia to Uganda and then across the Great Lakes region through planting material, infected plant parts, or contaminated tools |
| Cassava green mite | Monoychellus tanajoa (Insect) | Cassava | First detected in Uganda in 1971; presently widespread in Africa | Introduced from South America into Africa; path of spread not known |
| Cassava mealybug | Phenacoccus manihoti (Insect) | Cassava | First detected in the Democratic Republic of Congo in 1973; presently widespread in Africa | Possibly introduced from South America to Africa through plant parts, or containers |
| Cassava brown streak | Cassava brown streak ipomoviruses (Virus) | Cassava | Native species in Malawi, Mozambique, and Tanzania in Southern Africa; first recorded in East Africa in 2004 in an epidemic around the Great Lakes region Burundi, Comoros, DRC, Kenya, Rwanda, Uganda, and Zambia | Spread through exchange of infected stem cuttings and whitefly (Bemisia tabaci) vectors |
| Fall armyworm | Spodoptera frugiperda (Insect) | Maize and other crops | First detected in Nigeria in 2016; known to occur in all African countries | Path of spread not known; likely through plants, plant parts, or cargo containers. |
| Fruit fly | Bactrocera dorsalis
(Insect) | Mango and other crops | First reported in Mauritius in 1996; and on the mainland in Kenya in 2003; presently widespread in Africa and offshore islands | Pathway of spread not known; likely through plants, plant parts, or cargo containers |
| Maize lethal necrosis | Maize chlorotic mottle virus | Maize, sorghum, pearl millet | First detected in Kenya in 2011; then in Burundi, DRC, Ethiopia, Rwanda, Tanzania, and Uganda | Possibly spread from Southeast Asia |
| Panama disease—tropical race 4 | Fusarium oxysporum f. sp. Cubense Tropical Race 4 (Fungus) | Banana/plantain | First detected in 2010 in Mozambique; no reports of further spread in Africa | Introduced from South East Asia; possibly introduction through contaminated soil or planting material |
| Papaya mealybug | Paracoccus marginatus (Insect) | Papaya and several hosts | First detected in Ghana in 2009; spread to most African countries | Possibly spread from Southeast Asia /South Pacific islands through plants, plant parts, or other sources |
| Potato cyst nematode | Globodera pallida (Nematode) | Potato | First detected in Algeria in 2011; subsequently in Kenya in 2018; and then in neighboring countries in East Africa | Possibly spread from European region with seed potato |
| Taro blight | Phytophthora colocasiae (Oomycete) | Taro | First reported in Nigeria in 2011; Spread to most countries in sub-Saharan Africa | Possibly spread from South Pacific islands through infected corms |
| Tomato leaf miner | Tuta absoluta (Insect) | Tomato and other solanaceous crops | First reported from Benin in 2005; spread to most countries in Africa | Wind-borne spread of insects from the northern region |
| Wheat blast | Magnaporthe oryzae pathotype Triticum (Fungus) | Wheat | First reported in Zambia in 2020 | Possibly spread through contaminated seed/grain |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, P.L.; Cuervo, M.; Kreuze, J.F.; Muller, G.; Kulkarni, G.; Kumari, S.G.; Massart, S.; Mezzalama, M.; Alakonya, A.; Muchugi, A.; et al. Phytosanitary Interventions for Safe Global Germplasm Exchange and the Prevention of Transboundary Pest Spread: The Role of CGIAR Germplasm Health Units. Plants 2021, 10, 328. https://doi.org/10.3390/plants10020328
Kumar PL, Cuervo M, Kreuze JF, Muller G, Kulkarni G, Kumari SG, Massart S, Mezzalama M, Alakonya A, Muchugi A, et al. Phytosanitary Interventions for Safe Global Germplasm Exchange and the Prevention of Transboundary Pest Spread: The Role of CGIAR Germplasm Health Units. Plants. 2021; 10(2):328. https://doi.org/10.3390/plants10020328
Chicago/Turabian StyleKumar, P. Lava, Maritza Cuervo, J. F. Kreuze, Giovanna Muller, Gururaj Kulkarni, Safaa G. Kumari, Sebastien Massart, Monica Mezzalama, Amos Alakonya, Alice Muchugi, and et al. 2021. "Phytosanitary Interventions for Safe Global Germplasm Exchange and the Prevention of Transboundary Pest Spread: The Role of CGIAR Germplasm Health Units" Plants 10, no. 2: 328. https://doi.org/10.3390/plants10020328
APA StyleKumar, P. L., Cuervo, M., Kreuze, J. F., Muller, G., Kulkarni, G., Kumari, S. G., Massart, S., Mezzalama, M., Alakonya, A., Muchugi, A., Graziosi, I., Ndjiondjop, M.-N., Sharma, R., & Negawo, A. T. (2021). Phytosanitary Interventions for Safe Global Germplasm Exchange and the Prevention of Transboundary Pest Spread: The Role of CGIAR Germplasm Health Units. Plants, 10(2), 328. https://doi.org/10.3390/plants10020328






