Elicitors Modulate Young Sandalwood (Santalum album L.) Growth, Heartwood Formation, and Concrete Oil Synthesis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Growth Conditions and Selection of Materials
2.2. Treatment with Exogenous Chemical Elicitors
2.3. Anatomical Observations
2.4. Plant Height, Stem Diameter, Xylem Width, DBH, and Heartwood Extension Measurements
2.5. Concrete Oil Extraction from Heartwood
2.6. GC-MS Analysis
2.7. Statistical Analysis and Sample Replicates
3. Results
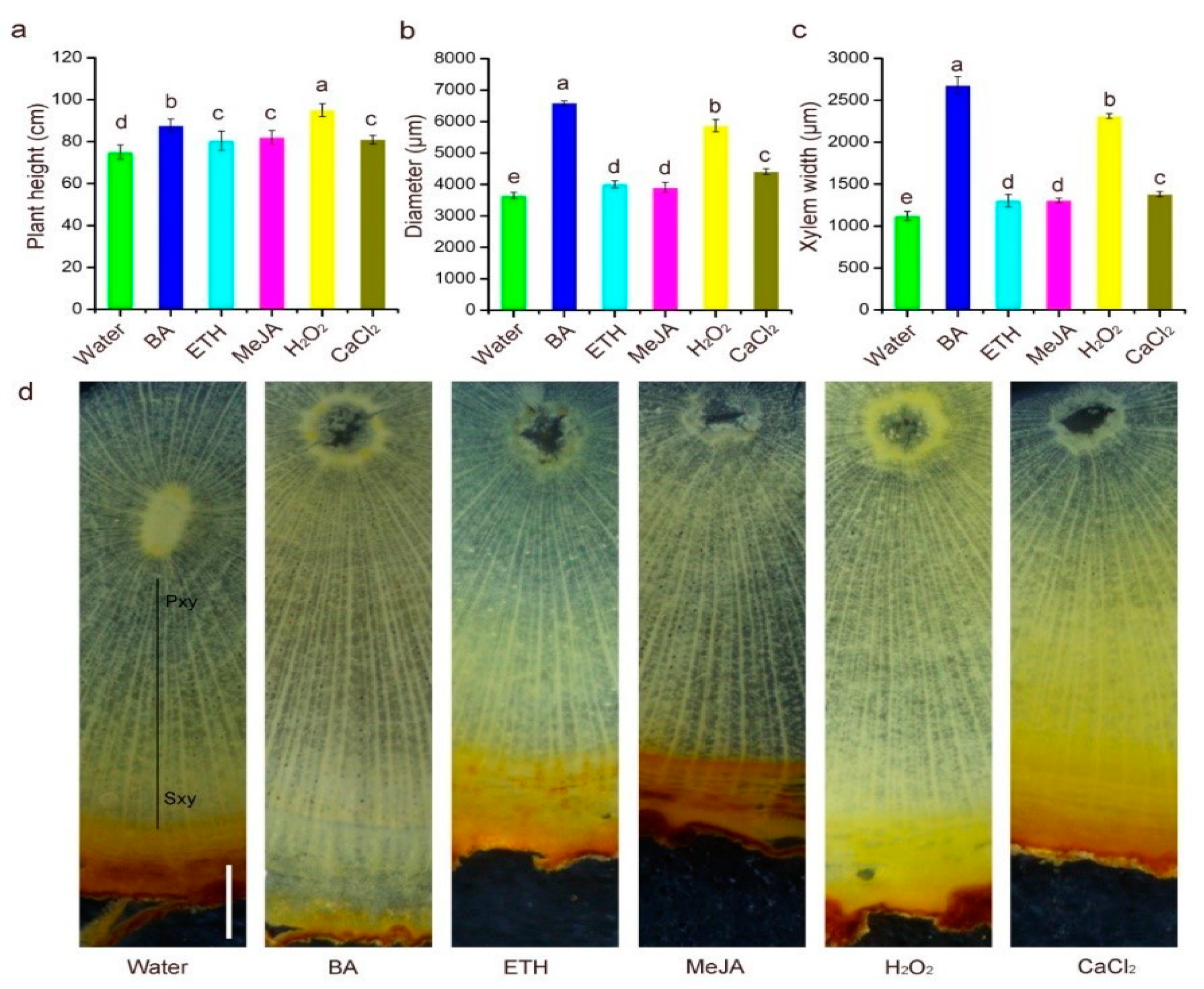
3.1. Effects of the Elicitors on Stem Cambium Cell Activity in 1-Year-Old Trees
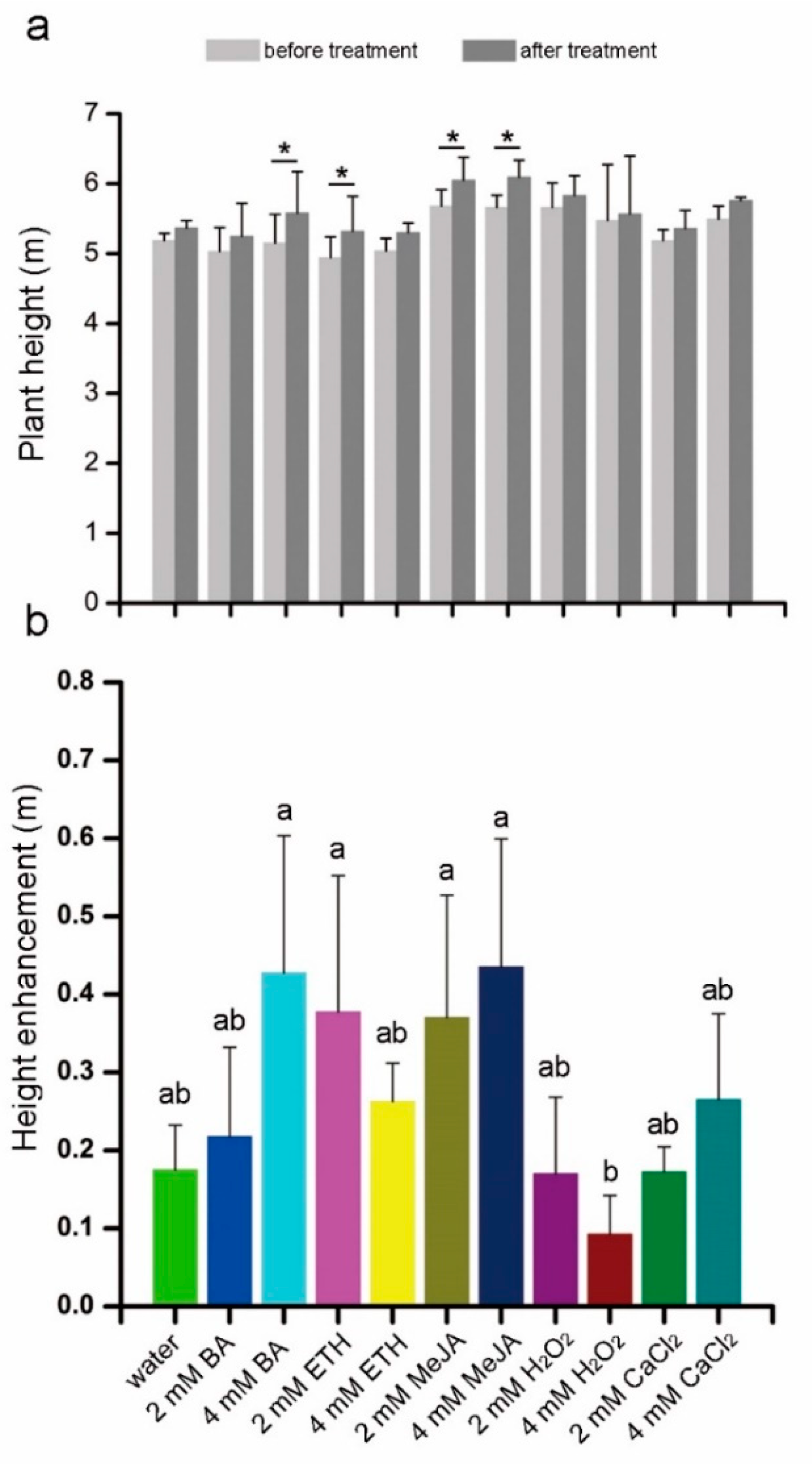
3.2. Effects of the Elicitors on Morphology, Height and Stem Diameter Changes of 5-Year-Old Sandalwood Trees
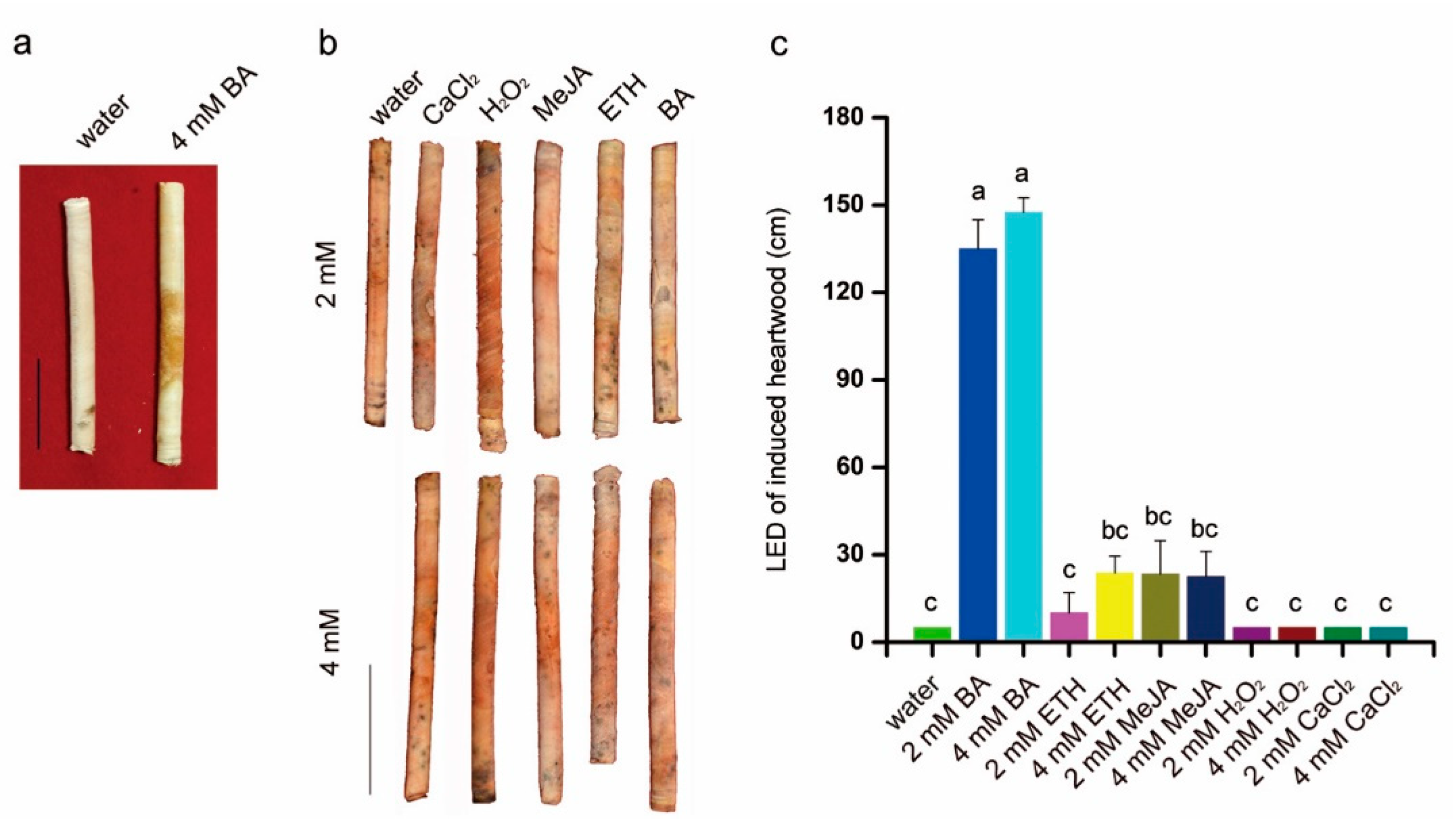
3.3. Chemical Elicitors Induced Heartwood Formation
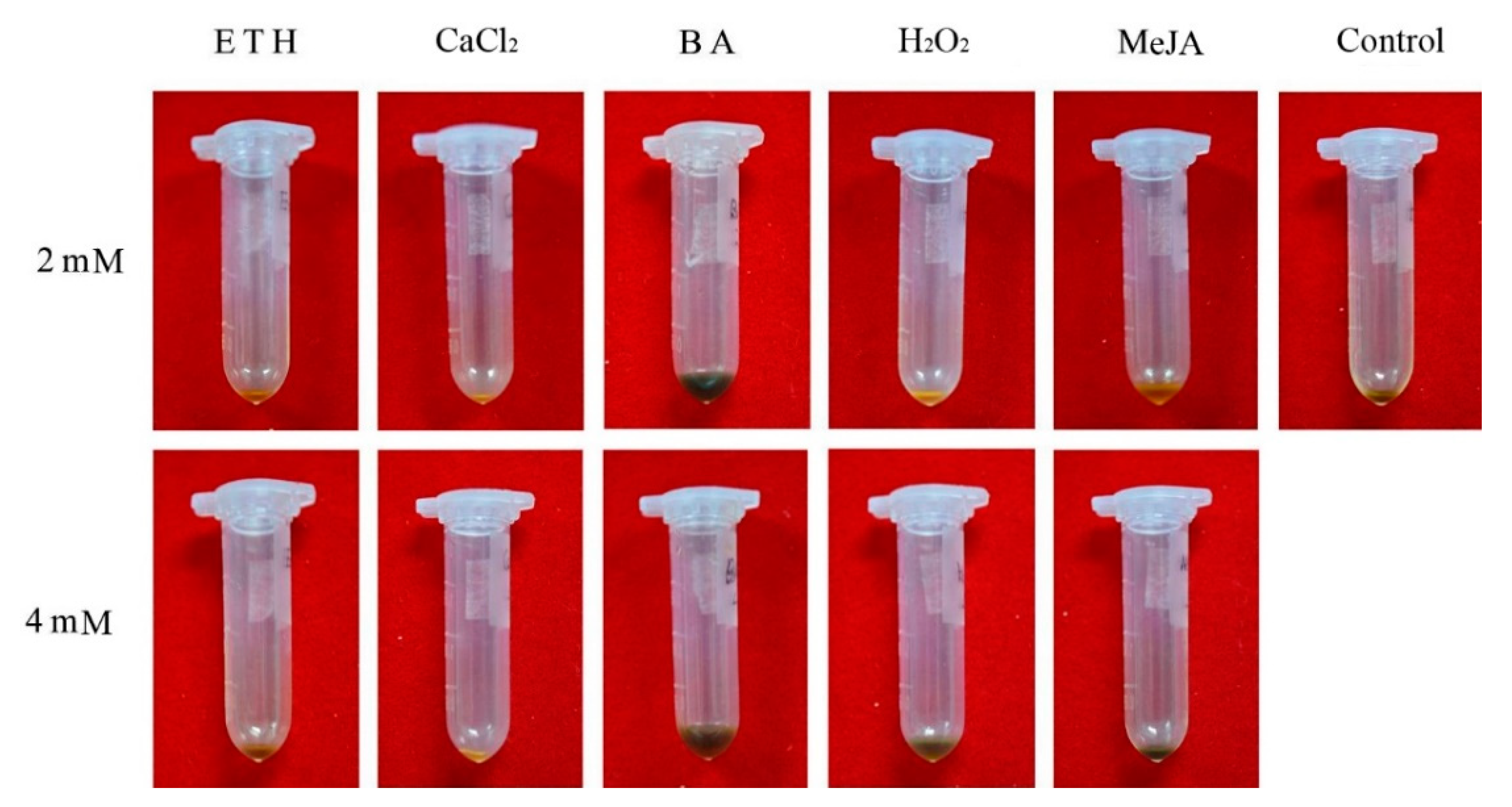
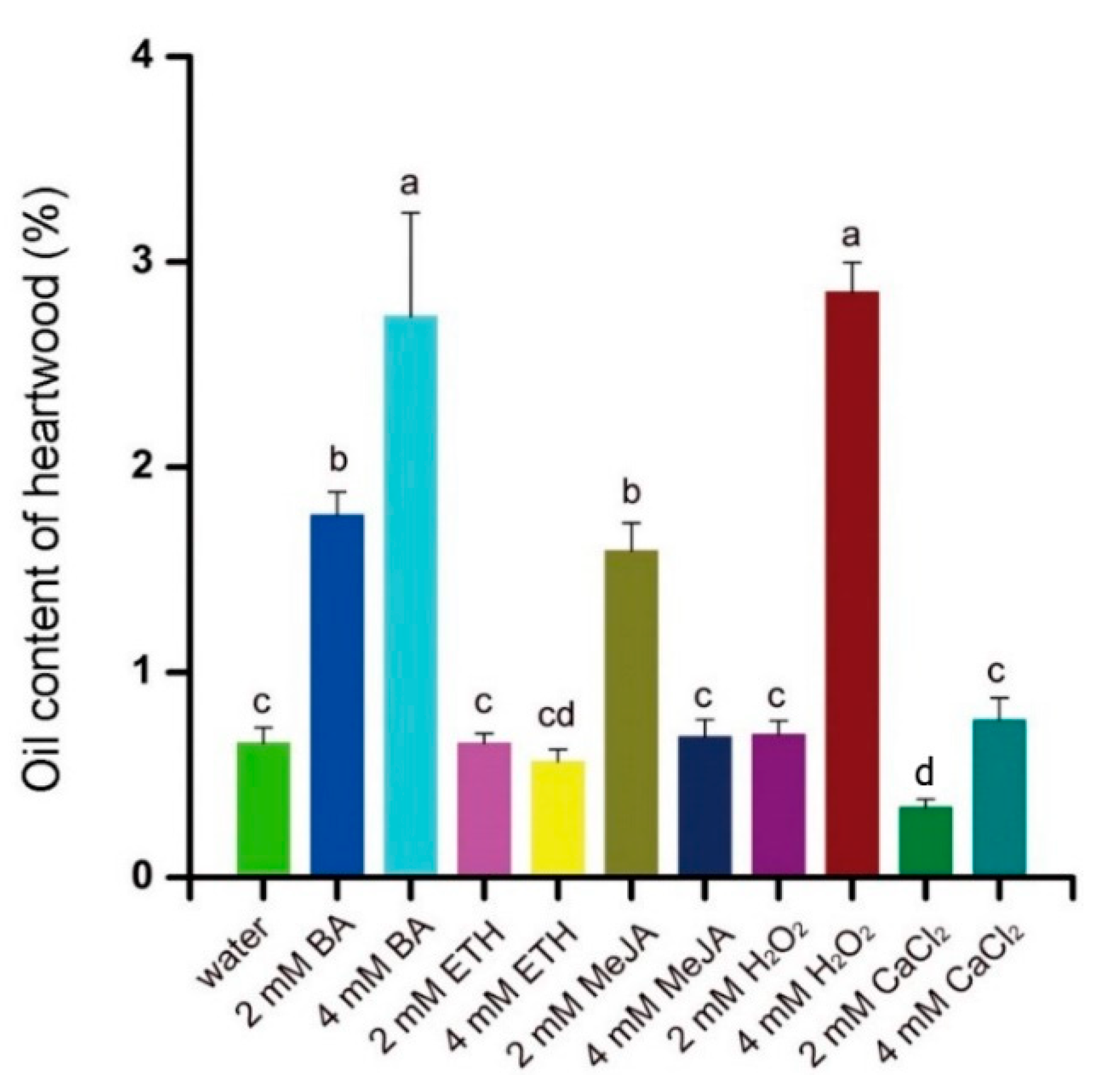
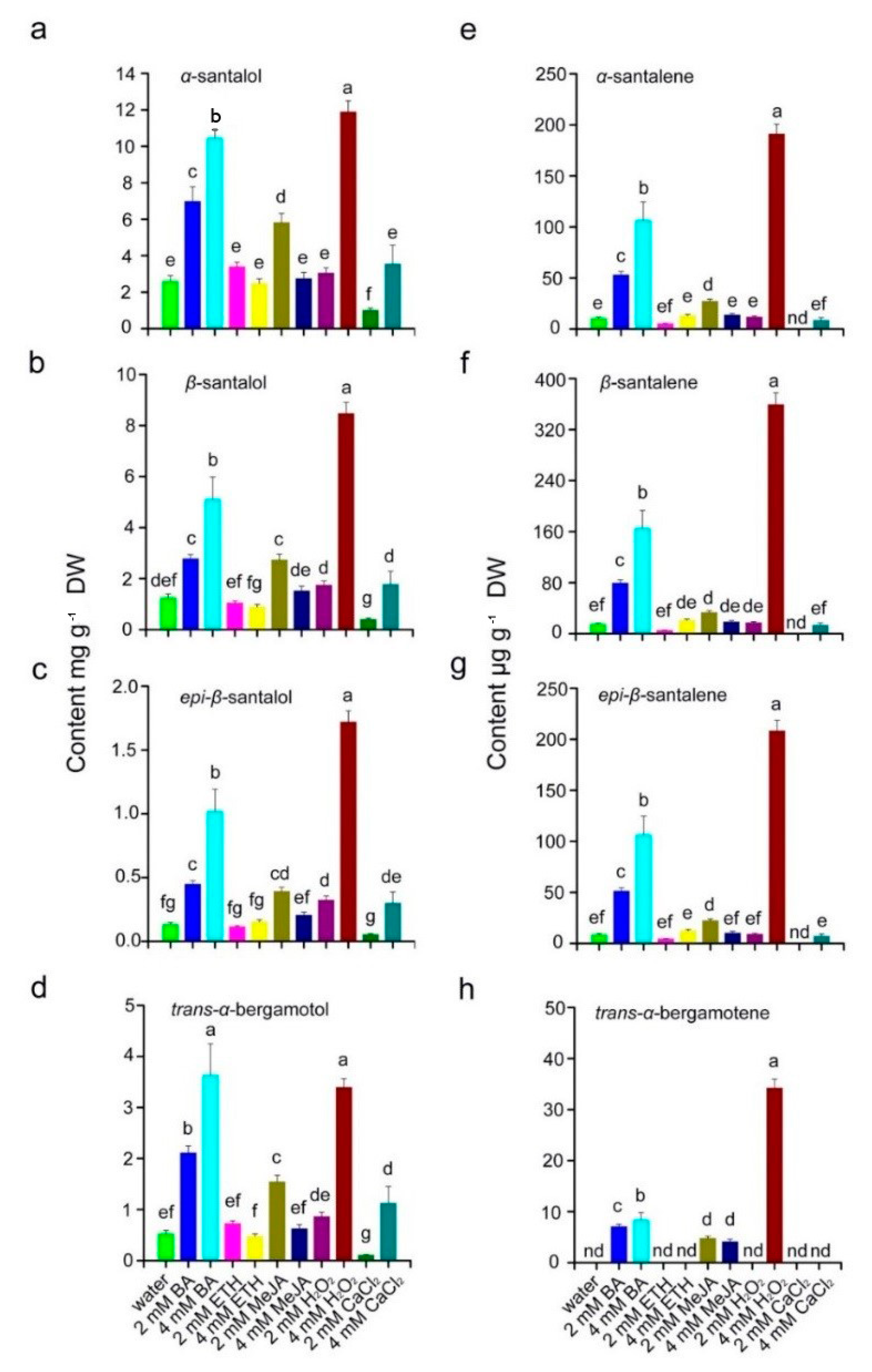
3.4. H2O2, BA and MeJA Affect Concrete Oil Content and Its Constituents
4. Discussion
4.1. Stem Cambium Cell Activity and Heartwood Formation: Response to Chemical Elicitors
4.2. Response of Plant Height and Diameter to Chemical Elicitors
4.3. Essential Oil Synthesis in Response to BA and H2O2
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Teixeira da Silva, J.A.; Kher, M.M.; Soner, D.; Ma, G.H. Sandalwood: Basic biology, tissue culture, and genetic transformation. Planta 2016, 243, 847–887. [Google Scholar] [CrossRef]
- Akhtar, R.; Shahzad, A. Morphology and ontogeny of directly differentiating shoot buds and somatic embryos in Santalum album L. J. For. Res. 2019, 30, 1179–1189. [Google Scholar] [CrossRef]
- Diaz-Chavez, M.L.; Moniodis, J.; Madilao, L.L. Biosynthesis of sandalwood oil: Santalum album CYP76F cytochromes P450 produce santalols and bergamotol. PLoS ONE 2013, 8, e75053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rai, S.N. Status and cultivation of sandalwood in India. In Proceedings of the Symposium on Sandalwood in the Pacific, Honolulu, HI, USA, 9–11 April 1990; Volume 122, pp. 66–71. [Google Scholar]
- Gillieson, D.; Page, T.; Silverman, J. An Inventory of Wild Sandalwood Stocks in Vanuatu; Australian Government: Canberra, ACT, Australia, 2008.
- Moniodis, J.; Renton, M.; Jones, C.G. Genetic and environmental parameters show associations with essential oil composition in West Australian sandalwood (Santalum spicatum). Aust. J. Bot. 2018, 66, 48–58. [Google Scholar] [CrossRef]
- Magel, E.; Jay-Allemand, C.; Ziegler, H. Formation of heartwood substances in the stemwood of Robinia pseudoacacia L. II. Distribution of nonstructural carbohydrates and wood extractives across the trunk. Trees 1994, 8, 165–171. [Google Scholar] [CrossRef]
- Magel, E.; Abdel-Latif, A.; Hampp, R. Non-structural carbohydrates and catalytic activities of sucrose metabolizing enzymes in trunks of two Juglans species and their role in heartwood formation. Holzforschung 2001, 55, 135–145. [Google Scholar] [CrossRef]
- Mayer, I.; Koch, G.; Puls, J. Topochemical investigations of wood extractives and their influence on colour changes in American black cherry (Prunus serotina Borkh.). Holzforschung 2006, 60, 589–594. [Google Scholar] [CrossRef]
- Kampe, A.; Magel, E. New insights into heartwood and heartwood formation. In Cellular Aspects of Wood Formation; Springer: Berlin/Heidelberg, Germany, 2013; pp. 71–95. [Google Scholar]
- Celedon, J.M.; Chiang, A.Y.; Macaire, M.S.; Diaz-Chavez, M.L.; Madilao, L.L.; Finnegan, P.M.; Barbour, E.L.; Bohlmann, J. Heartwood-specific transcriptome and metabolite signatures of tropical sandalwood (Santalum album) reveal the final step of (z)-santalol fragrance biosynthesis. Plant J. 2016, 86, 289–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Celedon, J.M.; Bohlmann, J. An extended model of heartwood secondary metabolism informed by functional genomics. Tree Physiol. 2017, 38, 311–319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tuteja, N.; Sopory, S.K. Chemical signaling under abiotic stress environment in plants. Plant Signal. Behav. 2008, 3, 525–536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dixon, R.A.; Paiva, N.L. Stress-induced phenylpropanoid metabolism. Plant Cell 1995, 7, 1085–1097. [Google Scholar] [CrossRef]
- Kadambi, K. Inducing heartwood formation in the Indian sandalwood tree, Santalum album Linn. Indian For. 1954, 80, 659–662. [Google Scholar]
- Li, Y.L. Study on Introduction of Sandalwood; Science Press: Beijing, China, 2003. [Google Scholar]
- Liu, X.J.; Xu, D.P.; Yang, Z.J.; Zhang, N.N. Effects of plant growth regulators on growth, heartwood formation and oil composition of young Santalum album. Sci. Silvae Sin. 2013, 49, 143–149. [Google Scholar]
- Liu, X.J.; Xu, D.P.; Yang, Z.J.; Zhang, N.N.; Pan, L.J. Investigation of exogenous benzyladenine on growth, biochemical composition, photosynthesis and antioxidant activity of Indian sandalwood (Santalum album L.) seedlings. J. Plant Growth Regul. 2018, 37, 1148–1158. [Google Scholar] [CrossRef]
- Radomiljac, A.M. The influence of pot host species, seedling age and supplementary nursery nutrition on Santalum album Linn. (Indian sandalwood) plantation establishment within the Ord River Irrigation Area, Western Australia. For. Ecol. Manag. 1998, 102, 193–201. [Google Scholar] [CrossRef]
- Ouyang, Y.; Zhang, X.H.; Chen, Y.L.; Teixeira da Silva, J.A.; Ma, G.H. Growth, photosynthesis and haustorial development of semiparasitic Santalum album L. penetrating into roots of three hosts: A comparative study. Trees 2016, 30, 317–328. [Google Scholar] [CrossRef]
- Külheim, C.; Jones, C.G.; Plummer, J.A. Foliar application of methyl jasmonate does not increase terpenoid accumulation, but weakly elicits terpenoid pathway genes in sandalwood (Santalum album L.) seedlings. Plant Biotechnol. 2014, 31, 585–591. [Google Scholar]
- Cheng, Q.W.; Xiong, Y.P.; Niu, M.Y.; Zhang, Y.Y.; Yan, H.F.; Liang, H.Z.; Guo, B.Y.; Zhang, X.H.; Teixeira da Silva, J.A.; Xiong, Y.H.; et al. Callus of East Indian sandalwood co-cultured with fungus Colletotrichum gloeosporioides accumulates santalenes and bisabolene. Trees 2019, 33, 305–312. [Google Scholar] [CrossRef]
- Ye, Z.H.; Zhong, R. Molecular control of wood formation in trees. J. Exp. Bot. 2015, 66, 4119–4131. [Google Scholar] [CrossRef] [Green Version]
- Nieminen, K.; Immanen, J.; Laxell, M. Cytokinin signaling regulates cambial development in poplar. Proc. Natl. Acad. Sci. USA 2008, 105, 20032–20037. [Google Scholar] [CrossRef] [Green Version]
- Hieno, A.; Naznin, H.A.; Inaba-Hasegawa, K. Transcriptome analysis and identification of a transcriptional regulatory network in the response to H2O2. Plant Physiol. 2009, 180, 1629–1646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanderauwera, S.; Zimmermann, P.; Rombauts, S. Genome-wide analysis of hydrogen peroxide-regulated gene expression in Arabidopsis reveals a high light-induced transcriptional cluster involved in anthocyanin biosynthesis. Plant Physiol. 2005, 139, 806–821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Černý, M.; Habánová, H.; Berka, M. Hydrogen peroxide: Its role in plant biology and crosstalk with signalling networks. Int. J. Mol. Sci. 2018, 19, 2812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neves, C.; Sá, M.C.; Amâncio, S. Histochemical detection of H2O2 by tissue printing as a precocious marker of rhizogenesis in grapevine. Plant Physiol. Biochem. 1998, 36, 817–824. [Google Scholar] [CrossRef]
- Potikha, T.S.; Collins, C.C.; Johnson, D.I. The involvement of hydrogen peroxide in the differentiation of secondary walls in cotton fibers. Plant Physiol. 1999, 119, 849–858. [Google Scholar] [CrossRef] [Green Version]
- Nair, M.N. B Wood anatomy and heartwood formation in neem (Azadirachta indica A. Juss.). Bot. J. Linn. Soc. 1988, 97, 79–90. [Google Scholar] [CrossRef]
- Magel, E.A.; Drouet, A.; Claudot, A.C.; Ziegler, H. Formation of heartwood substances in the stem of Robinia pseudoacacia L. Trees 1991, 5, 203–207. [Google Scholar] [CrossRef]
- Hillinger, C.; Höll, W.; Ziegler, H. Lipids and lipolytic enzymes in the trunk wood of Robinia pseudoacacia L. during heartwood formation. Trees 1996, 10, 366–375. [Google Scholar] [CrossRef]
- Taylor, A.M.; Gartner, B.L.; Morrell, J.J. Heartwood formation and natural durability. Wood Fiber Sci. 2002, 34, 587–611. [Google Scholar]
- Misra, B.B.; Dey, S. Immunolocalization of α-santalol in sandalwood. J. Essent. Oil Res. 2014, 26, 238–246. [Google Scholar] [CrossRef]
- Sudriá, C.; Palazón, J.; Cusidó, R. Effect of benzyladenine and indolebutyric acid on ultrastructure, glands formation, and essential oil accumulation in Lavandula dentata plantlets. Biol. Plant. 2001, 44, 1–6. [Google Scholar] [CrossRef]
- Lv, F.F.; Li, S.S.; Feng, J. Hydrogen peroxide burst triggers accumulation of jasmonates and salicylic acid inducing sesquiterpene biosynthesis in wounded Aquilaria sinesis. J. Plant Physiol. 2009, 234, 167–175. [Google Scholar] [CrossRef] [PubMed]


| Treatment | Stem Diameter at a Height of 30 cm above the Ground (cm) | Stem Diameter at a Height of 130 cm above the Ground (cm) | ||||
|---|---|---|---|---|---|---|
| Before Treatment (Mean ± SD) | After Treatment (Mean ± SD) | Change Range * (Mean ± SD) | Before Treatment (Mean ± SD) | After Treatment (Mean ± SD) | Change Range * (Mean ± SD) | |
| 2 mM BA | 7.51 ± 0.33 | 8.18 ± 0.31 | 0.67 ± 0.14 a | 6.10 ± 0.16 | 6.76 ± 0.27 | 0.66 ± 0.17 ab |
| 4 mM BA | 7.61 ± 0.42 | 8.45 ± 0.45 | 0.85 ± 0.05 a | 6.60 ± 0.27 | 7.33 ± 0.26 | 0.74 ± 0.15 ab |
| 2 mM ETH | 7.30 ± 0.11 | 7.73 ± 0.11 | 0.45 ± 0.10 ab | 6.01 ± 0.08 | 6.68 ± 0.11 | 0.67 ± 0.09 ab |
| 4 mM ETH | 6.75 ± 0.25 | 6.69 ± 0.30 | 0.23 ± 0.05 b | 5.99 ± 0.23 | 6.28 ± 0.24 | 0.30 ± 0.09 b |
| 2 mM MeJA | 7.29 ± 0.32 | 8.06 ± 0.58 | 0.78 ± 0.28 a | 6.26 ± 0.31 | 7.06 ± 0.29 | 0.80 ± 0.06 ab |
| 4 mM MeJA | 7.81 ± 0.02 | 8.70 ± 0.09 | 0.90 ± 0.09 a | 6.75 ± 0.04 | 7.39 ± 0.16 | 0.64 ± 0.14 ab |
| 2 mM H2O2 | 7.57 ± 0.19 | 8.47 ± 0.23 | 0.90 ± 0.11 a | 6.93 ± 0.51 | 7.42 ± 0.83 | 0.49 ± 0.17 ab |
| 4 mM H2O2 | 7.55 ± 0.28 | 8.41 ± 0.14 | 0.85 ± 0.23 a | 6.58 ± 0.45 | 7.10 ± 0.65 | 0.52 ± 0.23 ab |
| 2 mM CaCl2 | 7.88 ± 0.55 | 8.55 ± 0.60 | 0.70 ± 0.09 a | 6.68 ± 0.28 | 7.45 ± 0.27 | 0.77 ± 0.20 ab |
| 4 mM CaCl2 | 7.87 ± 0.33 | 8.75 ± 0.44 | 0.85 ± 0.12 a | 6.67 ± 0.18 | 7.76 ± 0.42 | 1.10 ± 0.25 a |
| Water control | 7.69 ± 0.22 | 8.52 ± 0.28 | 0.83 ± 0.10 a | 6.55 ± 0.11 | 7.25 ± 0.08 | 0.70 ± 0.19 ab |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Zhang, X.; Cheng, Q.; Teixeira da Silva, J.A.; Fang, L.; Ma, G. Elicitors Modulate Young Sandalwood (Santalum album L.) Growth, Heartwood Formation, and Concrete Oil Synthesis. Plants 2021, 10, 339. https://doi.org/10.3390/plants10020339
Li Y, Zhang X, Cheng Q, Teixeira da Silva JA, Fang L, Ma G. Elicitors Modulate Young Sandalwood (Santalum album L.) Growth, Heartwood Formation, and Concrete Oil Synthesis. Plants. 2021; 10(2):339. https://doi.org/10.3390/plants10020339
Chicago/Turabian StyleLi, Yuan, Xinhua Zhang, Qingwei Cheng, Jaime A. Teixeira da Silva, Lin Fang, and Guohua Ma. 2021. "Elicitors Modulate Young Sandalwood (Santalum album L.) Growth, Heartwood Formation, and Concrete Oil Synthesis" Plants 10, no. 2: 339. https://doi.org/10.3390/plants10020339
APA StyleLi, Y., Zhang, X., Cheng, Q., Teixeira da Silva, J. A., Fang, L., & Ma, G. (2021). Elicitors Modulate Young Sandalwood (Santalum album L.) Growth, Heartwood Formation, and Concrete Oil Synthesis. Plants, 10(2), 339. https://doi.org/10.3390/plants10020339





