Phytochemical and In Vitro Genotoxicity Studies of Standardized Ficus deltoidea var. kunstleri Aqueous Extract
Abstract
:1. Introduction
2. Results
2.1. Phytochemical Analysis
2.2. MTS Cytotoxicity Assay
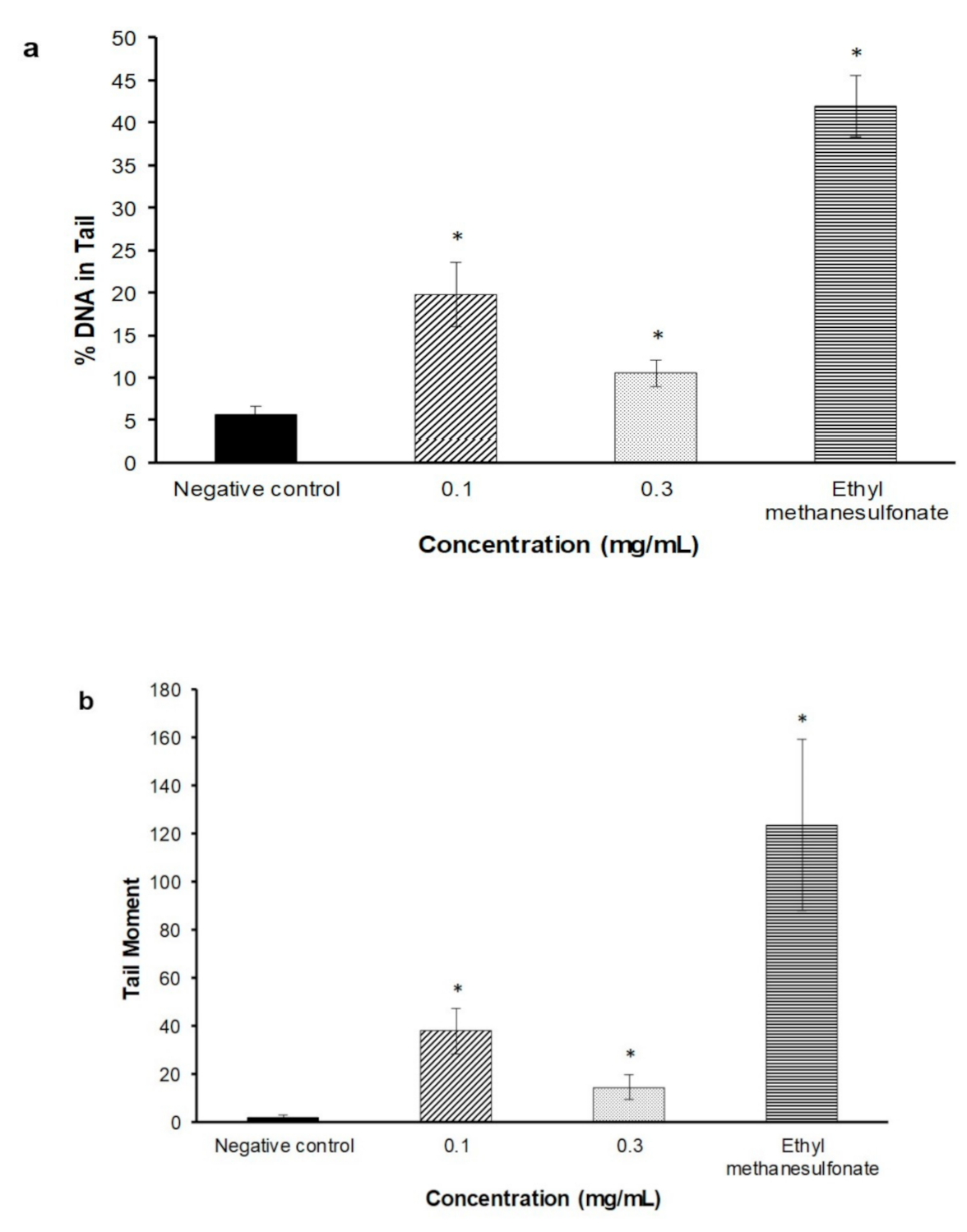
2.3. Alkaline Comet Assay
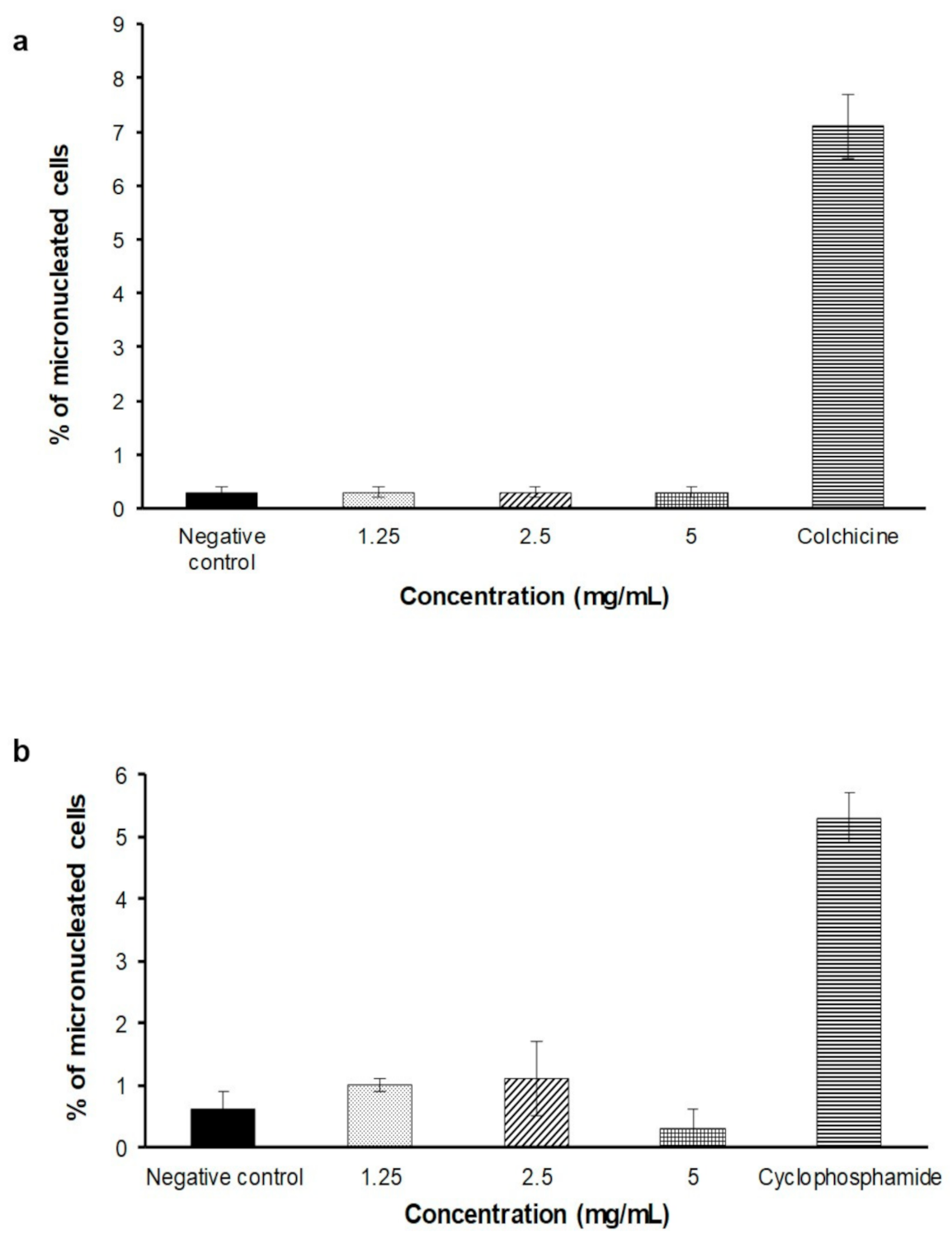
2.4. In Vitro Micronucleus Assay
2.5. Salmonella typhimurium/Microsome Assay
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Extract Preparation
4.2. MTS Cytotoxicity Assay
4.2.1. Cell Culture
4.2.2. Cytotoxicity Determination
4.3. Genotoxicity Assays
4.3.1. Alkaline Comet Assay
4.3.2. In Vitro Micronucleus Assay
4.3.3. Statistical Analysis
4.4. Salmonella typhimurium/Microsome Assay
4.4.1. Bacterial Strains and Metabolic Activation System (S9 Mixture)
4.4.2. Positive Control Mutagen
4.4.3. Mutagenicity Assay
4.4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shinde, V.M.; Dhalwal, K.; Potdar, M.; Mahadik, K.R. Application of Quality Control Principles to Herbal Drugs. Int. J. Phytomed. 2009, 1, 4–8. [Google Scholar]
- Builders, P.F. Introductory Chapter: Introduction to Herbal Medicine. In Herbal Medicine; IntechOpen: London, UK, 2018. [Google Scholar]
- De Smet, P.A. Health Risks of Herbal Remedies. Drug Saf. 1995, 13, 81–93. [Google Scholar]
- Roberts, S.M.; James, R.C.; Williams, P.L. Principles of Toxicology: Environmental and Industrial Applications, 3rd ed.; John Wiley & Sons: New York, NY, USA, 2014. [Google Scholar]
- Lansky, E.P.; Paavilainen, H.M. Traditional Herbal Medicines for Modern Times Figs: The Genus Ficus; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2011; pp. 13–50. [Google Scholar]
- Burkill, I.H.; Haniff, M. Malay Village Medicine. Gard. Bull. Straits Settl. 1930, 6, 167–332. [Google Scholar]
- Bunawan, H.; Amin, N.M.; Bunawan, S.N.; Baharum, S.N.; Mohd Noor, N. Ficus deltoidea Jack: A Review on Its Phytochemical and Pharmacological Importance. Evid. Based. Complement. Altern. Med. 2014, 2014, 902734. [Google Scholar]
- Harborne, J.B.; Williams, C.A. Advances in Flavonoid Research since 1992. Phytochemistry 2000, 55, 481–504. [Google Scholar]
- Havsteen, B.H. The Biochemistry and Medical Significance of the Flavonoids. Pharmacol. Ther. 2002, 96, 67–202. [Google Scholar]
- Skibola, C.F.; Smith, M.T. Potential Health Impacts of Excessive Flavonoid Intake. Free Radic. Biol. Med. 2000, 29, 375–383. [Google Scholar]
- Middleton, E., Jr. Effect of Plant Flavonoids on Immune and Inflammatory Cell Function. Adv. Exp. Med. Biol. 1998, 439, 175–182. [Google Scholar]
- Misbah, H.; Aziz, A.A.; Aminudin, N. Antidiabetic and antioxidant properties of Ficus deltoidea fruit extracts and fractions. BMC Complement. Altern. Med. 2013, 13, 118. [Google Scholar]
- Fernandes, L.M.; da Rosa Guterres, Z.; Almeida, I.V.; Vicentini, V.E.P. Genotoxicity and Antigenotoxicity Assessments of the Flavonoid Vitexin by the Drosophila melanogaster Somatic Mutation and Recombination Test. J. Med. Food 2017, 20, 601–609. [Google Scholar]
- Anter, J.; Romero-Jiménez, M.; Fernández-Bedmar, Z.; Villatoro-Pulido, M.; Analla, M.; Alonso-Moraga, A.; Muñoz-Serrano, A. Antigenotoxicity, Cytotoxicity, and Apoptosis Induction by Apigenin, Bisabolol, and Protocatechuic Acid. J. Med. Food 2011, 14, 276–283. [Google Scholar]
- Eghbaliferiz, S.; Iranshahi, M. Prooxidant Activity of Polyphenols, Flavonoids, Anthocyanins and Carotenoids: Updated Review of Mechanisms and Catalyzing Metals: Prooxidant Activity of Polyphenols and Carotenoids. Phytother. Res. 2016, 30, 1379–1391. [Google Scholar]
- Norrizah, J.S.; Norizan, A.; Sharipah, R.S.A.; Dzulsuhaim, D.; Nurul Hida, M.S. Cytotoxicity Activity and Reproductive Profiles of Male Rats Treated with Methanolic Extracts of Ficus deltoidea. Res. J. Med. Plant 2012, 6, 197–202. [Google Scholar]
- Azqueta, A.; Collins, A.R. The Essential Comet Assay: A Comprehensive Guide to Measuring DNA Damage and Repair. Arch. Toxicol. 2013, 87, 949–968. [Google Scholar]
- Sakihama, Y.; Cohen, M.F.; Grace, S.C.; Yamasaki, H. Plant Phenolic Antioxidant and Prooxidant Activities: Phenolics-Induced Oxidative Damage Mediated by Metals in Plants. Toxicology 2002, 177, 67–80. [Google Scholar]
- Oikawa, S.; Furukawaa, A.; Asada, H.; Hirakawa, K.; Kawanishi, S. Catechins Induce Oxidative Damage to Cellular and Isolated DNA through the Generation of Reactive Oxygen Species. Free Radic. Res. 2003, 37, 881–890. [Google Scholar]
- Srinivasan, P.; Vadhanam, M.V.; Arif, J.M.; Gupta, R.C. A rapid screening assay for antioxidant potential of natural and synthetic agents in vitro. Int. J. Oncol. 2002, 20, 983–986. [Google Scholar]
- Johnson, M.K.; Loo, G. Effects of Epigallocatechin Gallate and Quercetin on Oxidative Damage to Cellular DNA. Mutat. Res. DNA Repair 2000, 459, 211–218. [Google Scholar]
- Sugisawa, A.; Umegaki, K. Physiological Concentrations of (−)-Epigallocatechin-3-O-Gallate (EGCg) Prevent Chromosomal Damage Induced by Reactive Oxygen Species in WIL2-NS Cells. J. Nutr. 2002, 132, 1836–1839. [Google Scholar]
- Ogura, R.; Ikeda, N.; Yuki, K.; Morita, O.; Saigo, K.; Blackstock, C.; Nishiyama, N.; Kasamatsu, T. Genotoxicity Studies on Green Tea Catechin. Food Chem. Toxicol. 2008, 46, 2190–2200. [Google Scholar]
- Li, D.; Morimoto, K.; Takeshita, T.; Lu, Y. Arsenic induces DNA damage via reactive oxygen species in mammalian cells. Environ. Health Prev. Med. 2001, 6, 27–32. [Google Scholar]
- Schwerdtle, T.; Walter, I.; Mackiw, I.; Hartwig, A. Induction of Oxidative DNA Damage by Arsenite and Its Trivalent and Pentavalent Methylated Metabolites in Cultured Human Cells and Isolated DNA. Carcinogenesis 2003, 24, 967–974. [Google Scholar]
- Farsi, E.; Shafaei, A.; Hor, S.Y.; Ahamed, M.B.K.; Yam, M.F.; Asmawi, M.Z.; Ismail, Z. Genotoxicity and Acute and Subchronic Toxicity Studies of a Standardized Methanolic Extract of Ficus deltoidea Leaves. Clinics 2013, 68, 865–875. [Google Scholar]
- OECD. Test No. 487: In Vitro Mammalian Cell Micronucleus Test; OECD Guideline for Testing of Chemicals; OECD: Paris, France, 2010. [Google Scholar]
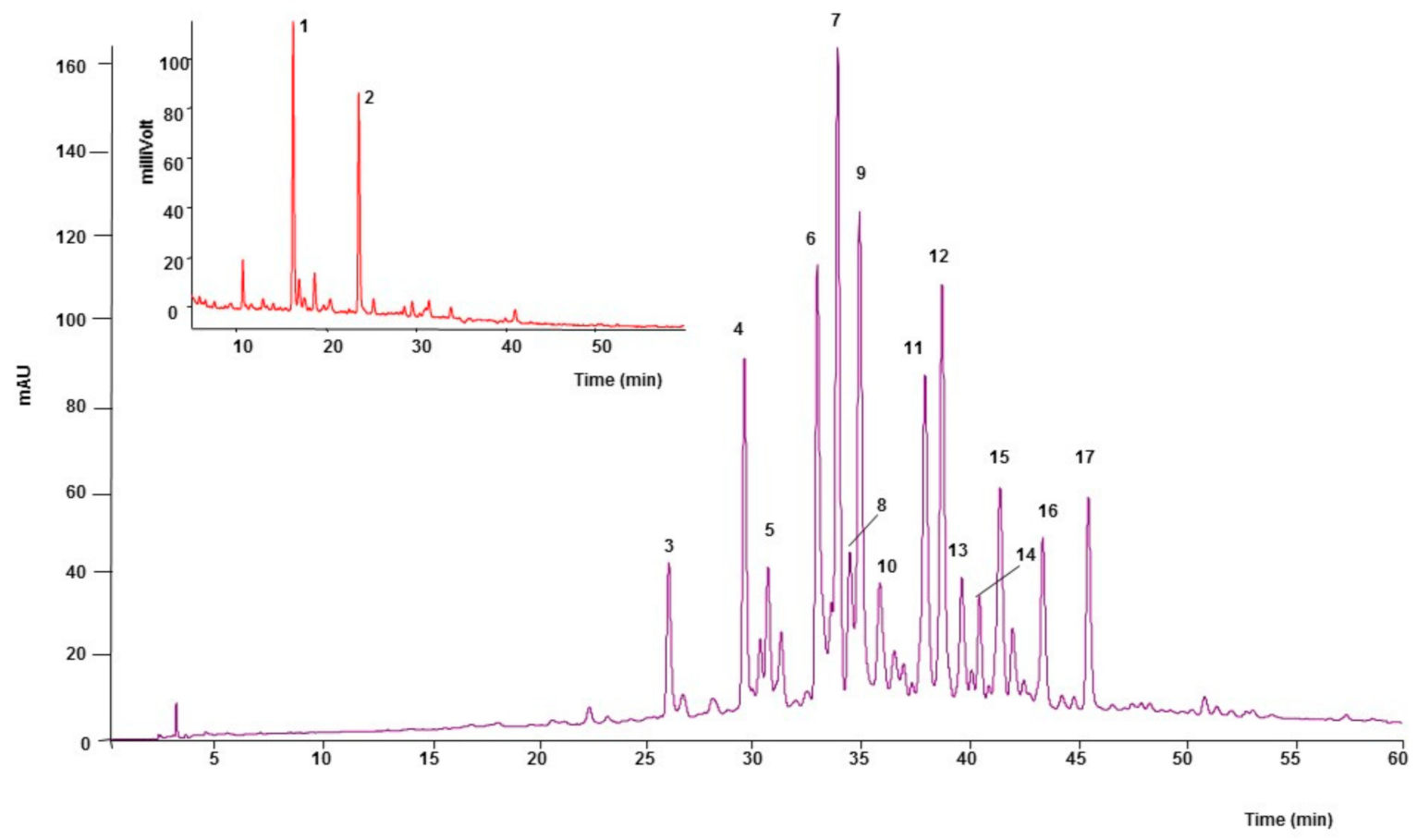

| Peak | tR | λmax | Compound | [M-H]− (m/z) | MS2 Fragments Ions (m/z) |
|---|---|---|---|---|---|
| 1 | 16.2 | 280 | (+)-Catechin | 289 | 245, 179 |
| 2 | 23.5 | 280 | (−)-Epicatechin | 289 | 245, 205, 179 |
| 3 | 25.9 | 350 | Luteolin-6-8-C-diglucoside (Lucenin-2) | 609 | 489, 519, 399 |
| 4 | 29.4 | 340 | Apigenin-6, 8-C-diglucoside (Vicenin-2) | 593 | 473, 503, 353 |
| 5 | 30.5 | 345 | Unidentified | 517 | 385, 205, 222 |
| 6 | 32.8 | 335 | Apigenin-6-C-ara-8-C-glucoside (Isoschaftoside) | 563 | 473, 443, 503 |
| 7 | 33.7 | 335 | Apigenin-6-C-glu-8-C-arabinoside (Schaftoside) | 563 | 473, 503, 443 |
| 8 | 34.3 | 345 | Luteolin 6-C-glucoside (Isoorientin) | 447 | 327, 357, 369 |
| 9 | 34.8 | 325 | Unidentified | 565 | 444, 474, 443 |
| 10 | 35.7 | 335 | Luteolin-8-C-diglucoside (Orientin) | 447 | 327, 429, 357 |
| 11 | 37.8 | 335 | Apigenin -8-C-glucoside (Vitexin) | 431 | 311, 341, 283 |
| 12 | 38.6 | 335 | Apigenin-6-C-pent-8-C-glucoside | 563 | 443, 473, 353 |
| 13 | 39.5 | 335 | Apigenin-6-C-pentosyl-8-C-pentoside | 533 | 443, 473, 515 |
| 14 | 40.3 | 335 | Apigenin-6-C-pentosyl-8-C-pentoside | 533 | 443, 473, 515 |
| 15 | 41.3 | 345 | Apigenin 6-C-glucoside (Isovitexin) | 431 | 311, 341, 413 |
| 16 | 43.3 | 335 | Unidentified | 535 | 443, 474, 516 |
| 17 | 45.4 | 335 | Chrysin-6-8-C-diglucoside | 577 | 457, 487, 353 |
| Dose (μg/plate) | Number of Revertants (Mean ± SD) | ||||
|---|---|---|---|---|---|
| Base-Pair Substitution Type | Frameshift Type | ||||
| TA100 | TA1535 | WP2uvrA | TA98 | TA1537 | |
| 0 | 136 ± 10 | 13 ± 2 | 57 ± 5 | 21 ± 4 | 8 ± 2 |
| 313 | 145 ± 12 | 14 ± 1 | 53 ± 3 | 21 ± 1 | 9 ± 2 |
| 625 | 135 ± 5 | 10 ± 2 | 58 ± 4 | 20 ± 3 | 9 ± 2 |
| 1250 | 130 ± 8 | 11 ± 3 | 48 ± 4 | 19 ± 1 | 11 ± 2 |
| 2500 | 134 ± 11 | 10 ± 1 | 55 ± 2 | 23 ± 4 | 10 ± 2 |
| 5000 | 166 ± 7 | 12 ± 1 | 53 ± 3 | 25 ± 2 | 11 ± 3 |
| Positive control | 627 ± 6 | 558 ± 6 | 180 ± 7 | 539 ± 4 | 608 ± 4 |
| Dose (μg/plate) | Number of Revertants (Mean ± SD) | ||||
|---|---|---|---|---|---|
| Base-Pair Substitution Type | Frameshift Type | ||||
| TA100 | TA1535 | WP2uvrA | TA98 | TA1537 | |
| 0 | 162 ± 6 | 17 ± 3 | 60 ± 4 | 24 ± 4 | 10 ± 1 |
| 313 | 151 ± 10 | 15 ± 2 | 63 ± 4 | 30 ± 4 | 11 ± 2 |
| 625 | 145 ± 12 | 14 ± 2 | 63 ± 2 | 28 ± 2 | 12 ± 2 |
| 1250 | 158 ± 5 | 15 ± 2 | 53 ± 4 | 32 ± 3 | 10 ± 2 |
| 2500 | 142 ± 3 | 14 ± 1 | 57 ± 5 | 28 ± 2 | 12 ± 2 |
| 5000 | 149 ± 6 | 14 ± 3 | 62 ± 8 | 29 ± 2 | 11 ± 1 |
| Positive control | 519 ± 4 | 165 ± 4 | 192 ± 4 | 232 ± 6 | 183 ± 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muhammad, H.; Omar, M.H.; Rasid, E.N.I.; Suhaimi, S.N.; Mohkiar, F.H.; Siu, L.M.; Awang, N. Phytochemical and In Vitro Genotoxicity Studies of Standardized Ficus deltoidea var. kunstleri Aqueous Extract. Plants 2021, 10, 343. https://doi.org/10.3390/plants10020343
Muhammad H, Omar MH, Rasid ENI, Suhaimi SN, Mohkiar FH, Siu LM, Awang N. Phytochemical and In Vitro Genotoxicity Studies of Standardized Ficus deltoidea var. kunstleri Aqueous Extract. Plants. 2021; 10(2):343. https://doi.org/10.3390/plants10020343
Chicago/Turabian StyleMuhammad, Hussin, Maizatul Hasyima Omar, Elda Nurafnie Ibnu Rasid, Shazlan Noor Suhaimi, Farah Huda Mohkiar, Lau Mei Siu, and Norizah Awang. 2021. "Phytochemical and In Vitro Genotoxicity Studies of Standardized Ficus deltoidea var. kunstleri Aqueous Extract" Plants 10, no. 2: 343. https://doi.org/10.3390/plants10020343
APA StyleMuhammad, H., Omar, M. H., Rasid, E. N. I., Suhaimi, S. N., Mohkiar, F. H., Siu, L. M., & Awang, N. (2021). Phytochemical and In Vitro Genotoxicity Studies of Standardized Ficus deltoidea var. kunstleri Aqueous Extract. Plants, 10(2), 343. https://doi.org/10.3390/plants10020343






