Plant Recovery after Metal Stress—A Review
Abstract
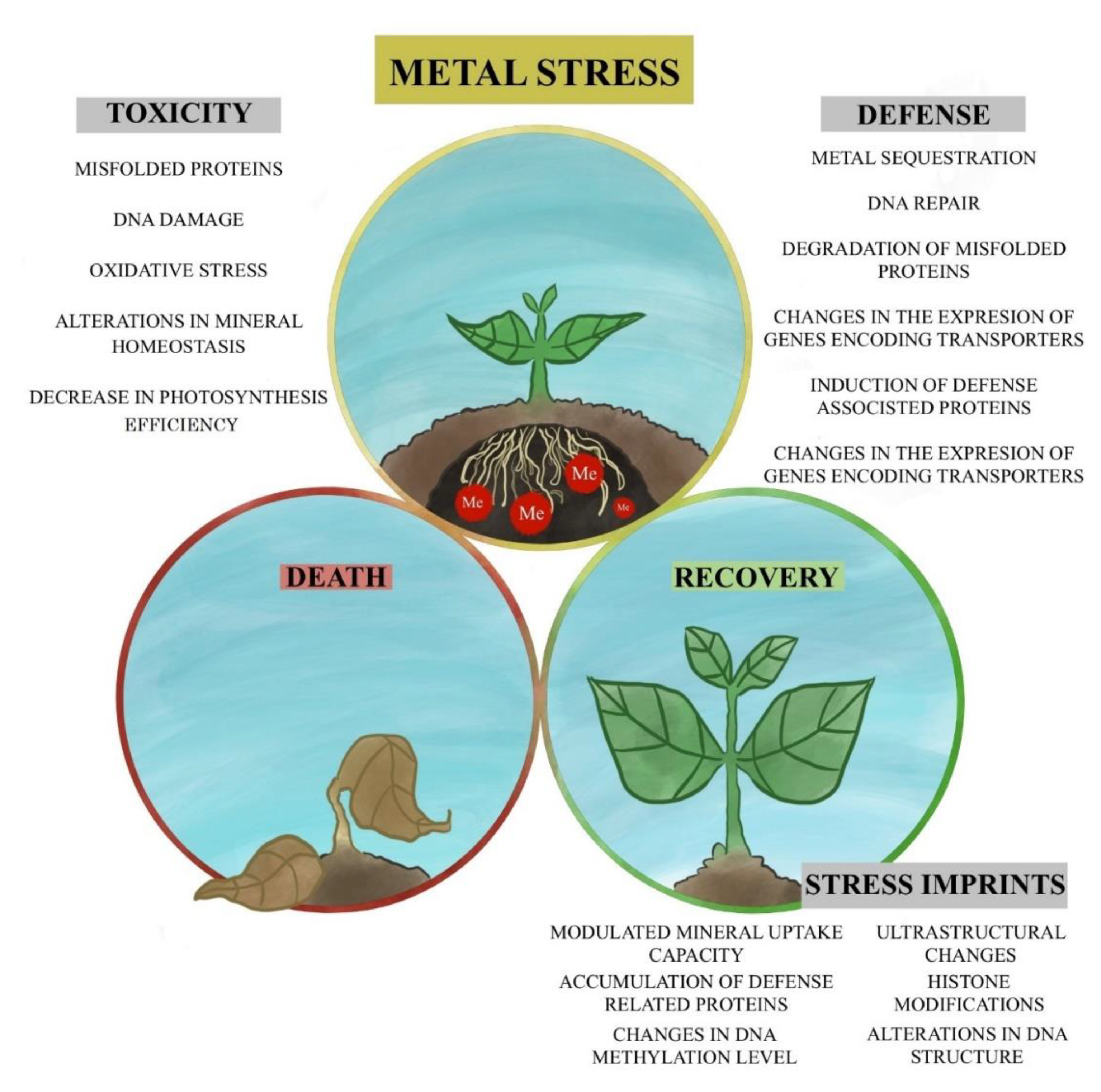
1. Introduction
2. Metal Toxicity in Plants
3. Post-Stress Recovery
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Tóth, G.; Hermann, T.; Szatmári, G.; Pásztor, L. Maps of heavy metals in the soil of European Union and proposed priority areas for detailed assessment. Sci. Total Environ. 2016, 565, 1054–1062. [Google Scholar] [CrossRef]
- Yabe, J.; Ishizuka, M.; Umemura, T. Current level of heavy metal pollution in Africa. J. Vet. Med. Sci. 2010, 72, 1257–1263. [Google Scholar] [CrossRef] [PubMed]
- Tong, S.; Li, H.; Wang, L.; Tudi, M.; Yang, L. Concentration, special distribution, contamination degree and human health risk assessment of heavy metals in urban soils across China between 2003 and 2019—A systematic review. Int. J. Environ. Res. Public Health 2020, 17, 3099. [Google Scholar] [CrossRef] [PubMed]
- Weissmanová, H.D.; Pavlovskỳ, J. Indices of soil contamination by heavy metals—Methodology of calculation for pollution assessment (minireview). Environ. Monit. Assess. 2017, 189, 616. [Google Scholar] [CrossRef] [PubMed]
- Afonne, O.J.; Ifebida, E.C. Heavy metals risks in plant foods—Need to step up precautionary measures. Curr. Opin. Toxicol. 2020, 22, 1–6. [Google Scholar] [CrossRef]
- Sharma, A.; Nagpal, A.K. Contamination of vegetables with heavy metals across the globe: Hampering food security goal. J. Food Sci. Technol. 2020, 57, 391–403. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Prasad, S.; Yadav, K.K.; Shrivastava, M.; Gupta, N.; Nagar, S.; Bach, Q.-V.; Kamyab, H.; Khana, S.S.; Yadav, S.; et al. Hazardous heavy metals contamination of vegetables and food chain: Role of sustainable remediation approaches—A review. Environ. Res. 2019, 179, 108792. [Google Scholar] [CrossRef]
- Striker, G.G. Time is on our side: The importance of considering a recovery periods when assessing flooding tolerance in plants. Ecol. Res. 2011, 27, 983–987. [Google Scholar] [CrossRef]
- Andresen, E.; Peiter, E.; Kűpper, H. Trace metal metabolism in plants. J. Exp. Bot. 2018, 5, 909–954. [Google Scholar] [CrossRef] [PubMed]
- Ghori, N.-H.; Ghori, T.; Hayat, M.Q.; Imadi, S.R.; Gul, A.; Altay, V.; Ozturk, M. Heavy metal stress and response in plants. Int. J. Environ. Sci. Technol. 2019, 16, 1807–1828. [Google Scholar] [CrossRef]
- Kűpper, H.; Andresen, E. Mechanisms of metal toxicity in plants. Metallomics 2016, 8, 269. [Google Scholar] [CrossRef] [PubMed]
- Tamás, M.J.; Sharma, S.K.; Ibstedt, S.; Jacobson, T.; Christen, P. Heavy metals and metalloids as a cause for protein misfolding and aggregation. Biomolecules 2014, 4, 252–267. [Google Scholar] [CrossRef] [PubMed]
- Rivetta, A.; Negrini, N.; Cocucci, M. Involvement of Ca2+-calmodulin in Cd2+ toxicity during the early phases of radish (Raphanus sativus L.) seed germination. Plant Cell Environ. 1997, 20, 600–608. [Google Scholar] [CrossRef]
- Kűpper, H.; Kűpper, F.; Spiller, M. Environmental relevance of heavy metal-substituted chlorophylls using example of water plants. J. Exp. Bot. 1996, 47, 259–266. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopulos, V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revising the crucial role of universal defense regulators. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef] [PubMed]
- Rucińska-Sobkowiak, R. Water relations in plants subjected to heavy metal stress. Acta Physiol. Plant. 2016, 38, 257. [Google Scholar] [CrossRef]
- Krzesłowska, M. The cell wall in plant cell wall response to trace metals: Polysaccharide remodelling and its role in defense strategy. Acta Physiol. Plant. 2011, 33, 35–51. [Google Scholar] [CrossRef]
- Rucińska-Sobkowiak, R.; Nowaczyk, G.; Krzesłowska, M.; Rabęda, I.; Jurga, S. Water status and water diffusion transport in lupine roots exposed to lead. Environ. Exp. Bot. 2013, 87, 100–109. [Google Scholar] [CrossRef]
- Samardakiewicz, S.; Krzesłowska, M.; Bilski, H.; Bartosiewicz, R.; Woźny, A. Is callose a barrier for lead ions entering Lemna minor L. root cells? Protoplasma 2012, 249, 347–351. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Przedpelska-Wasowicz, E.M.; Wierzbicka, M. Gating of aquaporins by heavy metals in Allium cepa L. epidermal cells. Protoplasma 2011, 248, 663–671. [Google Scholar] [CrossRef]
- Lamoreaux, R.J.; Chaney, W.R. Growth and water movement in silver maple seedlings affected by cadmium. J. Environ. Qual. 1997, 6, 201–205. [Google Scholar] [CrossRef]
- Kasim, W.A. Physiological consequences of structural and ultrastructural changes induced by Zn stress in Phaseolus vulgaris L. Growth and photosynthetic apparatus. Int. J. Bot. 2007, 3, 15–22. [Google Scholar] [CrossRef]
- De Silva, N.D.G.; Cholewa, E.; Ryser, P. Effects of combined drought and heavy metal stresses on xylem structure and hydraulic conductivity in red maple (Acer rubrum L.). J. Exp. Bot. 2012, 63, 5957–5966. [Google Scholar] [CrossRef]
- Rezvani, M.; Zaefarian, F.; Miransari, M.; Nematzadeh, G.A. Uptake and translocation of cadmium and nutrients by Aeluropus littoralis. Arch. Agron. Soil Sci. 2011, 58, 1413–1425. [Google Scholar] [CrossRef]
- Uzal, O.; Yasar, F. Effects of GA3 hormone treatments on ion uptake of pepper plants under cadmium stress. Appl. Ecol. Environ. Res. 2017, 15, 1347–1357. [Google Scholar] [CrossRef]
- Bankaji, I.; Caçador, I.; Sleimi, N. Physiological and biochemical response of Sueda fruticosa to cadmium and copper stress: Growth, nutrient uptake, antioxidant enzymes, phytochelatin, and glutathione levels. Environ. Sci. Pollut. Res. 2015, 22, 13058–13069. [Google Scholar] [CrossRef] [PubMed]
- Dobrikova, A.G.; Apostolova, E.L.; Hanć, A.; Yotsova, E.; Borisova, P.; Sperdouli, I.; Adamakis, J.-D.S.; Moustakas, M. Cadmium toxicity in Salvia sclarea L.: An integrative response of element uptake, oxidative stress markers, leaf structure and photosynthesis. Ecotoxicol. Environ. Saf. 2021, 209, 111851. [Google Scholar] [CrossRef]
- Andresen, E.; Lyubenova, L.; Hubáček, T.; Bokhari, S.N.H.; Matoušková, S.; Mijovilovich, A.; Jan Rohovec, J.; Küpper, H. Chronic exposure of soybean plants to nanomolar cadmium reveals specific additional high-affinity targets of cadmium toxicity. J. Exp. Bot. 2020, 71, 1628–1644. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Singh, A.K.; Tiwari, M.K.; Uttam, K.N. Prompt Screening of the Alterations in Biochemical and Mineral Profile of Wheat Plants Treated with Chromium Using Attenuated Total Reflectance Fourier Transform Infrared Spectroscopy and X-ray Fluorescence Excited by Synchrotron Radiation. Ann. Lett. 2020, 53, 482–508. [Google Scholar] [CrossRef]
- Doostikhah, N.; Panahpoor, E.; Nadian, H.; Gholam, A. Tomato (Lycopersicon esculentum L.) nutrient and lead uptake affected by zeolite and DTPA in a lead polluted soil. Plant Biol. 2019, 22, 317–322. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, V.; Shahzad, B.; Ramakrishnan, M.; Sidhu, G.R.S.; Bali, A.S.; Handa, N.; Kapoor, D.; Yadav, P.; Khanna, K.; et al. Photosynthetic response of plants under different abiotic stresses: A review. J. Plant Growth Regul. 2020, 39, 509–531. [Google Scholar] [CrossRef]
- Sarangthem, J.; Jain, M.; Gadre, R. Inhibition of δ-aminolevulinic acid dehydratase activity by cadmium in excised etiolated maize leaf segments during greening. Plant Soil Environ. 2011, 57, 332–337. [Google Scholar] [CrossRef]
- Skrebsky, E.C.; Tabaldi, L.A.; Pereira, L.B.; Rauber, R.; Maldaner, J.; Cargnelutti, D.; Gonçalves, J.F.; Castro, G.Y.; Shetinger, M.R.; Nicoloso, F.T. Effect of cadmium on growth, micronutrient concentration, and δ-aminolevulinic acid dehydratase and acid phosphatase activities in plants of Pfaffia glomerata. Braz. J. Plant Physiol. 2008, 20, 285–294. [Google Scholar] [CrossRef]
- Gonçalves, J.; Nicoloso, F.; Becker, A.; Pereira, L.; Tabaldi, L.; Cargnelutti, D.; Pelegrin, C.; Dressler, V.; Rocha, J.; Schetinger, M. Photosynthetic pigments content, δ-aminolevulinic acid dehydratase and acid phosphatase activities and mineral nutrients concentration in cadmium-exposed Cucumis sativus L. Biologia 2009, 64, 310–318. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, L.; Huang, X.; Zhou, Y.; Quan, Q.; Li, Y.; Zhu, X. Response of photosynthesis to different concentrations of heavy metals in Davidia involucrata. PLoS ONE 2020, 15, e0228563. [Google Scholar] [CrossRef] [PubMed]
- Garg, P.; Chandra, P.; Devi, S. Chromium (VI) induced morphological changes in Limnanthemum cristatum Griseb: A possible bioindicator. Phytomorphology 1994, 44, 201–206. [Google Scholar]
- Gratăo, P.L.; Monteiro, C.C.; Rossi, M.L.; Martinelli, A.P.; Peres, L.E.; Medici, L.O.; Lea, P.J.; Azevedo, R.A. Differential ultrastructural changes in tomato hormonal mutants exposed to cadmium. Environ. Exp. Bot. 2009, 67, 387–394. [Google Scholar] [CrossRef]
- Najeeb, U.; Jilani, G.; Ali, S.; Sarwar, M.; Xu, L.; Zhou, W. Insights into cadmium induced physiological and ultra-structural disorders in Juncus effusus L. and its remediation through exogenous citric acid. J. Hazard. Mater. 2011, 186, 565–574. [Google Scholar] [CrossRef]
- Santos, C.L.V.; Purrut, B.; de Oliveira, J.M.P.F. The use of comet assay in plant toxicology: Recent advacnes. Front. Genet. 2015, 6, 216. [Google Scholar] [CrossRef]
- Rucińska, R.; Sobkowiak, R.; Gwóźdź, A.E. Genotoxicity of lead in lupin root cells as evaluated by the comet assay. Cell. Mol. Biol. Lett. 2004, 9, 519–528. [Google Scholar]
- Batir, M.B.; Candan, F.; Bűtűk, Ï. Determination of the DNA changes in the artichoke seedlings (Cynara scolymus L.) subjected to lead and copper stresses. Plant Soil Environ. 2016, 3, 143–149. [Google Scholar] [CrossRef]
- Malar, S.; Sahi, S.V.; Favas, P.J.C.; Venkatachalam, P. Assessment of mercury heavy metal toxicity-induced physiochemical and molecular changes in Sesbania grandiflora L. Int. J. Environ. Sci. Technol. 2015, 12, 3273–3282. [Google Scholar] [CrossRef]
- Ozyigit, I.I.; Dogan, I.; Igdelioglu, S.; Filiz, E.; Karadeniz, S.; Uzunova, Z. Screening of damage induced by lead (Pb) in rye (Secale cereale L.)—A genetic and physiological approach. Biotechnol. Biotechnol. Equip. 2016, 30, 489–496. [Google Scholar] [CrossRef]
- Erturk, F.A.; Agar, G.; Arslan, E.; Nardemir, G. Analysis of genetic and epigentic effects of maize seeds in response to heavy metal (Zn) stress. Environ. Sci. Pollut. Res. 2015, 22, 10291–10297. [Google Scholar] [CrossRef] [PubMed]
- Pizzaia, D.; Nogueira, M.L.; Mondin, M.; Carvalho, M.E.A.; Piotto, F.A.; Rosario, M.F.; Azevedo, R.A. Cadmium toxicity and its relationship with disturbances in the cytoskeleton, cell cycle and chromosome stability. Ecotoxicology 2019, 28, 1046–1055. [Google Scholar] [CrossRef] [PubMed]
- Fargašová, A. Plants as models as models for chromium and nickel risk assessment. Ecotoxicology 2012, 21, 1476–1483. [Google Scholar] [CrossRef]
- Liu, D.; Xue, P.; Meng, Q.; Zou, J.; Gu, J.; Jiang, W. Pb/Cd effects on the organization of microtubule cytoskeleton in interphase and mitotic cells of Allium sativum L. Plant Cell Rep. 2009, 28, 695–702. [Google Scholar] [CrossRef]
- Gzyl, J.; Chmielowska-Bąk, J.; Przymusiński, R. Gamma-tubulin distribution and ultrastructural changes in root cells of soybean (Glycine max L.) seedlings under cadmium stress. Environ. Exp. Bot. 2017, 143, 82–90. [Google Scholar] [CrossRef]
- Gzyl, J.; Chmielowska-Bąk, J.; Przymusiński, R.; Gwóźdź, E.A. Cadmium affects microtubule organization and post-translational modifications of tubulin in seedlings of soybean (Glycine max L.). Front. Plant Sci. 2015, 6, 937. [Google Scholar] [CrossRef] [PubMed]
- Kranner, I.; Colville, L. Metals and seeds: Biochemical and molecular implications and their significance for seed germination. Environ. Exp. Bot. 2011, 72, 93–105. [Google Scholar] [CrossRef]
- Abbas, A.M.; Hammad, S.; Soliman, W.S. Influence of copper and lead on germination of three Mimosoideae plant species. Asian J. Agric. Biol. 2017, 5, 320–327. [Google Scholar]
- Chmielowska-Bąk, J.; Holubek, R.; Frontasyeva, M.; Zinicovscaia, I.; Îşidoǧru, S.; Deckert, J. Tough Sprouting—Impact of Cadmium on Physiological State and Germination Rate of Soybean Seeds. Acta Soc. Bot. Pol. 2020, 89, 8923. [Google Scholar] [CrossRef]
- Drost, W.; Matzke, M.; Backhaus, T. Heavy metal toxicity in Lemna minor: Studies on the time dependence of growth inhibition and recovery after exposure. Chemosphere 2007, 67, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Fojtová, M.; Fulnečková, J.; Fajkus, J.; Kovařík, A. Recovery of tobacco cells from cadmium stress is accompanied by DNA repair and increased telomerase activity. J. Exp. Bot. 2002, 53, 2151–2158. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bazihizina, N.; Taiti, C.; Serre, N.; Nocci, C.; Spinelli, F.; Nissim, W.G.; Azzarello, E.; Marti, L.; Redwan, M.; Gonelli, C.; et al. Awaiting better times: A quiescence response and adventitious root primordia formation prolong survival under cadmium stress in Tetradenia riparia (Hochs) Codd. Envrion. Exp. Bot. 2016, 130, 1–10. [Google Scholar] [CrossRef]
- Holubek, R.; Deckert, J.; Zinicovscaia, I.; Yushin, N.; Vergel, K.; Frontasyeva, M.; Sirotkin, V.A.; Samdumu, D.; Chmielowska-Bąk, J. The recovery of soybean plants after short term cadmium stress. Plants 2020, 9, 782. [Google Scholar] [CrossRef]
- Tripathi, B.N.; Mehta, S.K.; Gaur, J.P. Recovery of uptake and assimilation of nitrate in Scenedesmus sp. previously exposed to elevated levels of Cu2+ and Zn2+. J. Plant Physiol. 2004, 161, 543–549. [Google Scholar] [CrossRef]
- Mohanty, S.; Das, A.B.; Das, P.; Mohanty, P. Effect of a low dose of aluminum on mitotic and meiotic activity, 4C DNA content, and pollen sterility in rice, Oryza sativa L. cv. Lalat. Ecotoxicol. Environ. Saf. 2004, 59, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Boquete, M.T.; Lang, I.; Weidinger, M.; Richards, C.L.; Alosno, C. Patterns and mechanisms of heavy metal accumulation in two terrestrial moss species with contrasting habitat specialization. Environ. Exp. Bot. 2021, 182, 104336. [Google Scholar] [CrossRef]
- Becket, R.P.; Brown, D.H. The relationship between cadmium uptake and heavy metal tolerance in the lichen genus Peltigera. New Phytol. 1984, 97, 301–311. [Google Scholar] [CrossRef]
- Pawlik-Skowrońska, B.; Wójciak, H. Heavy metal accumulation, resistance and physiological status of some epigeic and epiphytic lichens inhabiting Zn and Pb polluted areas. Pol. J. Ecol. 2008, 56, 195–207. [Google Scholar]
- Sharma, S.S.; Dietz, K.-J.; Mimura, T. Vacuolar compartmentalization as indispensable component of heavy metal detoxification in plants. Plant Cell Environ. 2016, 39, 1112–1126. [Google Scholar] [CrossRef]
- Faé, M.; Balestrazzi, A.; Confalonieri, M.; Doná, M.; Macovei, A.; Valassi, A.; Giraffa, G.; Carbonera, D. Copper-mediated genotoxic stress is attenuated by the overexpression of the DNA repair gene MtTdp2 (tyrosyl-DNA phosphodiesterase 2) in Medicago truncatula plants. Plant Cell Rep. 2014, 33, 1071–1180. [Google Scholar] [CrossRef]
- Macovei, A.; Balestrazzi, A.; Confalonieri, M.; Buttafava, A.; Carbonera, D. The TFIIS and TFIIS-like genes from Medicago truncatula are involved in oxidative stress response. Gene 2011, 470, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Hossein, Z.; Komatsu, S. Contribution of proteomic studies towards understanding plant heavy metal stress response. Front Plant Sci. 2013, 3, 310. [Google Scholar] [CrossRef]
- Chaudhary, R.; Baranwal, V.K.; Kumar, R.; Sircar, D.; Chauhan, H. Genome-wide identification and expression analysis of HSP70, HSP90, and HSP100, heat shock protein genes in barley under stress conditions and reproductive development. Funct. Integr. Genom. 2019, 19, 1007–1022. [Google Scholar] [CrossRef] [PubMed]
- Decou, R.; Laloi, G.; Zouari, M.; Labrousse, P.; Delmail, D. Evaluation of the relevance of Myriphyllum alterniflroum (Haloragaceae) cadmium-sensitive biomarkers for ecotoxicological surveys. Bull Environ. Contamin Toxicol. 2018, 101, 458–466. [Google Scholar] [CrossRef]
- Huang, J.; Wu, X.; Tian, F.; Chen, Q.; Luo, P.; Zhang, F.; Wan, X.; Zhong, Y.; Liu, Q.; Lin, T. Changes in Proteome and Protein Phosphorylation Reveal the Protective Roles of Exogenous Nitrogen in Alleviating Cadmium Toxicity in Poplar Plants. Int. J. Mol. Sci. 2020, 21, 278. [Google Scholar] [CrossRef] [PubMed]
- Tkalec, M.; Prebeg, T.; Roje, V.; Pevalek-Kozlina, B.; Ljubešić, N. Cadmium-induced responses in duckweed Lemna minor L. Acta Physiol. Plant 2008, 30, 881–890. [Google Scholar] [CrossRef]
- Chmielowska, J.; Veloso, J.; Gutiérrez, J.; Silvar, C.; Díaz, J. Cross-protection of pepper plants stressed by copper against a vascular pathogen is accompanied by the induction of a defence response. Plant Sci. 2010, 178, 176–182. [Google Scholar] [CrossRef]
- Arasimowicz-Jelonek, M.; Floryszak-Weiczorek, J.; Drzewiecka, K.; Chemilowska-Bąk, J.; Abramowski, D.; Izbiańska, K. Aluminum induced cross-resistance of potato to Phytophtora Infestans. Planta 2013, 239, 679–694. [Google Scholar] [CrossRef]
- Barrientos, E.Y.; Wrobel, K.; Torres, A.L.; Corona, F.G.; Wrobel, K. Application of reversed-phase high-performance liquid chromatography with fluorimetric detection for simultaneous assessment of global DNA and total RNA methylation in Lepidium sativum: Effect of plant exposure to Cd(II) and Se(IV). Anal. Bioanal. Chem. 2013, 405, 2397–2404. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.J.; Liu, X.S.; Tao, H.; Tan, S.K.; Chu, S.S.; Oono, Y.; Zhang, X.D.; Chen, J.; Yang, Z.M. Variation of DNA methylation patterns associated with gene expression in rice (Oryza sativa) exposed to cadmium. Plant Cell Environ. 2016, 39, 2629–2649. [Google Scholar] [CrossRef]
- Greco, M.; Chiappetta, A.; Bruno, L.; Bitonti, M.B. In Posidonia oceanica cadmium induces changes in DNA methylation and chromatin patterning. J. Exp. Bot 2012, 63, 695–709. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, Z.; Chen, R.; Li, X.; Tai, P.; Gong, Z.; Jia, C.; Liu, W. DNA damage and genetic methylation changes caused by Cd in Arabidopsis thaliana seedlings. Environ. Toxicol. Chem. 2015, 9, 2095–2103. [Google Scholar] [CrossRef] [PubMed]
- Shafiq, S.; Zeb, Q.; Ali, A.; Sajjad, Y.; Nazir, R.; Widemann, E.; Liu, L. Lead, cadmium and zinc phytotoxicity alter DNA methylation levels to confer heavy metal tolerance in wheat. Int. J. Mol. Sci. 2019, 20, 4676. [Google Scholar] [CrossRef] [PubMed]
- Yagci, S.; Yildirim, E.; Yildirim, N.; Shams, M.; Agar, G. Nitric oxide alleviates the effects of copper-induced DNA methylation, genomic instability, LTR retrotransposon polymorphism and enzyme activity in lettuce. Plant Physiol. Rep. 2019, 24, 289–295. [Google Scholar] [CrossRef]
- Iwasaki, M.; Paszkowski, J. Epigenetic memory in plants. EMBO J. 2014, 33, 1987–1998. [Google Scholar] [CrossRef]
- Kinoshita, T.; Seki, M. Epigenetic memory for stress response and adaptation in plants. Plant Cell Physiol. 2014, 55, 1859–1863. [Google Scholar] [CrossRef]
- Hassan, S.S.; Jin, H.-Z.; Abu-Izneid, T.; Rauf, A.; Ishaq, M.; Suleria, H.A.R. Stress-driven discovery in the natural products: A gateway towards new drugs. BioMed. Pharamcol. 2019, 109, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Yu, L.; Wu, X.; Chen, J. New CuCl2-induced glucoside esters and other constituents from Portucala oleracea. Carbohydr. Res. 2012, 352, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, H.; Fatima, N.; Ahmad, I.Z. Evaluation of antioxidant and antibacterial potential of Nigella sativa L. suspension culture under elicitation. BioMed Res. Int. 2015, 5, 708691. [Google Scholar]
| Metal/Concentration | Treatment Duration | Plant Species | Recovery Time | References |
|---|---|---|---|---|
| Cd (50 µM) | up to 4 days (96 h) | Tobacco suspension cells (Nicotiana tabacum) | 4 days | 54 |
| Cd (30 and 150 µM) | 35 days (5 weeks) | Tetradenia riparia | 21 days | 55 |
| Cd (10 and 25 mg/L; 89 and 223 µM) | 2 days (48 h) | Soybean (Glycine max) | 7 days | 56 |
| Zn (1026.4 µM) Cu (12.1 µM) Ni (81.8 µM) Cd (3.5 µM) | 3 days (72 h) | Duckweed (Lemna minor) | 7 days | 53 |
| Cu (1000 and 2000 ppm) Pb (1000 and 2000 ppm) Cu + Pb (500 and 1000 ppm each metal) | 20 days | Three species belonging to the Memosoideae family: Acacia tortilis, Acaica raddiana, Prosopis juliflora | 20 days | 51 |
| Cu (0.5–50 μM), Zn (1–100 μM) | 2 days (48 h) | Green alga Scenedesmus sp. | 4 days | 57 |
| Al (50 µM) | 1 day (24 h) | Rice (Oryza sativa) | 15 days | 58 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chmielowska-Bąk, J.; Deckert, J. Plant Recovery after Metal Stress—A Review. Plants 2021, 10, 450. https://doi.org/10.3390/plants10030450
Chmielowska-Bąk J, Deckert J. Plant Recovery after Metal Stress—A Review. Plants. 2021; 10(3):450. https://doi.org/10.3390/plants10030450
Chicago/Turabian StyleChmielowska-Bąk, Jagna, and Joanna Deckert. 2021. "Plant Recovery after Metal Stress—A Review" Plants 10, no. 3: 450. https://doi.org/10.3390/plants10030450
APA StyleChmielowska-Bąk, J., & Deckert, J. (2021). Plant Recovery after Metal Stress—A Review. Plants, 10(3), 450. https://doi.org/10.3390/plants10030450







