Suitability of Habitats in Nepal for Dactylorhiza hatagirea Now and under Predicted Future Changes in Climate
Abstract
1. Introduction
- (a)
- using recent data from field excursions;
- (b)
- mapping the potential suitable habitat using more predictors than Kunwar et al. [59] by adding geological substrate and soil properties, which makes the predictions more realistic;
- (c)
- making predictions about how the current potential distribution of this species will change under future climate change scenarios.
2. Results
2.1. Environmental Variables Associated with the Localities Where D. hatagirea Currently Occurs
2.2. Distribution and Habitat Suitability of D. hatagirea under Current Climate and Various Future Climate Change Scenarios
2.3. Numbers of Suitable Grid Cells of D. hatagirea under Current Climate vs. Those under Different Future Climate Change Scenarios
2.4. Percentage Change in Habitat Suitability of D. hatagirea under Current Climate and under Different Future Climate Change Scenarios
2.5. Change in Altitude of Potential Suitable Areas of D. hatagirea under Current Climate and under Different Future Climate Change Scenarios
3. Discussion
4. Materials and Methods
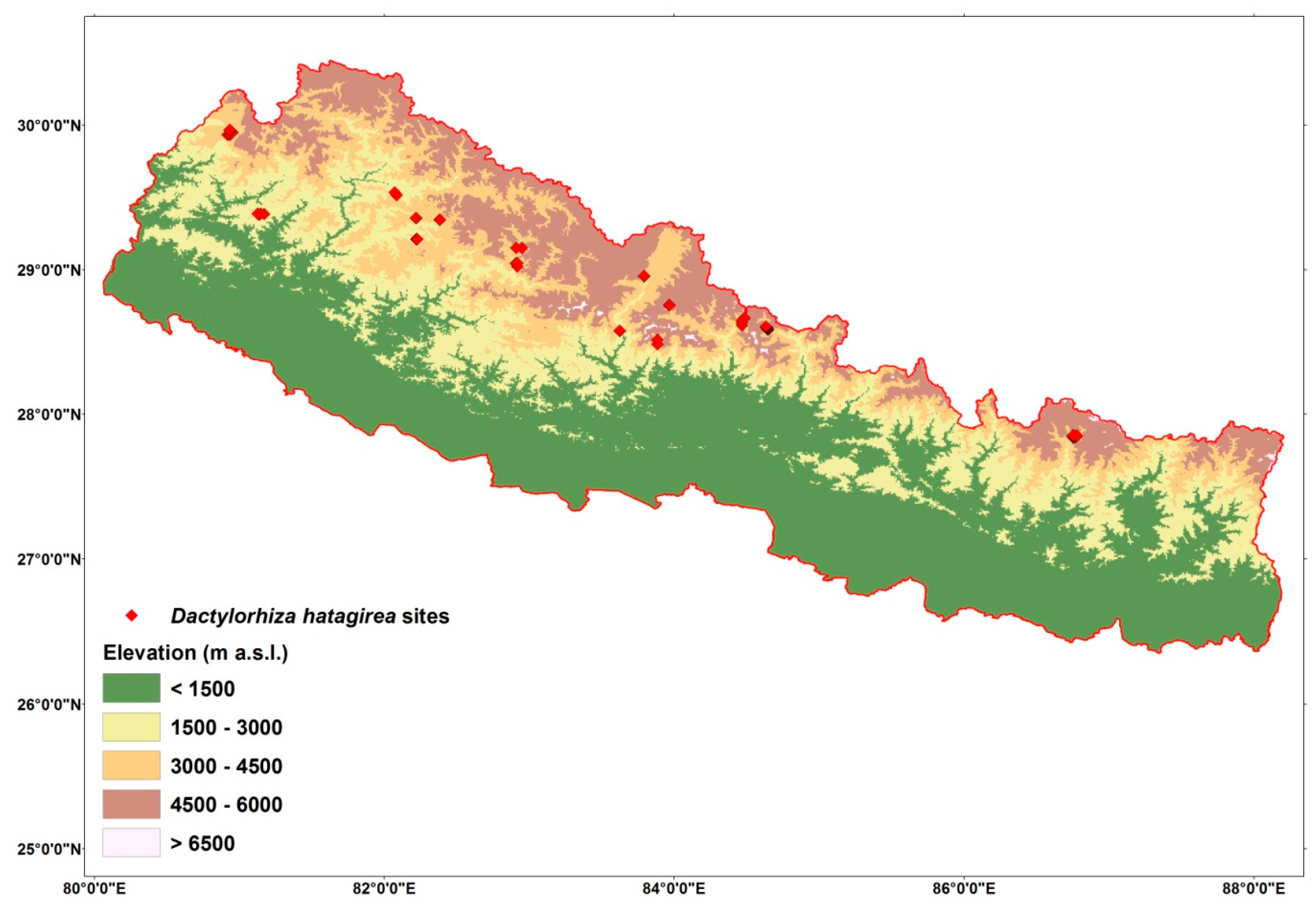
4.1. Study Area
4.2. Sampling and GPS Presence Locations
4.3. Predictive Distribution Model
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Intergovernmental Panel on Climate Change. Part B: Regional Aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. In Climate Change 2014: Impacts, Adaptation, and Vulnerability; Barros, V.R., Field, C.B., Dokken, D.J., Mastrandrea, M.D., Mach, K.J., Bilir, T.E., Chatterjee, M., Ebi, K.L., Estrada, Y.O., Genova, R.C., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2014; pp. 1–669. [Google Scholar]
- Hanski, I.; Zurita, G.A.; Bellocq, M.I.; Rybicki, J. Species-fragmented area relationship. Proc. Natl. Acad. Sci. USA 2013, 110, 12715–12720. [Google Scholar] [CrossRef] [PubMed]
- Qin, A.; Liu, B.; Guo, Q.; Bussmann, R.W.; Ma, F.; Jian, Z.; Xu, G.; Pei, S. Maxent modeling for predicting impacts of climate change on the potential distribution of Thuja sutchuenensis Franch., an extremely endangered conifer from southwestern China. Glob. Ecol. Conserv. 2017, 10, 139–146. [Google Scholar] [CrossRef]
- Antúnez, P.; Suárez-Mota, M.E.; Valenzuela-Encinas, C.; Ruiz-Aquino, F. The potential distribution of tree species in three periods of time under a climate change scenario. Forests 2018, 9, 628. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, H.; Yue, J.; Xie, T.; Xu, Y.; Tian, Y. Predicting shifts in the suitable climatic distribution of walnut (Juglans regia L.) in China: Maximum entropy model paves the way to forest management. Forests 2018, 9, 103. [Google Scholar]
- Pramanik, M.; Paudel, U.; Mondal, B.; Chakraborti, S.; Deb, P. Predicting climate change impacts on the distribution of the threatened Garcinia indica in the Western Ghats, India. Clim. Risk Manag. 2018, 19, 94–105. [Google Scholar] [CrossRef]
- Zhang, L.; Jing, Z.; Li, Z.; Liu, Y.; Fang, S. Predictive modeling of suitable habitats for Cinnamomum Camphora (L.) presl using maxent model under climate change in China. Int. J. Environ. Res. 2019, 16, 3185. [Google Scholar] [CrossRef] [PubMed]
- Cotrina Sánchez, D.A.; Barboza Castillo, E.; Rojas Briceño, N.B.; Oliva, M.; Torres Guzman, C.; Amasifuen Guerra, C.A.; Bandopadhyay, S. Distribution Models of Timber Species for Forest Conservation and Restoration in the Andean-Amazonian Landscape, North of Peru. Sustainability 2020, 12, 7945. [Google Scholar] [CrossRef]
- Gilani, H.; Arif Goheer, M.; Ahmad, H.; Hussain, K. Under predicted climate change: Distribution and ecological niche modelling of six native tree species in Gilgit-Baltistan, Pakistan. Ecol. Indic. 2020, 111, 106049. [Google Scholar] [CrossRef]
- Rojas, N.B.; Cotrina, D.A.; Castillo, E.B.; Oliva, M.; Salas, R. Current and Future Distribution of Five Timber Forest Species in Amazonas, Northeast Peru: Contributions towards a Restoration Strategy. Diversity 2020, 12, 305. [Google Scholar] [CrossRef]
- Guo, Y.; Wei, H.; Lu, C.; Gao, B.; Gu, W. Predictions of potential geographical distribution and quality of Schisandra sphenanthera under climate change. PeerJ 2016, 4, e2554. [Google Scholar] [CrossRef]
- Rana, S.K.; Rana, H.K.; Ghimire, S.K.; Shrestha, K.K.; Ranjitkar, S. Predicting the impact of climate change on the distribution of two threatened Himalayan medicinal plants of Liliaceae in Nepal. J. Mt. Sci. 2017, 14, 558–570. [Google Scholar] [CrossRef]
- Bai, Y.; Wei, X.; Li, X. Distributional dynamics of a vulnerable species in response to past and future climate change: A window for conservation prospects. PeerJ 2018, 6, e4287. [Google Scholar] [CrossRef] [PubMed]
- Abolmaali, S.M.R.; Tarkesh, M.; Bashari, H. Maxent modeling for predicting suitable habitats and identifying the effects of climate change on a threatened species, Daphne mucronata, in central Iran. Ecol. Inform. 2018, 43, 116–123. [Google Scholar] [CrossRef]
- You, J.; Qin, X.; Ranjitkar, S.; Lougheed, S.C.; Wang, M.; Zhou, W.; Ouyang, D.; Zhou, Y.; Xu, J.; Zhang, W.; et al. Response to climate change of montane herbaceous plants in the genus Rhodiola predicted by ecological niche modelling. Sci. Rep. 2018, 8, 5879. [Google Scholar] [CrossRef]
- Zhao, Q.; Li, R.; Gao, Y.; Yao, Q.; Guo, X.; Wang, W. Modeling impacts of climate change on the geographic distribution of medicinal plant Fritillaria cirrhosa D. Don. Plant Biosyst. 2018, 152, 349–355. [Google Scholar] [CrossRef]
- Abdelaal, M.; Fois, M.; Fenu, G.; Bacchetta, G. Using Maxent modeling to predict the potential distribution of the endemic plant Rosa arabica Crép. Egypt. Ecol. Inform. 2019, 50, 68–75. [Google Scholar] [CrossRef]
- Yuan, H.S.; Wei, Y.L.; Wang, X.G. Maxent modeling for predicting the potential distribution of Sanghuang, an important group of medicinal fungi in China. Fungal Ecol. 2015, 17, 140–145. [Google Scholar] [CrossRef]
- Shrestha, U.B.; Sharma, K.P.; Devkota, A.; Siwakoti, M.; Shrestha, B.B. Potential impact of climate change on the distribution of six invasive alien plants in Nepal. Ecol. Indic. 2018, 95, 99–107. [Google Scholar] [CrossRef]
- Kariyawasam, C.S.; Kumar, L.; Ratnayake, S.S. Invasive plant species establishment and range dynamics in Sri Lanka under climate change. Entropy 2019, 21, 571. [Google Scholar] [CrossRef]
- Ongaro, S.; Martellos, S.; Bacaro, G.; Agostini, A.D.; Cogoni, A.; Cortis, P. Distributional pattern of Sardinian orchids under a climate change scenario. Community Ecol. 2018, 19, 223–232. [Google Scholar] [CrossRef]
- Cavaliere, C. The effects of climate change on medicinal and aromatic plants. Herb. Gram. 2008, 81, 44–57. [Google Scholar]
- Kelly, A.E.; Goulden, M.L. Rapid shifts in plant distribution with recent climate change. Proc. Natl. Acad. Sci. USA 2008, 105, 11823–11826. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.C.; Hill, J.K.; Ohlemüller, R.; Roy, D.B.; Thomas, C.D. Rapid range shifts of species associated with high levels of climate warming. Science 2011, 333, 1024–1026. [Google Scholar] [CrossRef] [PubMed]
- Lenoir, J.; Svenning, J.C. Latitudinal and elevational Range Shifts under contemporary Climate Change. In Encyclopedia of Biodiversity, 2nd ed.; Levin, S., Ed.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 599–611. [Google Scholar]
- Suggitt, A.J.; Platts, P.J.; Barata, I.M.; Bennie, J.; Burgess, M.D.; Bystriakova, N.; Duffield, S.; Ewing, S.R.; Gillingham, P.K.; Harper, A.B.; et al. Conducting robust ecological analyses with climate data. Oikos 2017, 126, 1533–1541. [Google Scholar] [CrossRef]
- Štípková, Z.; Romportl, D.; Černocká, V.; Kindlmann, P. Factors associated with the distributions of orchids in the Jeseníky Mountains, Czech Republic. Eur. J. Environ. Sci. 2017, 7, 135–145. [Google Scholar] [CrossRef]
- La Sorte, F.A.; Jetz, W. Projected range contractions of montane biodiversity under global warming. Proc. R. Soc. Lond. 2010, 277, 3401–3410. [Google Scholar] [CrossRef] [PubMed]
- Intergovernmental Panel on Climate Change. The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. In Climate Change 2007; Solomon, S., Qin, D., Manning, M., Chen, Z., Marquis, M., Averyt, K.B., Tignor, M., Miller, H.L., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2007; pp. 1–996. [Google Scholar]
- Shrestha, U.B.; Gautam, S.; Bawa, K.S. Widespread climate change in the Himalayas and associated changes in local ecosystems. PLoS ONE 2012, 7, e36741. [Google Scholar] [CrossRef]
- Xu, J.; Grumbine, R.E.; Shrestha, A.; Eriksson, M.; Yang, X.; Wang, Y.; Wilkes, A. The melting Himalayas: Cascading effects of climate change on water, biodiversity, and livelihoods. Conserv. Biol. 2009, 23, 520–530. [Google Scholar] [CrossRef]
- González-Salazar, C.; Stephens, C.R.; Marquet, P.A. Comparing the relative contributions of biotic and abiotic factors as mediators of species’ distributions. Ecol. Model. 2013, 248, 57–70. [Google Scholar] [CrossRef]
- Telwala, Y.; Brook, B.W.; Manish, K.; Pandit, M.K. Climate-induced elevational range shifts and increase in plant species richness in a Himalayan biodiversity epicentre. PLoS ONE 2013, 8, e57103. [Google Scholar] [CrossRef]
- Thomas, C.D.; Cameron, A.; Green, R.E.; Bakkenes, M.; Beaumont, L.J.; Collingham, Y.C.; Erasmus, B.F.N.; de Siqueira, M.F.; Grainger, A.; Hannah, L.; et al. Extinction risk from climate change. Nature 2004, 42, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Dixon, K.W.; Kell, S.P.; Barrett, R.L.; Cribb, P.J. Orchid Conservation; Sabah Natural History Publications: Kota Kinabalu, Malaysia, 2003. [Google Scholar]
- Sharma, P.K.; Sharita, S.; Prell, J. Dactylor hizahatagirea (D. Don) Soo—A West Himalayan Orchid in Peril. Curr. Sci. 2005, 89, 610–612. [Google Scholar]
- Swarts, N.D.; Dixon, K.W. Terrestrial orchid conservation in the age of extinction. Ann. Bot. 2009, 104, 543–556. [Google Scholar] [CrossRef]
- Aggarwal, S.; Zettler, L.W. Reintroduction of an endangered terrestrial orchid Dactylorhiza hatagirea (D. Don) Soo, assisted by symbiotic seed germination: First report from the Indian subcontinent. Nat. Sci. 2010, 8, 139–145. [Google Scholar]
- Duffy, K.J.; Johnson, S.D. Specialized mutualisms may constrain the geographical distribution of flowering plants. Proc. R. Soc. B 2017, 284, 1841. [Google Scholar] [CrossRef] [PubMed]
- Kolanowska, M.; Jakubska-Busse, A. Is the lady’s-slipper orchid (Cypripedium calceolus) likely to shortly become extinct in Europe?—Insights based on ecological niche modelling. PLoS ONE 2020, 15, e0228420. [Google Scholar]
- Tsiftsis, S.; Djordjević, V. Modelling sexually deceptive orchid species distributions under future climates: The importance of plant–pollinator interactions. Sci. Rep. 2020, 10, 10623. [Google Scholar] [CrossRef]
- Conservation Assessment and Management Plan. Executive Summary Report of the Conservation Assessment and Management Plan (CAMP) of the Biodiversity Conservation Prioritization Project on Selected Medicinal Plants of Northern, North-Eastern and Central India. Available online: http://msubiology.info/vesna/nauka/pillon2006.pdf (accessed on 29 March 2014).
- Samant, S.S.; Dhar, U.; Rawal, R.S. (Eds.) Himalayan Medicinal Plants- Potential and Prospects; Gyanodaya Prakashan: Nainital, India, 2001. [Google Scholar]
- Go, N. Protected Plants of Nepal: Its amendments. Kathmandu, Nepal: Ministry of Forests and Soil Conservation. Nepal Gaz. 2011, 60, 38–45. [Google Scholar]
- Raskoti, B.B. The Orchids of Nepal; Bhakta Bahadur Raskoti and Rita Ale: Kathmandu, Nepal, 2009. [Google Scholar]
- Bhattarai, P.; Pandey, B.; Gautam, K.R.; Chhetri, R. Ecology and Conservation Status of Threatened Orchid Dactylorhiza hatagirea (D. Don) Soo in Manaslu Conservation Area, Central Nepal. Am. J. Plant Sci. 2014, 5, 3483–3491. [Google Scholar] [CrossRef]
- Shrestha, B.; Kindlmann, P.; Jnawali, S.R. Interactions between the Himalayan tahr, livestock and snow leopards in the Sagarmatha National Park. In Himalayan Biodiversity in the Changing World; Kindlmann, P., Ed.; Springer: Dordrecht, The Netherlands, 2012; pp. 115–145. [Google Scholar]
- Khadka, C.; Hammet, A.; Singh, A.; Balla, M.; Timilsina, Y. Ecological status and diversity indices of Panchaule (Dactylorhiza hatagirea) and its associates in Lete village of Mustang district, Nepal. Banko Janakari 2016, 26, 45–52. [Google Scholar] [CrossRef][Green Version]
- Chhetri, H.B.; Gupta, V.N.P. A survey of non-timber forest products (NTFPS) in Upper Mustang. Sci. World 2007, 5, 89–94. [Google Scholar] [CrossRef]
- International Union for Nature Conservation Nepal. National Register of Medicinal and Aromatic Plants; International Union for Nature Conservation Nepal: Kathmandu, Nepal, 2004. [Google Scholar]
- Pant, S.; Tsewang, R. Dactylorhiza hatagirea: A high value medicinal orchid. J. Med. Plant Res. 2012, 6, 3522–3524. [Google Scholar]
- Chamoli, K.P.; Sharan, H. Ethno-medicinal properties of Dactylorhiza hatagirea in higher Himalayan villages of Rudraprayag district of Uttarakhand. J. Mt. Res. 2019, 14, 85–88. [Google Scholar]
- Thakur, M.; Dixit, V.K. Aphrodisiac Activity of Dactylorhiza hatagirea (D. Don) Soo in Male Albino Rats. Evid. Based Complementary Altern. Med. 2007, 4, 29–31. [Google Scholar] [CrossRef] [PubMed]
- Popli, D. Elicitation of Dactylorhin–E and Studying Anti-Cancerous Potential of Dactylorhiza Hatagirea (D. Don). Master’s Dissertation, Biotechnology, Jaypee University of Information and Technology, Waknaghat, India, 2017. [Google Scholar]
- Kindlmann, P.; Kull, T.; Whigham, D.F.; Willems, J. Ecology and population dynamics of terrestrial orchids: An introduction. Folia Geobot. 2006, 41, 1–2. [Google Scholar] [CrossRef]
- Dubuis, A.; Pottier, J.; Rion, V.; Pellissier, L.; Theurillat, J.P.; Guisan, A. Predicting spatial patterns of plant species richness: A comparison of direct macroecological and species stacking approaches. Divers. Distrib. 2011. [Google Scholar] [CrossRef]
- Kaky, E.; Gilbert, F. Using species distribution models to assess the importance of Egypt’s protected areas for the conservation of medicinal plants. J. Arid Environ. 2016, 135, 140–146. [Google Scholar] [CrossRef]
- Pandey, B.; Timilsina, A.; Pandey, B.; Thapa, C.L.; Nepali, K.B.; Neupane, P.; Thapa, R.; Gaire, S.K.; Siwakoti, M. Peoples’ perception and conservation of Dactylorhiza hatagirea (D. Don) Soó in Manaslu Conservation Area, Central Nepal. Am. J. Plant Sci. 2016, 7, 1662–1672. [Google Scholar] [CrossRef]
- Kunwar, R.M.; Rimal, B.; Sharma, H.P.; Poudel, R.C.; Pyakurel, D.; Tiwari, A.; Magar, S.T.; Karki, G.; Bhandari, G.S.; Pandey, P.; et al. Distribution and habitat modeling of Dactylorhiza hatagirea (D. Don) Soo, Paris polyphylla Sm. and Taxus species in Nepal Himalaya. J. Appl. Res. Med. Aromat. Plants 2021, 20, 100274. [Google Scholar]
- Djordjević, V.; Tsiftsis, S. The Role of Ecological Factors in Distribution and Abundance of Terrestrial Orchids. In Orchids Phytochemistry, Biology and Horticulture; Mérillon, J.M., Kodja, H., Eds.; Springer: Cham, Switerland, 2020; pp. 1–71. [Google Scholar]
- Pearson, R.G.; Raxworthy, C.; Nakamura, M.; Peterson, A. Predicting species distributions from small numbers of occurrence records: A test case using cryptic geckos in Madagascar. J. Biogeogr. 2006, 34, 102–117. [Google Scholar] [CrossRef]
- Hernandez, P.A.; Graham, C.H.; Master, L.L.; Albert, D.L. The effect of sample size and species characteristics on performance of different species distribution modelling methods. Ecography 2006, 29, 773–785. [Google Scholar] [CrossRef]
- Bhattarai, S.; Chaudhary, R.P.; Taylor, R.S.L. Prioritization and trade of ethnomedicinal plants by the people of Manang district, central Nepal. In Vegetation and Society: Their Interaction in the Himalayas; Chaudhary, R.P., Subedi, B.P., Vetaas, O., Eds.; Tribhuvan University: Kathmandu, Nepal; University of Bergen Norway: Bergen, Norway, 2002; pp. 151–169. [Google Scholar]
- Subedi, A.; Kunwar, B.; Choi, Y.; Dai, Y.; van Andel, T.; Chaudhary, R.P.; de Boer, H.J.; Gravendeel, B. Collection and trade of wild-harvested orchids in Nepal. J. Ethnobiol. Ethnomed. 2013, 9, 64. [Google Scholar] [CrossRef] [PubMed]
- Thakur, R.B.; Yadav, R.P.; Thakur, N.P. Enumerating the status of orchid species of Makawanpur district. Hamro Kalpabricha 2010, 20, 1–18. [Google Scholar]
- Zaniewski, A.E.; Lehman, A.; Overton, J. Predicting species spatial distributions using presence-only data: A case study of native New Zealand ferns. Ecol. Model. 2002, 157, 261–280. [Google Scholar] [CrossRef]
- de Mesquita, C.P.B.; Tillmann, L.S.; Bernard, C.D.; Katherine, C.R.; Noah, P.M.; Suding, K.N. Topographic heterogeneity explains patterns of vegetation response to climate change (1972–2008) across a mountain landscape, Niwot Ridge, Colorado. Arct. Antarct. Alp. Res. 2018, 50, e1504492. [Google Scholar] [CrossRef]
- Amagai, Y.; Kudo, G.; Sato, K. Changes in alpine plant communities under climate change: Dynamics of snow-meadow vegetation in northern Japan over the last 40 years. Appl. Veg. Sci. 2018, 21, 561–571. [Google Scholar] [CrossRef]
- Shrestha, K.B.; Hofgaard, A.; Vandvik, V. Recent treeline dynamics are similarbetween dry and mesic areas of Nepal, central Himalaya. J. Plant Ecol. 2015, 8, 347–358. [Google Scholar] [CrossRef]
- Gaire, N.P.; Koirala, M.; Bhuju, D.R.; Carrer, M. Site- and species-specific treeline responses to climatic variability in eastern Nepal Himalaya. Dendrochronologia 2017, 41, 44–56. [Google Scholar] [CrossRef]
- Sakai, A.; Larcher, W. Frost Survival of Plants: Responses and Adaptation to Freezing Stress; Springer: Berlin, Germany, 1987. [Google Scholar]
- Thakur, D.; Rathore, M.; Sharma, M.K.; Chawla, A. Enhanced reproductive success revealed key strategy for persistence of devastated populations in Himalayan food-deceptive orchid, Dactylorhiza hatagirea. Plant Species Biol. 2018, 33, 191–202. [Google Scholar] [CrossRef]
- Memmott, J.; Craze, P.G.; Waser, N.M.; Price, M.V. Global warming and the disruption of plant–pollinator interactions. Ecol. Lett. 2007, 10, 710–717. [Google Scholar] [CrossRef]
- Kosanic, A.; Anderson, K.; Frère, C.H.; Harrison, S. Regional vegetation change and implications for local conservation: An example from West Cornwall (United Kingdom). Glob. Ecol. Conserv. 2015, 4, 405–413. [Google Scholar] [CrossRef]
- Magar, M.; Dhital, S.; Yamada, T.; Pun, U. Dactylorhiza hatagirea: A Critical Issue for Research and Development in Nepal. Nepal J. Sci. Technol. 2020, 19, 26–38. [Google Scholar] [CrossRef]
- Pearson, R.G. Species’ distribution modeling for conservation educators and practitioners. Lessons Conserv. 2010, 3, 54–89. [Google Scholar]
- Phillips, S.J.; Dudík, M. Modeling of species distributions with Maxent: New extensions and a comprehensive evaluation. Ecography 2008, 31, 161–175. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Dudík, M.; Schapire, R.E.; Blair, M.E. Opening the black box: An open-source release of Maxent. Ecography 2017, 40, 887–893. [Google Scholar] [CrossRef]
- Corsi, F.; De Leeuw, J.; Skidmore, A. Modelling species distribution with GIS. In Research Techniques in Animal Ecology; Controversies and Consequences; Boitan, L., Fuller, T.K., Eds.; Columbia University Press: New York, NY, USA, 2000; pp. 389–434. [Google Scholar]
- Elith, J.; Graham, C.H.; Anderson, R.P.; Dudik, M.; Ferrier, S.; Guisan, A.; Hijmans, R.J.; Huettmann, F.; Leathwick, J.R.; Lehmann, A.; et al. Novel methods improve prediction of species’ distributions from occurrence data. Ecography 2006, 29, 129–151. [Google Scholar] [CrossRef]
- Guisan, A.; Zimmerman, N.E. Predictive habitat distribution models in ecology. Ecol. Model. 2000, 135, 147–186. [Google Scholar] [CrossRef]
- Scott, J.M.; Heglund, P.J.; Morrison, M.L.; Haufler, J.B.; Raphael, M.G.; Wall, W.A.; Samson, F.B. (Eds.) Predicting Species Occurrences: Issues of Scale and Accuracy; Island Press: Washington, DC, USA, 2002. [Google Scholar]
- Franklin, J. Mapping Species Distributions: Spatial Inference and Prediction; Cambridge University Press: Cambridge, UK, 2009. [Google Scholar]
- Boyce, M.; McDonald, L. Relating populations to habitats using resource selection functions. Trends Ecol. Evol. 1999, 14, 268–272. [Google Scholar] [CrossRef]
- Manly, B.F.J.; McDonald, L.L.; Thomas, D.L.; MacDonald, T.L.; Erickson, W.P. (Eds.) Resource selection by animals. In Statistical Design and Analysis for Field Studies, 2nd ed.; Kluwer Academic Publisher: London, UK, 2002. [Google Scholar]
- McCullagh, P.; Nelder, J.A. Generalized Linear Models, 2nd ed.; Chapman and Hall/CRC: Washington, DC, USA, 1989. [Google Scholar]
- Ripley, B.D. Pattern Recognition and Neural Networks; Cambridge University Press: Cambridge, UK, 1996. [Google Scholar]
- Elith, J.; Leathwick, J. The contribution of species distribution modelling to conservation prioritization. In Spatial Conservation Prioritization. Quantitative Methods & Computational Tools; Moilanen, A., Wilson, A.K., Possingham, H.P., Eds.; Oxford University Press Inc.: New York, NY, USA, 2009; pp. 70–93. [Google Scholar]
- David, O.A.; Akomolafe, G.F.; Onwusiri, K.C.; Fabolude, G.O. Predicting the distribution of the invasive species Hyptis suaveolens in Nigeria. Eur. J. Environ. Sci. 2020, 10, 98–106. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Clim. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Dijkshoorn, J.A.; Huting, J.R.M. Soil and Terrain Database for Nepal; ISRIC—World Soil Information: Wageningen, The Netherlands, 2009; Available online: http://www.isric.org (accessed on 9 April 2019).
- Warren, D.L.; Glor, R.E.; Turelli, M. ENMTools: A toolbox for comparative studies of environmental niche models. Ecography 2010, 33, 607–611. [Google Scholar] [CrossRef]
- Warren, D.L.; Seifert, S.N. Ecological niche modeling in Maxent: The importance of model complexity and the performance of model selection criteria. Ecol. Appl. 2011, 21, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Liu, T.; Li, L.; Zhao, Y.; Pei, L.; Zhao, J. Predicting the potential distribution of Polygala tenuifolia Willd. under climate change in China. PLoS ONE 2016, 11, e0163718. [Google Scholar] [CrossRef] [PubMed]
- Freeman, E.A.; Moisen, G.G. A comparison of the performance of threshold criteria for binary classification in terms of predicted prevalence and kappa. Ecol. Model. 2008, 217, 48–58. [Google Scholar] [CrossRef]
- Kremen, C.A.; Cameron, A.; Moilanen, S.J.; Phillips, C.D.; Thomas, H.; Beentje, J.; Dransfield, B.L.; Fisher, F.; Glaw, T.C.; Good, G.J.; et al. Aligning conservation priorities across taxa in Madagascar with high-resolution planning tools. Science 2008, 320, 222–226. [Google Scholar] [CrossRef] [PubMed]
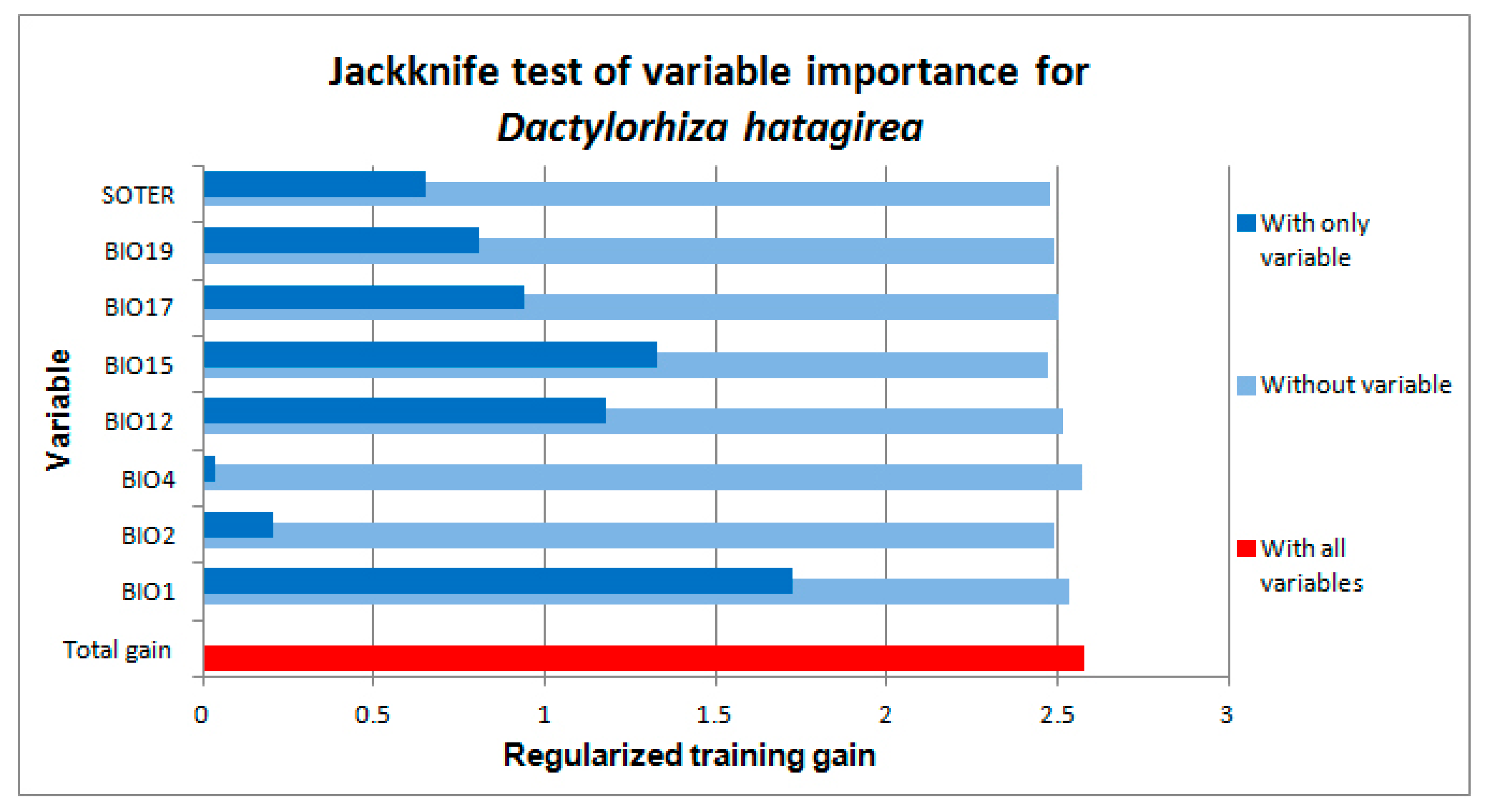


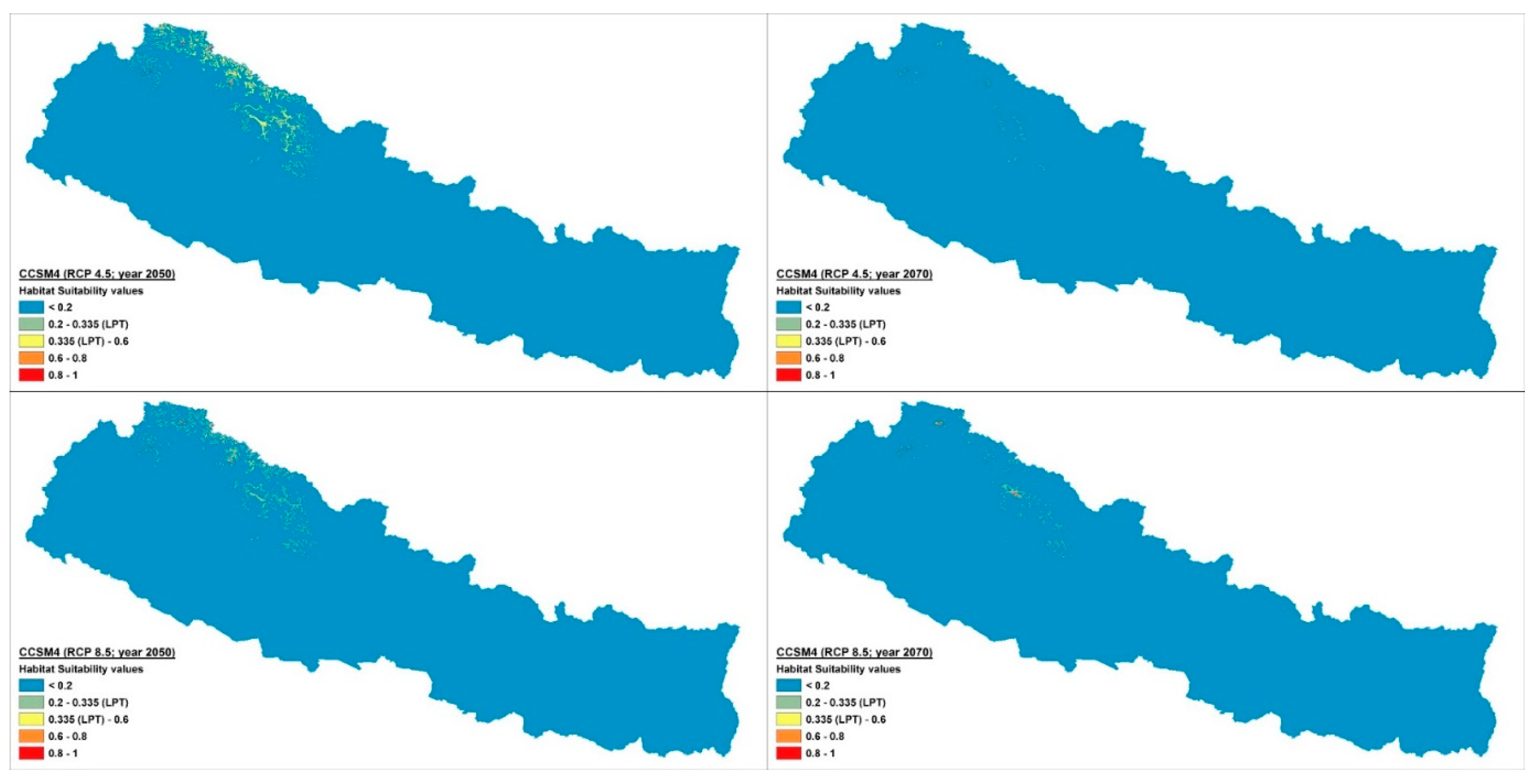

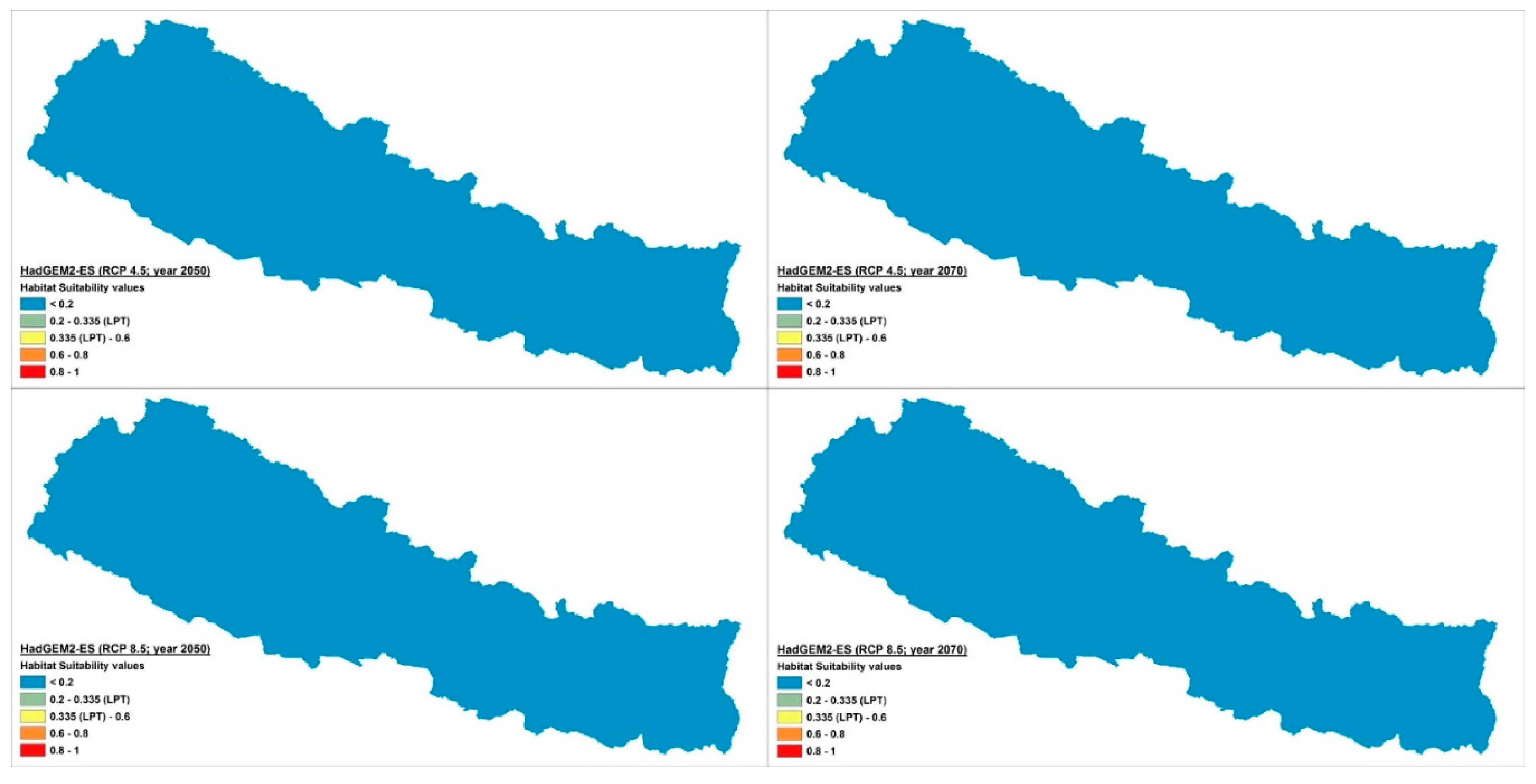
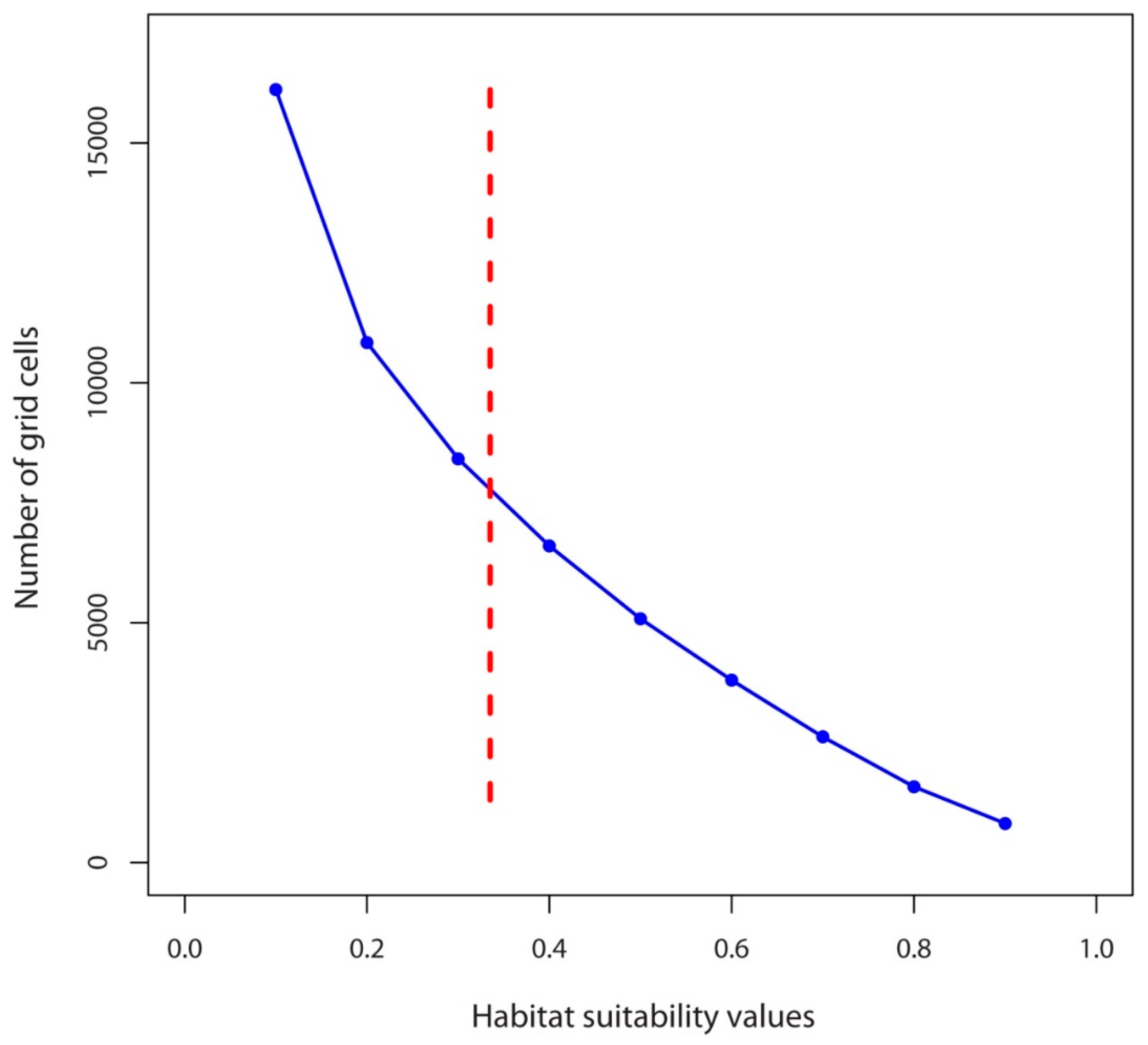





| Bio1 | Bio2 | Bio3 | Bio4 | Bio5 | Bio6 | Bio7 | Bio8 | Bio9 | Bio10 | Bio11 | Bio12 | Bio13 | Bio14 | Bio15 | Bio16 | Bio17 | Bio18 | Bio19 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bio1 | 0 | −0.04 | 0.62 | −0.69 | 0.99 | 0.99 | −0.58 | 0.98 | 0.96 | 0.99 | 0.99 | 0.78 | 0.84 | −0.16 | 0.78 | 0.83 | −0.06 | 0.69 | −0.66 |
| Bio2 | 0 | 0 | 0.19 | 0.32 | 0.05 | −0.13 | 0.54 | −0.03 | −0.04 | 0.003 | −0.08 | −0.18 | −0.10 | −0.33 | 0.01 | −0.11 | −0.15 | −0.24 | −0.03 |
| Bio3 | 0 | 0 | 0 | −0.81 | 0.56 | 0.67 | −0.68 | 0.55 | 0.49 | 0.56 | 0.67 | 0.55 | 0.51 | 0.10 | 0.32 | 0.54 | 0.15 | 0.51 | −0.47 |
| Bio4 | 0 | 0 | 0 | 0 | −0.59 | −0.78 | 0.95 | −0.61 | −0.57 | −0.61 | −0.75 | −0.65 | −0.57 | −0.25 | −0.31 | −0.60 | −0.20 | −0.63 | 0.49 |
| Bio5 | 0 | 0 | 0 | 0 | 0 | 0.96 | −0.46 | 0.97 | 0.96 | 0.99 | 0.97 | 0.77 | 0.82 | −0.23 | 0.81 | 0.81 | −0.11 | 0.66 | −0.66 |
| Bio6 | 0 | 0 | 0 | 0 | 0 | 0 | −0.69 | 0.96 | 0.93 | 0.97 | 0.99 | 0.82 | 0.83 | −0.08 | 0.73 | 0.82 | −0.01 | 0.72 | −0.64 |
| Bio7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | −0.51 | −0.47 | −0.50 | −0.65 | −0.60 | −0.51 | −0.33 | −0.25 | −0.54 | −0.26 | −0.60 | 0.36 |
| Bio8 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.95 | 0.98 | 0.96 | 0.79 | 0.83 | −0.18 | 0.81 | 0.82 | −0.09 | 0.69 | −0.65 |
| Bio9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.97 | 0.94 | 0.76 | 0.81 | −0.22 | 0.78 | 0.79 | −0.13 | 0.65 | −0.61 |
| Bio10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.98 | 0.79 | 0.83 | −0.21 | 0.81 | 0.82 | −0.09 | 0.67 | −0.65 |
| Bio11 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.81 | 0.83 | −0.11 | 0.74 | 0.82 | −0.03 | 0.71 | −0.66 |
| Bio12 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.97 | −0.03 | 0.74 | 0.98 | −0.10 | 0.95 | −0.61 |
| Bio13 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | −0.14 | 0.84 | 0.99 | −0.18 | 0.91 | −0.64 |
| Bio14 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | −0.41 | −0.13 | 0.81 | −0.03 | 0.54 |
| Bio15 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.82 | −0.38 | 0.67 | −0.67 |
| Bio16 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | −0.18 | 0.94 | −0.66 |
| Bio17 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | −0.19 | 0.67 |
| Bio18 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | −0.63 |
| Bio19 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Model | Year | RCP Pathway | −1 (Range Expansion) | 0 (Absence in Both) | 1 (Present in Both) | 2 (Range Contraction) | Coverage Change |
|---|---|---|---|---|---|---|---|
| BCC-CSM1-1 | 2050 | 4.5 | 2,085,974 | 136,487,198 | 3805 | 8,143,194 | −74.35% |
| 8.5 | 1,133,826 | 137,439,346 | 12,366 | 8,134,633 | −85.93% | ||
| 2070 | 4.5 | 2,818,395 | 135,754,777 | 15,219 | 8,131,780 | −65.22% | |
| 8.5 | 1,890,028 | 136,683,145 | 12,366 | 8,134,633 | −76.65% | ||
| CCSM4 | 2050 | 4.5 | 2,330,432 | 136,242,741 | 3805 | 8,143,194 | −71.35% |
| 8.5 | 1,502,891 | 137,070,282 | 1902 | 8,145,096 | −81.53% | ||
| 2070 | 4.5 | 91,315 | 138,481,858 | 0951 | 8,146,048 | −98.87% | |
| 8.5 | 389,991 | 138,183,182 | 5707 | 8,141,292 | −95.14% | ||
| HadGEM2-ES | 2050 | 4.5 | 4756 | 138,568,416 | 0 | 8,146,999 | −99.94% |
| 8.5 | 0 | 138,573,172 | 0 | 8,146,999 | −100.00% | ||
| 2070 | 4.5 | 0 | 138,573,172 | 0 | 8,146,999 | −100.00% | |
| 8.5 | 0 | 138,573,172 | 0 | 8,146,999 | −100.00% | ||
| MIROC5 | 2050 | 4.5 | 1,776,835 | 136,796,337 | 3805 | 8,143,194 | −78.14% |
| 8.5 | 1,385,893 | 137,187,279 | 8561 | 8,138,438 | −82.88% | ||
| 2070 | 4.5 | 605,912 | 137,967,260 | 3805 | 8,143,194 | −92.52% | |
| 8.5 | 1,122,412 | 137,450,760 | 16,170 | 8,130,828 | −86.02% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shrestha, B.; Tsiftsis, S.; Chapagain, D.J.; Khadka, C.; Bhattarai, P.; Kayastha Shrestha, N.; Alicja Kolanowska, M.; Kindlmann, P. Suitability of Habitats in Nepal for Dactylorhiza hatagirea Now and under Predicted Future Changes in Climate. Plants 2021, 10, 467. https://doi.org/10.3390/plants10030467
Shrestha B, Tsiftsis S, Chapagain DJ, Khadka C, Bhattarai P, Kayastha Shrestha N, Alicja Kolanowska M, Kindlmann P. Suitability of Habitats in Nepal for Dactylorhiza hatagirea Now and under Predicted Future Changes in Climate. Plants. 2021; 10(3):467. https://doi.org/10.3390/plants10030467
Chicago/Turabian StyleShrestha, Bikram, Spyros Tsiftsis, Deep Jyoti Chapagain, Chhatra Khadka, Prakash Bhattarai, Neelima Kayastha Shrestha, Marta Alicja Kolanowska, and Pavel Kindlmann. 2021. "Suitability of Habitats in Nepal for Dactylorhiza hatagirea Now and under Predicted Future Changes in Climate" Plants 10, no. 3: 467. https://doi.org/10.3390/plants10030467
APA StyleShrestha, B., Tsiftsis, S., Chapagain, D. J., Khadka, C., Bhattarai, P., Kayastha Shrestha, N., Alicja Kolanowska, M., & Kindlmann, P. (2021). Suitability of Habitats in Nepal for Dactylorhiza hatagirea Now and under Predicted Future Changes in Climate. Plants, 10(3), 467. https://doi.org/10.3390/plants10030467








