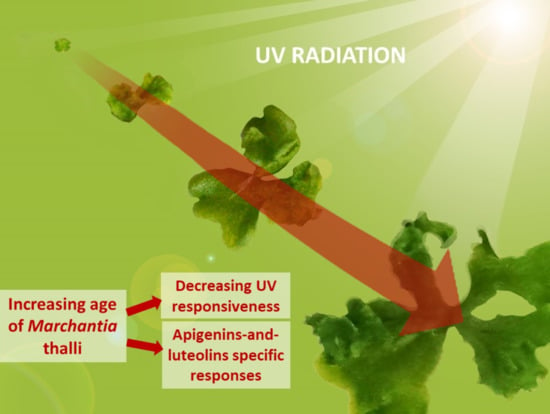
Developmental Stage Determines the Accumulation Pattern of UV-Absorbing Compounds in the Model Liverwort Marchantia polymorpha subsp. ruderalis under Controlled Conditions
Abstract
1. Introduction
2. Results
2.1. Individual UV-Absorbing Compounds Found
2.2. Influence of the Radiation Regime and Developmental Stage on the Physiological Variables
2.3. Principal Components Analysis (PCA) Ordination of Samples
3. Discussion
3.1. Phenolic Composition of the Liverwort Samples
| No Subspecies Assigned | Subsp. polymorpha | Subsp. montivagans | Subsp. ruderalis | ||||
|---|---|---|---|---|---|---|---|
| Tak-1 Accession | Local Wild Accession | Sey-1 and Aud-2 Accessions | No Accession Assigned | ||||
| Apigenin | [20,33] | [34] | - | [23,26] | [28] | - | [34] |
| Apigenin 7-O-glucoside | [33] | - | - | - | - | - | - |
| Apigenin 7-O-glucuronide | [20] | [27,29,34] | [29] | [26] (this study) | [28] | [21,22] | [29,34] |
| Apigenin 7,4′-di-O-glucuronide | [20,33] | [27,29,34] | [29] | [26] (this study) | [28] | - | [29,34] |
| Apigenin glucuronide | - | - | - | [23] | - | - | - |
| Luteolin | [20] | [34] | - | [26] | [28] | - | [34] |
| Luteolin 3′-O-glucuronide | [20] | [29,34] | [29] | [26] (this study) | [28] | [22] | [29,34] |
| Luteolin 4′-O-glucuronide | [20] | - | - | [26] (this study) | - | [21] | - |
| Luteolin 7-O-glucuronide | [20] | [27,29,34] | [29] | [23,26] (this study) | [28] | [21,22] | [29,34] |
| Luteolin 3′,4′-di-O-glucuronide | - | [29,34] | [29] | - | - | - | [29,34] |
| Luteolin 7,3′-di-O-glucuronide | [20] | [29,34] | [29] | [23,26] (this study) | [28] | [21,22] | [29,34] |
| Luteolin 7,4′-di-O-glucuronide | [20] | [27,29,34] | [29] | [23] | [28] | - | [29,34] |
| Luteolin 7,3′,4′-tri-O-glucuronide | - | [34] | - | - | - | - | [29,34] |
| Baicalein 6,7-di-O-glucopyranuronoside | [33] | - | - | - | - | - | - |
| Chrysoeriol 7-O-neohesperidoside | [33] | - | - | - | - | - | - |
3.2. Effects of UV Radiation
3.3. Effects of Developmental Stage
3.4. Ordination of Samples by PCA
4. Materials and Methods
4.1. Plant Material and Culture Conditions
- P (only PAR), using XT Vitroflex 395 Solarium Incoloro (Polimer Tecnic, Girona, Spain), which cut off UV radiation.
- PAB (PAR + UV-A + UV-B), using Ultraphan 295 (Digefra GmbH, Munich, Germany), which cut off UV-C radiation.
4.2. Variables Measured
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Housti, F.; Andary, C.; Gargadennec, A.; Amssa, M. Effects of wounding and salicylic acid on hydroxycinnamoylmalic acids in Thunbergia alata. Plant Physiol. Biochem. 2002, 40, 761–769. [Google Scholar] [CrossRef]
- Bates, J.W. The relationship between physiological vitality and age in shoot segments of Pleurozium schreberi (Brid.) Mitt. J. Bryol. 1979, 10, 339–351. [Google Scholar] [CrossRef]
- Rütten, D.; Santarius, K.A. Age-related differences in frost sensitivity of the photosynthetic apparatus of two Plagiomnium species. Planta 1992, 187, 224–229. [Google Scholar] [CrossRef]
- Arróniz-Crespo, M.; Phoenix, G.; Núñez-Olivera, E.; Martínez-Abaigar, J. Age-specific physiological responses to UV radiation in the aquatic liverwort Jungermannia exsertifolia subsp. cordifolia. Cryptogam. Bryol. 2008, 29, 115–126. [Google Scholar]
- Rühling, A.; Tyler, G. Sorption and retention of heavy metals in the woodland moss Hylocomium splendens (Hedw.) Br. et Sch. Oikos 1970, 21, 92–97. [Google Scholar] [CrossRef]
- Chapin, F.S., III; Oechel, W.C.; Van Cleve, K.; Lawrence, W. The role of mosses in the phosphorus cycling of an Alaskan black spruce forest. Oecologia 1987, 74, 310–315. [Google Scholar] [CrossRef]
- Martínez-Abaigar, J.; García-Álvaro, M.A.; Beaucourt, N.; Núñez-Olivera, E. Combined seasonal and longitudinal variations of element concentrations in two aquatic mosses (Fontinalis antipyretica and F. squamosa). Nova Hedwigia 2002, 74, 349–364. [Google Scholar] [CrossRef]
- Boquete, M.T.; Aboal, J.R.; Carballeira, A.; Fernandez, J.A. Effect of age on the heavy metal concentration in segments of Pseudoscleropodium purum and the biomonitoring of atmospheric deposition of metals. Atmos. Environ. 2014, 86, 28–34. [Google Scholar] [CrossRef]
- Kälviäinen, E.; Karunen, P.; Ekman, R. Age-related contents of polymerized lipids in the ectohydric forest mosses Pleurozium schreberi and Hylocomium splendens. Physiol. Plant. 1985, 65, 269–274. [Google Scholar] [CrossRef]
- Xu, S.J.; Jiang, P.A.; Wang, Z.W.; Wang, Y. Crystal structures and chemical composition of leaf surface wax depositions on the desert moss Syntrichia caninervis. Biochem. Syst. Ecol. 2009, 37, 723–730. [Google Scholar] [CrossRef]
- Majer, P.; Hideg, E. Developmental stage is an important factor that determines the antioxidant responses of young and old grapevine leaves under UV irradiation in a green-house. Plant Physiol. Biochem. 2012, 50, 15–23. [Google Scholar] [CrossRef]
- Csepregi, K.; Coffey, A.; Cunningham, N.; Prinsen, E.; Hideg, E.; Jansen, M.A.K. Developmental age and UV-B exposure co-determine antioxidant capacity and flavonol accumulation in Arabidopsis leaves. Environ. Exp. Bot. 2017, 140, 19–25. [Google Scholar] [CrossRef]
- Holub, P.; Nezval, J.; Štroch, M.; Špunda, V.; Urban, O.; Jansen, M.A.K.; Klem, K. Induction of phenolic compounds by UV and PAR is modulated by leaf ontogeny and barley genotype. Plant Physiol. Biochem. 2019, 134, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Nazari, M.; Zarinkamar, F. Ultraviolet-B induced changes in Mentha aquatica (a medicinal plant) at early and late vegetative growth stages: Investigations at molecular and genetic levels. Ind. Crops Prod. 2020, 154, 112618. [Google Scholar] [CrossRef]
- Reifenrath, K.; Müller, C. Species-specific and leaf-age dependent effects of ultraviolet radiation on two Brassicaceae. Phytochemistry 2007, 68, 875–885. [Google Scholar] [CrossRef] [PubMed]
- Klem, K.; Ac, A.; Holub, P.; Kovac, D.; Spunda, V.; Robson, T.M.; Urban, O. Interactive effects of PAR and UV radiation on the physiology, morphology and leaf optical properties of two barley varieties. Environ. Exp. Bot. 2012, 75, 52–64. [Google Scholar] [CrossRef]
- Müller, V.; Lankes, C.; Albert, A.; Barbro Winkler, J.; Zimmermann, B.F.; Noga, G.; Hunsche, M. Concentration of hinokinin, phenolic acids and flavonols in leaves and stems of Hydrocotyle leucocephala is differently influenced by PAR and ecologically relevant UV-B level. J. Plant Physiol. 2015, 173, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Robson, T.M.; Aphalo, P.J.; Banas, A.K.; Barnes, P.W.; Brelsford, C.C.; Jenkins, G.I.; Kotilainen, T.K.; Labuz, J.; Martínez-Abaigar, J.; Morales, L.O.; et al. A perspective on ecologically relevant plant-UV research and its practical application. Photochem. Photobiol. Sci. 2019, 18, 970–988. [Google Scholar] [CrossRef]
- Bowman, J.L.; Araki, T.; Kohchi, T. Marchantia: Past, Present and Future. Plant Cell Physiol. 2016, 57, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Markham, K.R.; Ryan, K.G.; Bloor, S.J.; Mitchell, K.A. An increase in the luteolin:apigenin ratio in Marchantia polymorpha on UV-B enhancement. Phytochemistry 1998, 48, 791–794. [Google Scholar] [CrossRef]
- Albert, N.W.; Thrimawithana, A.H.; McGhie, T.K.; Clayton, W.A.; Deroles, S.C.; Schwinn, K.E.; Bowman, J.L.; Jordan, B.R.; Davies, K.M. Genetic analysis of the liverwort Marchantia polymorpha reveals that R2R3MYB activation of flavonoid production in response to abiotic stress is an ancient character in land plants. New Phytol. 2018, 218, 554–566. [Google Scholar] [CrossRef]
- Clayton, W.A.; Albert, N.W.; Thrimawithana, A.H.; McGhie, T.K.; Deroles, S.C.; Schwinn, K.E.; Warren, B.A.; McLachlan, A.R.G.; Bowman, J.L.; Jordan, B.R.; et al. UVR8-mediated induction of flavonoid biosynthesis for UVB tolerance is conserved between the liverwort Marchantia polymorpha and flowering plants. Plant J. 2018, 96, 503–517. [Google Scholar] [CrossRef]
- Kubo, H.; Nozawa, S.; Hiwatashi, T.; Kondou, Y.; Nakabayashi, R.; Mori, T.; Saito, K.; Takanashi, K.; Kohchi, T.; Ishizaki, K. Biosynthesis of riccionidins and marchantins is regulated by R2R3-MYB transcription factors in Marchantia polymorpha. J. Plant Res. 2018, 131, 849–864. [Google Scholar] [CrossRef]
- Soriano, G.; Cloix, C.; Heilmann, M.; Núñez-Olivera, E.; Martínez-Abaigar, J.; Jenkins, G.I. Evolutionary conservation of structure and function of the UVR8 photoreceptor from the liverwort Marchantia polymorpha and the moss Physcomitrella patens. New Phytol. 2018, 217, 151–162. [Google Scholar] [CrossRef]
- Kondou, Y.; Miyagi, Y.; Morito, T.; Fujihira, K.; Miyauchi, W.; Moriyama, A.; Terasawa, T.; Ishida, S.; Iwabuchi, K.; Kubo, H.; et al. Physiological function of photoreceptor UVR8 in UV-B tolerance in the liverwort Marchantia polymorpha. Planta 2019, 249, 1349–1364. [Google Scholar] [CrossRef] [PubMed]
- Soriano, G.; Del-Castillo-Alonso, M.Á.; Monforte, L.; Tomás-Las-Heras, R.; Martínez-Abaigar, J.; Núñez-Olivera, E. Photosynthetically-active radiation, UV-A and UV-B, causes both common and specific damage and photoprotective responses in the model liverwort Marchantia polymorpha subsp. ruderalis. Photochem. Photobiol. Sci. 2019, 18, 400–412. [Google Scholar] [CrossRef] [PubMed]
- Soriano, G.; Del-Castillo-Alonso, M.Á.; Monforte, L.; Núñez-Olivera, E.; Martínez-Abaigar, J. Acclimation of Bryophytes to Sun Conditions, in Comparison to Shade Conditions, Is Influenced by Both Photosynthetic and Ultraviolet Radiations. Front. Plant Sci. 2019, 10, 998. [Google Scholar] [CrossRef]
- Soriano, G.; Del-Castillo-Alonso, M.Á.; Monforte, L.; Núñez-Olivera, E.; Martínez-Abaigar, J. Phenolic compounds from different bryophyte species and cell compartments respond specifically to ultraviolet radiation, but not particularly quickly. Plant Physiol. Biochem. 2019, 134, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Campbell, E.O.; Markham, K.R.; Moore, N.A.; Porter, L.J.; Wallace, J.W. Taxonomic and phylogenetic implications of the comparative flavonoid chemistry of species in the family Marchantiaceae. J. Hatt. Bot. Lab. 1979, 45, 185–199. [Google Scholar]
- Martens, S.; Mithöfer, A. Flavones and flavone synthases. Phytochemistry 2005, 66, 2399–2407. [Google Scholar] [CrossRef]
- Agati, G.; Brunetti, C.; Fini, A.; Gori, A.; Guidi, L.; Landi, M.; Sebastiani, F.; Tattini, M. Are Flavonoids Effective Antioxidants in Plants? Twenty Years of Our Investigation. Antioxidants 2020, 9, 1098. [Google Scholar] [CrossRef] [PubMed]
- Gendrisch, F.; Esser, P.R.; Schempp, C.M.; Wolfle, U. Luteolin as a modulator of skin aging and inflammation. Biofactors 2021. [Google Scholar] [CrossRef]
- Wang, X.; Cao, J.G.; Wu, Y.H.; Wang, Q.X.; Xiao, J.B. Flavonoids, Antioxidant Potential, and Acetylcholinesterase Inhibition Activity of the Extracts from the Gametophyte and Archegoniophore of Marchantia polymorpha L. Molecules 2016, 21, 360. [Google Scholar] [CrossRef] [PubMed]
- Markham, K.R.; Porter, I.J. Flavonoids of the liverwort Marchantia polymorpha. Phytochemistry 1974, 13, 1937–1942. [Google Scholar] [CrossRef]
- Bischler-Causse, H.; Boisselier-Dubayle, M.C. Lectotypification of Marchantia polymorpha L. J. Bryol. 1991, 16, 361–365. [Google Scholar] [CrossRef]
- Bischler-Causse, H. Marchantia L. The European and African Taxa; J. Cramer: Berlin, Germany, 1993; pp. 1–129. [Google Scholar]
- Shimamura, M. Marchantia polymorpha: Taxonomy, Phylogeny and Morphology of a Model System. Plant Cell Physiol. 2016, 57, 230–256. [Google Scholar] [CrossRef]
- Linde, A.M.; Sawangproh, W.; Cronberg, N.; Szovenyi, P.; Lagercrantz, U. Evolutionary History of the Marchantia polymorpha Complex. Front. Plant Sci. 2020, 11, 829. [Google Scholar] [CrossRef]
- Hodgetts, N.G.; Söderström, L.; Blockeel, T.L.; Caspari, S.; Ignatov, M.S.; Konstantinova, N.A.; Lockhart, N.; Papp, B.; Schröck, C.; Sim-Sim, M.; et al. An annotated checklist of bryophytes of Europe, Macaronesia and Cyprus. J. Bryol. 2020, 42, 1–116. [Google Scholar] [CrossRef]
- Häder, D.P.; Lebert, M.; Schuster, M.; Del Campo, L.; Helbling, E.W.; McKenzie, R. ELDONET—A decade of monitoring solar radiation on five continents. Photochem. Photobiol. 2007, 83, 1348–1357. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Abaigar, J.; Núñez-Olivera, E. Aquatic bryophytes under ultraviolet radiation. In Bryophyte Ecology and Climate Change; Tuba, Z., Slack, N.G., Stark, L.R., Eds.; Cambridge University Press: New York, NY, USA, 2011; pp. 115–146. [Google Scholar]
- Searles, P.S.; Flint, S.D.; Caldwell, M.M. A meta-analysis of plant field studies simulating stratospheric ozone depletion. Oecologia 2001, 127, 1–10. [Google Scholar] [CrossRef]
- Newsham, K.K.; Robinson, S.A. Responses of plants in polar regions to UVB exposure: A meta-analysis. Glob. Chang. Biol. 2009, 15, 2574–2589. [Google Scholar] [CrossRef]
- Di Ferdinando, M.; Brunetti, C.; Agati, G.; Tattini, M. Multiple functions of polyphenols in plants inhabiting unfavorable Mediterranean areas. Environ. Exp. Bot. 2014, 103, 107–116. [Google Scholar] [CrossRef]
- Yang, B.X.; Guan, Q.J.; Tian, J.K.; Komatsu, S. Transcriptomic and proteomic analyses of leaves from Clematis terniflora DC. under high level of ultraviolet-B irradiation followed by dark treatment. J. Proteom. 2017, 150, 323–340. [Google Scholar] [CrossRef] [PubMed]
- Righini, S.; Rodriguez, E.J.; Berosich, C.; Grotewold, E.; Casati, P.; Falcone Ferreyra, M.L. Apigenin produced by maize flavone synthase I and II protects plants against UV-B-induced damage. Plant Cell Environ. 2019, 42, 495–508. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, D.; Yang, Z.; Zeng, Q.W.; Luo, Y.W.; He, N.J. Flavones Produced by Mulberry Flavone Synthase Type I Constitute a Defense Line against the Ultraviolet-B Stress. Plants 2020, 9, 215. [Google Scholar] [CrossRef]
- Ryan, K.G.; Burne, A.; Seppelt, R.D. Historical ozone concentrations and flavonoid levels in herbarium specimens of the Antarctic moss Bryum argenteum. Glob. Chang. Biol. 2009, 15, 1694–1702. [Google Scholar] [CrossRef]
- Wang, H.J.; Liu, S.H.; Wang, T.L.; Liu, H.W.; Xu, X.H.; Chen, K.S.; Zhang, P.Y. The moss flavone synthase I positively regulates the tolerance of plants to drought stress and UV-B radiation. Plant Sci. 2020, 298, 110591. [Google Scholar] [CrossRef]
- Davies, K.M.; Jibran, R.; Zhou, Y.; Albert, N.W.; Brummell, D.A.; Jordan, B.R.; Bowman, J.L.; Schwinn, K.E. The Evolution of Flavonoid Biosynthesis: A Bryophyte Perspective. Front. Plant Sci. 2020, 11, 7. [Google Scholar] [CrossRef]
- Del-Castillo-Alonso, M.A.; Diago, M.P.; Tomás-Las-Heras, R.; Monforte, L.; Soriano, G.; Martínez-Abaigar, J.; Núñez-Olivera, E. Effects of ambient solar UV radiation on grapevine leaf physiology and berry phenolic composition along one entire season under Mediterranean field conditions. Plant Physiol. Biochem. 2016, 109, 374–386. [Google Scholar] [CrossRef]
- Del-Castillo-Alonso, M.Á.; Monforte, L.; Tomás-Las-Heras, R.; Martínez-Abaigar, J.; Núñez-Olivera, E. Phenolic characteristics acquired by berry skins of Vitis vinifera cv. Tempranillo in response to close-to-ambient solar ultraviolet radiation are mostly reflected in the resulting wines. J. Sci. Food Agric. 2020, 100, 401–409. [Google Scholar] [CrossRef]
- Furst-Jansen, J.M.R.; De Vries, S.; De Vries, J. Evo-physio: On stress responses and the earliest land plants. J. Exp. Bot. 2020, 71, 3254–3269. [Google Scholar] [CrossRef]
- Li, D.D.; Ni, R.; Wang, P.P.; Zhang, X.S.; Wang, P.Y.; Zhu, T.T.; Sun, C.J.; Liu, C.J.; Lou, H.X.; Cheng, A.X. Molecular Basis for Chemical Evolution of Flavones to Flavonols and Anthocyanins in Land Plants. Plant Physiol. 2020, 184, 1731–1743. [Google Scholar] [CrossRef]
- Wolf, L.; Rizzini, L.; Stracke, R.; Ulm, R.; Rensing, S.A. The Molecular and Physiological Responses of Physcomitrella patens to Ultraviolet-B Radiation. Plant Physiol. 2010, 153, 1123–1134. [Google Scholar] [CrossRef]
- Suzuki, T.; Honda, Y.; Mukasa, Y. Effects of UV-B radiation, cold and desiccation stress on rutin concentration and rutin glucosidase activity in tartary buckwheat (Fagopyrum tataricum) leaves. Plant Sci. 2005, 168, 1303–1307. [Google Scholar] [CrossRef]
- Lambdon, P.W.; Hassall, M.; Boar, R.R.; Mithen, R. Asynchrony in the nitrogen and glucosinolate leaf-age profiles of Brassica: Is this a defensive strategy against generalist herbivores? Agric. Ecosyst. Environ. 2003, 97, 205–214. [Google Scholar] [CrossRef]
- Ibañez, S.; Rosa, M.; Hilal, M.; Gonzalez, J.A.; Prado, F.E. Leaves of Citrus aurantifolia exhibit a different sensibility to solar UV-B radiation according to development stage in relation to photosynthetic pigments and UV-B absorbing compounds production. J. Photochem. Photobiol. B Biol. 2008, 90, 163–169. [Google Scholar] [CrossRef]
- Sroka, Z. Antioxidative and antiradical properties of plant phenolics. Z. Naturforsch. Sect. C J. Biosci. 2005, 60, 833–843. [Google Scholar] [CrossRef]
- Brunetti, C.; Di Ferdinando, M.; Fini, A.; Pollastri, S.; Tattini, M. Flavonoids as Antioxidants and Developmental Regulators: Relative Significance in Plants and Humans. Int. J. Mol. Sci. 2013, 14, 3540–3555. [Google Scholar] [CrossRef]
- Caldwell, M.M. Solar UV irradiation and the growth and development of higher plants. In Photophysiology: Current Topics in Photobiology and Photochemistry; Giese, A.C., Ed.; Academic Press: New York, NY, USA, 1971; Volume 6, pp. 131–177. [Google Scholar]
- Flint, S.D.; Caldwell, M.M. A biological spectral weighting function for ozone depletion research with higher plants. Physiol. Plant. 2003, 117, 137–144. [Google Scholar] [CrossRef]
- Fabón, G.; Monforte, L.; Tomás-Las-Heras, R.; Núñez-Olivera, E.; Martínez-Abaigar, J. Dynamic response of UV-absorbing compounds, quantum yield and the xanthophyll cycle to diel changes in UV-B and photosynthetic radiations in an aquatic liverwort. J. Plant Physiol. 2012, 169, 20–26. [Google Scholar] [CrossRef]




| p—Rad | p—DS | p—Rad × DS | post-hoc (DS) | |||
|---|---|---|---|---|---|---|
| Water Content | ns | *** | ns | G a | T1 b | T2 c |
| SUVAC | *** | *** | *** | G a | T1 b | T2 b |
| Apigenin 7-O-glucuronide | *** | *** | *** | G a | T1 b | T2 c |
| Luteolin 3′-O-glucuronide | *** | *** | *** | G a | T1 a | T2 b |
| Luteolin 4′-O-glucuronide | *** | *** | *** | G a | T1 b | T2 c |
| Luteolin 7-O-glucuronide | *** | ns | *** | G a | T1 b | T2 a |
| Apigenin 7,4′-di-O-glucuronide | *** | *** | *** | G a | T1 b | T2 c |
| Luteolin 7,3′-di-O-glucuronide | *** | *** | *** | G a | T1 b | T2 c |
| Radiation | P | PAB |
|---|---|---|
| PAR (μmol m−2 s−1) | 67.1 | 66.5 |
| PAR (W m−2) | 13.9 | 13.9 |
| UV-A (W m−2) | 0.06 | 3.27 |
| UV-B (W m−2) | 0.00 | 1.38 |
| UV-BBE (W m−2) | 0.00 | 0.17 |
| UVBE (W m−2) | 0.00 | 0.19 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soriano, G.; Del-Castillo-Alonso, M.-Á.; Monforte, L.; Tomás-Las-Heras, R.; Martínez-Abaigar, J.; Núñez-Olivera, E. Developmental Stage Determines the Accumulation Pattern of UV-Absorbing Compounds in the Model Liverwort Marchantia polymorpha subsp. ruderalis under Controlled Conditions. Plants 2021, 10, 473. https://doi.org/10.3390/plants10030473
Soriano G, Del-Castillo-Alonso M-Á, Monforte L, Tomás-Las-Heras R, Martínez-Abaigar J, Núñez-Olivera E. Developmental Stage Determines the Accumulation Pattern of UV-Absorbing Compounds in the Model Liverwort Marchantia polymorpha subsp. ruderalis under Controlled Conditions. Plants. 2021; 10(3):473. https://doi.org/10.3390/plants10030473
Chicago/Turabian StyleSoriano, Gonzalo, María-Ángeles Del-Castillo-Alonso, Laura Monforte, Rafael Tomás-Las-Heras, Javier Martínez-Abaigar, and Encarnación Núñez-Olivera. 2021. "Developmental Stage Determines the Accumulation Pattern of UV-Absorbing Compounds in the Model Liverwort Marchantia polymorpha subsp. ruderalis under Controlled Conditions" Plants 10, no. 3: 473. https://doi.org/10.3390/plants10030473
APA StyleSoriano, G., Del-Castillo-Alonso, M.-Á., Monforte, L., Tomás-Las-Heras, R., Martínez-Abaigar, J., & Núñez-Olivera, E. (2021). Developmental Stage Determines the Accumulation Pattern of UV-Absorbing Compounds in the Model Liverwort Marchantia polymorpha subsp. ruderalis under Controlled Conditions. Plants, 10(3), 473. https://doi.org/10.3390/plants10030473