Climate-Driven Plant Response and Resilience on the Tibetan Plateau in Space and Time: A Review
Abstract
:1. Introduction
1.1. Climate Has Been Warming on the Tibetan Plateau
1.2. The Changing Climate Has Affected Alpine Ecosystems and the Effect Magnitude Varies with Scale
1.3. Individual Species and Community Response to Global Changes and the Underlying Mechanism
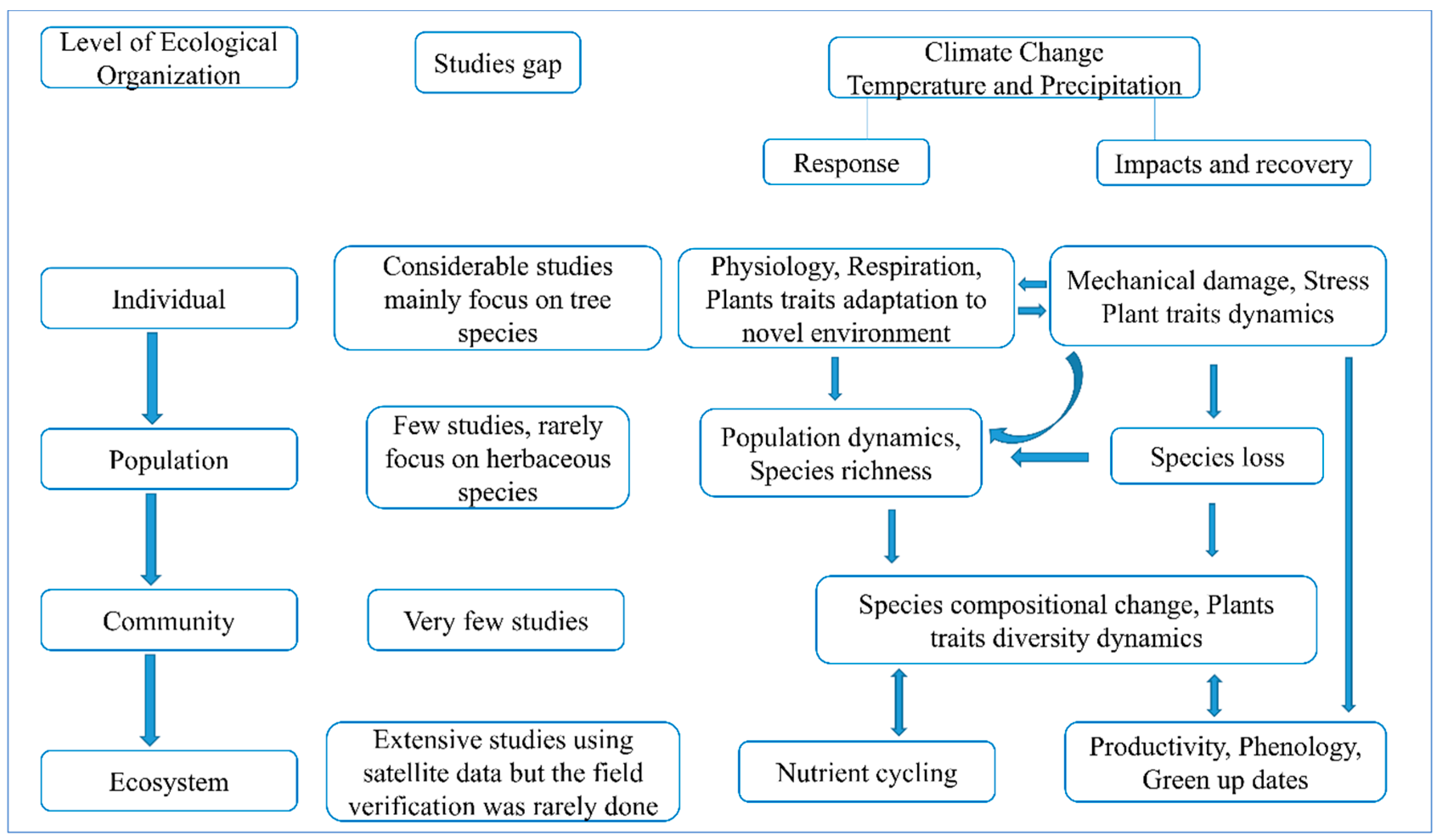
1.4. Why Is It Necessary for Us to Investigate across Scale Responses of Plants to Global Changes?
2. Spatiotemporal Variation in Cross-Scale Ecological Organizations and Climate Indicators
2.1. Driving Factors on Spatial–Temporal Variations of Vegetation Dynamics on the TP
2.1.1. Driving Factors in Vegetation Dynamics on Ecosystems Scale
2.1.2. Driving Factors in Vegetation Dynamics on Individual and Population Scales
3. Individual and Population Level Responses to Climate Dynamics
3.1. Photosynthetic Rates Accelerate as the Function of Increasing Temperature
3.2. Species Traits Determine the Responses
3.3. Species Reproductive Phenology
4. Community Responses to Global Changes
Community Structures and Species Composition Responses to Global Changes
5. Ecosystem Processes Response to Climate Dynamics
5.1. Phenology Responses to Global Changes
5.2. Ecosystem Productivity Response to Global Changes
6. Response to Global Changes among Scales
7. Concluding Remarks and Further Direction
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IPCC. Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.-K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013; p. 1535. [Google Scholar]
- Wang, W.; Jia, M.; Wang, G.; Zhu, W.; McDowell, N.G. Rapid warming forces contrasting growth trends of subalpine fir (Abies fabri) at higher-and lower-elevations in the eastern Tibetan Plateau. For. Ecol. Manag. 2017, 402, 135–144. [Google Scholar] [CrossRef]
- Liu, X.; Chen, B. Climatic warming in the Tibetan Plateau during recent decades. Int. J. Climatol. J. R. Meteorol. Soc. 2000, 20, 1729–1742. [Google Scholar] [CrossRef]
- Xu, Z.; Gong, T.; Li, J. Decadal trend of climate in the Tibetan Plateau—Regional temperature and precipitation. Hydrol. Process. Int. J. 2008, 22, 3056–3065. [Google Scholar] [CrossRef]
- Sala, O.E.; Chapin, F.S.; Armesto, J.J.; Berlow, E.; Bloomfield, J.; Dirzo, R.; Huber-Sanwald, E.; Huenneke, L.F.; Jackson, R.B.; Kinzig, A. Global biodiversity scenarios for the year 2100. Science 2000, 287, 1770–1774. [Google Scholar] [CrossRef] [PubMed]
- Garcia, R.A.; Cabeza, M.; Rahbek, C.; Araújo, M.B. Multiple dimensions of climate change and their implications for biodiversity. Science 2014, 344, 1247579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhatta, K.P.; Grytnes, J.A.; Vetaas, O.R. Scale sensitivity of the relationship between alpha and gamma diversity along an alpine elevation gradient in central Nepal. J. Biogeogr. 2018, 45, 804–814. [Google Scholar] [CrossRef]
- Goring, S.J.; Williams, J.W. Effect of historical land-use and climate change on tree-climate relationships in the upper Midwestern United States. Ecol. Lett. 2017, 20, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.C.; Anand, M. Probing for the influence of atmospheric CO2 and climate change on forest ecosystems across biomes. Glob. Ecol. Biogeogr. 2013, 22, 83–92. [Google Scholar] [CrossRef]
- Körner, C. Alpine Plant Life: Functional Plant Ecology of High Mountain Ecosystems with 47 Tables; Springer Science & Business Media: Berlin/Heiderberg, Germany, 2003. [Google Scholar]
- Smith, M.D. An ecological perspective on extreme climatic events: A synthetic definition and framework to guide future research. J. Ecol. 2011, 99, 656–663. [Google Scholar] [CrossRef]
- Felton, A.J.; Smith, M.D. Integrating plant ecological responses to climate extremes from individual to ecosystem levels. Philos. Trans. R. Soc. B Biol. Sci. 2017, 372, 20160142. [Google Scholar] [CrossRef] [Green Version]
- Alexander, J.M.; Diez, J.M.; Levine, J.M. Novel competitors shape species’ responses to climate change. Nature 2015, 525, 515. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Yangjian, Z.; Holzapfel, C.; Ke, H.; Ning, C.; Jian, T.; Juntao, Z. Vegetation pattern in Northern Tibet in relation to environmental and geo-spatial factors. J. Resour. Ecol. 2018, 9, 526–537. [Google Scholar] [CrossRef]
- Chapin, F.S., III. Effects of plant traits on ecosystem and regional processes: A conceptual framework for predicting the consequences of global change. Ann. Bot. 2003, 91, 455–463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, J.; Jiang, L.; Zhang, Y. Relationships between functional diversity and aboveground biomass production in the Northern Tibetan alpine grasslands. Sci. Rep. 2016, 6, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Ravenscroft, C.H.; Whitlock, R.; Fridley, J.D. Rapid genetic divergence in response to 15 years of simulated climate change. Glob. Chang. Biol. 2015, 21, 4165–4176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klein, J.A.; Harte, J.; Zhao, X.-Q. Decline in medicinal and forage species with warming is mediated by plant traits on the Tibetan Plateau. Ecosystems 2008, 11, 775–789. [Google Scholar] [CrossRef]
- Menéndez, R.; Megías, A.G.; Hill, J.K.; Braschler, B.; Willis, S.G.; Collingham, Y.; Fox, R.; Roy, D.B.; Thomas, C.D. Species richness changes lag behind climate change. Proc. R. Soc. B Biol. Sci. 2006, 273, 1465–1470. [Google Scholar] [CrossRef] [Green Version]
- Klein, J.A.; Harte, J.; Zhao, X.Q. Experimental warming causes large and rapid species loss, dampened by simulated grazing, on the Tibetan Plateau. Ecol. Lett. 2004, 7, 1170–1179. [Google Scholar] [CrossRef]
- Levin, S.A. The problem of pattern and scale in ecology: The Robert, H. MacArthur award lecture. Ecology 1992, 73, 1943–1967. [Google Scholar] [CrossRef]
- Shi, C.; Silva, L.C.; Zhang, H.; Zheng, Q.; Xiao, B.; Wu, N.; Sun, G. Climate warming alters nitrogen dynamics and total non-structural carbohydrate accumulations of perennial herbs of distinctive functional groups during the plant senescence in autumn in an alpine meadow of the Tibetan Plateau, China. Agric. For. Meteorol. 2015, 200, 21–29. [Google Scholar] [CrossRef]
- Ren, Z.; Li, Q.; Chu, C.; Zhao, L.; Zhang, J.; Ai, D.; Yang, Y.; Wang, G. Effects of resource additions on species richness and ANPP in an alpine meadow community. J. Plant Ecol. 2009, 3, 25–31. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Duan, J.; Xu, G.; Wang, Y.; Zhang, Z.; Rui, Y.; Luo, C.; Xu, B.; Zhu, X.; Chang, X. Effects of warming and grazing on soil N availability, species composition, and ANPP in an alpine meadow. Ecology 2012, 93, 2365–2376. [Google Scholar] [CrossRef]
- Huston, M. A general hypothesis of species diversity. Am. Nat. 1979, 113, 81–101. [Google Scholar] [CrossRef]
- Castro-Díez, P. Functional traits analyses: Scaling-up from species to community level. Plant Soil 2012, 357, 9–12. [Google Scholar] [CrossRef]
- Suding, K.N.; Lavorel, S.; Chapin Iii, F.; Cornelissen, J.H.; DIAz, S.; Garnier, E.; Goldberg, D.; Hooper, D.U.; Jackson, S.T.; Navas, M.L. Scaling environmental change through the community-level: A trait-based response-and-effect framework for plants. Glob. Chang. Biol. 2008, 14, 1125–1140. [Google Scholar] [CrossRef] [Green Version]
- Anderegg, W.R.; Martinez-Vilalta, J.; Cailleret, M.; Camarero, J.J.; Ewers, B.E.; Galbraith, D.; Gessler, A.; Grote, R.; Huang, C.-y.; Levick, S.R. When a tree dies in the forest: Scaling climate-driven tree mortality to ecosystem water and carbon fluxes. Ecosystems 2016, 19, 1133–1147. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, Q.; He, J.; Lu, Y.; Ji, L.; Xiao, J.; Luo, T. Carbon dynamics of terrestrial ecosystems on the Tibetan Plateau during the 20th century: An analysis with a process-based biogeochemical model. Glob. Ecol. Biogeogr. 2010, 19, 649–662. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Z.; Piao, S.; Fang, J. Regional differences in the timing of recent air warming during the past four decades in China. Chin. Sci. Bull. 2010, 55, 1968–1973. [Google Scholar] [CrossRef]
- Dong, M.; Jiang, Y.; Zheng, C.; Zhang, D. Trends in the thermal growing season throughout the Tibetan Plateau during 1960–2009. Agric. For. Meteorol. 2012, 166, 201–206. [Google Scholar] [CrossRef]
- Chen, H.; Zhu, Q.; Peng, C.; Wu, N.; Wang, Y.; Fang, X.; Gao, Y.; Zhu, D.; Yang, G.; Tian, J. The impacts of climate change and human activities on biogeochemical cycles on the Q inghai-T ibetan P lateau. Glob. Chang. Biol. 2013, 19, 2940–2955. [Google Scholar] [CrossRef]
- Shen, M.; Tang, Y.; Chen, J.; Zhu, X.; Zheng, Y. Influences of temperature and precipitation before the growing season on spring phenology in grasslands of the central and eastern Qinghai-Tibetan Plateau. Agric. For. Meteorol. 2011, 151, 1711–1722. [Google Scholar] [CrossRef]
- Yu, H.; Xu, J.; Okuto, E.; Luedeling, E. Seasonal response of grasslands to climate change on the Tibetan Plateau. PLoS ONE 2012, 7, e49230. [Google Scholar] [CrossRef]
- Ding, M.; Zhang, Y.; Sun, X.; Liu, L.; Wang, Z.; Bai, W. Spatiotemporal variation in alpine grassland phenology in the Qinghai-Tibetan Plateau from 1999 to 2009. Chin. Sci. Bull. 2013, 58, 396–405. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Peng, S.; Lin, X.; Chang, J. Declining snow cover may affect spring phenological trend on the Tibetan Plateau. Proc. Natl. Acad. Sci. USA 2013, 110, E2854–E2855. [Google Scholar] [CrossRef] [Green Version]
- Shen, M.; Piao, S.; Cong, N.; Zhang, G.; Jassens, I.A. Precipitation impacts on vegetation spring phenology on the T ibetan P lateau. Glob. Chang. Biol. 2015, 21, 3647–3656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, M.; Piao, S.; Chen, X.; An, S.; Fu, Y.H.; Wang, S.; Cong, N.; Janssens, I.A. Strong impacts of daily minimum temperature on the green-up date and summer greenness of the Tibetan Plateau. Glob. Chang. Biol. 2016, 22, 3057–3066. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, S.; Dong, S.; Su, X.; Wang, X.; Wu, X.; Wu, L.; Zhang, X. Analysis of vegetation change associated with human disturbance using MODIS data on the rangelands of the Qinghai-Tibet Plateau. Rangel. J. 2015, 37, 77–87. [Google Scholar] [CrossRef]
- Zhong, L.; Ma, Y.; Salama, M.S.; Su, Z. Assessment of vegetation dynamics and their response to variations in precipitation and temperature in the Tibetan Plateau. Clim. Chang. 2010, 103, 519–535. [Google Scholar] [CrossRef]
- Li, H.; Li, Y.; Shen, W.; Li, Y.; Lin, J.; Lu, X.; Xu, X.; Jiang, J. Elevation-dependent vegetation greening of the Yarlung Zangbo River basin in the southern Tibetan Plateau, 1999–2013. Remote Sens. 2015, 7, 16672–16687. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Li, Y.; Gao, Y.; Zou, C.; Yan, S.; Gao, J. Human impact on vegetation dynamics around Lhasa, southern Tibetan Plateau, China. Sustainability 2016, 8, 1146. [Google Scholar] [CrossRef] [Green Version]
- Pang, G.; Wang, X.; Yang, M. Using the NDVI to identify variations in, and responses of, vegetation to climate change on the Tibetan Plateau from 1982 to 2012. Quat. Int. 2017, 444, 87–96. [Google Scholar] [CrossRef]
- Cong, N.; Shen, M.; Yang, W.; Yang, Z.; Zhang, G.; Piao, S. Varying responses of vegetation activity to climate changes on the Tibetan Plateau grassland. Int. J. Biometeorol. 2017, 61, 1433–1444. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, Y.; Liu, L.; Wu, J.; Wang, Z.; Li, S.; Zhang, H.; Zu, J.; Ding, M.; Paudel, B. Spatiotemporal patterns of vegetation greenness change and associated climatic and anthropogenic drivers on the Tibetan Plateau during 2000–2015. Remote Sens. 2018, 10, 1525. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Zhao, H.; Su, X.; Deng, L.; Dong, S.; Zhang, X. Spatio-temporal variability in rangeland conditions associated with climate change in the Altun Mountain National Nature Reserve on the Qinghai-Tibet Plateau over the past 15 years. Rangel. J. 2015, 37, 67–75. [Google Scholar] [CrossRef]
- Xu, W.; Gu, S.; Zhao, X.; Xiao, J.; Tang, Y.; Fang, J.; Zhang, J.; Jiang, S. High positive correlation between soil temperature and NDVI from 1982 to 2006 in alpine meadow of the Three-River Source Region. on the Qinghai-Tibetan Plateau. Int. J. Appl. Earth Obs. Geoinf. 2011, 13, 528–535. [Google Scholar] [CrossRef]
- Zhang, Y.; Qi, W.; Zhou, C.; Ding, M.; Liu, L.; Gao, J.; Bai, W.; Wang, Z.; Zheng, D. Spatial and temporal variability in the net primary production of alpine grassland on the Tibetan Plateau since 1982. J. Geogr. Sci. 2014, 24, 269–287. [Google Scholar] [CrossRef]
- Wang, X.; Yi, S.; Wu, Q.; Yang, K.; Ding, Y. The role of permafrost and soil water in distribution of alpine grassland and its NDVI dynamics on the Qinghai-Tibetan Plateau. Glob. Planet. Chang. 2016, 147, 40–53. [Google Scholar] [CrossRef]
- Xu, H.-J.; Wang, X.-P.; Zhang, X.-X. Impacts of climate change and human activities on the aboveground production in alpine grasslands: A case study of the source region of the Yellow river, China. Arab. J. Geosci. 2017, 10, 17. [Google Scholar] [CrossRef]
- Lin, X.; Zhang, Z.; Wang, S.; Hu, Y.; Xu, G.; Luo, C.; Chang, X.; Duan, J.; Lin, Q.; Xu, B. Response of ecosystem respiration to warming and grazing during the growing seasons in the alpine meadow on the Tibetan plateau. Agric. For. Meteorol. 2011, 151, 792–802. [Google Scholar] [CrossRef]
- Tan, K.; Ciais, P.; Piao, S.; Wu, X.; Tang, Y.; Vuichard, N.; Liang, S.; Fang, J. Application of the ORCHIDEE global vegetation model to evaluate biomass and soil carbon stocks of Qinghai-Tibetan grasslands. Glob. Biogeochem. Cycles 2010, 24. [Google Scholar] [CrossRef]
- Yao, T.; Thompson, L.; Yang, W.; Yu, W.; Gao, Y.; Guo, X.; Yang, X.; Duan, K.; Zhao, H.; Xu, B. Different glacier status with atmospheric circulations in Tibetan Plateau and surroundings. Nat. Clim. Chang. 2012, 2, 663. [Google Scholar] [CrossRef]
- Wu, T.; Zhao, L.; Li, R.; Wang, Q.; Xie, C.; Pang, Q. Recent ground surface warming and its effects on permafrost on the central Qinghai-Tibet Plateau. Int. J. Climatol. 2013, 33, 920–930. [Google Scholar] [CrossRef]
- Kang, S.; Xu, Y.; You, Q.; Flügel, W.-A.; Pepin, N.; Yao, T. Review of climate and cryospheric change in the Tibetan Plateau. Environ. Res. Lett. 2010, 5, 015101. [Google Scholar] [CrossRef]
- Kuang, X.; Jiao, J.J. Review on climate change on the Tibetan Plateau during the last half century. J. Geophys. Res. Atmos. 2016, 121, 3979–4007. [Google Scholar] [CrossRef]
- Latif, A.; Ilyas, S.; Zhang, Y.; Xin, Y.; Zhou, L.; Zhou, Q. Review on global change status and its impacts on the Tibetan Plateau environment. J. Plant Ecol. 2019, 12, 917–930. [Google Scholar] [CrossRef]
- Yang, K.; Wu, H.; Qin, J.; Lin, C.; Tang, W.; Chen, Y. Recent climate changes over the Tibetan Plateau and their impacts on energy and water cycle: A review. Glob. Planet. Chang. 2014, 112, 79–91. [Google Scholar] [CrossRef]
- Harris, R.B. Rangeland degradation on the Qinghai-Tibetan plateau: A review of the evidence of its magnitude and causes. J. Arid Environ. 2010, 74, 1–12. [Google Scholar] [CrossRef]
- Yao, T.; Masson-Delmotte, V.; Gao, J.; Yu, W.; Yang, X.; Risi, C.; Sturm, C.; Werner, M.; Zhao, H.; He, Y. A review of climatic controls on δ18O in precipitation over the Tibetan Plateau: Observations and simulations. Rev. Geophys. 2013, 51, 525–548. [Google Scholar] [CrossRef]
- Yang, M.; Nelson, F.E.; Shiklomanov, N.I.; Guo, D.; Wan, G. Permafrost degradation and its environmental effects on the Tibetan Plateau: A review of recent research. Earth-Sci. Rev. 2010, 103, 31–44. [Google Scholar] [CrossRef]
- Lehnert, L.; Wesche, K.; Trachte, K.; Reudenbach, C.; Bendix, J. Climate variability rather than overstocking causes recent large scale cover changes of Tibetan pastures. Sci. Rep. 2016, 6, 24367. [Google Scholar] [CrossRef] [Green Version]
- Chen, B.; Zhang, X.; Tao, J.; Wu, J.; Wang, J.; Shi, P.; Zhang, Y.; Yu, C. The impact of climate change and anthropogenic activities on alpine grassland over the Qinghai-Tibet Plateau. Agric. For. Meteorol. 2014, 189, 11–18. [Google Scholar] [CrossRef]
- Fan, J.-W.; Shao, Q.-Q.; Liu, J.-Y.; Wang, J.-B.; Harris, W.; Chen, Z.-Q.; Zhong, H.-P.; Xu, X.-L.; Liu, R.-G. Assessment of effects of climate change and grazing activity on grassland yield in the Three Rivers Headwaters Region. of Qinghai–Tibet Plateau, China. Environ. Monit. Assess. 2010, 170, 571–584. [Google Scholar] [CrossRef]
- Piao, S.; Tan, K.; Nan, H.; Ciais, P.; Fang, J.; Wang, T.; Vuichard, N.; Zhu, B. Impacts of climate and CO2 changes on the vegetation growth and carbon balance of Qinghai–Tibetan grasslands over the past five decades. Glob. Planet. Chang. 2012, 98, 73–80. [Google Scholar] [CrossRef]
- Wang, B.; Chen, T.; Xu, G.; Liu, X.; Wang, W.; Wu, G.; Zhang, Y. Alpine timberline population dynamics under climate change: A comparison between Qilian juniper and Qinghai spruce tree species in the middle Qilian Mountains of northeast Tibetan Plateau. Boreas 2016, 45, 411–422. [Google Scholar] [CrossRef]
- Huang, K.; Zhang, Y.; Zhu, J.; Liu, Y.; Zu, J.; Zhang, J. The influences of climate change and human activities on vegetation dynamics in the Qinghai-Tibet Plateau. Remote Sens. 2016, 8, 876. [Google Scholar] [CrossRef] [Green Version]
- Tao, J.; Xu, T.; Dong, J.; Yu, X.; Jiang, Y.; Zhang, Y.; Huang, K.; Zhu, J.; Dong, J.; Xu, Y. Elevation-dependent effects of climate change on vegetation greenness in the high mountains of southwest China during 1982–2013. Int. J. Climatol. 2018, 38, 2029–2038. [Google Scholar] [CrossRef]
- Gao, Q.; Li, Y.; Wan, Y.; Qin, X.; Jiangcun, W.; Liu, Y. Dynamics of alpine grassland NPP and its response to climate change in Northern Tibet. Clim. Chang. 2009, 97, 515. [Google Scholar] [CrossRef]
- Liu, H.; Mi, Z.; Lin, L.; Wang, Y.; Zhang, Z.; Zhang, F.; Wang, H.; Liu, L.; Zhu, B.; Cao, G. Shifting plant species composition in response to climate change stabilizes grassland primary production. Proc. Natl. Acad. Sci. USA 2018, 115, 4051–4056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, Y.; Wang, Y.; Ma, Y.; Ma, W.; Liang, C.; Flynn, D.; Schmid, B.; Fang, J.; He, J.-S. Field-based observations of regional-scale, temporal variation in net primary production in Tibetan alpine grasslands. Biogeosciences 2014, 11, 2003–2016. [Google Scholar] [CrossRef] [Green Version]
- Pan, T.; Zou, X.; Liu, Y.; Wu, S.; He, G. Contributions of climatic and non-climatic drivers to grassland variations on the Tibetan Plateau. Ecol. Eng. 2017, 108, 307–317. [Google Scholar] [CrossRef]
- Wu, J.; Feng, Y.; Zhang, X.; Wurst, S.; Tietjen, B.; Tarolli, P.; Song, C. Grazing exclusion by fencing non-linearly restored the degraded alpine grasslands on the Tibetan Plateau. Sci. Rep. 2017, 7, 15202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, Y.; Zhou, X.; Wang, Q.; Wang, C.; Zhan, Z.; Chen, L.; Yan, J.; Qu, R. Vegetation net primary productivity and its response to climate change during 2001–2008 in the Tibetan Plateau. Sci. Total Environ. 2013, 444, 356–362. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, G.; Yang, L.; Guo, J. Effects of drought and warming on biomass, nutrient allocation, and oxidative stress in Abies fabri in eastern Tibetan Plateau. J. Plant Growth Regul. 2013, 32, 298–306. [Google Scholar] [CrossRef]
- Piao, S.; Friedlingstein, P.; Ciais, P.; de Noblet-Ducoudré, N.; Labat, D.; Zaehle, S. Changes in climate and land use have a larger direct impact than rising CO2 on global river runoff trends. Proc. Natl. Acad. Sci. USA 2007, 104, 15242–15247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, Z.; Zhuang, Q.; He, J.-S.; Luo, T.; Shi, Y. Phenology shift from 1989 to 2008 on the Tibetan Plateau: An analysis with a process-based soil physical model and remote sensing data. Clim. Chang. 2013, 119, 435–449. [Google Scholar] [CrossRef]
- Piao, S.; Cui, M.; Chen, A.; Wang, X.; Ciais, P.; Liu, J.; Tang, Y. Altitude and temperature dependence of change in the spring vegetation green-up date from 1982 to 2006 in the Qinghai-Xizang Plateau. Agric. For. Meteorol. 2011, 151, 1599–1608. [Google Scholar] [CrossRef]
- Yu, H.; Luedeling, E.; Xu, J. Winter and spring warming result in delayed spring phenology on the Tibetan Plateau. Proc. Natl. Acad. Sci. USA 2010, 107, 22151–22156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, G.; Zhang, Y.; Dong, J.; Xiao, X. Green-up dates in the Tibetan Plateau have continuously advanced from 1982 to 2011. Proc. Natl. Acad. Sci. USA 2013, 110, 4309–4314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, E.; Wang, Y.; Eckstein, D.; Luo, T. Little change in the fir tree-line position on the southeastern Tibetan Plateau after 200 years of warming. New Phytol. 2011, 190, 760–769. [Google Scholar] [CrossRef] [PubMed]
- Harsch, M.A.; Hulme, P.E.; McGlone, M.S.; Duncan, R.P. Are treelines advancing? A global meta-analysis of treeline response to climate warming. Ecol. Lett. 2009, 12, 1040–1049. [Google Scholar] [CrossRef]
- Xu, X.; Chen, H.; Levy, J.K. Spatiotemporal vegetation cover variations in the Qinghai-Tibet Plateau under global climate change. Chin. Sci. Bull. 2008, 53, 915–922. [Google Scholar] [CrossRef] [Green Version]
- Liang, E.; Wang, Y.; Piao, S.; Lu, X.; Camarero, J.J.; Zhu, H.; Zhu, L.; Ellison, A.M.; Ciais, P.; Peñuelas, J. Species interactions slow warming-induced upward shifts of treelines on the Tibetan Plateau. Proc. Natl. Acad. Sci. USA 2016, 113, 4380–4385. [Google Scholar] [CrossRef] [Green Version]
- Yu, D.; Liu, J.; Zhou, L.; Zhou, W.; Fang, X.; Wei, Y.; Jiang, S.; Dai, L. Spatial variation and temporal instability in the climate–growth relationship of Korean pine in the Changbai Mountain region of Northeast. China. For. Ecol. Manag. 2013, 300, 96–105. [Google Scholar] [CrossRef]
- Lu, X.; Liang, E.; Wang, Y.; Babst, F.; Leavitt, S.W.; Julio Camarero, J. Past the climate optimum: Recruitment is declining at the world’s highest juniper shrublines on the Tibetan Plateau. Ecology 2019, 100, e02557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graae, B.J.; Alsos, I.G.; Ejrnaes, R. The impact of temperature regimes on development, dormancy breaking and germination of dwarf shrub seeds from arctic, alpine and boreal sites. Plant Ecol. 2008, 198, 275–284. [Google Scholar] [CrossRef]
- Milbau, A.; Graae, B.J.; Shevtsova, A.; Nijs, I. Effects of a warmer climate on seed germination in the subarctic. Ann. Bot. 2009, 104, 287–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kullman, L. Treeline (Pinus sylvestris) landscape evolution in the Swedish Scandes—A 40-year demographic effort viewed in a broader temporal context. Norsk Geografisk Tidsskr.-Nor. J. Geogr. 2014, 68, 155–167. [Google Scholar] [CrossRef]
- Xu, C.; Liu, H.; Anenkhonov, O.A.; Korolyuk, A.Y.; Sandanov, D.V.; Balsanova, L.D.; Naidanov, B.B.; Wu, X. Long-term forest resilience to climate change indicated by mortality, regeneration, and growth in semiarid southern S iberia. Glob. Chang. Biol. 2017, 23, 2370–2382. [Google Scholar] [CrossRef]
- Baker, B.; Moseley, R. Advancing treeline and retreating glaciers: Implications for conservation in Yunnan, PR China. Arct. Antarct. Alp. Res. 2007, 39, 200–209. [Google Scholar] [CrossRef] [Green Version]
- Rustad, L.; Campbell, J.; Marion, G.; Norby, R.; Mitchell, M.; Hartley, A.; Cornelissen, J.; Gurevitch, J. A meta-analysis of the response of soil respiration, net nitrogen mineralization, and aboveground plant growth to experimental ecosystem warming. Oecologia 2001, 126, 543–562. [Google Scholar] [CrossRef]
- Fang, O.; Zhang, Q.B. Tree resilience to drought increases in the Tibetan Plateau. Glob. Chang. Biol. 2019, 25, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Chapin Iii, F.S.; Zavaleta, E.S.; Eviner, V.T.; Naylor, R.L.; Vitousek, P.M.; Reynolds, H.L.; Hooper, D.U.; Lavorel, S.; Sala, O.E.; Hobbie, S.E. Consequences of changing biodiversity. Nature 2000, 405, 234–242. [Google Scholar] [CrossRef]
- Croft, H.; Chen, J.M.; Luo, X.; Bartlett, P.; Chen, B.; Staebler, R.M. Leaf chlorophyll content as a proxy for leaf photosynthetic capacity. Glob. Chang. Biol. 2017, 23, 3513–3524. [Google Scholar] [CrossRef]
- Slaney, M.; Wallin, G.; Medhurst, J.; Linder, S. Impact of elevated carbon dioxide concentration and temperature on bud burst and shoot growth of boreal Norway spruce. Tree Physiol. 2007, 27, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Bronson, D.R.; Gower, S.T.; Tanner, M.; Van Herk, I. Effect of ecosystem warming on boreal black spruce bud burst and shoot growth. Glob. Chang. Biol. 2009, 15, 1534–1543. [Google Scholar] [CrossRef]
- Xu, Z.; Hu, T.; Zhang, Y. Effects of experimental warming on phenology, growth and gas exchange of treeline birch (Betula utilis) saplings, Eastern Tibetan Plateau, China. Eur. J. For. Res. 2012, 131, 811–819. [Google Scholar] [CrossRef]
- Jordan, D.B.; Ogren, W.L. The CO2/O2 specificity of ribulose 1, 5-bisphosphate carboxylase/oxygenase. Planta 1984, 161, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Long, S.P.; Drake, B.G. Effect of the long-term elevation of CO2 concentration in the field on the quantum yield of photosynthesis of the C3 sedge, Scirpus olneyi. Plant Physiol. 1991, 96, 221–226. [Google Scholar] [CrossRef] [Green Version]
- Wan, S.; Hui, D.; Wallace, L.; Luo, Y. Direct and indirect effects of experimental warming on ecosystem carbon processes in a tallgrass prairie. Glob. Biogeochem. Cycles 2005, 19. [Google Scholar] [CrossRef]
- Saxe, H.; Cannell, M.G.; Johnsen, Ø.; Ryan, M.G.; Vourlitis, G. Tree and forest functioning in response to global warming. New Phytol. 2001, 149, 369–399. [Google Scholar] [CrossRef]
- Yin, C.; Berninger, F.; Li, C. Photosynthetic responses of Populus przewalski subjected to drought stress. Photosynthetica 2006, 44, 62–68. [Google Scholar] [CrossRef]
- Smith, N.G.; Dukes, J.S. Plant respiration and photosynthesis in global-scale models: Incorporating acclimation to temperature and CO2. Glob. Chang. Biol. 2013, 19, 45–63. [Google Scholar] [CrossRef]
- Lloyd, A.H.; Fastie, C.L. Spatial and temporal variability in the growth and climate response of treeline trees in Alaska. Clim. Chang. 2002, 52, 481–509. [Google Scholar] [CrossRef]
- Barber, V.A.; Juday, G.P.; Finney, B.P.; Wilmking, M. Reconstruction of summer temperatures in interior Alaska from tree-ring proxies: Evidence for changing synoptic climate regimes. Clim. Chang. 2004, 63, 91–120. [Google Scholar] [CrossRef]
- Nijs, I.; Teughels, H.; Blum, H.; Hendrey, G.; Impens, I. Simulation of climate change with infrared heaters reduces the productivity of Lolium perenne L. in summer. Environ. Exp. Bot. 1996, 36, 271–280. [Google Scholar] [CrossRef]
- Luomala, E.M.; Laitinen, K.; Kellomäki, S.; Vapaavuori, E. Variable photosynthetic acclimation in consecutive cohorts of Scots pine needles during 3 years of growth at elevated CO2 and elevated temperature. Plant Cell Environ. 2003, 26, 645–660. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, J.; Wang, G.; Yang, L.; Yang, Y. Effects of drought and nitrogen addition on photosynthetic characteristics and resource allocation of Abies fabri seedlings in eastern Tibetan Plateau. New For. 2012, 43, 505–518. [Google Scholar] [CrossRef]
- Millard, P. The accumulation and storage of nitrogen by herbaceous plants. Plant Cell Environ. 1988, 11, 1–8. [Google Scholar] [CrossRef]
- Loik, M.E.; Still, C.J.; Huxman, T.E.; Harte, J. In situ photosynthetic freezing tolerance for plants exposed to a global warming manipulation in the Rocky Mountains, Colorado, USA. New Phytol. 2004, 162, 331–341. [Google Scholar] [CrossRef]
- Chen, J.; Li, Y.; Luo, Y.; Tu, W.; Wan, T. Drought differently affects growth properties, leaf ultrastructure, nitrogen absorption and metabolism of two dominant species of Hippophae in Tibet Plateau. Acta Physiol. Plant. 2019, 41, 1–12. [Google Scholar] [CrossRef]
- Kessler, M.; Siorak, Y.; Wunderlich, M.; Wegner, C. Patterns of morphological leaf traits among pteridophytes along humidity and temperature gradients in the Bolivian Andes. Funct. Plant Biol. 2007, 34, 963–971. [Google Scholar] [CrossRef]
- Liu, Y.; Reich, P.B.; Li, G.; Sun, S. Shifting phenology and abundance under experimental warming alters trophic relationships and plant reproductive capacity. Ecology 2011, 92, 1201–1207. [Google Scholar] [CrossRef]
- Shen, H.; Wang, S.; Tang, Y. Grazing alters warming effects on leaf photosynthesis and respiration in Gentiana straminea, an alpine forb species. J. Plant Ecol. 2013, 6, 418–427. [Google Scholar] [CrossRef] [Green Version]
- Ren, F.; Zhou, H.-k.; Zhao, X.-Q.; Han, F.; Shi, L.-N.; Duan, J.-C.; Zhao, J.-Z. Influence of simulated warming using OTC on physiological–biochemical characteristics of Elymus nutans in alpine meadow on Qinghai-Tibetan plateau. Acta Ecol. Sin. 2010, 30, 166–171. [Google Scholar] [CrossRef]
- Kliber, A.; Eckert, C.G. Sequential decline in allocation among flowers within inflorescences: Proximate mechanisms and adaptive significance. Ecology 2004, 85, 1675–1687. [Google Scholar] [CrossRef]
- Sherry, R.A.; Zhou, X.; Gu, S.; Arnone III, J.A.; Johnson, D.W.; Schimel, D.S.; Verburg, P.S.; Wallace, L.L.; Luo, Y. Changes in duration of reproductive phases and lagged phenological response to experimental climate warming. Plant Ecol. Divers. 2011, 4, 23–35. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, Y.; Wang, W. Interactions between warming and soil moisture increase overlap in reproductive phenology among species in an alpine meadow. Biol. Lett. 2016, 12, 20150749. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Mu, J.; Niklas, K.J.; Li, G.; Sun, S. Global warming reduces plant reproductive output for temperate multi-inflorescence species on the Tibetan plateau. New Phytol. 2012, 195, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Dorji, T.; Totland, Ø.; Moe, S.R.; Hopping, K.A.; Pan, J.; Klein, J.A. Plant. functional traits mediate reproductive phenology and success in response to experimental warming and snow addition in Tibet. Glob. Chang. Biol. 2013, 19, 459–472. [Google Scholar] [CrossRef] [PubMed]
- Noss, R.F. Indicators for monitoring biodiversity: A hierarchical approach. Conserv. Biol. 1990, 4, 355–364. [Google Scholar] [CrossRef]
- Wu, J.; Wurst, S.; Zhang, X. Plant. functional trait diversity regulates the nonlinear response of productivity to regional climate change in Tibetan alpine grasslands. Sci. Rep. 2016, 6, 35649. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.; Zhang, Y.; Zhu, J.; Tao, J.; Zhang, T.; Xi, Y. Effects of community structure on precipitation-use efficiency of grasslands in northern Tibet. J. Veg. Sci. 2017, 28, 281–290. [Google Scholar] [CrossRef]
- Poorter, H.; Lambers, H. Is interspecific variation in relative growth rate positively correlated with biomass allocation to the leaves? Am. Nat. 1991, 138, 1264–1268. [Google Scholar] [CrossRef]
- Zhang, Y.; Welker, J.M. Tibetan alpine tundra responses to simulated changes in climate: Aboveground biomass and community responses. Arct. Alp. Res. 1996, 28, 203–209. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Gao, Q.; Dong, S.; Liu, S.; Wang, X.; Su, X.; Li, Y.; Tang, L.; Wu, X.; Zhao, H. Effects of grazing and climate warming on plant diversity, productivity and living state in the alpine rangelands and cultivated grasslands of the Qinghai-Tibetan Plateau. Rangel. J. 2015, 37, 57–65. [Google Scholar] [CrossRef]
- Elmendorf, S.C.; Henry, G.H.; Hollister, R.D.; Björk, R.G.; Boulanger-Lapointe, N.; Cooper, E.J.; Cornelissen, J.H.; Day, T.A.; Dorrepaal, E.; Elumeeva, T.G. Plot-scale evidence of tundra vegetation change and links to recent summer warming. Nat. Clim. Chang. 2012, 2, 453. [Google Scholar] [CrossRef]
- Na, L.; Genxu, W.; Yan, Y.; Yongheng, G.; Guangsheng, L. Plant. production, and carbon and nitrogen source pools, are strongly intensified by experimental warming in alpine ecosystems in the Qinghai-Tibet Plateau. Soil Biol. Biochem. 2011, 43, 942–953. [Google Scholar] [CrossRef]
- Peng, F.; Xue, X.; Xu, M.; You, Q.; Jian, G.; Ma, S. Warming-induced shift towards forbs and grasses and its relation to the carbon sequestration in an alpine meadow. Environ. Res. Lett. 2017, 12, 044010. [Google Scholar] [CrossRef] [Green Version]
- Welker, J.; Rykiel, E.; Briske, D.; Goeschl, J. Carbon import among vegetative tillers within two bunchgrasses: Assessment with carbon-11 labelling. Oecologia 1985, 67, 209–212. [Google Scholar] [CrossRef]
- Welker, J.; Briske, D. Clonal biology of the temperate, caespitose, graminoid Schizachyrium scoparium: A synthesis with reference to climate change. Oikos 1992, 63, 357–365. [Google Scholar] [CrossRef]
- Briske, D.; Butler, J. Density-dependent regulation of ramet populations within the bunchgrass Schizachyrium scoparium: Interclonal versus intraclonal interference. J. Ecol. 1989, 77, 963–974. [Google Scholar] [CrossRef]
- Rogers, C.; Oldroyd, G.E. Synthetic biology approaches to engineering the nitrogen symbiosis in cereals. J. Exp. Bot. 2014, 65, 1939–1946. [Google Scholar] [CrossRef] [Green Version]
- Klanderud, K. Species-specific responses of an alpine plant community under simulated environmental change. J. Veg. Sci. 2008, 19, 363–372. [Google Scholar] [CrossRef]
- Jägerbrand, A.K.; Alatalo, J.M.; Chrimes, D.; Molau, U. Plant. community responses to 5 years of simulated climate change in meadow and heath ecosystems at a subarctic-alpine site. Oecologia 2009, 161, 601–610. [Google Scholar] [CrossRef]
- Tilman, D.; Downing, J.A. Biodiversity and stability in grasslands. Nature 1994, 367, 363. [Google Scholar] [CrossRef]
- Kahmen, A.; Perner, J.; Buchmann, N. Diversity-dependent productivity in semi-natural grasslands following climate perturbations. Funct. Ecol. 2005, 19, 594–601. [Google Scholar] [CrossRef]
- Schwartz, M.W.; Iverson, L.R.; Prasad, A.M.; Matthews, S.N.; O’Connor, R.J. Predicting extinctions as a result of climate change. Ecology 2006, 87, 1611–1615. [Google Scholar] [CrossRef]
- Memmott, J.; Craze, P.G.; Waser, N.M.; Price, M.V. Global warming and the disruption of plant–pollinator interactions. Ecol. Lett. 2007, 10, 710–717. [Google Scholar] [CrossRef]
- Shaver, G.R.; Canadell, J.; Chapin, F.S.; Gurevitch, J.; Harte, J.; Henry, G.; Ineson, P.; Jonasson, S.; Melillo, J.; Pitelka, L. Global Warming and Terrestrial Ecosystems: A Conceptual Framework for Analysis: Ecosystem responses to global warming will be complex and varied. Ecosystem warming experiments hold great potential for providing insights on ways terrestrial ecosystems will respond to upcoming decades of climate change. Documentation of initial conditions provides the context for understanding and predicting ecosystem responses. BioScience 2000, 50, 871–882. [Google Scholar]
- Welker, J.; Fahnestock, J.; Jones, M. Annual CO2 flux in dry and moist arctic tundra: Field responses to increases in summer temperatures and winter snow depth. Clim. Chang. 2000, 44, 139–150. [Google Scholar] [CrossRef]
- Hobbie, S.E.; Chapin, F.S., III. The response of tundra plant biomass, aboveground production, nitrogen, and CO2 flux to experimental warming. Ecology 1998, 79, 1526–1544. [Google Scholar]
- Hollister, R.D.; Flaherty, K.J. Above-and below-ground plant biomass response to experimental warming in northern Alaska. Appl. Veg. Sci. 2010, 13, 378–387. [Google Scholar] [CrossRef]
- Lim, H.; Oren, R.; Näsholm, T.; Strömgren, M.; Lundmark, T.; Grip, H.; Linder, S. Boreal forest biomass accumulation is not increased by two decades of soil warming. Nat. Clim. Chang. 2019, 9, 49–52. [Google Scholar] [CrossRef]
- Forrest, J.; Miller-Rushing, A.J. Toward a synthetic understanding of the role of phenology in ecology and evolution. R. Soc. 2010. [Google Scholar] [CrossRef] [Green Version]
- Zu, J.; Zhang, Y.; Huang, K.; Liu, Y.; Chen, N.; Cong, N. Biological and climate factors co-regulated spatial-temporal dynamics of vegetation autumn phenology on the Tibetan Plateau. Int. J. Appl. Earth Obs. Geoinf. 2018, 69, 198–205. [Google Scholar] [CrossRef]
- Wang, C.; Guo, H.; Zhang, L.; Liu, S.; Qiu, Y.; Sun, Z. Assessing phenological change and climatic control of alpine grasslands in the Tibetan Plateau with MODIS time series. Int. J. Biometeorol. 2015, 59, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Wang, G.; Liu, G.; Li, T.; Ren, D.; Wang, Y.; Cheng, H.; Wang, J. Influences of alpine ecosystem degradation on soil temperature in the freezing-thawing process on Qinghai–Tibet Plateau. Environ. Geol. 2009, 57, 1391–1397. [Google Scholar] [CrossRef]
- Chen, H.; Zhu, Q.; Wu, N.; Wang, Y.; Peng, C.-H. Delayed spring phenology on the Tibetan Plateau may also be attributable to other factors than winter and spring warming. Proc. Natl. Acad. Sci. USA 2011, 108, E93. [Google Scholar] [CrossRef] [Green Version]
- Xia, J.; Ma, M.; Liang, T.; Wu, C.; Yang, Y.; Zhang, L.; Zhang, Y.; Yuan, W. Estimates of grassland biomass and turnover time on the Tibetan Plateau. Environ. Res. Lett. 2018, 13, 014020. [Google Scholar] [CrossRef]
- Fu, G.; Zhang, X.; Zhang, Y.; Shi, P.; Li, Y.; Zhou, Y.; Yang, P.; Shen, Z. Experimental warming does not enhance gross primary production and above-ground biomass in the alpine meadow of Tibet. J. Appl. Remote Sens. 2013, 7, 073505. [Google Scholar] [CrossRef] [Green Version]
- Walther, G.-R. Community and ecosystem responses to recent climate change. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 2019–2024. [Google Scholar] [CrossRef]
- Chapin III, F.S.; Randerson, J.T.; McGuire, A.D.; Foley, J.A.; Field, C.B. Changing feedbacks in the climate–biosphere system. Front. Ecol. Environ. 2008, 6, 313–320. [Google Scholar] [CrossRef]
- Luo, Y. Terrestrial carbon–cycle feedback to climate warming. Annu. Rev. Ecol. Evol. Syst. 2007, 38, 683–712. [Google Scholar] [CrossRef] [Green Version]
- Margalef, R. Diversity, Stability and Maturity in Natural Ecosystems, in Unifying Concepts in Ecology; Springer: Berlin/Heidelberg, Germany, 1975; pp. 151–160. [Google Scholar]
- Wang, P.; Zhang, Q.; Wang, Y.; Wang, T.; Li, X.; Li, Y.; Ding, L.; Jiang, G. Altitude dependence of polychlorinated biphenyls (PCBs) and polybrominated diphenyl ethers (PBDEs) in surface soil from Tibetan Plateau, China. Chemosphere 2009, 76, 1498–1504. [Google Scholar] [CrossRef] [PubMed]
- Yunfei, F.; Xianzhou, Z.; Peili, S.; Gang, F.; Yangjian, Z.; Guangshuai, Z.; Chaoxu, Z.; Jing, Z. Livestock dynamic responses to climate change in alpine grasslands on the Northern Tibetan Plateau: Forage consumption and time-lag effects. J. Resour. Ecol. 2017, 8, 88–96. [Google Scholar] [CrossRef]
- Sun, J.; Cheng, G.; Li, W.; Sha, Y.; Yang, Y. On the variation of NDVI with the principal climatic elements in the Tibetan Plateau. Remote Sens. 2013, 5, 1894–1911. [Google Scholar] [CrossRef] [Green Version]
- Reed, B.C.; Schwartz, M.D.; Xiao, X. Remote sensing phenology. In Phenology of Ecosystem Processes; Springer: Berlin/Heidelberg, Germany, 2009; pp. 231–246. [Google Scholar]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhattarai, P.; Zheng, Z.; Bhatta, K.P.; Adhikari, Y.P.; Zhang, Y. Climate-Driven Plant Response and Resilience on the Tibetan Plateau in Space and Time: A Review. Plants 2021, 10, 480. https://doi.org/10.3390/plants10030480
Bhattarai P, Zheng Z, Bhatta KP, Adhikari YP, Zhang Y. Climate-Driven Plant Response and Resilience on the Tibetan Plateau in Space and Time: A Review. Plants. 2021; 10(3):480. https://doi.org/10.3390/plants10030480
Chicago/Turabian StyleBhattarai, Prakash, Zhoutao Zheng, Kuber Prasad Bhatta, Yagya Prasad Adhikari, and Yangjian Zhang. 2021. "Climate-Driven Plant Response and Resilience on the Tibetan Plateau in Space and Time: A Review" Plants 10, no. 3: 480. https://doi.org/10.3390/plants10030480
APA StyleBhattarai, P., Zheng, Z., Bhatta, K. P., Adhikari, Y. P., & Zhang, Y. (2021). Climate-Driven Plant Response and Resilience on the Tibetan Plateau in Space and Time: A Review. Plants, 10(3), 480. https://doi.org/10.3390/plants10030480






