Comparative Study of Chemical Composition and Antioxidant Activity of Essential Oils and Crude Extracts of Four Characteristic Zingiberaceae Herbs
Abstract
:1. Introduction
2. Results and Discussion
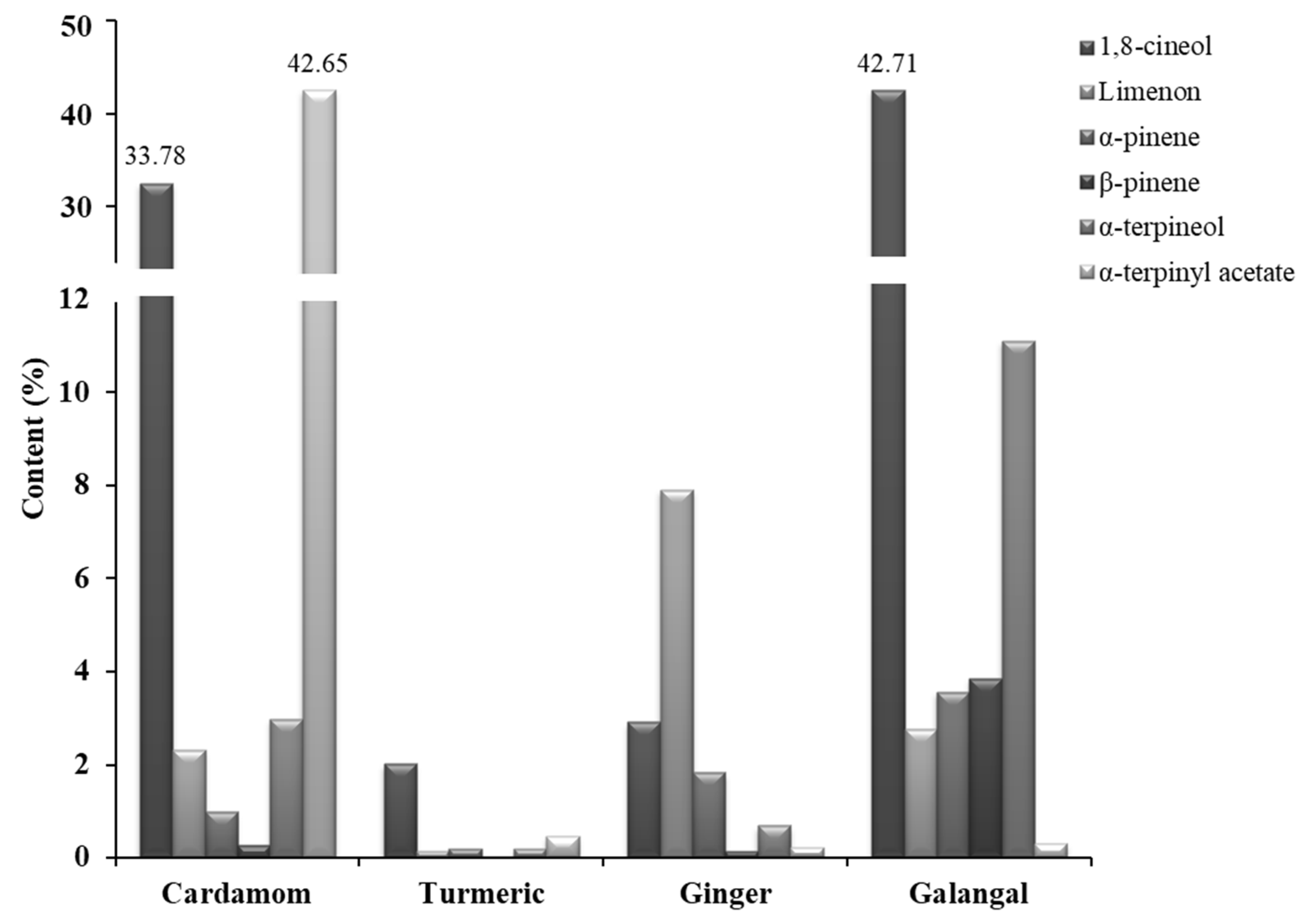
2.1. Essential Oils (EOs) Composition
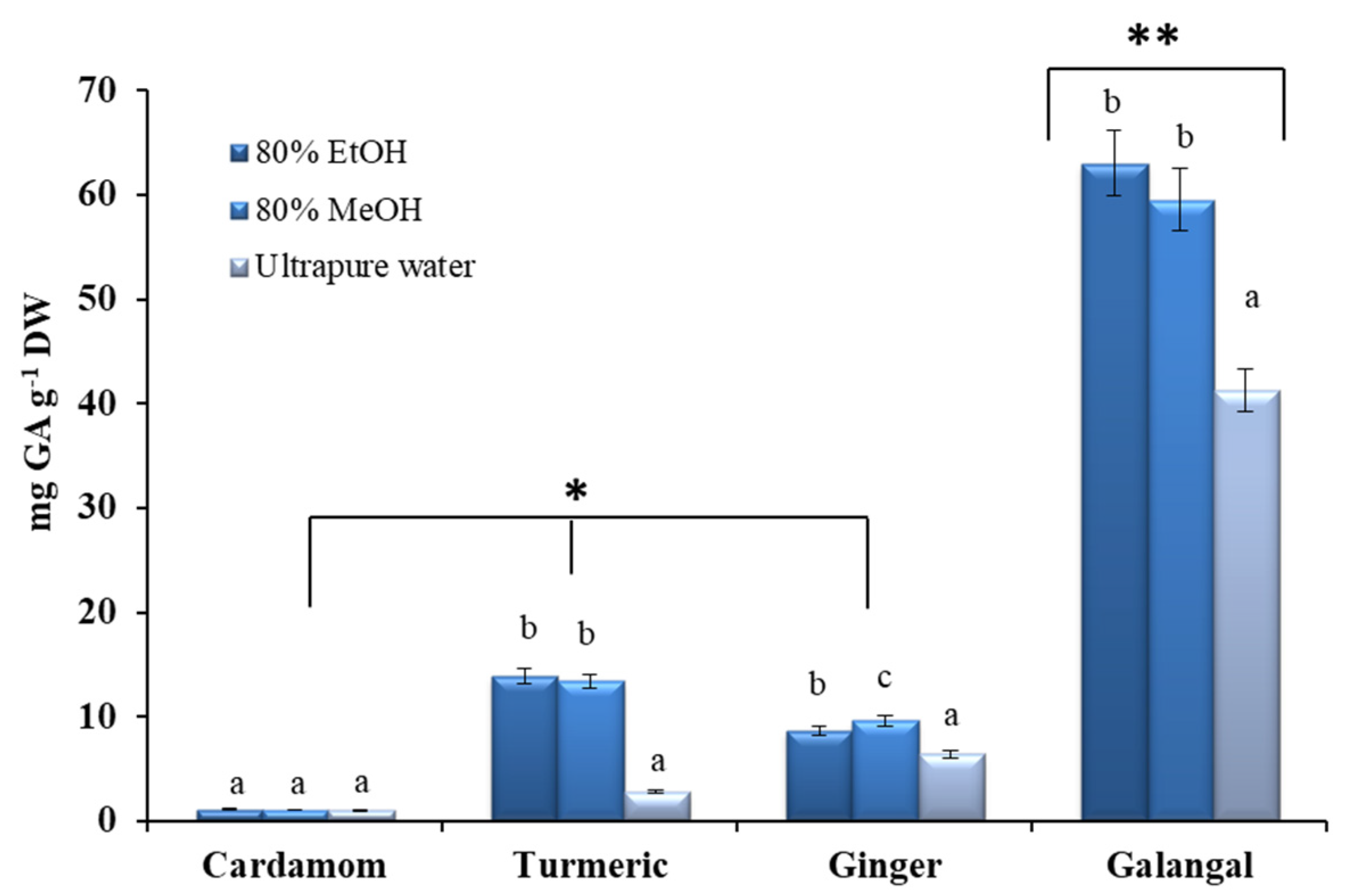
2.2. Total Phenolic Content (TPC) of the Crude Extracts
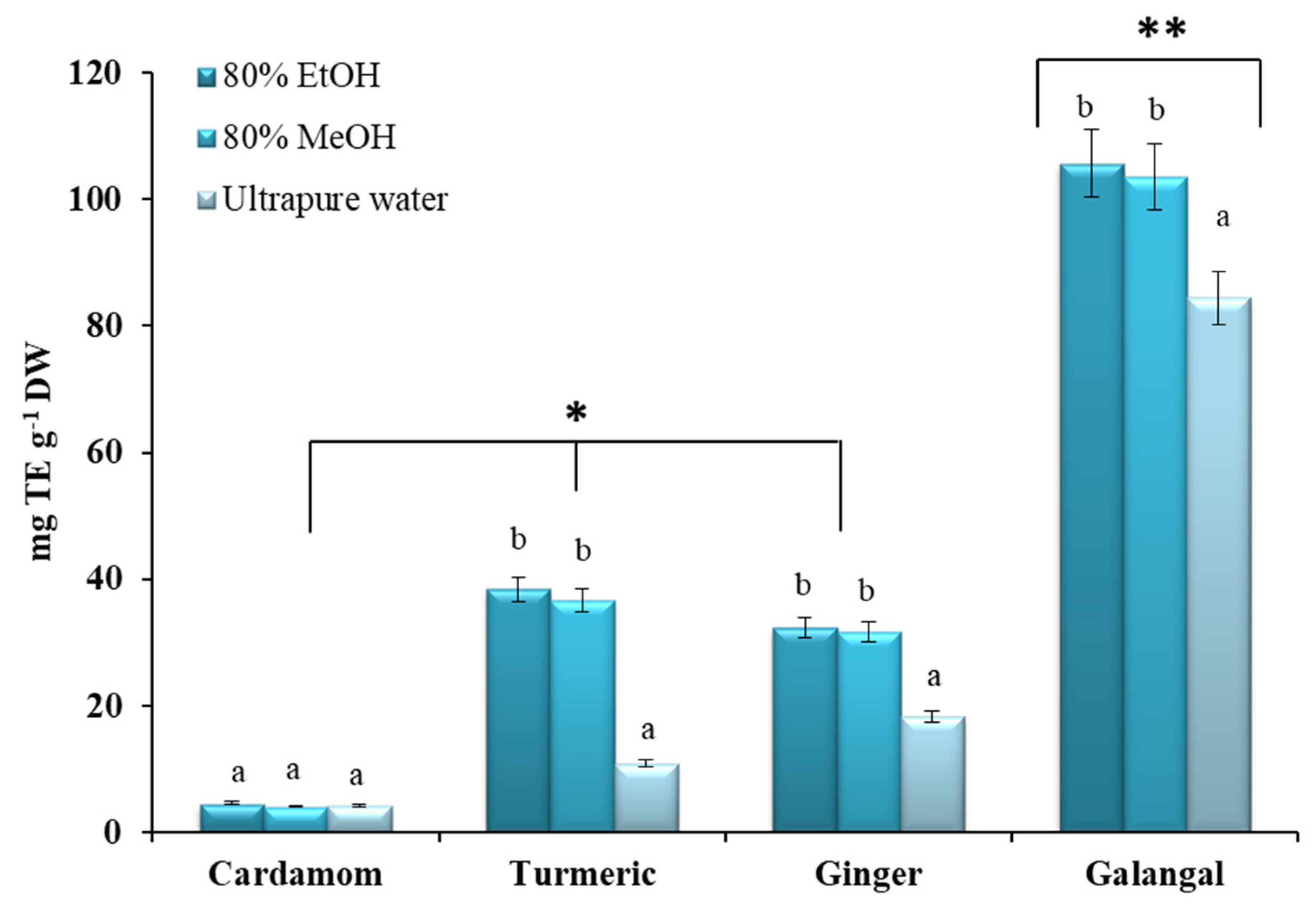
2.3. Antioxidant Properties of Crude Extracts and Essential Oils of Zingiberaceae Species
2.3.1. FRAP Assay
2.3.2. ABTS Assay
3. Materials and Methods
3.1. Chemicals and Plant Material
3.2. Hydrodistillation of eEssential Oils from the Selected Species of Zingiberaceae Family
3.3. GC and GC-MS/MS Analysis of the Essential Oils
3.4. Preparation of Crude Solvent Extracts by Ultrasound-Assisted Extraction (UAE)
3.5. Determination of Total Phenolic Content (TPC) of Crude Solvent Extracts
3.6. Determination of Antioxidant Activity of Crude Extracts and Essential Oils
3.6.1. ABTS Radical Scavenging Assay
3.6.2. Ferric Reducing Antioxidant Power Test (FRAP Assay)
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Barbosa, G.B.; Jayasinghe, N.S.; Natera, S.H.; Inutan, E.D.; Peteros, N.P.; Roessner, U. From common to rare Zingiberaceae plants—A metabolomics study using GC-MS. Phytochemistry 2017, 140, 141–150. [Google Scholar] [CrossRef]
- Zhang, L.; Liang, X.; Ou, Z.; Ye, M.; Shi, Y.; Chen, Y.; Zhao, J.; Zheng, D.; Xiang, H. Screening of chemical composition, anti-arthritis, antitumor and antioxidant capacities of essential oils from four Zingiberaceae herbs. Ind. Crop. Prod. 2020, 149, 112342. [Google Scholar] [CrossRef]
- Ashokkumar, K.; Murugan, M.; Dhanya, M.; Warkentin, T.D. Botany, traditional uses, phytochemistry and biological activities of cardamom [Elettaria cardamomum (L.) Maton]—A critical review. J. Ethnopharmacol. 2020, 246, 112244. [Google Scholar] [CrossRef]
- Ding, P.; Yang, L.; Feng, C.; Xian, J.-C. Research and application of Alpinia officinarum in medicinal field. Chin. Herb. Med. 2019, 11, 132–140. [Google Scholar] [CrossRef]
- Zhang, L.; Pan, C.; Ou, Z.; Liang, X.; Shi, Y.; Chi, L.; Zhang, Z.; Zheng, X.; Li, C.; Xiang, H. Chemical profiling and bioactivity of essential oils from Alpinia officinarum Hance from ten localities in China. Ind. Crop. Prod. 2020, 153, 112583. [Google Scholar] [CrossRef]
- Jugreet, B.S.; Suroowan, S.; Rengasamy, R.K.; Mahomoodally, M.F. Chemistry, bioactivities, mode of action and industrial applications of essential oils. Trends Food Sci. Technol. 2020, 101, 89–105. [Google Scholar] [CrossRef]
- Ma, Y.-N.; Xu, F.-R.; Chen, C.-J.; Li, Q.-Q.; Wang, M.-Z.; Cheng, Y.-X.; Dong, X. The beneficial use of essential oils from buds and fruit of Syzygium aromaticum to combat pathogenic fungi of Panax notoginseng. Ind. Crop. Prod. 2019, 133, 185–192. [Google Scholar] [CrossRef]
- Pateiro, M.; Munekata, P.E.; Sant’Ana, A.S.; Domínguez, R.; Rodríguez-Lázaro, D.; Lorenzo, J.M. Application of essential oils as antimicrobial agents against spoilage and pathogenic microorganisms in meat products. Int. J. Food Microbiol. 2021, 337, 108966. [Google Scholar] [CrossRef]
- Pinto, L.; Cefola, M.; Bonifacio, M.; Cometa, S.; Bocchino, C.; Pace, B.; De Giglio, E.; Palumbo, M.; Sada, A.; Logrieco, A.; et al. Effect of red thyme oil (Thymus vulgaris L.) vapours on fungal decay, quality parameters and shelf-life of oranges during cold storage. Food Chem. 2021, 336, 127590. [Google Scholar] [CrossRef]
- Pinto, L.; Bonifacio, M.A.; De Giglio, E.; Cometa, S.; Logrieco, A.F.; Baruzzi, F. Unravelling the antifungal effect of red thyme oil (Thymus vulgaris L.) compounds in vapor phase. Molecules 2020, 25, 4761. [Google Scholar] [CrossRef]
- Baldin, V.P.; Bertin de Lima Scodro, R.; Mariano Fernandez, C.M.; Ieque, A.L.; Caleffi-Ferracioli, K.R.; Dias Siqueira, V.L.; de Almeida, A.L.; Gonçalves, J.E.; Garcia Cortez, D.A.; Cardoso, R.F. Ginger essential oil and fractions against Mycobacterium spp. J. Ethnopharmacol. 2019, 244, 112095. [Google Scholar] [CrossRef]
- Anwar, F.; Abbas, A.; Alkharfy, K.M.; Gilani, A.-U.-H. Cardamom (Elettaria cardamomum Maton) Oils; Elsevier: Amsterdam, The Netherlands, 2016; ISBN 9780124166448. [Google Scholar]
- Jamil, B.; Abbasi, R.; Abbasi, S.; Imran, M.; Khan, S.U.; Ihsan, A.; Javed, S.; Bokhari, H. Encapsulation of cardamom essential oil in chitosan nano-composites: In-vitro efficacy on antibiotic-resistant bacterial pathogens and cytotoxicity studies. Front. Microbiol. 2016, 7, 1580. [Google Scholar] [CrossRef]
- Sengottuvelu, S. Cardamom (Elettaria cardamomum Linn. Maton) seeds in health. In Nuts and Seeds in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2011; pp. 285–291. ISBN 9780123756886. [Google Scholar]
- Cui, H.; Zhang, C.; Li, C.; Lin, L. Inhibition mechanism of cardamom essential oil on methicillin-resistant Staphylococcus aureus biofilm. LWT 2020, 122, 109057. [Google Scholar] [CrossRef]
- Avanço, G.B.; Ferreira, F.D.; Bomfim, N.S.; Rodriques dos Santos, P.A.d.S.; Peralta, R.M.; Brugnari, T.; Mallann, A.; Filho, B.A.d.A.; Graton Mikcha, J.M.; Machinski, M., Jr. Curcuma longa L. essential oil composition, antioxidant effect, and effect on Fusarium verticillioides and fumonisin production. Food Control. 2017, 73, 806–813. [Google Scholar] [CrossRef]
- Mishra, R.; Gupta, A.K.; Kumar, A.; Lal, R.K.; Saikia, D.; Chanotiya, C.S. Genetic diversity, essential oil composition, and in vitro antioxidant and antimicrobial activity of Curcuma longa L. germplasm collections. J. Appl. Res. Med. Aromat. Plants 2018, 10, 75–84. [Google Scholar] [CrossRef]
- Pal, K.; Chowdhury, S.; Dutta, S.K.; Chakraborty, S.; Chakraborty, M.; Pandit, G.K.; Dutta, S.; Paul, P.K.; Choudhury, A.; Majumder, B.; et al. Analysis of rhizome colour content, bioactive compound profiling and ex-situ conservation of turmeric genotypes (Curcuma longa L.) from sub-Himalayan terai region of India. Ind. Crop. Prod. 2020, 150, 112401. [Google Scholar] [CrossRef]
- Ahmad, M.; Kamran, S.H.; Mobasher, A. Protective effect of crude Curcuma longa and its methanolic extract in alloxanized rabbits. Pak. J. Pharm. Sci. 2014, 27, 121–128. [Google Scholar]
- Kim, Y.; You, Y.; Yoon, H.-G.; Lee, Y.-H.; Kim, K.; Lee, J.; Kim, M.S.; Kim, J.-C.; Jun, W. Hepatoprotective effects of fermented Curcuma longa L. on carbon tetrachloride-induced oxidative stress in rats. Food Chem. 2014, 151, 148–153. [Google Scholar] [CrossRef]
- Zeng, C.; Zhong, P.; Zhao, Y.; Kanchana, K.; Zhang, Y.; Khan, Z.A.; Chakrabarti, S.; Wu, L.; Wang, J.; Liang, G. Curcumin protects hearts from FFA-induced injury by activating Nrf2 and inactivating NF-κB both in vitro and in vivo. J. Mol. Cell. Cardiol. 2015, 79, 1–12. [Google Scholar] [CrossRef]
- Ko, M.-J.; Nam, H.-H.; Chung, M.-S. Conversion of 6-gingerol to 6-shogaol in ginger (Zingiber officinale) pulp and peel during subcritical water extraction. Food Chem. 2019, 270, 149–155. [Google Scholar] [CrossRef]
- Mahboubi, M. Zingiber officinale Rosc. essential oil, a review on its composition and bioactivity. Clin. Phytoscience 2019, 5, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Ali, H.A.; Mohamed, S.H.; Alharbi, H.F.; Algheshairy, R.M. Synergism between probiotics and herbs to manage type 2 diabetes in rats. Int. J. Pharm. Pharm. Sci. 2020, 12, 26–35. [Google Scholar] [CrossRef]
- Kazeem, M.; Akanji, M.; Yakubu, M.; Ashafa, A. Antiglycation and hypolipidemic effects of polyphenols from Zingiber officinale Roscoe (Zingiberaceae) in streptozotocin-induced diabetic rats. Trop. J. Pharm. Res. 2015, 14, 55–61. [Google Scholar] [CrossRef] [Green Version]
- Abubakar, I.B.; Malami, I.; Yahaya, Y.; Sule, S.M. A review on the ethnomedicinal uses, phytochemistry and pharmacology of Alpinia officinarum Hance. J. Ethnopharmacol. 2018, 224, 45–62. [Google Scholar] [CrossRef]
- Sun, D.-J.; Zhu, L.-J.; Zhao, Y.-Q.; Zhen, Y.-Q.; Zhang, L.; Lin, C.-C.; Chen, L.-X. Diarylheptanoid: A privileged structure in drug discovery. Fitoterapia 2020, 142, 104490. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Dou, J.; Zhang, S.; Liang, Q.; Meng, Q. Chemical composition and antioxidant properties of the essential oil and methanol extracts of rhizoma Alpinia officinarum from China in vitro. Afr. J. Biotechnol. 2010, 9, 4414–4421. [Google Scholar]
- Raina, A.P.; Verma, S.; Abraham, Z. Volatile constituents of essential oils isolated from Alpinia galanga Willd. (L.) and A. officinarum Hance rhizomes from North East India. J. Essent. Oil Res. 2014, 26, 24–28. [Google Scholar] [CrossRef]
- Martinez-Correa, H.A.; Paula, J.T.; Kayano, A.C.A.; Queiroga, C.L.; Magalhães, P.M.; Costa, F.T.; Cabral, F.A. Composition and antimalarial activity of extracts of Curcuma longa L. obtained by a combination of extraction processes using supercritical CO2, ethanol and water as solvents. J. Supercrit. Fluids 2017, 119, 122–129. [Google Scholar] [CrossRef]
- Mesomo, M.C.; Corazza, M.L.; Ndiaye, P.M.; Dalla Santa, O.R.; Cardozo, L.; Scheer, A.D.P. Supercritical CO2 extracts and essential oil of ginger (Zingiber officinale R.): Chemical composition and antibacterial activity. J. Supercrit. Fluids 2013, 80, 44–49. [Google Scholar] [CrossRef]
- Morsy, N.F. A short extraction time of high quality hydrodistilled cardamom (Elettaria cardamomum L. Maton) essential oil using ultrasound as a pretreatment. Ind. Crop. Prod. 2015, 65, 287–292. [Google Scholar] [CrossRef]
- Przygodzka, M.; Zielińska, D.; Ciesarová, Z.; Kukurová, K.; Zieliński, H. Comparison of methods for evaluation of the antioxidant capacity and phenolic compounds in common spices. LWT Food Sci. Technol. 2014, 58, 321–326. [Google Scholar] [CrossRef]
- Guo, J.-B.; Fan, Y.; Zhang, W.-J.; Wu, H.; Du, L.-M.; Chang, Y.-X. Extraction of gingerols and shogaols from ginger (Zingiber officinale Roscoe) through microwave technique using ionic liquids. J. Food Compos. Anal. 2017, 62, 35–42. [Google Scholar] [CrossRef]
- Abu-Orabi, S.T.; Al-Qudah, M.A.; Saleh, N.R.; Bataineh, T.T.; Obeidat, S.M.; Al-Sheraideh, M.S.; Al-Jaber, H.I.; Tashtoush, H.I.; Lahham, J.N. Antioxidant activity of crude extracts and essential oils from flower buds and leaves of Cistus creticus and Cistus salviifolius. Arab. J. Chem. 2020, 13, 6256–6266. [Google Scholar] [CrossRef]
- Al-Dhahli, A.S.; Al-Hassani, F.A.; Mohammed Alarjani, K.; Mohamed Yehia, H.; Al Lawati, W.M.; Najmul Hejaz Azmi, S.; Alam Khan, S. Essential oil from the rhizomes of the Saudi and Chinese Zingiber officinale cultivars: Comparison of chemical composition, antibacterial and molecular docking studies. J. King Saud Univ. Sci. 2020, 32, 3343–3350. [Google Scholar] [CrossRef]
- Köse, L.P.; Gülçin, I.; Gören, A.C.; Namiesnik, J.; Martinez-Ayala, A.L.; Gorinstein, S. LC–MS/MS analysis, antioxidant and anticholinergic properties of galanga (Alpinia officinarum Hance) rhizomes. Ind. Crop. Prod. 2015, 74, 712–721. [Google Scholar] [CrossRef]
- Feng, Y.; Dunshea, F.R.; Suleria, H.A.R. LC-ESI-QTOF/MS characterization of bioactive compounds from black spices and their potential antioxidant activities. J. Food Sci. Technol. 2020, 57, 4671–4687. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Yuan, B.; Zeng, M.; Chen, J. Antioxidant capacity and major phenolic compounds of spices commonly consumed in China. Food Res. Int. 2011, 44, 530–536. [Google Scholar] [CrossRef]
- Elguindy, N.M.; Yacout, G.A.; El Azab, E.F.; Maghraby, H.K. Chemoprotective effect of elettaria cardamomum against chemically induced hepatocellular carcinoma in rats by inhibiting NF-κB, oxidative stress, and activity of ornithine decarboxylase. S. Afr. J. Bot. 2016, 105, 251–258. [Google Scholar] [CrossRef]
- Yashin, A.; Yashin, Y.; Xia, X.; Nemzer, B. Antioxidant activity of spices and their impact on human health: A review. Antioxidants 2017, 6, 70. [Google Scholar] [CrossRef] [Green Version]
- Singh, G.; Kiran, S.; Marimuthu, P.; Isidorov, V.; Vinogorova, V. Antioxidant and antimicrobial activities of essential oil and various oleoresins ofElettaria cardamomum (seeds and pods). J. Sci. Food Agric. 2007, 88, 280–289. [Google Scholar] [CrossRef]
- Kuyumcu Savan, E.; Küçükbay, F.Z. Essential oil composition of elettaria cardamomum maton. J. Appl. Biol. Sci. 2013, 7, 42–45. [Google Scholar]
- Chowdhury, S.; Kumar, S. Alpha-terpinyl acetate: A natural monoterpenoid from Elettaria cardamomum as multi-target directed ligand in Alzheimer’s disease. J. Funct. Foods 2020, 68, 103892. [Google Scholar] [CrossRef]
- Jakab, E.; Blazsó, M.; Barta-Rajnai, E.; Babinszki, B.; Sebestyén, Z.; Czégény, Z.; Nicol, J.; Clayton, P.; McAdam, K.; Liu, C. Thermo-oxidative decomposition of lime, bergamot and cardamom essential oils. J. Anal. Appl. Pyrolysis 2018, 134, 552–561. [Google Scholar] [CrossRef]
- Kizhakkayil, J.; Thomas, E.; Zachariah, T.; Syamkumar, S.; Sasikumar, B. A comparative quality appraisal of exported cardamoms of India, Sri Lanka and Guatemala. Indian J. Nat. Prod. Resour. 2006, 5, 361–365. [Google Scholar]
- Naz, S.; Ilyas, S.; Parveen, Z.; Javed, S. Chemical analysis of essential oils from turmeric (Curcuma longa) rhizome through GC-MS. Asian J. Chem. 2010, 22, 3153–3158. [Google Scholar]
- Zhang, L.; Yang, Z.; Chen, F.; Su, P.; Chen, D.; Pan, W.; Fang, Y.; Dong, C.; Zheng, X.; Du, Z. Composition and bioactivity assessment of essential oils of Curcuma longa L. collected in China. Ind. Crop. Prod. 2017, 109, 60–73. [Google Scholar] [CrossRef]
- Gounder, D.K.; Lingamallu, J. Comparison of chemical composition and antioxidant potential of volatile oil from fresh, dried and cured turmeric (Curcuma longa) rhizomes. Ind. Crop. Prod. 2012, 38, 124–131. [Google Scholar] [CrossRef]
- Mastinu, A.; Bonini, S.; Premoli, M.; Maccarinelli, G.; Mac Sweeney, E.; Zhang, L.; Lucini, L.; Memo, M. Protective effects of Gynostemma pentaphyllum (var. Ginpent) against lipopolysaccharide-induced inflammation and motor alteration in mice. Molecules 2021, 26, 570. [Google Scholar] [CrossRef] [PubMed]
- Dincer, C.; Topuz, A.; Sahin-Nadeem, H.; Özdemir, K.S.; Cam, I.B.; Tontul, I.; Göktürk, R.S.; Ay, S.T. A comparative study on phenolic composition, antioxidant activity and essential oil content of wild and cultivated sage (Salvia fruticosa Miller) as influenced by storage. Ind. Crop. Prod. 2012, 39, 170–176. [Google Scholar] [CrossRef]
- Usai, M.; Marchetti, M.; Foddai, M.; Del Caro, A.; Desogus, R.; Sanna, I.; Piga, A. Influence of different stabilizing operations and storage time on the composition of essential oil of thyme (Thymus officinalis L.) and rosemary (Rosmarinus officinalis L.). LWT Food Sci. Technol. 2011, 44, 244–249. [Google Scholar] [CrossRef]
- Kumar, A.; Agarwal, K.; Singh, M.; Saxena, A.; Yadav, P.; Maurya, A.K.; Yadav, A.; Tandon, S.; Chanda, D.; Bawankule, D.U. Essential oil from waste leaves of Curcuma longa L. alleviates skin inflammation. Inflammopharmacology 2018, 26, 1245–1255. [Google Scholar] [CrossRef] [PubMed]
- Yue, G.G.-L.; Kwok, H.-F.; Lee, J.K.-M.; Jiang, L.; Chan, K.-M.; Cheng, L.; Wong, E.C.-W.; Leung, P.-C.; Fung, K.-P.; Lau, C.B.-S. Novel anti-angiogenic effects of aromatic-turmerone, essential oil isolated from spice turmeric. J. Funct. Foods 2015, 15, 243–253. [Google Scholar] [CrossRef]
- Park, S.Y.; Kim, Y.H.; Kim, Y.; Lee, S.J. Aromatic-turmerone attenuates invasion and expression of MMP-9 and COX-2 through inhibition of NF-κB activation in TPA-induced breast cancer cells. J. Cell. Biochem. 2012, 113, 3653–3662. [Google Scholar] [CrossRef]
- Camero, M.; Lanave, G.; Catella, C.; Capozza, P.; Gentile, A.; Fracchiolla, G.; Britti, D.; Martella, V.; Buonavoglia, C.; Tempesta, M. Virucidal activity of ginger essential oil against caprine alphaherpesvirus-1. Vet. Microbiol. 2019, 230, 150–155. [Google Scholar] [CrossRef]
- Amalraj, A.; Haponiuk, J.T.; Thomas, S.; Gopi, S. Preparation, characterization and antimicrobial activity of polyvinyl alcohol/gum arabic/chitosan composite films incorporated with black pepper essential oil and ginger essential oil. Int. J. Biol. Macromol. 2020, 151, 366–375. [Google Scholar] [CrossRef] [PubMed]
- dos Santos Reis, N.; de Santana, N.B.; de Carvalho Tavares, I.M.; Lessa, O.A.; dos Santos, L.R.; Pereira, N.E.; Soares, G.A.; Oliveira, R.A.; Oliveira, J.R.; Franco, M. Enzyme extraction by lab-scale hydrodistillation of ginger essential oil (Zingiber officinale Roscoe): Chromatographic and micromorphological analyses. Ind. Crop. Prod. 2020, 146, 112210. [Google Scholar] [CrossRef]
- De Melo, G.A.N.; Grespan, R.; Fonseca, J.P.; Farinha, T.O.; Da Silva, E.L.; Romero, A.L.; Bersani-Amado, C.A.; Cuman, R.K.N. Inhibitory effects of ginger (Zingiber officinale Roscoe) essential oil on leukocyte migration in vivo and in vitro. J. Nat. Med. 2011, 65, 241–246. [Google Scholar] [CrossRef]
- Mahomoodally, M.; Aumeeruddy, M.; Rengasamy, K.R.; Roshan, S.; Hammad, S.; Pandohee, J.; Hu, X.; Zengin, G. Ginger and its active compounds in cancer therapy: From folk uses to nano-therapeutic applications. Semin. Cancer Biol. 2019. [Google Scholar] [CrossRef]
- Bruchhage, K.-L.; Koennecke, M.; Drenckhan, M.; Plötze-Martin, K.; Pries, R.; Wollenberg, B. 1,8-cineol inhibits the Wnt/β-catenin signaling pathway through GSK-3 dephosphorylation in nasal polyps of chronic rhinosinusitis patients. Eur. J. Pharmacol. 2018, 835, 140–146. [Google Scholar] [CrossRef]
- Juergens, U.R. Anti-inflammatory properties of the monoterpene 1.8-cineole: Current evidence for co-medication in inflammatory airway diseases. Drug Res. 2014, 64, 638–646. [Google Scholar] [CrossRef]
- Zhang, J.; Pan, H.; Chen, Y.; Tanveer, Z.I.; Liu, L.; Cai, Z.; Hong, Y.; Wu, Y. Application of pharmacodynamics-based optimization to the extraction of bioactive compounds from Chansu. Microchem. J. 2020, 159, 105552. [Google Scholar] [CrossRef]
- Rajan, M.; Rajkumar, G.; Farias Lima Guedes, T.J.; Chagas Barros, R.G.; Barros, R.G.C.; Narain, N. Performance of different solvents on extraction of bioactive compounds, antioxidant and cytotoxic activities in Phoenix loureiroi Kunth leaves. J. Appl. Res. Med. Aromat. Plants 2020, 17, 100247. [Google Scholar] [CrossRef]
- Pimentel-Moral, S.; Borrás-Linares, I.; Lozano-Sánchez, J.; Alañón, M.; Arráez-Román, D.; Segura-Carretero, A. Pressurized GRAS solvents for the green extraction of phenolic compounds from hibiscus sabdariffa calyces. Food Res. Int. 2020, 137, 109466. [Google Scholar] [CrossRef]
- Carbone, K.; Macchioni, V.; Petrella, G.; Cicero, D.O. Exploring the potential of microwaves and ultrasounds in the green extraction of bioactive compounds from Humulus lupulus for the food and pharmaceutical industry. Ind. Crop. Prod. 2020, 156, 112888. [Google Scholar] [CrossRef]
- Anwer, M.K.; Shakeel, F. Measurement and correlation of solubility of diosmin in four pure solvents and β-cyclodextrin solution at 298.15 K to 333.15 K. Chin. J. Chem. Eng. 2015, 23, 812–815. [Google Scholar] [CrossRef]
- Suresh, K.; Nangia, A. Curcumin: Pharmaceutical solids as a platform to improve solubility and bioavailability. CrystEngComm 2018, 20, 3277–3296. [Google Scholar] [CrossRef]
- Yang, Q.-Q.; Cheng, L.-Z.; Zhang, T.; Yaron, S.; Jiang, H.-X.; Sui, Z.-Q.; Corke, H. Phenolic profiles, antioxidant, and antiproliferative activities of turmeric (Curcuma longa). Ind. Crop. Prod. 2020, 152, 112561. [Google Scholar] [CrossRef]
- Hester, F.; Verghese, M.; Sunkara, R.; Willis, S.; Walker, L.T. A Comparison of the antioxidative and anti-diabetic potential of thermally treated garlic, turmeric, and ginger. Food Nutr. Sci. 2019, 10, 207–219. [Google Scholar] [CrossRef] [Green Version]
- Avci, G.A.; Avci, E.; Cilak, G.O.; Cehver, S.C. Antimicrobial and antioxidant activities of Zingiber officinale (ginger) and Alpina officinarum (galangal). Hittite J. Sci. Eng. 2020, 7, 45–49. [Google Scholar] [CrossRef]
- Ibáñez, M.D.; Blázquez, M.A. Curcuma longa L. rhizome essential oil from extraction to its agri-food applications. A review. Plants 2020, 10, 44. [Google Scholar] [CrossRef]
- Sana, S.; Arshad, M.U.; Farhan, S.; Ahmad, R.; Ali, I.; Tabussam, T. Nutritional characterization of cinnamon and turmeric with special reference to their antioxidant profile. Int. J. Biosci. 2019, 15, 178–187. [Google Scholar]
- Razboršek, M.I.; Ivanović, M.; Krajnc, P.; Kolar, M. Choline chloride based natural deep eutectic solvents as extraction media for extracting phenolic compounds from chokeberry (Aronia melanocarpe). Molecules 2020, 25, 1619. [Google Scholar] [CrossRef] [Green Version]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morgenstern, A.; Ekholm, A.; Scheewe, P.; Rumpunen, K. Changes in content of major phenolic compounds during leaf development of sea buckthorn (Hippophaë rhamnoides L.). Agric. Food Sci. 2014, 23, 207–219. [Google Scholar] [CrossRef] [Green Version]



| No. | Chemical Compound | Peak Area Percentage (%) | Classification | |||
|---|---|---|---|---|---|---|
| Cardamom | Turmeric | Ginger | Galangal | |||
| Elettaria cardamomum L. Maton | Curcuma longa L. | Zingiber officinale Roscoe | Alpinia officinarum Hance | |||
| 1 | 4-acetyl-1-methyl-1-cyclohexene | - | 0.03 | - | - | other |
| 2 | p-acetyl toluene | - | 0.08 | - | - | other |
| 3 | trans-anethole | - | 0.04 | - | 0.42 | other |
| 4 | cis-α-bergamotene | - | - | 0.11 | - | sesquiterpene |
| 5 | trans-α-bergamotene | - | 0.09 | 0.08 | 0.22 | sesquiterpene |
| 6 | Borneol | - | - | 0.04 | 0.06 | monoterpene |
| 7 | β-bisabolene | - | 3.04 | 7.55 | 0.38 | sesquiterpene |
| 8 | Butyl isobutyrate | - | - | - | 0.34 | other |
| 9 | sec-butyl isobutyrate | - | - | - | 0.19 | other |
| 10 | Benzyl acetone | - | - | - | 0.14 | other |
| 11 | 3-carene | - | 0.03 | - | - | monoterpene |
| 12 | o-cymene | - | 0.01 | - | - | monoterpene |
| 13 | p-cymene | 0.11 | 3.36 | 0.10 | 0.52 | monoterpene |
| 14 | Camphor | - | 0.01 | 0.10 | 2.24 | monoterpene |
| 15 | p-cymenol | - | 0.17 | 0.03 | 0.03 | other |
| 16 | Carvone | - | 0.06 | - | 0.34 | monoterpene |
| 17 | Carvacrol | - | 0.22 | - | / | monoterpene |
| 18 | β-caryophyllene | - | 0.85 | 0.06 | 0.49 | sesquiterpene |
| 19 | ar-curcumene | - | 11.42 | 11.63 | 0.94 | sesquiterpene |
| 20 | Caryophyllene oxide | - | 0.69 | - | 0.43 | sesquiterpene |
| 21 | 1-(3-cyclopentylpropyl)-2,4-dimethylbenzene | - | 1.47 | - | - | other |
| 22 | Cedrenol | - | 0.73 | - | - | sesquiterpene |
| 23 | Curlone | - | 7.59 | - | - | sesquiterpene |
| 24 | 1,8-cineol/eucalyptol | 33.78 | 2.01 | 2.92 | 42.71 | monoterpene |
| 25 | Camphene | - | - | 5.84 | 4.58 | monoterpene |
| 26 | Citronellol | - | - | 0.07 | - | monoterpene |
| 27 | Citronellyl acetate | - | - | 0.17 | - | monoterpene |
| 28 | α-copaene | - | - | 0.21 | 0.05 | sesquiterpene |
| 29 | δ-cadinene | - | - | 0.19 | 0.38 | sesquiterpene |
| 30 | α-calacorene | - | - | - | 0.63 | sesquiterpene |
| 31 | β-calacorene | - | - | - | 0.08 | sesquiterpene |
| 32 | Cubenol | - | - | - | 1.26 | sesquiterpene |
| 33 | Cadalene | - | - | - | 0.74 | sesquiterpene |
| 34 | Dimethyl styrene | - | 0.05 | - | - | other |
| 35 | 4,4-dimethyl-3,4-dihydro coumarin | - | 0.63 | - | - | other |
| 36 | Ethyl dihydrocinnamate | - | - | - | 0.10 | other |
| 37 | β-elemene | 0.11 | - | 0.36 | - | sesquiterpene |
| 38 | δ-elemene | - | - | 0.03 | - | sesquiterpene |
| 39 | α-farnesene | - | - | 2.26 | - | sesquiterpene |
| 40 | endo-fenchol | - | - | - | 0.03 | monoterpene |
| 41 | exo-fenchol | - | - | - | 0.12 | monoterpene |
| 42 | Fenchyl acetate | - | - | - | 0.55 | monoterpene |
| 43 | D-germacrene | - | - | 2.79 | 1.89 | sesquiterpene |
| 44 | Geranial | 0.08 | - | 0.04 | - | monoterpene |
| 45 | Geraniol | 0.24 | - | - | - | monoterpene |
| 46 | Geranyl acetate | 0.23 | - | 1.23 | - | monoterpene |
| 47 | α-humulene | - | 0.26 | - | 0.17 | sesquiterpene |
| 48 | 2-heptanol | - | - | 0.04 | - | other |
| 49 | Isobutyl isovalerate | - | - | - | 0.08 | other |
| 50 | Isoamyl isovalerate | - | - | - | 0.04 | other |
| 51 | Isoamyl-2-methylbutyrate | - | - | - | 0.06 | other |
| 52 | Isoborneol | - | 0.01 | 1.42 | 0.60 | monoterpene |
| 53 | Isobornyl acetate | - | - | 0.58 | 0.22 | monoterpene |
| 54 | Isobutyl benzoate | - | - | - | 0.21 | other |
| 55 | Limonene | 2.32 | 0.15 | 7.90 | 2.76 | monoterpene |
| 56 | Linalool | 2.72 | 0.02 | 0.41 | 0.41 | monoterpene |
| 57 | Linalyl acetate | 0.67 | - | - | - | monoterpene |
| 58 | Myrcene | 0.78 | 0.05 | 0.43 | 0.20 | monoterpene |
| 59 | 6-methyl-5-hepten-2-one | - | 0.01 | 0.13 | 0.41 | other |
| 60 | Methyl isovalerate | - | - | - | 0.05 | other |
| 61 | Neral | - | - | 0.36 | - | monoterpene |
| 62 | Neryl acetate | 0.08 | - | - | - | monoterpene |
| 63 | Ocimene | 0.06 | - | - | - | monoterpene |
| 64 | α-pinene | 0.98 | 0.20 | 1.83 | 3.56 | monoterpene |
| 65 | β-pinene | 0.26 | 0.03 | 0.15 | 3.85 | monoterpene |
| 66 | Phenethyl isobutyrate | - | - | - | 0.56 | other |
| 67 | Phenethyl isovalerate | - | - | - | 0.44 | other |
| 68 | α-phellandrene | - | 1.33 | 0.40 | - | monoterpene |
| 69 | α-selinene | - | - | - | 0.72 | sesquiterpene |
| 70 | β-selinene | - | - | 0.38 | 0.49 | sesquiterpene |
| 71 | Sabinene | 1.82 | 0.01 | 0.08 | - | monoterpene |
| 72 | β-sesquiphellandrene | - | 10.44 | 10.08 | - | sesquiterpene |
| 73 | Sabinyl acetate | - | 0.20 | - | - | monoterpene |
| 74 | α-thujene | 0.14 | 0.02 | 0.01 | 0.07 | monoterpene |
| 75 | α-terpinene | 0.36 | 0.03 | 0.03 | 0.25 | monoterpene |
| 76 | γ-terpinene | 0.61 | 0.03 | 0.04 | 0.31 | monoterpene |
| 77 | Terpinolene | 0.32 | 0.26 | 0.14 | 0.20 | monoterpene |
| 78 | 4-terpineol | 1.85 | 0.08 | 0.21 | 2.29 | monoterpene |
| 79 | α-terpineol | 2.98 | 0.20 | 0.69 | 11.11 | monoterpene |
| 80 | α-terpinyl acetate | 42.65 | 0.46 | 0.21 | 0.31 | monoterpene |
| 81 | ar-turmerone | - | 12.28 | - | - | sesquiterpene |
| 82 | β-turmerone | - | 25.77 | - | - | sesquiterpene |
| 83 | Tricyclene | - | - | 0.12 | 0.11 | monoterpene |
| 84 | Thymol | - | 0.48 | - | - | monoterpene |
| 85 | 2-undecanone | - | - | 0.38 | - | other |
| 86 | Valencene | - | - | - | 0.35 | sesquiterpene |
| 87 | α-zingiberene | - | 5.13 | 22.69 | - | sesquiterpene |
| Total monoterpenes (%) | 93.04 | 9.26 | 25.52 | 77.40 | ||
| Total sesquiterpenes (%) | 0.11 | 78.29 | 58.42 | 9.22 | ||
| Other compounds (%) | - | 2.48 | 0.58 | 3.07 | ||
| Total compounds identified (%) | 93.15 | 90.03 | 84.52 | 89.69 | ||
| Common Name | Latin Name | Plant Part | Origin |
|---|---|---|---|
| Cardamom | Elettaria cardamomum L. Maton | fruits | Guatemala |
| Turmeric | Curcuma longa L. | rhizome | India |
| Ginger | Zingiber officinale Roscoe | rhizome | Madagascar |
| Galangal | Alpinia officinarum Hance | rhizome | China |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivanović, M.; Makoter, K.; Islamčević Razboršek, M. Comparative Study of Chemical Composition and Antioxidant Activity of Essential Oils and Crude Extracts of Four Characteristic Zingiberaceae Herbs. Plants 2021, 10, 501. https://doi.org/10.3390/plants10030501
Ivanović M, Makoter K, Islamčević Razboršek M. Comparative Study of Chemical Composition and Antioxidant Activity of Essential Oils and Crude Extracts of Four Characteristic Zingiberaceae Herbs. Plants. 2021; 10(3):501. https://doi.org/10.3390/plants10030501
Chicago/Turabian StyleIvanović, Milena, Kaja Makoter, and Maša Islamčević Razboršek. 2021. "Comparative Study of Chemical Composition and Antioxidant Activity of Essential Oils and Crude Extracts of Four Characteristic Zingiberaceae Herbs" Plants 10, no. 3: 501. https://doi.org/10.3390/plants10030501
APA StyleIvanović, M., Makoter, K., & Islamčević Razboršek, M. (2021). Comparative Study of Chemical Composition and Antioxidant Activity of Essential Oils and Crude Extracts of Four Characteristic Zingiberaceae Herbs. Plants, 10(3), 501. https://doi.org/10.3390/plants10030501







