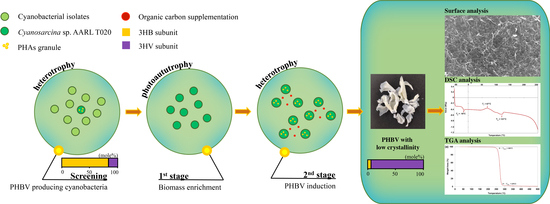
Low Crystallinity of Poly(3-Hydroxybutyrate-co-3-Hydroxyvalerate) Bioproduction by Hot Spring Cyanobacterium Cyanosarcina sp. AARL T020
Abstract
1. Introduction
2. Results
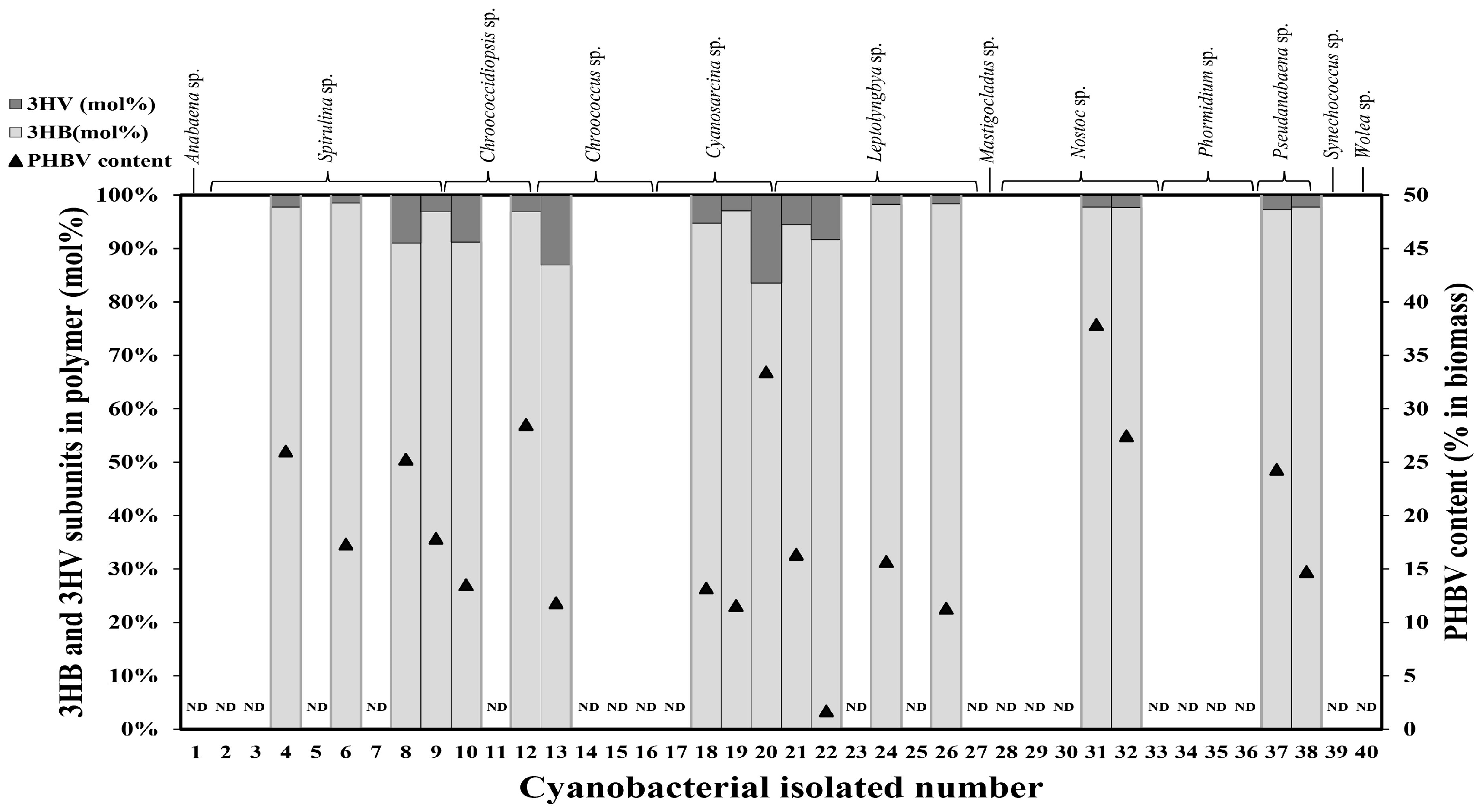
2.1. Screening of Cyanobacteria for PHBV Production
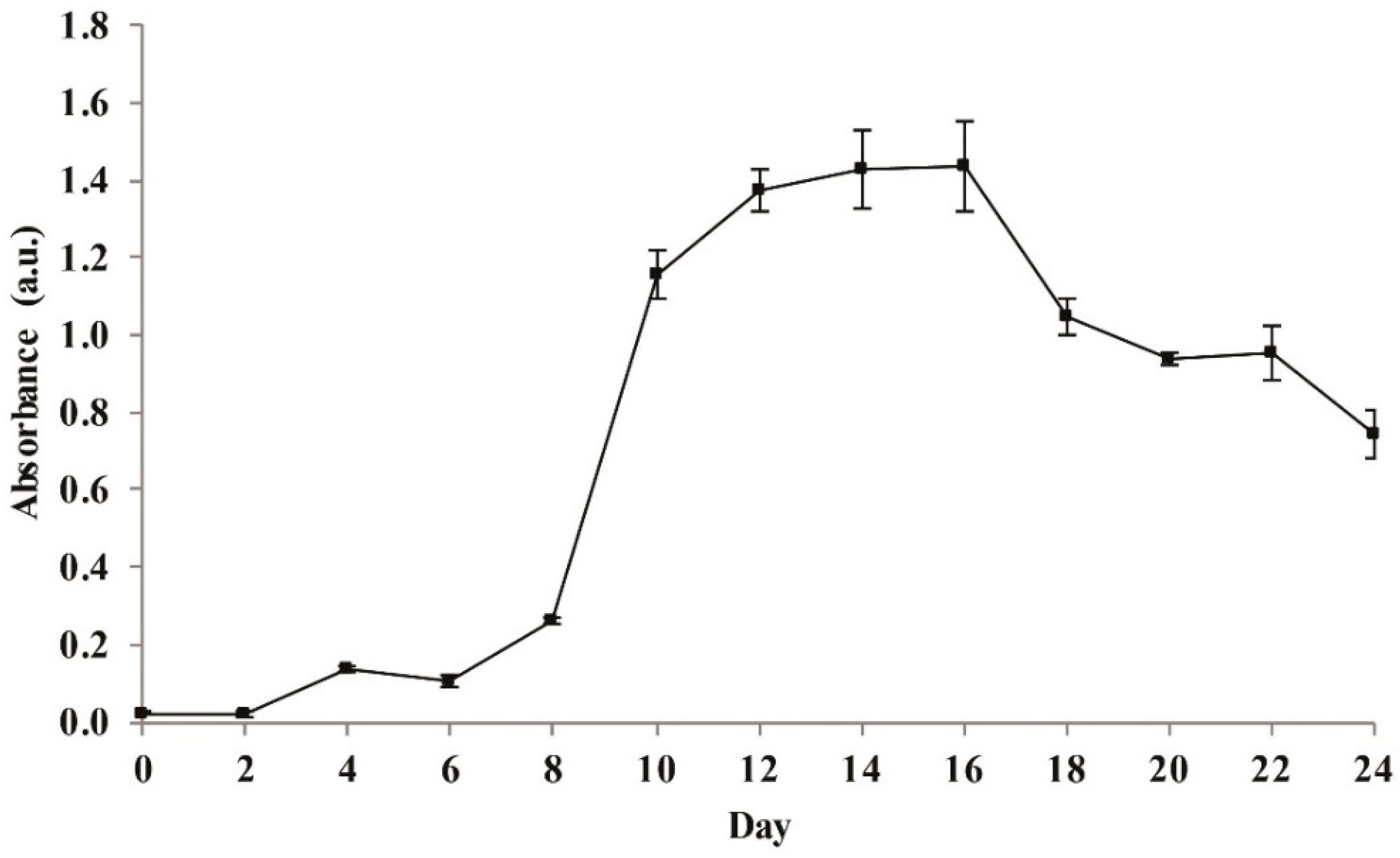
2.2. The Growth of Cyanobacteria
2.3. Model Fitting of First-Stage Cultivation
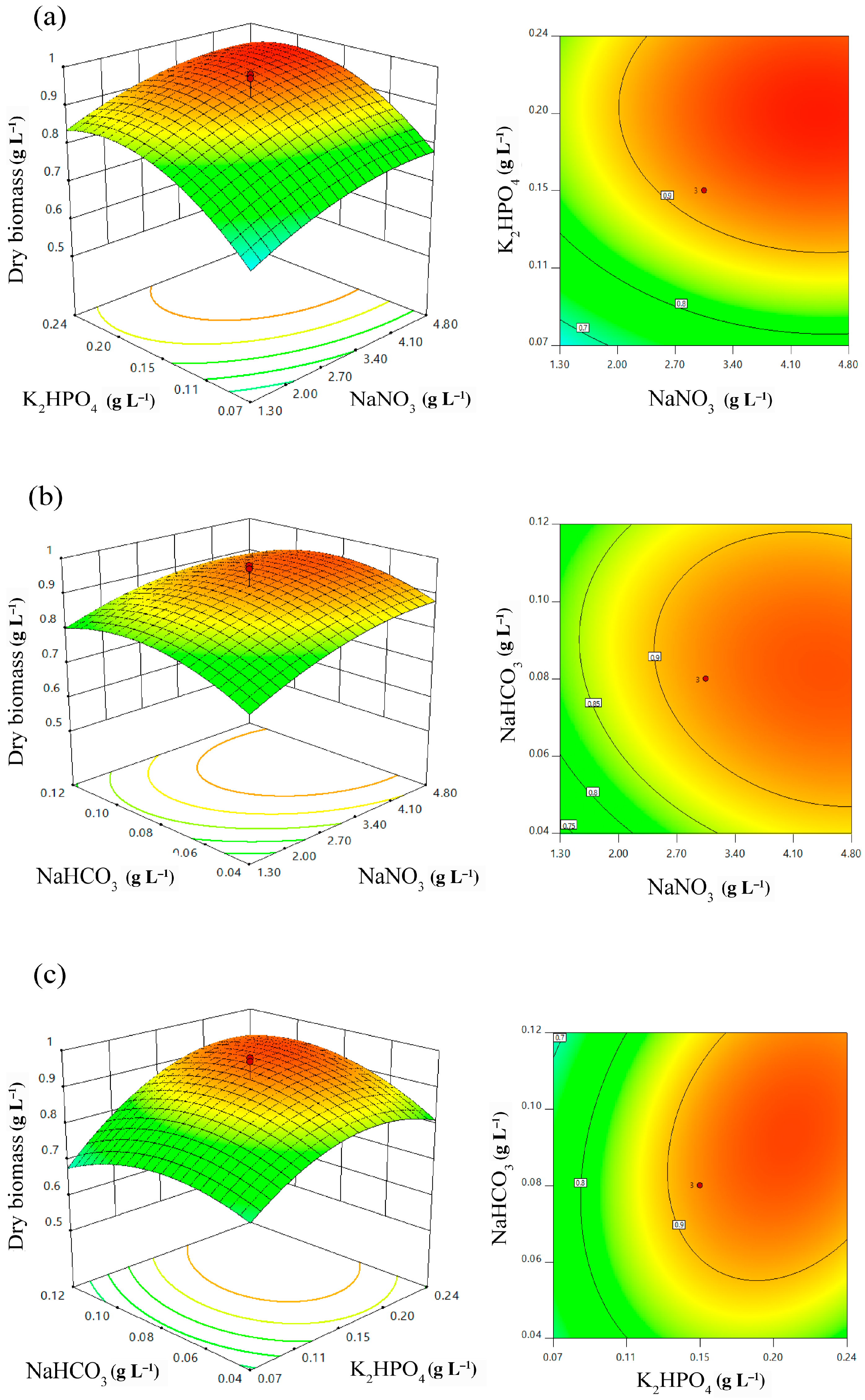
2.4. Effect of Independent Variables and Model Verification
2.5. Second-Stage Cultivation with PHBV Production Parameters
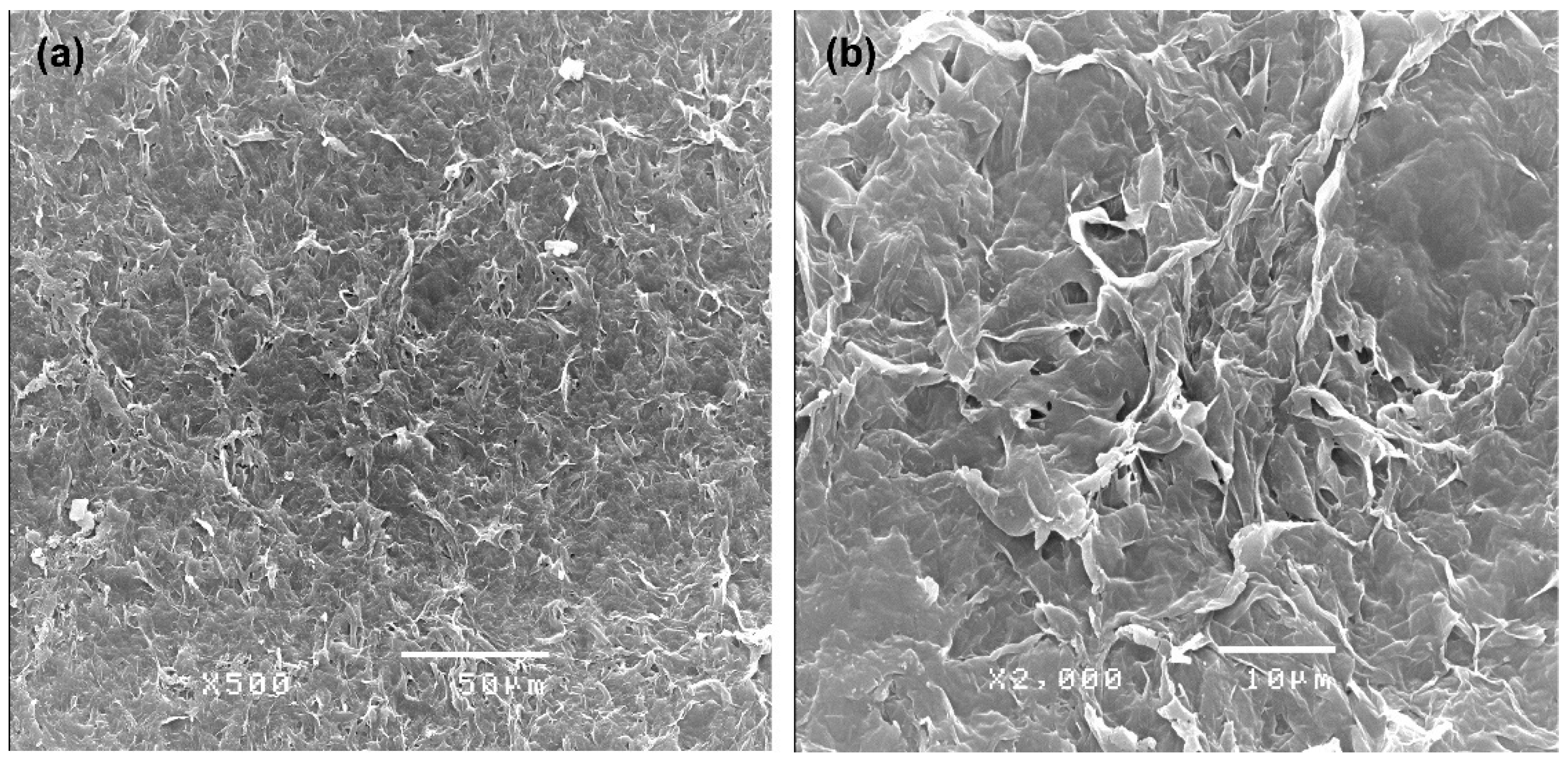
2.6. Surface Analysis of Extracted PHBV
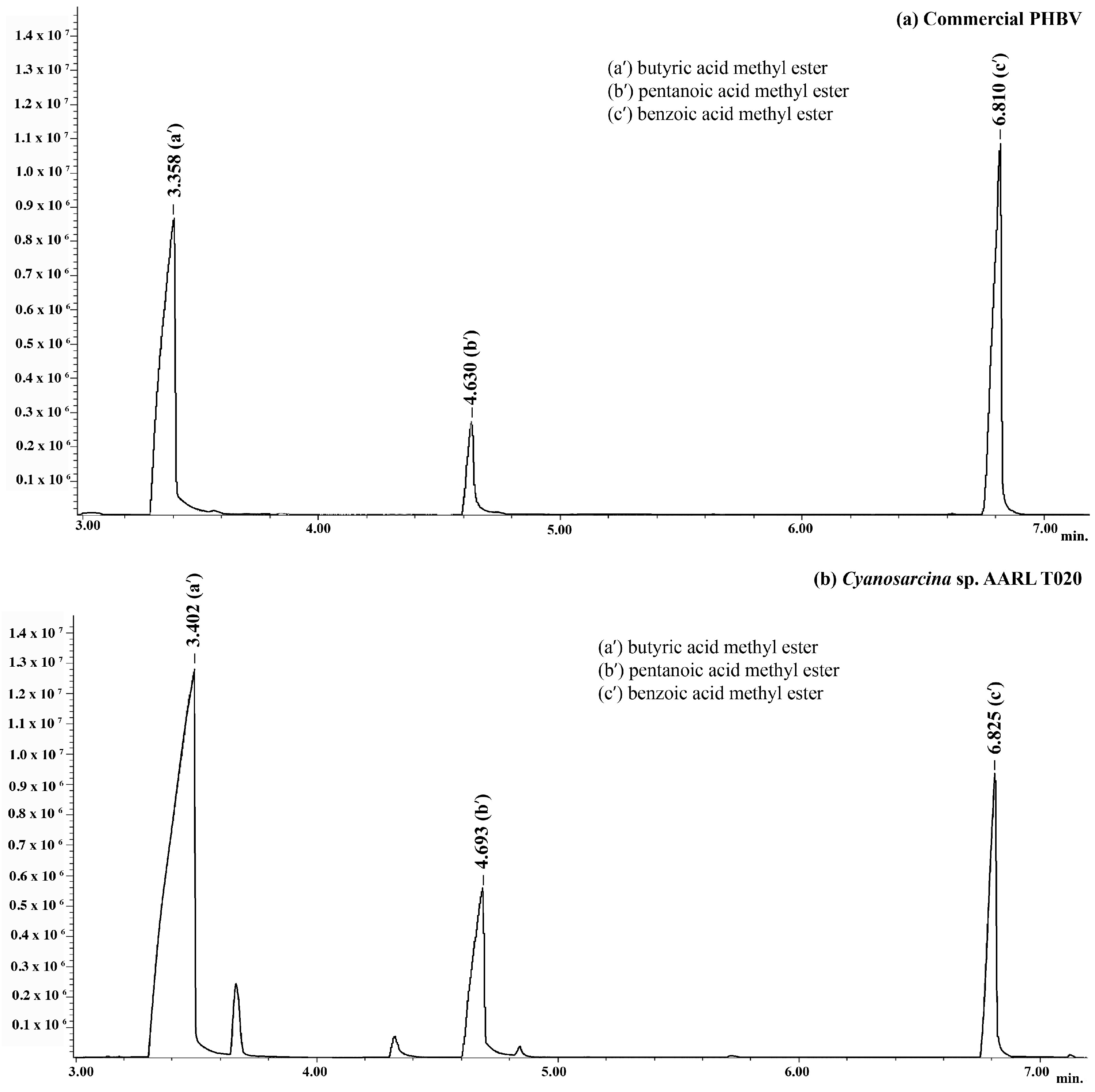
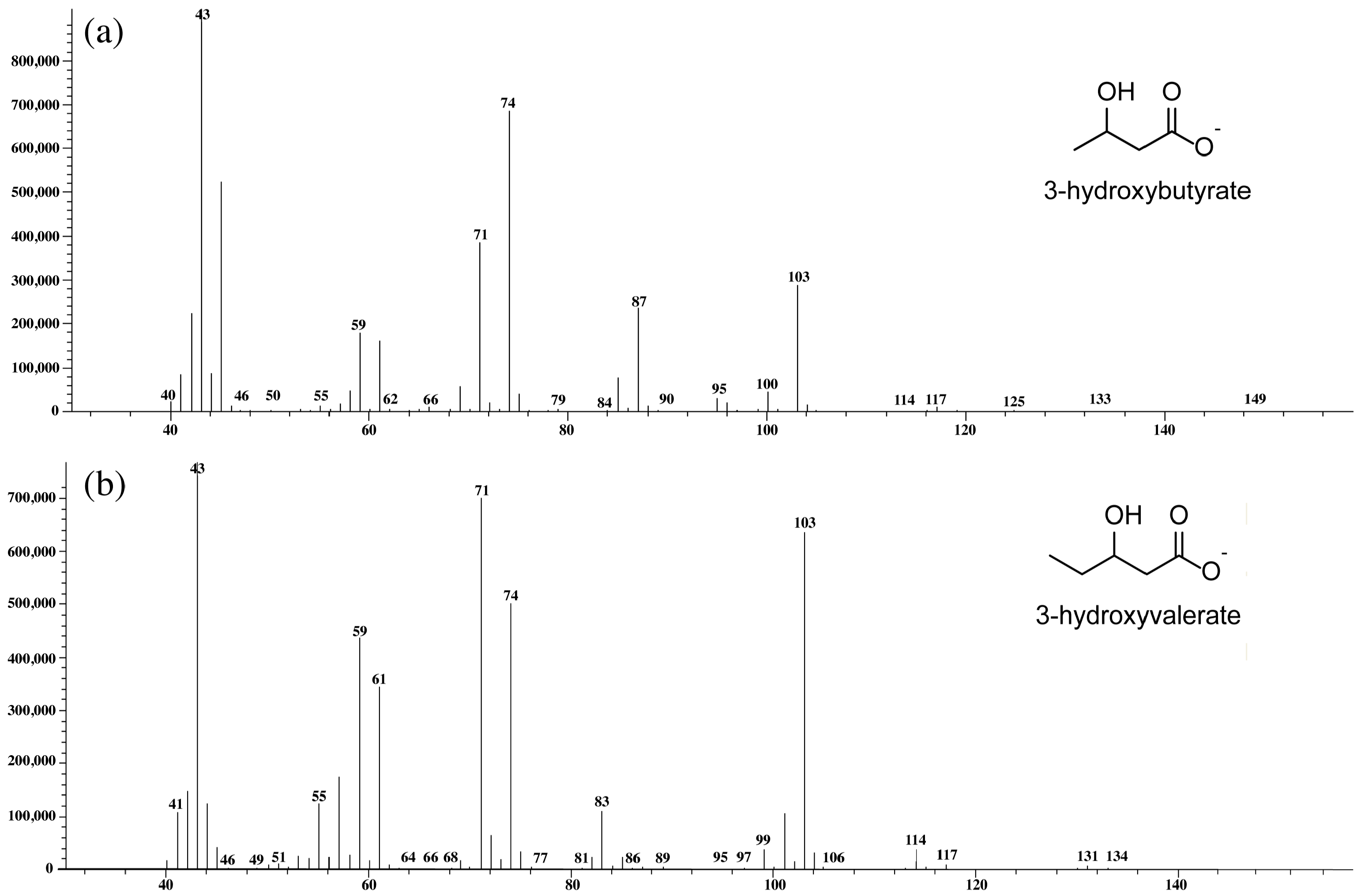
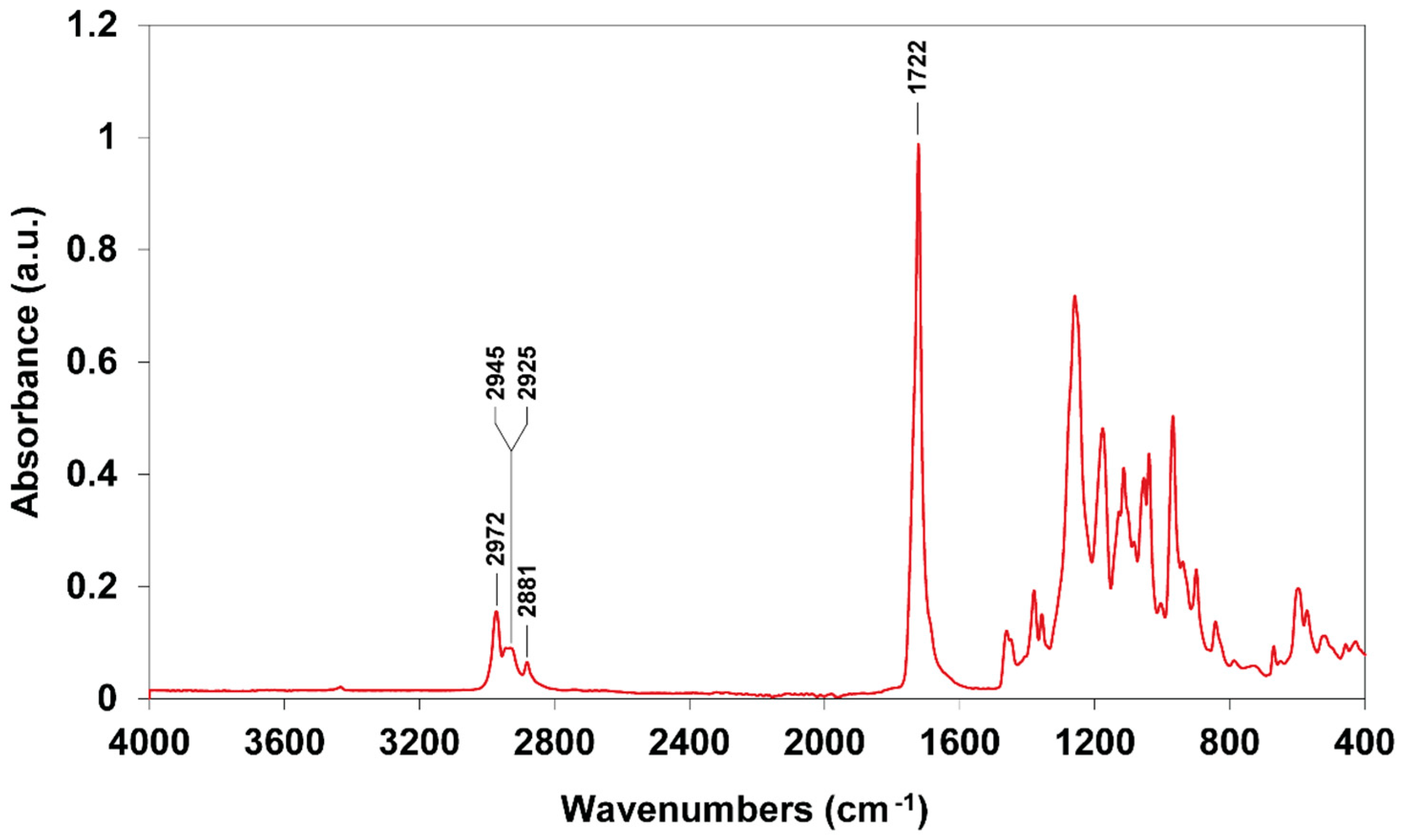
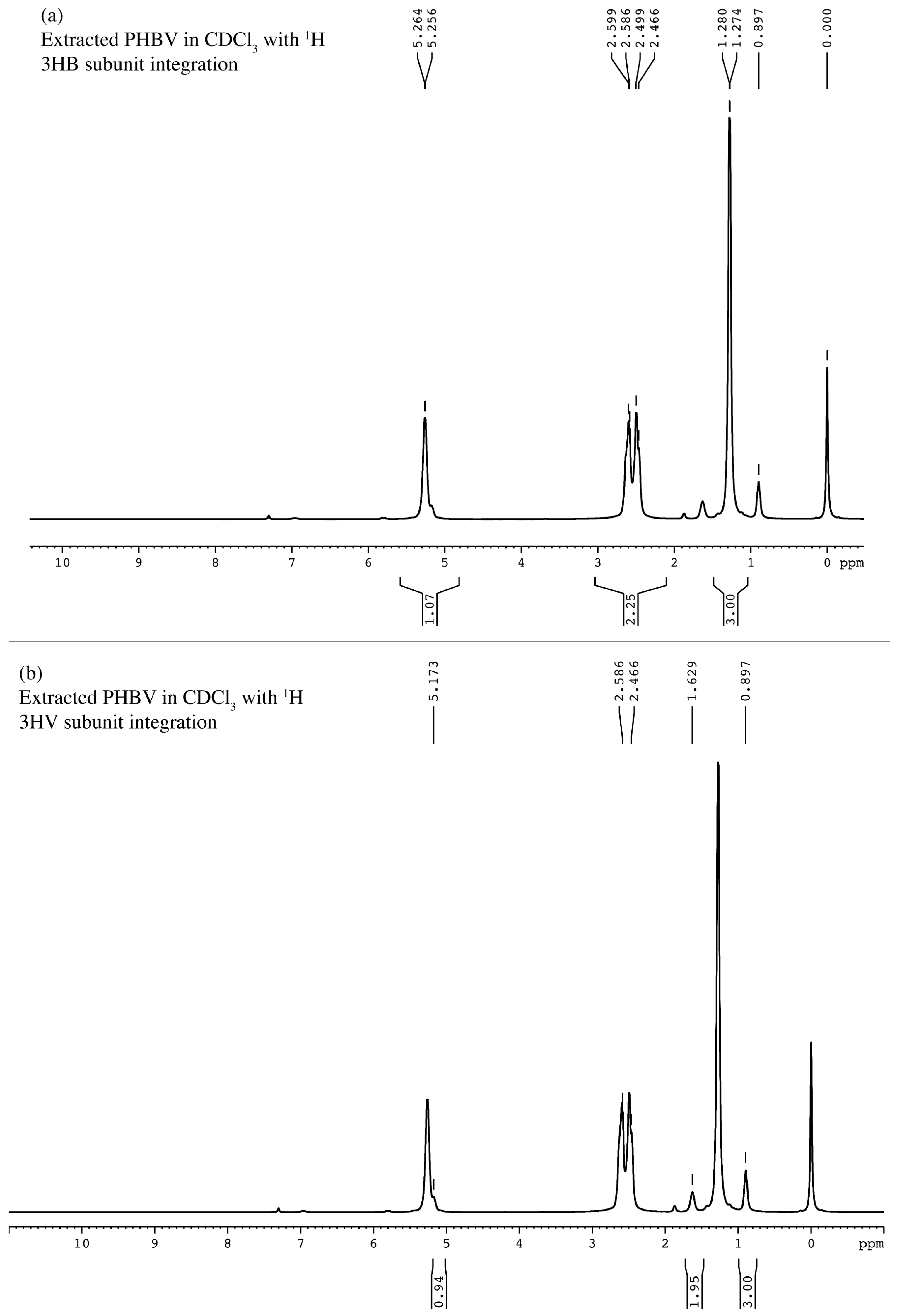
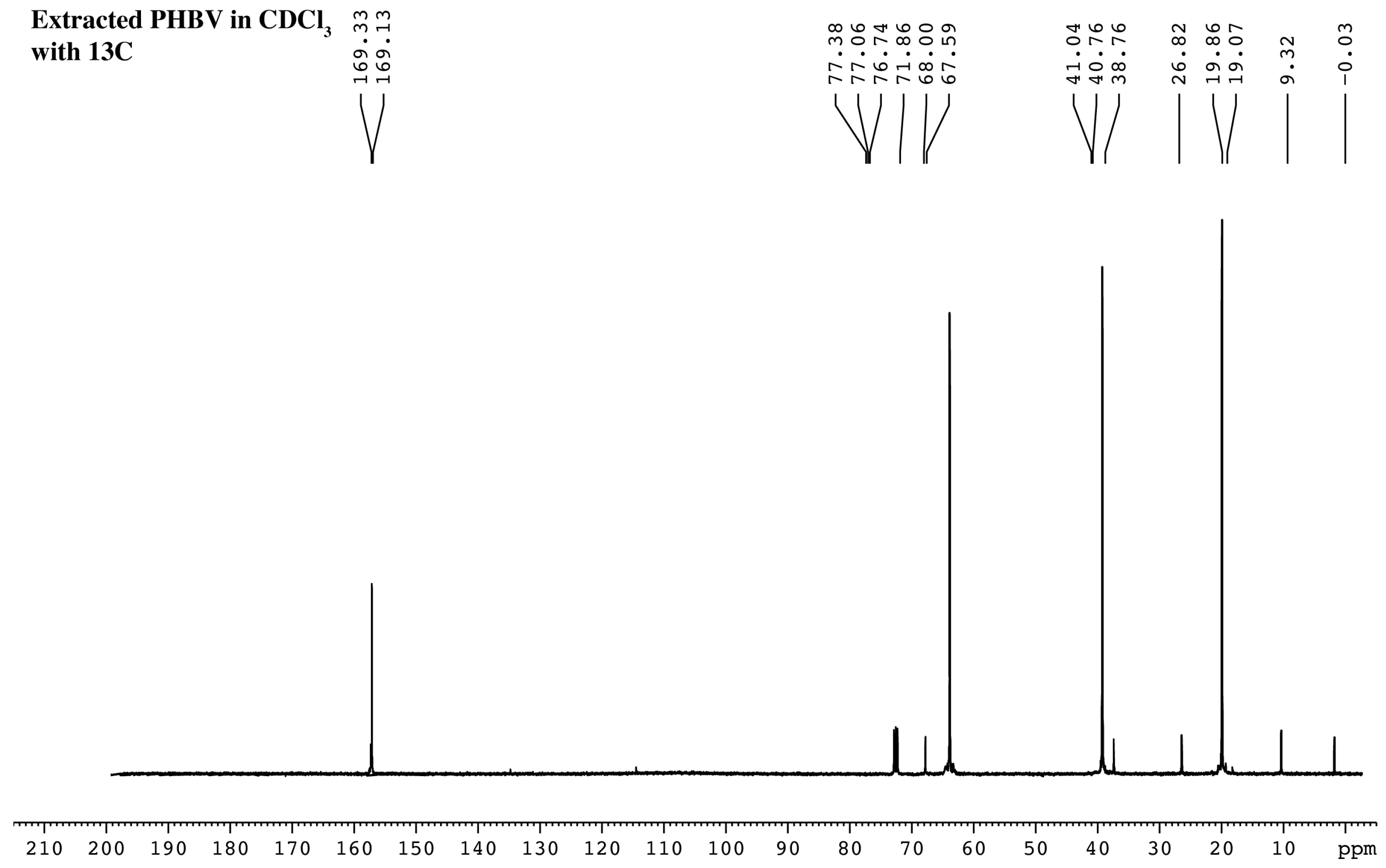
2.7. Chemical Characterization of Extracted PHBV
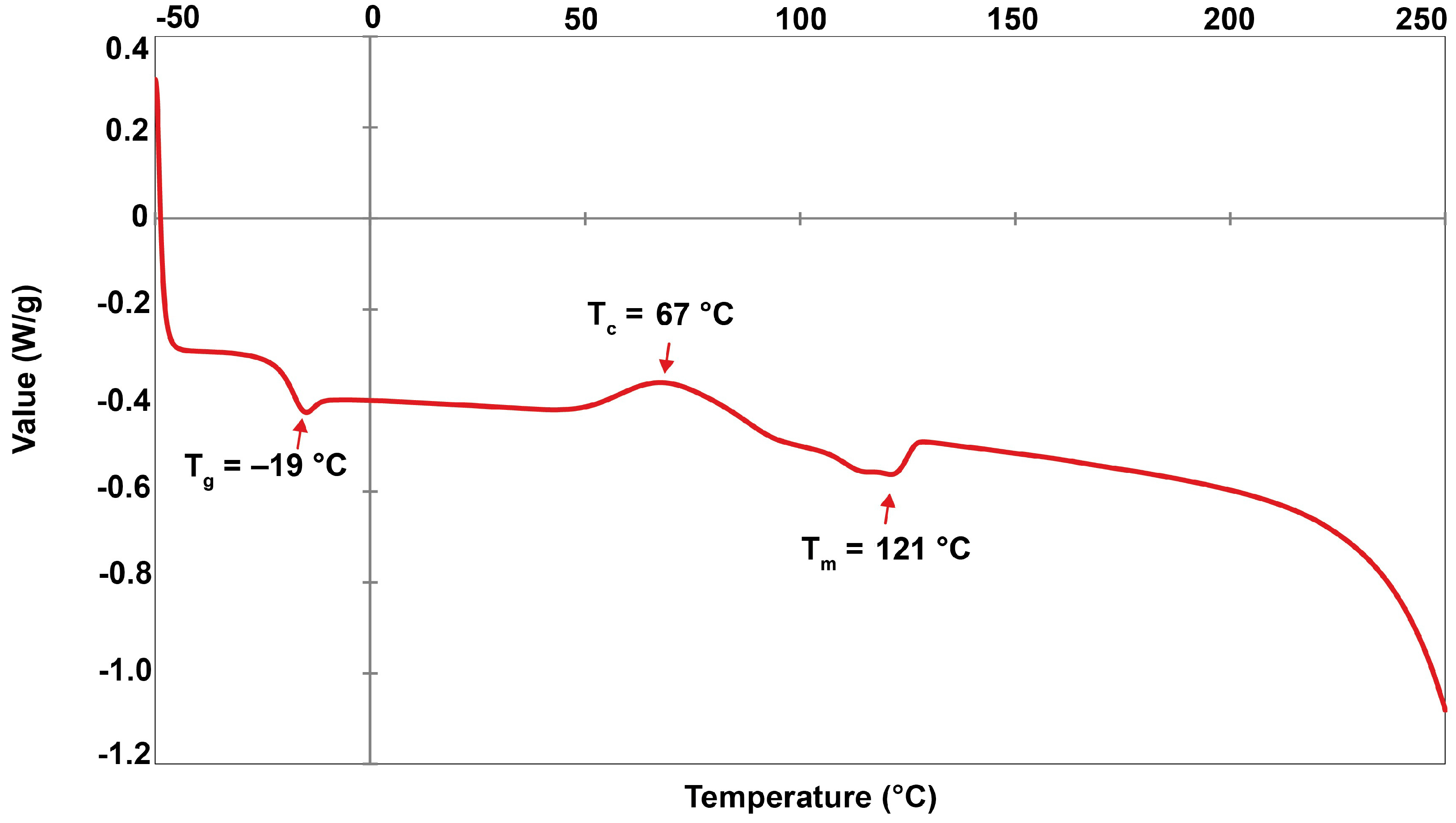
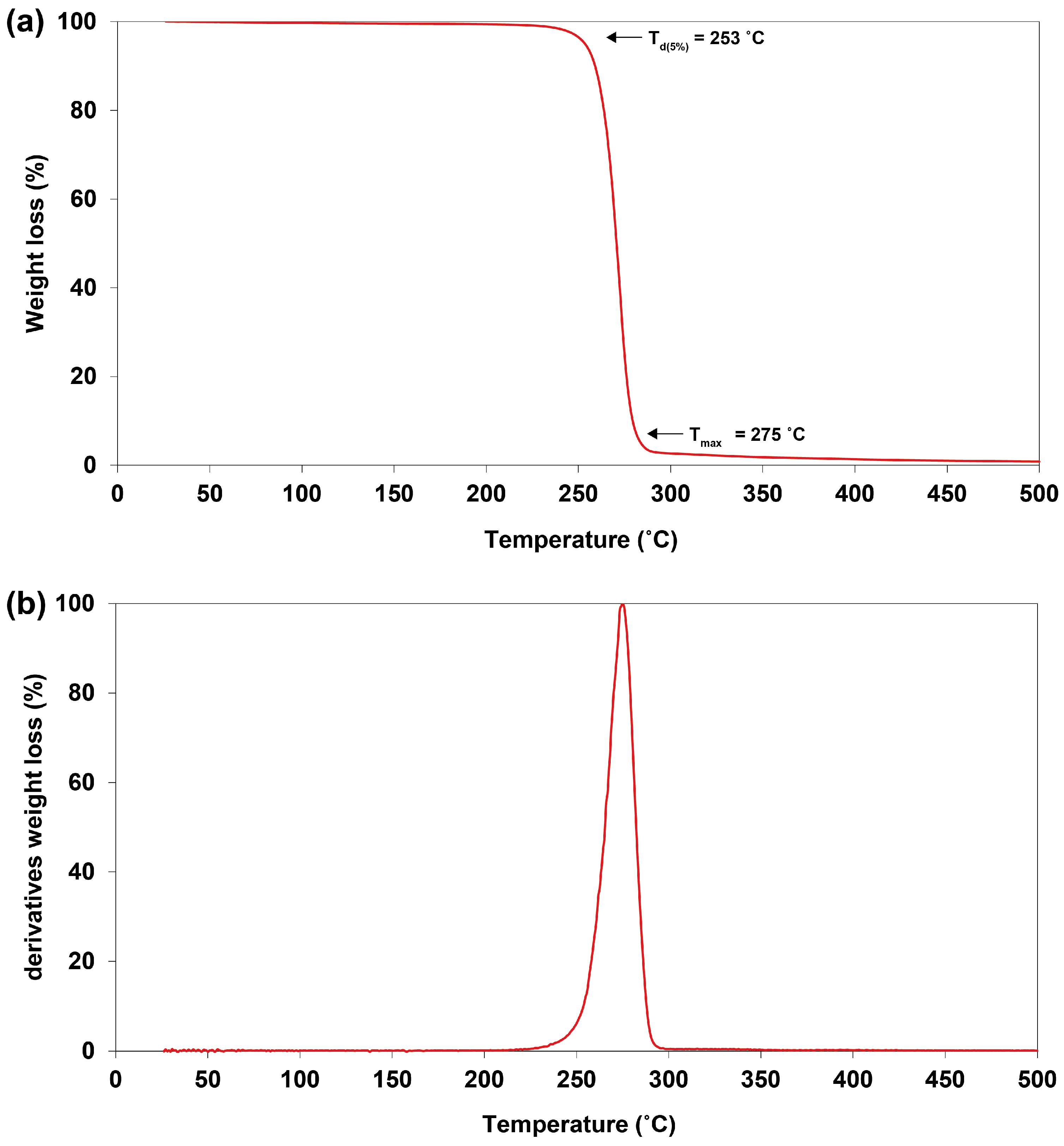
2.8. Thermal Properties of PHBV
3. Discussion
4. Materials and Methods
4.1. Cyanobacterial Strain
4.2. Screening of Cyanobacteria for PHBV Production
4.3. Quantification of PHBV
4.4. Growth and Biomass Measurement
4.5. First-stage Cultivation: Biomass Optimization
4.6. Mathematical Analysis of the Three Factors
4.7. Extraction of PHBV
4.8. Scanning Electron Microscopy (SEM) Analysis
4.9. Mass Spectrometry Analysis
4.10. H-NMR and 13C-NMR Analysis
4.11. Fourier-Transform Infrared (FTIR) Spectroscopy
4.12. Gel Permeation Chromatography (GPC)
4.13. Thermal Properties
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hottle, T.A.; Bilec, M.M.; Landis, A.E. Sustainability assessments of bio-based polymers. Polym. Degrad. Stab. 2013, 98, 1898–1907. [Google Scholar] [CrossRef]
- Gao, X.; Chen, J.C.; Wu, Q.; Chen, G.Q. Polyhydroxyalkanoates as a source of chemicals, polymers, and biofuels. Curr. Opin. Biotechnol. 2011, 22, 768–774. [Google Scholar] [CrossRef]
- Meng, D.C.; Shen, R.; Yao, H.; Chen, J.C.; Wu, Q.; Chen, G.Q. Engineering the diversity of polyesters. Curr. Opin. Biotechnol. 2014, 29, 24–33. [Google Scholar] [CrossRef]
- Steinbüchel, A.; Valentin, H.E. Diversity of bacterial polyhydroxyalkanoic acids. FEMS Microbiol. Lett. 1995, 128, 219–228. [Google Scholar] [CrossRef]
- Sankhla, I.S.; Bhati, R.; Singh, A.K.; Mallick, N. Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) co-polymer production from a local isolate, Brevibacillus invocatus MTCC 9039. Bioresour. Technol. 2010, 101, 1947–1953. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.; Jho, E.H.; Kim, M.; Nam, K. Increased 3HV Concentration in the Bacterial Production of 3-Hydroxybutyrate (3HB) and 3-Hydroxyvalerate (3HV) Copolymer with Acid-Digested Rice Straw Waste. J. Polym. Environ. 2016, 24, 98–103. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, H.; Deng, B.; Zhao, X. Poly(3-hydroxybutyrate) and Poly(3-hydroxybutyrate-co-3-hydroxyvalerate): Structure, Property, and Fiber. Int. J. Polym. Sci. 2014, 2014, 374368. [Google Scholar] [CrossRef]
- Braunegg, G.; Lefebvre, G.; Genser, K.F. Polyhydroxyalkanoates, biopolyesters from renewable resources: Physiological and engineering aspects. J. Biotechnol. 1998, 65, 127–161. [Google Scholar] [CrossRef]
- Valentin, H.E.; Berger, P.A.; Gruys, K.J.; Filomena de Andrade Rodrigues, M.; Steinbüchel, A.; Tran, M.; Asrar, J. Biosynthesis and Characterization of Poly(3-hydroxy-4-pentenoic acid). Macromolecules 1999, 32, 7389–7395. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Liu, G.M.; Weng, W.Q.; Ding, J.Y.; Liu, S.J. Engineering of Ralstonia eutropha for the production of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) from glucose. J. Biotechnol. 2015, 195, 82–88. [Google Scholar] [CrossRef]
- Chanprateep, S. Current trends in biodegradable polyhydroxyalkanoates. J. Biosci. Bioeng. 2010, 110, 621–632. [Google Scholar] [CrossRef]
- Lee, S.Y. Bacterial polyhydroxyalkanoates. Biotechnol. Bioeng. 1996, 49, 1–14. [Google Scholar] [CrossRef]
- Chanprateep, S.; Kulpreecha, S. Production and characterization of biodegradable terpolymer poly(3-hydroxybutyrate-co-3-hydroxyvalerate-co-4-hydroxybutyrate) by Alcaligenes sp. A-04. J. Biosci. Bioeng. 2006, 101, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Bhati, R.; Samantaray, S.; Sharma, L.; Mallick, N. Poly-beta-hydroxybutyrate accumulation in cyanobacteria under photoautotrophy. Biotechnol. J. 2010, 5, 1181–1185. [Google Scholar] [CrossRef]
- Kaewbai-Ngam, A.; Incharoensakdi, A.; Monshupanee, T. Increased accumulation of polyhydroxybutyrate in divergent cyanobacteria under nutrient-deprived photoautotrophy: An efficient conversion of solar energy and carbon dioxide to polyhydroxybutyrate by Calothrix scytonemicola TISTR 8095. Bioresour. Technol. 2016, 212, 342–347. [Google Scholar] [CrossRef]
- Bhati, R.; Mallick, N. Production and characterization of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) co-polymer by a N2-fixing cyanobacterium, Nostoc muscorum Agardh. J. Chem. Technol. Biotechnol. 2012, 87, 505–512. [Google Scholar] [CrossRef]
- Samantaray, S.; Mallick, N. Role of cultural variables in tailoring poly(3-hydroxybutyrate-co-3-hydroxyvalerate) copolymer synthesis in the diazotrophic cyanobacterium Aulosira fertilissima CCC 444. J. Appl. Phycol. 2014, 27, 197–203. [Google Scholar] [CrossRef]
- Taepucharoen, K.; Tarawat, S.; Puangcharoen, M.; Incharoensakdi, A.; Monshupanee, T. Production of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) under photoautotrophy and heterotrophy by non-heterocystous N2-fixing cyanobacterium. Bioresour. Technol. 2017, 239, 523–527. [Google Scholar] [CrossRef]
- Tarawat, S.; Incharoensakdi, A.; Monshupanee, T. Cyanobacterial production of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) from carbon dioxide or a single organic substrate: Improved polymer elongation with an extremely high 3-hydroxyvalerate mole proportion. J. Appl. Phycol. 2020, 32, 1095–1102. [Google Scholar] [CrossRef]
- Kourmentza, C.; Kornaros, M. Biotransformation of volatile fatty acids to polyhydroxyalkanoates by employing mixed microbial consortia: The effect of pH and carbon source. Bioresour. Technol. 2016, 222, 388–398. [Google Scholar] [CrossRef]
- Mallick, N.; Gupta, S.; Panda, B.; Sen, R. Process optimization for poly(3-hydroxybutyrate-co-3-hydroxyvalerate) co-polymer production by Nostoc muscorum. Biochem. Eng. J. 2007, 37, 125–130. [Google Scholar] [CrossRef]
- Bhati, R.; Mallick, N. Carbon dioxide and poultry waste utilization for production of polyhydroxyalkanoate biopolymers by Nostoc muscorum Agardh: A sustainable approach. J. Appl. Phycol. 2015, 28, 161–168. [Google Scholar] [CrossRef]
- Bhati, R.; Mallick, N. Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) copolymer production by the diazotrophic cyanobacterium Nostoc muscorum Agardh: Process optimization and polymer characterization. Algal Res. 2015, 7, 78–85. [Google Scholar] [CrossRef]
- Chen, J.; Li, W.; Zhang, Z.-Z.; Tan, T.-W.; Li, Z.-J. Metabolic engineering of Escherichia coli for the synthesis of polyhydroxyalkanoates using acetate as a main carbon source. Microb. Cell Factories 2018, 17, 102. [Google Scholar] [CrossRef] [PubMed]
- Aramvash, A.; Hajizadeh-Turchi, S.; Moazzeni-zavareh, F.; Gholami-Banadkuki, N.; Malek-sabet, N.; Akbari-Shahabi, Z. Effective enhancement of hydroxyvalerate content of PHBV in Cupriavidus necator and its characterization. Int. J. Biol. Macromol. 2016, 87, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Verlinden, R.A.; Hill, D.J.; Kenward, M.A.; Williams, C.D.; Radecka, I. Bacterial synthesis of biodegradable polyhydroxyalkanoates. J. Appl. Microbiol. 2007, 102, 1437–1449. [Google Scholar] [CrossRef] [PubMed]
- Monshupanee, T.; Nimdach, P.; Incharoensakdi, A. Two-stage (photoautotrophy and heterotrophy) cultivation enables efficient production of bioplastic poly-3-hydroxybutyrate in auto-sedimenting cyanobacterium. Sci. Rep. 2016, 6, 37121. [Google Scholar] [CrossRef]
- Collier, J.L.; Herbert, S.K.; Fork, D.C.; Grossman, A.R. Changes in the cyanobacterial photosynthetic apparatus during acclimation to macronutrient deprivation. Photosynth. Res. 1994, 42, 173–183. [Google Scholar] [CrossRef]
- Jeppesen, E.; Kronvang, B.; Meerhoff, M.; Sondergaard, M.; Hansen, K.M.; Andersen, H.E.; Lauridsen, T.L.; Liboriussen, L.; Beklioglu, M.; Ozen, A.; et al. Climate change effects on runoff, catchment phosphorus loading and lake ecological state, and potential adaptations. J. Environ. Qual. 2009, 38, 1930–1941. [Google Scholar] [CrossRef]
- Schindler, D.W. Evolution of phosphorus limitation in lakes. Science 1977, 195, 260–262. [Google Scholar] [CrossRef]
- Klausmeier, C.A.; Litchman, E.; Daufresne, T.; Levin, S.A. Optimal nitrogen-to-phosphorus stoichiometry of phytoplankton. Nature 2004, 429, 171–174. [Google Scholar] [CrossRef]
- Xie, L.; Xie, P.; Li, S.; Tang, H.; Liu, H. The low TN:TP ratio, a cause or a result of Microcystis blooms? Water Res. 2003, 37, 2073–2080. [Google Scholar] [CrossRef]
- Chislock, M.F.; Sharp, K.L.; Wilson, A.E. Cylindrospermopsis raciborskii dominates under very low and high nitrogen-to-phosphorus ratios. Water Res. 2014, 49, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.G.; Canvin, D.T. Glycolaldehyde Inhibits CO2 Fixation in the Cyanobacterium Synechococcus UTEX 625 without Inhibiting the Accumulation of Inorganic Carbon or the Associated Quenching of Chlorophyll a Fluorescence. Plant Physiol. 1989, 91, 1044–1049. [Google Scholar] [CrossRef] [PubMed]
- Suttle, C.A.; Stockner, J.G.; Shortreed, K.S.; Harrison, P.J. Time-courses of size-fractionated phosphate uptake: Are larger cells better competitors for pulses of phosphate than smaller cells? Oecologia 1988, 74, 571–576. [Google Scholar] [CrossRef]
- Dolman, A.M.; Rucker, J.; Pick, F.R.; Fastner, J.; Rohrlack, T.; Mischke, U.; Wiedner, C. Cyanobacteria and cyanotoxins: The influence of nitrogen versus phosphorus. PLoS ONE 2012, 7, e38757. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Li, Q.; Yan, G.; Zhou, D.; Crittenden, J.C. Hormesis effects of phosphorus on the viability of Chlorella regularis cells under nitrogen limitation. Biotechnol. Biofuels 2019, 12, 121. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhou, W.; Chen, H.; Zhan, J.; He, C.; Wang, Q. Ammonium Nitrogen Tolerant Chlorella Strain Screening and Its Damaging Effects on Photosynthesis. Front. Microbiol. 2019, 9. [Google Scholar] [CrossRef]
- Carlos Eduardo de Farias, S.; Barbara, G.; Eleonora, S.; Nicoletta La, R.; Alberto, B. Effects of Sodium Bicarbonate on Biomass and Carbohydrate Production in Synechococcus PCC 7002. Chem. Eng. Trans. 2016, 49, 241–246. [Google Scholar]
- Morowvat, M.H.; Ghasemi, Y. Culture medium optimization for enhanced beta-carotene and biomass production by Dunaliella salina in mixotrophic culture. Biocatal. Agric. Biotechnol. 2016, 7, 217–223. [Google Scholar] [CrossRef]
- Zhai, J.; Li, X.T.; Li, W.; Rahaman, M.H.; Zhao, Y.T.; Wei, B.B.; Wei, H.X. Optimization of biomass production and nutrients removal by Spirulina platensis from municipal wastewater. Ecol. Eng. 2017, 108, 83–92. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, R.; Cai, J.; Liu, Z.; Zheng, Y.; Wang, H.; Li, Q.; He, N. Biosynthesis and thermal properties of PHBV produced from levulinic acid by Ralstonia eutropha. PLoS ONE 2013, 8, e60318. [Google Scholar] [CrossRef]
- Rand, J.M.; Pisithkul, T.; Clark, R.L.; Thiede, J.M.; Mehrer, C.R.; Agnew, D.E.; Campbell, C.E.; Markley, A.L.; Price, M.N.; Ray, J.; et al. A metabolic pathway for catabolizing levulinic acid in bacteria. Nat. Microbiol. 2017, 2, 1624–1634. [Google Scholar] [CrossRef]
- Toh, P.; Jau, M.-H.; Yew, S.-P.; Abed, R.; Sudesh, K. Comparison of polyhydroxyalkonates biosynthesis, mobilization and the effects of cellular morphology in Spirulina platensis and Synechocystis sp. UNIWG. J. Biosci. 2008, 19, 21–38. [Google Scholar]
- Sun, C.; Webb, C.; Theodoropoulos, C. Dynamic Metabolic Modelling of Cupriavidus necator DSM 545 in PHB Production from Glycerol. In Computer Aided Chemical Engineering; Kravanja, Z., Bogataj, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; Volume 38, pp. 2217–2222. [Google Scholar]
- Khetkorn, W.; Incharoensakdi, A.; Lindblad, P.; Jantaro, S. Enhancement of poly-3-hydroxybutyrate production in Synechocystis sp. PCC 6803 by overexpression of its native biosynthetic genes. Bioresour. Technol. 2016, 214, 761–768. [Google Scholar] [CrossRef]
- Kamravamanesh, D.; Kovacs, T.; Pflügl, S.; Druzhinina, I.; Kroll, P.; Lackner, M.; Herwig, C. Increased poly-β-hydroxybutyrate production from carbon dioxide in randomly mutated cells of cyanobacterial strain Synechocystis sp. PCC 6714: Mutant generation and characterization. Bioresour. Technol. 2018, 266, 34–44. [Google Scholar] [CrossRef]
- Miyake, M.; Erata, M.; Asada, Y. A thermophilic cyanobacterium, Synechococcus sp. MA19, capable of accumulating poly-β-hydroxybutyrate. J. Ferment. Bioeng. 1996, 82, 512–514. [Google Scholar] [CrossRef]
- Nishioka, M.; Nakai, K.; Miyake, M.; Asada, Y.; Taya, M. Production of poly-β-hydroxybutyrate by thermophilic cyanobacterium, Synechococcus sp. MA19, under phosphate-limited conditions. Biotechnol. Lett. 2001, 23, 1095–1099. [Google Scholar] [CrossRef]
- Samantaray, S.; Mallick, N. Production and characterization of poly-β-hydroxybutyrate (PHB) polymer from Aulosira fertilissima. J. Appl. Phycol. 2012, 24, 803–814. [Google Scholar] [CrossRef]
- Corre, Y.-M.; Bruzaud, S.; Audic, J.-L.; Grohens, Y. Morphology and functional properties of commercial polyhydroxyalkanoates: A comprehensive and comparative study. Polym. Test. 2012, 31, 226–235. [Google Scholar] [CrossRef]
- Wei, L.; Stark, N.M.; McDonald, A.G. Interfacial improvements in biocomposites based on poly(3-hydroxybutyrate) and poly(3-hydroxybutyrate-co-3-hydroxyvalerate) bioplastics reinforced and grafted with α-cellulose fibers. Green Chem. 2015, 17, 4800–4814. [Google Scholar] [CrossRef]
- Scandola, M.; Focarete, M.L.; Adamus, G.; Sikorska, W.; Baranowska, I.; Świerczek, S.; Gnatowski, M.; Kowalczuk, M.; Jedliński, Z. Polymer Blends of Natural Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) and a Synthetic Atactic Poly(3-hydroxybutyrate). Characterization and Biodegradation Studies. Macromolecules 1997, 30, 2568–2574. [Google Scholar] [CrossRef]
- Díaz, E.; Valle, M.; Ribeiro, S.; Lanceros-Mendez, S.; Barandiarán, J. Development of Magnetically Active Scaffolds for Bone Regeneration. Nanomaterials 2018, 8, 678. [Google Scholar] [CrossRef]
- Taddei, P.; Di Foggia, M.; Causa, F.; Ambrosio, L.; Fagnano, C. In vitro Bioactivity of Poly(∊-Caprolactone)-Apatite (PCL-AP) Scaffolds for Bone Tissue Engineering: The Influence of the PCL/AP Ratio. Int. J. Artif. Organs 2006, 29, 719–725. [Google Scholar] [CrossRef]
- Savenkova, L.; Gercberga, Z.; Bibers, I.; Kalnin, M. Effect of 3-hydroxy valerate content on some physical and mechanical properties of polyhydroxyalkanoates produced by Azotobacter chroococcum. Process. Biochem. 2000, 36, 445–450. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, Y.; Diao, M.; Wang, W.; Hua, W.; Wu, S.; Chen, P.; Ruan, R.; Cheng, Y. Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) Production by Rhodospirillum rubrum Using a Two-step Culture Strategy. J. Chem. 2019, 2019, 1–8. [Google Scholar] [CrossRef]
- Huang, L.; Gao, B.; Wu, M.; Wang, F.; Zhang, C. Comparative transcriptome analysis of a long-time span two-step culture process reveals a potential mechanism for astaxanthin and biomass hyper-accumulation in Haematococcus pluvialis JNU35. Biotechnol. Biofuels 2019, 12, 18. [Google Scholar] [CrossRef] [PubMed]
- Chentir, I.; Hamdi, M.; Doumandji, A.; HadjSadok, A.; Ouada, H.B.; Nasri, M.; Jridi, M. Enhancement of extracellular polymeric substances (EPS) production in Spirulina (Arthrospira sp.) by two-step cultivation process and partial characterization of their polysaccharidic moiety. Int. J. Biol. Macromol. 2017, 105 Pt 2, 1412–1420. [Google Scholar] [CrossRef]
- Rumiani, L.A.; Jalili, H.; Amrane, A. Enhanced docosahexaenoic acid production by Crypthecodinium cohnii under combined stress in two-step cultivation with date syrup based medium. Algal Res. 2018, 34, 75–81. [Google Scholar] [CrossRef]
- Chen, H.H.; Xue, L.L.; Liang, M.H.; Jiang, J.G. Sodium azide intervention, salinity stress and two-step cultivation of Dunaliella tertiolecta for lipid accumulation. Enzym. Microb. Technol. 2019, 127, 1–5. [Google Scholar] [CrossRef]
- Ashby, R.D.; Solaiman, D.K.; Strahan, G.D.; Zhu, C.; Tappel, R.C.; Nomura, C.T. Glycerine and levulinic acid: Renewable co-substrates for the fermentative synthesis of short-chain poly(hydroxyalkanoate) biopolymers. Bioresour. Technol. 2012, 118, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Bozell, J.J.; Moens, L.; Elliott, D.C.; Wang, Y.; Neuenscwander, G.G.; Fitzpatrick, S.W.; Bilski, R.J.; Jarnefeld, J.L. Production of levulinic acid and use as a platform chemical for derived products. Resour. Conserv. Recycl. 2000, 28, 227–239. [Google Scholar] [CrossRef]
- Cha, J.Y.; Hanna, M.A. Levulinic acid production based on extrusion and pressurized batch reaction. Ind. Crop. Prod. 2002, 16, 109–118. [Google Scholar] [CrossRef]
- Hartweg, M.; Becer, C.R. Levulinic Acid as Sustainable Feedstock in Polymer Chemistry. In Green Polymer Chemistry: New Products, Processes, and Applications; American Chemical Society: Washington, DC, USA, 2018; Volume 1310, pp. 331–338. [Google Scholar]
- Castrillo, M.; Lucas-Salas, L.M.; Rodríguez-Gil, C.; Martínez, D. High pH-induced flocculation–sedimentation and effect of supernatant reuse on growth rate and lipid productivity of Scenedesmus obliquus and Chlorella vulgaris. Bioresour. Technol. 2013, 128, 324–329. [Google Scholar] [CrossRef]
- Kotteswari, M.; Subbiah, M.; Rk, R. Phycoremediation of dairy effluent by using the mcroalgae Nostoc sp. Int. J. Environ. Res. Dev. 2012, 2, 35–43. [Google Scholar]
- Stanier, R.Y.; Kunisawa, R.; Mandel, M.; Cohen-Bazire, G. Purification and properties of unicellular blue-green algae (order Chroococcales). Bacteriol. Rev. 1971, 35, 171–205. [Google Scholar] [CrossRef] [PubMed]
- Monshupanee, T.; Incharoensakdi, A. Enhanced accumulation of glycogen, lipids and polyhydroxybutyrate under optimal nutrients and light intensities in the cyanobacterium Synechocystis sp. PCC 6803. J. Appl. Microbiol. 2014, 116, 830–838. [Google Scholar] [CrossRef]
- Khazi, M.I.; Demirel, Z.; Liaqat, F.; Dalay, M.C. Analytical Grade Purification of Phycocyanin from Cyanobacteria. Methods Mol. Biol. 2020, 1980, 173–179. [Google Scholar]
- Yellore, V.; Desai, A. Production of poly-3-hydroxybutyrate from lactose and whey by Methylobacterium sp. ZP24. Lett. Appl. Microbiol. 1998, 26, 391–394. [Google Scholar] [CrossRef]
- Penkhrue, W.; Jendrossek, D.; Khanongnuch, C.; Pathom-Aree, W.; Aizawa, T.; Behrens, R.L.; Lumyong, S. Response surface method for polyhydroxybutyrate (PHB) bioplastic accumulation in Bacillus drentensis BP17 using pineapple peel. PLoS ONE 2020, 15, e0230443. [Google Scholar] [CrossRef]
- Glossop, J.R.; Cartmell, S.H. Tensile strain and magnetic particle force application do not induce MAP3K8 and IL-1B differential gene expression in a similar manner to fluid shear stress in human mesenchymal stem cells. J. Tissue Eng. Regen. Med. 2010, 4, 577–579. [Google Scholar] [CrossRef]
| Independent Variables | Symbol Coded | Level of Variables | ||||
|---|---|---|---|---|---|---|
| (−α) | −1 | 0 | +1 | (+α) | ||
| NaNO3 (g L−1) | X1 | 0.10 | 1.30 | 3.05 | 4.80 | 6.00 |
| K2HPO4 (g L−1) | X2 | 0.01 | 0.07 | 0.15 | 0.24 | 0.30 |
| NaHCO3 (g L−1) | X3 | 0.01 | 0.04 | 0.08 | 0.12 | 0.15 |
| Source | Sum of Squares | df | Mean Square | F Value | p-Value Prob > F | |
|---|---|---|---|---|---|---|
| Model | 0.25 | 9 | 0.028 | 7.37 | 0.0077 | Significant |
| A | 0.045 | 1 | 0.045 | 11.83 | 0.0108 | Significant |
| B | 0.099 | 1 | 0.099 | 26.24 | 0.0014 | Significant |
| C | 4.727 × 10−3 | 1 | 4.727 × 10−3 | 1.25 | 0.3007 | |
| A2 | 0.014 | 1 | 0.014 | 3.66 | 0.0973 | |
| B2 | 0.074 | 1 | 0.074 | 19.54 | 0.0031 | Significant |
| C2 | 0.040 | 1 | 0.040 | 10.65 | 0.0138 | Significant |
| AB | 1.125 × 10−4 | 1 | 1.125 × 10−4 | 0.030 | 0.8680 | |
| AC | 1.513 × 10−3 | 1 | 1.513 × 10−3 | 0.40 | 0.5475 | |
| BC | 0.011 | 1 | 0.011 | 2.78 | 0.1396 | |
| Residual | 0.027 | 7 | 3.787 × 10−3 | |||
| Lack of Fit | 8.307 × 10−3 | 5 | 1.661 × 10−3 | 0.18 | 0.9450 | Not significant |
| Pure Error | 0.018 | 2 | 9.100 × 10−3 | |||
| Cor Total | 0.28 | 16 |
| Independent Variables (g L−1) | Before Optimization | After Optimization | Biomass Production (g L−1) | |
|---|---|---|---|---|
| Before optimization | After optimization | |||
| X1: NaNO3 | 1.50 | 4.35 | 0.250 ± 0.03 | 1.220 ± 0.07 |
| X2: K2HPO4 | 0.04 | 0.20 | ||
| X3: NaHCO3 | 0.02 | 0.09 | ||
| Condition /Supplementation | Polymer Type | Polymer Content (%) | 3HB Fraction (mol%) | 3HV Fraction (mol %) | Dry Biomass (mg L−1) | PHBV or PHB Productivity (mg L−1 day−1) |
|---|---|---|---|---|---|---|
| Control | ND | ND | ND | ND | 958.00 ± 83.80 b | ND |
| Nitrogen limitation | ND | ND | ND | ND | 919.17 ± 191.44 b | ND |
| Phosphorus limitation | ND | ND | ND | ND | 970.33 ± 139.50 b | ND |
| Glucose | PHB | 2.83 ± 0.28 a | 100 a | ND | 556.00 ± 115.61 a | 1.11 ± 0.17 a |
| Sodium acetate | PHB | 12.34 ± 0.83 b | 100 a | ND | 1519.67 ± 38.42 d | 13.41 ± 1.20 b |
| Glycerol | PHB | 17.92 ± 0.79 c | 100 a | ND | 1207.67 ± 49.80 c | 15.43 ± 0.20 b |
| Sodium propionate | PHBV | 3.28 ± 0.71 a | 20.92 ± 13.32 c | 79.08 ±13.32 a | 774.67 ± 24.40 ab | 1.81 ± 0.39 a |
| Levulinic acid | PHBV | 69.18 ± 2.06 d | 5.91 ± 0.72 b | 94.09 ± 0.72 b | 1641.33 ± 128.59 d | 81.30 ± 8.80 c |
| Organisms | Condition/Supplementation | PHBV Production | Thermal Properties | Crystallinity Index | Molecular Weight | References | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PHBV Content (%) | PHBV Productivity (mg L−1 day−1) | 3HB Fraction (mol%) | 3HV Fraction (mol %) | Tm (°C) | Tg (°C) | Tc (°C) | Td(5%) (°C) | Tmax (°C) | ΔHm (J g−1) | Xc (%) | Mw (kDa) | Mn (kDa) | Mw/Mn | |||
| Commercial PHBV a | NA | NA | NA | 92 | 8.00 | 167.1 | 24.9 | 115.7 | NA | 290 | 131.6 | 90.1 | NA | NA | NA | [51] |
| Oscillatoria okeni | Nitrogen limitation, acetate | 42.00 | 66.83 | 93.50 | 6.50 | 168 | −3.3 | 53 | NA | NA | 65 | 44 | 54.3 | 32.1 | 1.70 | [18] |
| Nostoc muscorum | Acetate and propionate | 42.40 | 8.90 | 78.00 | 22.00 | 148.8 | −4.3 | NA | NA | NA | NA | NA | NA | NA | NA | [16] |
| Aulosira fertilisima | valerate | 24.80 | 29.84 | 45.90 | 54.10 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | [17] |
| Nostoc muscorum | Phosphorus limitation, acetate, glucose and valerate | 71.00 | 98.30 | 72.80 | 27.20 | 145 | −4.7 | NA | 275 | 291 | 57.7 | 39.5 | NA | NA | NA | [23] |
| Nostoc microscopicum | Nitrogen limitation, acetate | 3.40 | 7.83 | 3.80 | 96.20 | 101 | −9 | 70 | NA | NA | 33 | 22.6 | 49.2 | 26.0 | 1.90 | [19] |
| Cyanosarcina sp. AARL T020 | Levulinic acid | 69.18 | 81.30 | 5.91 | 94.09 | 121 | −19 | 67 | 253 | 284 | 7.07 | 4.84 | 63.9 | 42.0 | 1.52 | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chotchindakun, K.; Pathom-Aree, W.; Dumri, K.; Ruangsuriya, J.; Pumas, C.; Pekkoh, J. Low Crystallinity of Poly(3-Hydroxybutyrate-co-3-Hydroxyvalerate) Bioproduction by Hot Spring Cyanobacterium Cyanosarcina sp. AARL T020. Plants 2021, 10, 503. https://doi.org/10.3390/plants10030503
Chotchindakun K, Pathom-Aree W, Dumri K, Ruangsuriya J, Pumas C, Pekkoh J. Low Crystallinity of Poly(3-Hydroxybutyrate-co-3-Hydroxyvalerate) Bioproduction by Hot Spring Cyanobacterium Cyanosarcina sp. AARL T020. Plants. 2021; 10(3):503. https://doi.org/10.3390/plants10030503
Chicago/Turabian StyleChotchindakun, Kittipat, Wasu Pathom-Aree, Kanchana Dumri, Jetsada Ruangsuriya, Chayakorn Pumas, and Jeeraporn Pekkoh. 2021. "Low Crystallinity of Poly(3-Hydroxybutyrate-co-3-Hydroxyvalerate) Bioproduction by Hot Spring Cyanobacterium Cyanosarcina sp. AARL T020" Plants 10, no. 3: 503. https://doi.org/10.3390/plants10030503
APA StyleChotchindakun, K., Pathom-Aree, W., Dumri, K., Ruangsuriya, J., Pumas, C., & Pekkoh, J. (2021). Low Crystallinity of Poly(3-Hydroxybutyrate-co-3-Hydroxyvalerate) Bioproduction by Hot Spring Cyanobacterium Cyanosarcina sp. AARL T020. Plants, 10(3), 503. https://doi.org/10.3390/plants10030503