Abstract
Allelopathic potential of buckwheat roots on the radicle growth of the broomrape weed species Orobanche cumana and Phelipanche ramosa was studied. Buckwheat root exudates induced a significant growth inhibition in P. ramosa radicles but radicles of O. cumana were not affected. Among the metabolites present in the root organic extract we identified the flavonol quercetin and the stilbene p-coumaric acid methyl ester with only quercetin showing inhibitory effect on P. ramosa. The activity of quercetin was compared with other two similar flavanoids, the flavone apigenin and the dihydroflavanol 3-O-acetylpadmatin extracted respectively from Lavandula stoechas and Dittrichia viscosa plants. In this comparative assay only 3-O-acetylpadmatin besides quercetin, showed inhibition activity of radicle growth while apigenin was inactive. These results indicated that the presence of two ortho-free hydroxy groups of C ring, like catechol, could be an important feature to impart activity while the carbon skeleton of B ring and substituents of both A and B rings are not essential. Besides reduction of radicle growth, haustorium induction was observed at the tip of P. ramosa radicles treated with quercetin which swelled and a layer of papillae was formed. Activity of quercetin on haustorium induction in P. ramosa was assayed in comparison with the known haustorium-inducing factor 2,6-dimethoxy-p-benzoquinone (DMBQ) and a three partial methyl ether derivatives semisynthetized from quercetin. Results indicated that P. ramosa haustorium was induced by DMBQ at concentrations of 1–0.5 mM and quercetin and its derivatives at concentration range 0.1–0.05 mM.
1. Introduction
The parasitic broomrape weeds (Orobanche and Phelipanche species) have not functional roots nor photosynthetic activity obtaining all nutrients and water from the crop root by haustorial connections with the vascular system [1]. Their expansion in Mediterranean Basin and Asia is uncontrolled infecting crops in the Apiaceae, Asteraceae, Brassicaceae, Fabaceae or Solanaceae becoming a threat to food security [1,2]. Broomrapes are one of the most difficult-to-control of all weeds, because the difficult application of methods that can kill the broomrapes without damaging the crop to which they are physically and biochemically overlapped through the haustorium. Additional factors challenging broomrape control is their high fecundity, persistent seedbank, and rapid responses to changes in agricultural practices, adapting to new hosts with increased aggressiveness [3].
Weed management is essential for agricultural production, and it has relayed on traditional chemical herbicides for decades with efficient control but carrying the long-term problems of agroecosystem contamination, undesirable health effects and the emergence of herbicide-resistant weed populations [4]. Due to the undesirable effects of herbicides, the number of old herbicides authorized are in constant decline with few novel herbicides in development [5,6] which prompt the need for the development of novel nature-inspired bioherbicides containing either microbial strains or toxins from microbial or plant origin. From the screening of toxins of microbial and plant origins new compounds with antagonistic activity against parasitic weeds have been discovered [7,8,9,10,11,12,13,14,15]. In many cases the natural herbicidal substances lack appropriate physicochemical properties for field application and their use in agriculture depends on the development of formulations that increase the solubility in water [16,17] or the development of strategies for the application of the whole organic extract of allelopathic plants or incorporating them into biofilms [18].
Buckwheat (Fagopyrum esculentum Moench) is a short life cycle crop from the Polygonaceae with activity in weed suppression [19,20]. Despite being the subject of many studies there is no conclusive evidence of which compounds are responsible for the allelopathic suppression of weed growth, although it has been suggested that phenolic acids and flavonoids could be responsible [21,22]. Buckwheat roots exudate to the rhizosphere allelochemicals with inhibitory effect on weeds mainly palmitic and gallic acid [23,24]. The roots extracts of buckwheat contain allelopathic flavonoids mainly catechin, and isoquercitrin [22]. To the best of our knowledge, there are currently no reports on the effects of buckwheat on parasitic weeds. From a previous field screening of the USDA buckwheat germplasm collection, the buckwheat accession PI 658422 from Nepal was selected in our lab for allelopathic activity. This manuscript reports the allelopathic activity of roots of buckwheat accession PI 658422 on the radicle growth of Phelipanche ramosa and the isolation and identification of the flavanol quercetin with inhibitory activity. The activity of quercetin was compared with similar flavonoids previously isolated in our laboratory from different plant origins, i.e., the flavone apigenin and the dihydroflavanol 3-O-acetylpadmatin extracted, respectively, from Lavandula stoechas [25] and Dittrichia viscosa [26]. In addition, quercetin activity was compared with that of semisynthetic methyl ether derivatives of quercetin to elucidate structure activity relationships (SAR).
2. Results and Discussion
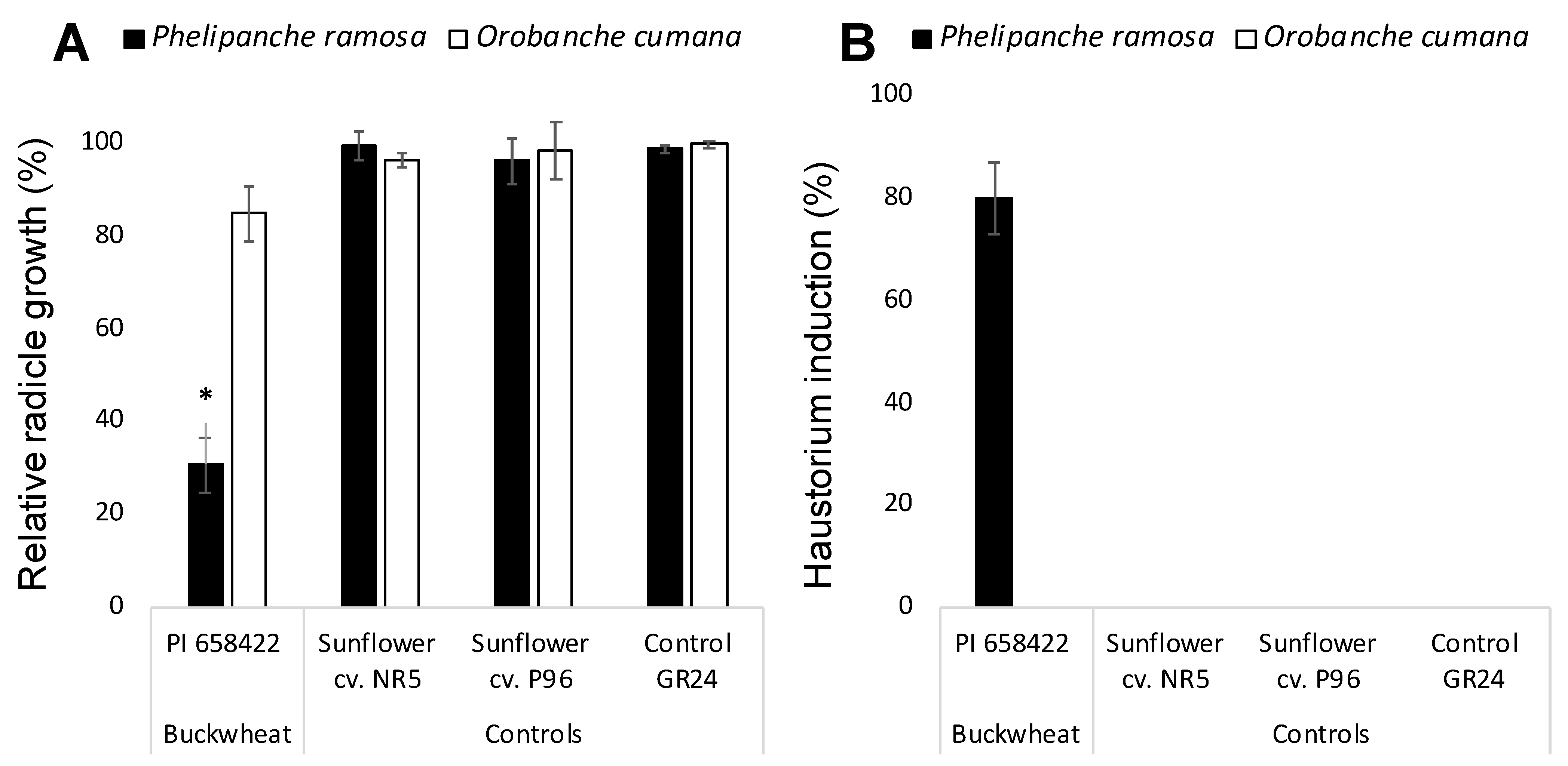
Allelopathic potential of buckwheat root exudates was assayed on radicle development of two broomrape species P. ramosa and O. cumana and the effect of buckwheat compared with the effect of two sunflower cultivars NR5 and P96 and the negative control GR24. Broomrape seeds only germinate upon detection of germination stimulants exuded by host roots. For allelopathic bioassays, broomrape seeds require the induction of germination with the synthetic germination stimulant GR24 active both in O. cumana and P. ramosa that act as a negative control for radicle growth inhibition [12,14]. Growth of O. cumana radicles treated with a combination of buckwheat root exudates and GR24 was not significantly different from the growth of GR24-treated O. cumana control radicles nor the growth of radicles treated with combination of sunflower root exudates and GR24. However, P. ramosa seeds treated with the combination of buckwheat root exudate and GR24 displayed shorter radicles with a swelled tip and a layer of papillae in its surface which indicates that haustorium was formed. The radicle growth cessation and haustorium formation was not observed in P. ramosa radicles when treated with the negative control GR24 nor when treated with the combination of sunflower root exudates and GR24 (Figure 1A,B).
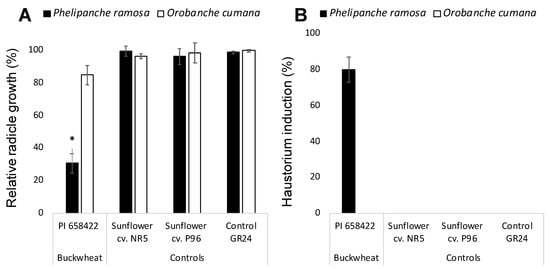
Figure 1.
Allelopathic effect of hydroponically collected buckwheat and sunflower root exudates on growth (A) and haustorium formation (B) in radicles of Phelipanche ramosa and Orobanche cumana. * Indicates differences at the 0.05 level compared with the control GR24. Error bars represent the standard error of the mean.
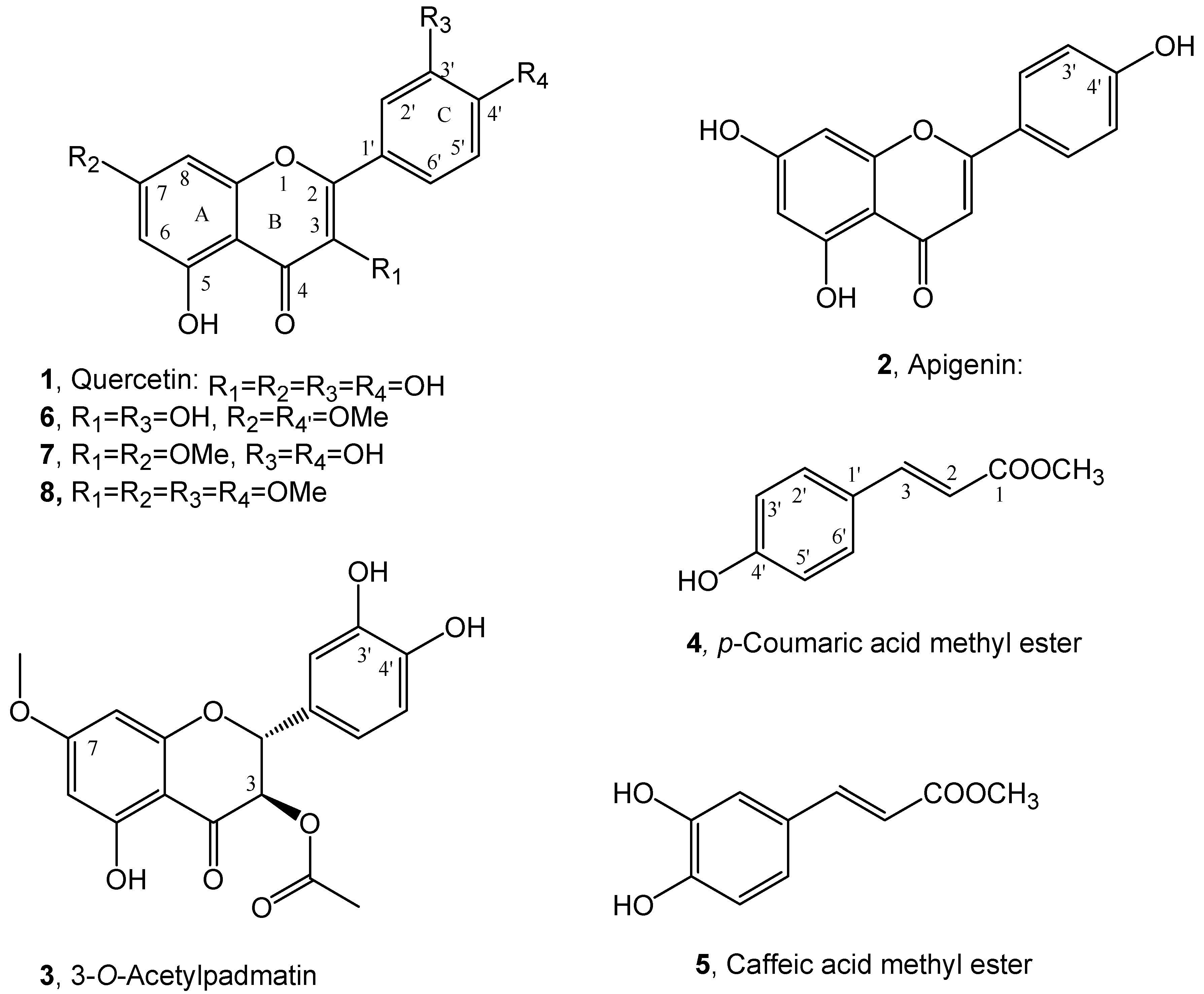
The purification of the organic extract obtained from dried roots of buckwheat (Fagopyrum esculentum) by combined column and TLC chromatography, as detailed reported in the Materials and Methods section, afforded quercetin and the methyl ester of p-coumaric acid (1 and 4, Figure 2). They were characterized by comparison of their spectroscopic data (essentially 1H NMR and ESI MS) with those reported in literature for 1 by Grande at al. [26] and for 4 by Karthikeyan et al. [27] and by data reported in Materials and Methods section and SI. Compounds 1 and 4 belong to cynnamic acids and flavonols groups of natural occurring substances and are both biosynthesized via shikimic acid pathway [28]. They are already reported as plant [29,30,31] and fungi [32] bioactive metabolites. Although quercetin is found in many vegetables, it is present in low amounts in Polygonaceae family [22]. Golisz et al. [33] identified quercetin in buckwheat among eight allelochemicals including rutin, (+)-catechin, (−)-epicatechin, chlorogenic acid, caffeic acid, ferulic acid, and gallic acid. The presence of quercetin is low in aerial vegetative organs of buckwheat [33] but its concentration increases in leaves at plant maturity [22]. The content of quercetin in buckwheat roots has been described as low and only detected at buckwheat flowering stage [22]. Previous studies of buckwheat root deposits in soil did not detect quercetin while it was detected in agar plates after buckwheat germination [24]. Several studies indicate that there is a wide variation in contents of allelopathic flavonoids depending on the variety, phenological stage, and environmental conditions [34,35,36,37,38,39,40,41]. Levels of quercetin in buckwheat increase in drier [37] and sunnier [35] weathers. Recently p-coumaric acid methyl ester and its close analogue methyl ester of caffeic acid (5, Figure 2) were isolated together with two new copaane sesquiterpenoids, named stoechanones A and B, from Lavandula stoechas whose organic extract showed strong herbicidal activity against the noxious weed Amarantus retroflexus [25]. Caffeic acid methyl ester (5) was identified comparing its spectroscopic data (essentially 1H NMR and ESI MS) with those reported in literature [42]. From the same plant organic extract also apigenin (2, Figure 2), a flavanone which belongs to another subgroup of flavonoids and thus close to 1 was isolated and identified by comparing its spectroscopic data (essentially 1H NMR and MS) with those reported in literature [43] (see Materials and Methods section and SI). Independently working on Dittrichia viscosa, as potential allelopathic plant, four new phytotoxic sesquiterpenoins, named inuloxins A-D and α-costic acid were firstly isolated [15] and successively also 3-O-acetylpadmatin (3, Figure 2), a dihydroflavonol which belongs to another subgroup of flavonoids and thus close to 1 and 2. Compound 3 was identified by comparing its physic and spectroscopic (essentially specific optical rotation and 1H and 13C NMR and ESI MS) data with those reported in literature [26]. In particular, the correlations observed in the HMBC NMR spectrum were essential to assign the oxygenated and not quaternary sp2 carbons [44] (see Materials and Methods and SI).
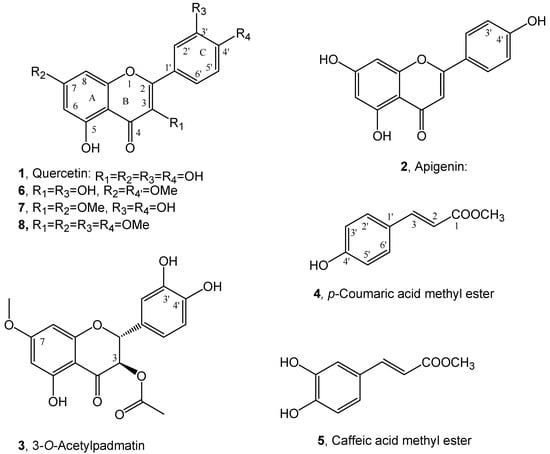
Figure 2.
Structure of quercetin, apigenin and 3-O-acetylpadmatin (1–3), p-coumaric and caffeic acid methyl esters (4 and 5) and the two dimethyl (6 and 7) and tetramethyl (8) derivatives of quercetin.
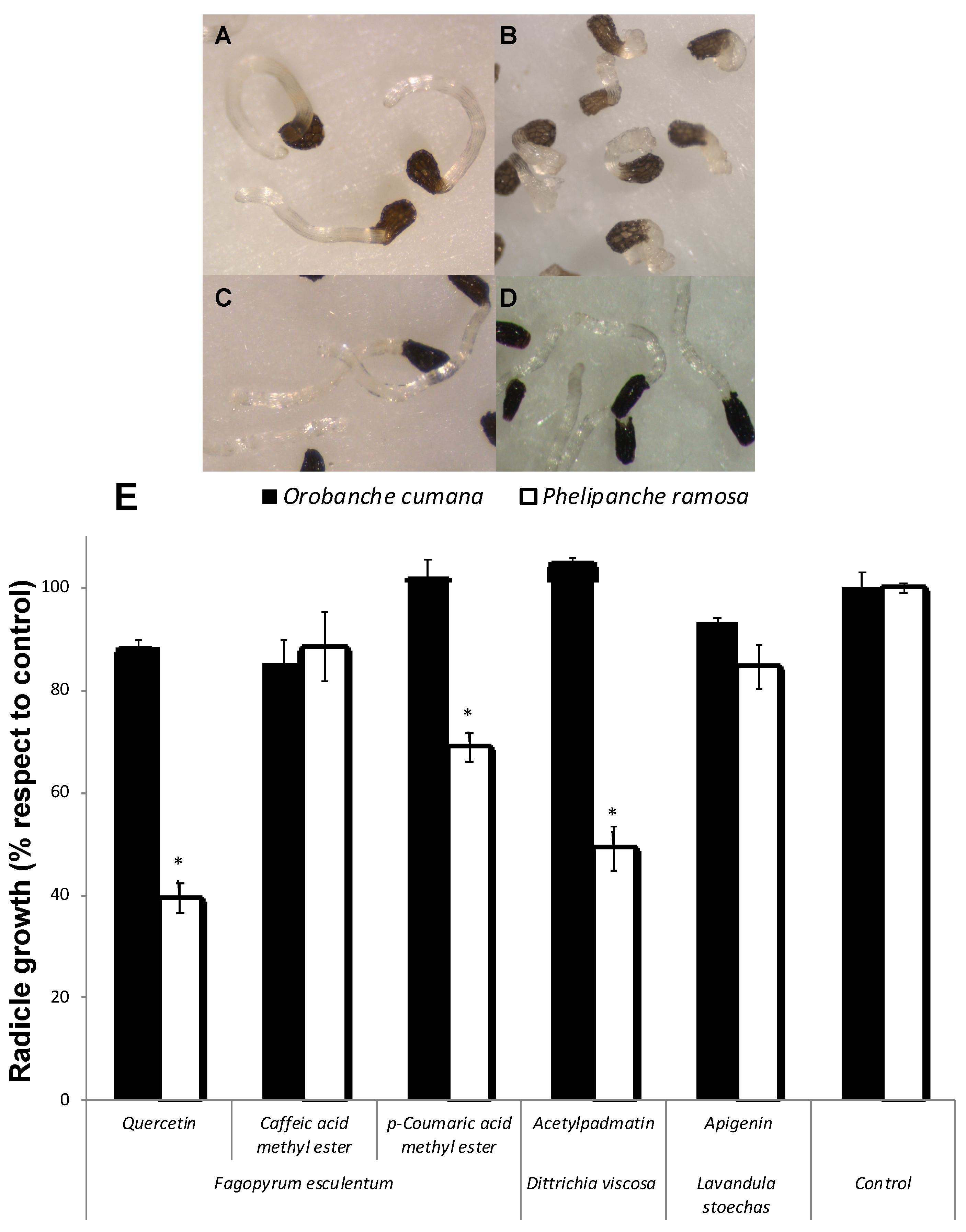
Allelopathic effects of the flavonoids quercetin, apigenin and 3-O-acetylpadmatin (1-3), and caffeic acid and coumaric acid methyl esters were assayed on P. ramosa and O. cumana seedlings (Figure 3). Quercetin (1) showed a strong inhibition of the radicle growth of P. ramosa seedlings in comparison with the control (Figure 3A,B,E). Flavonoids have well known growth inhibitory activities being frequently involved in root allelopathy. Golisz et al. [33] observed the quercetin inhibitory activity of root growth in lettuce seedlings. Inhibition of P. ramosa radicle growth was also induced by acetylpadmatin (3) while apigenin (2) and caffeic acid were inactive (Figure 3E). The allelopathic effect of 1 and 3 in P. ramosa radicles was associated with a cessation of the radicle elongation and not associated with darkening or any other visible sign of toxicity in the radicle tissue. In addition, the tip of the P. ramosa radicles treated with 1 and 3 became swallowed into a spherical form and differentiated the haustorial organ while P. ramosa radicles treated with the rest of the compounds or with the control did not. A slight but significant reduction in radicle growth without haustorium formation was observed in p-coumaric-treated P. ramosa radicles. Quercetin had no allelopathic effect on O. cumana radicle in comparison with the control (Figure 3C–E), nor the rest of the compounds tested (Figure 3E). Species-specific differences in allelopathic effects have been described for species of Orobanche and Phelipanche genera. They have different host ranges, and they differ in their capacity for signal perception and sensibility to inhibition by allelochemicals [8,9,12,14].
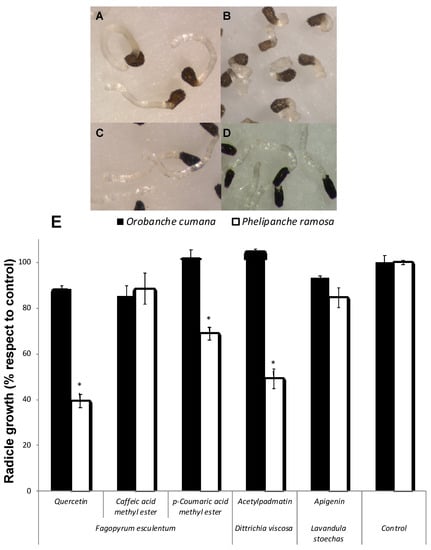
Figure 3.
Inhibition of radicle growth of broomrape species. P. ramosa radicles treated with control (A) and with quercetin (B); O. cumana radicles treated with control (C) and quercetin (D). Allelopathic effect of quercetin, caffeic acid and p-coumaric acid methyl esters, apigenin and acetylpadmatin on radicle growth of P. ramosa and O. cumana (E). * Indicates differences at the 0.05 level compared with the control. Error bars represent the standard error of the mean.
Quercetin is the most widely distributed flavone in the plant kingdom playing several roles in the rhizosphere during plant-microbial associations [45]. Quercetin was reported with stimulatory activity on the hyphal growth of Glomus margarita and the authors hypothesized that the hydroxyl group in position 3 is essential to confer stimulatory activity, and concluded, that flavonols in general should be more stimulatory than flavones. Among the flavonols tested by these authors, quercetin, with hydroxyl groups on positions 3′ and 4′, gave the greatest stimulation of hyphal growth. Quercetin-3-O-galactoside was found to be the dominant flavonoid released from alfalfa seeds promoting spore germination of G. etunicatum and G. macrocarpum [46]. Quercetin also enhances nodulation by Rhizobium etli or R. tropici in bean roots [47]. Other activities of quercetin during plant-microbe associations have also been described such the induction of resistance against Pseudomonas syringae by increasing H2O2 and callose production [48]. Reactive oxygen species generation is indispensable for haustorium formation in Striga, being observed a strong accumulation of H2O2 [49]. Quercetin induced haustoria in Triphysaria versicolor roots [50]. The effect of 1 and 3 in P. ramosa radicles may be associated with the presence of the two ortho-free hydroxy group of C ring, like catechol, are an important feature to impart activity and the carbon skeleton of B ring and substituents on both A and B ring are not essential. The result does not surprise as the presence of two ortho-hydroxy phenolic groups (like catechol) as well as that of two para hydroxy phenolic group (as hydroquinone) represents of an oxidoreductive couples. The relation between the quinone/hydroquinone skeleton and its biological activity is already known. In fact, in previous structure activity relationships study using sphaeropsidone and episphaerosidone and some of their derivatives testing their ability to initiate haustorium development in Striga and Orobanche species was demonstrated that the conversion of the natural sphaeropsidones, their analogues, and hemisynthetic derivatives in the corresponding 3-methoxyquinone and this finally, by reductive opening of the epoxy group followed by water nucleophilic elimination into the 3-methoxyquinone, is fundamental to impart activity [51]. This hypothesis is in full agreement with the activity observed by quinones as sorghum xenognosin and dimethoxybenzoquinones. The latter is very close to the 3-methoxyquinone, which as above explained could be generated from oxidation of sphaeropsidone, could play a role in the chemistry in host recognition parasitic angiosperms. Thus, quinone/hydroquinone structures serve as cofactors in many metabolic pathways, playing critical chemical roles in oxidation/reduction processes [28,52,53]. This mode of action could also operate in the haustorium-induction in broomrape [51]. Similar structure activity relationships were also observed in additional studies carried out by assaying epi-epoformin, a phytotoxic cyclohexene epoxide isolated from the Diplodia quercivora, responsible Quercus canariensis declining in Tunisia [54], and some of its semisynthetic derivatives in an etiolated wheat coleoptile bioassay [55]. In addition, the importance of the quinone/hydroquinone skeleton was also recently observed testing the phytotoxicity, on host and non-host plants of three new anthraquinones, named lentiquinone A-C and the already known lentisone, pachybasin, ω-hydroxypachybasin, 1,7-dihydroxy-3-methylanthracene- 9,10-dione, and phomarin isolated from Ascochyta lentis, the causal agent of ascochyta blight on lentil [56,57].
To confirm these SAR results three methyl derivatives of quercetin were prepared from 1 by reaction with diazomethane. The crude reaction mixture was purified as detailed reported in the Materials and Methods section and the main derivatives isolated were the 7,4′-O,O′- and 3,7-O,O′- dimethyl (6 and 7, Figure 2) and the 3,7,3′,4′-O,O′,O″, O‴-tetramethyl (8, Figure 2) derivatives of quercetin. The 1H NMR and ESI data of 6 and 7 were reported in Materials and Methods and SI. These data agree with those previously reported for 6 by Haraguchi et al., [58] and for 7 by Valesi et al., [59]. The unambiguously location of the methoxy groups at C-7 and C-4′ and C-3 and C-7 in 6 and 7, respectively, was obtained recording their NOESY spectra [60] (see SI). The 1H NMR and ESI MS data of 8 are reported in Materials and Methods and SI and are in agreement to those previously reported [59].
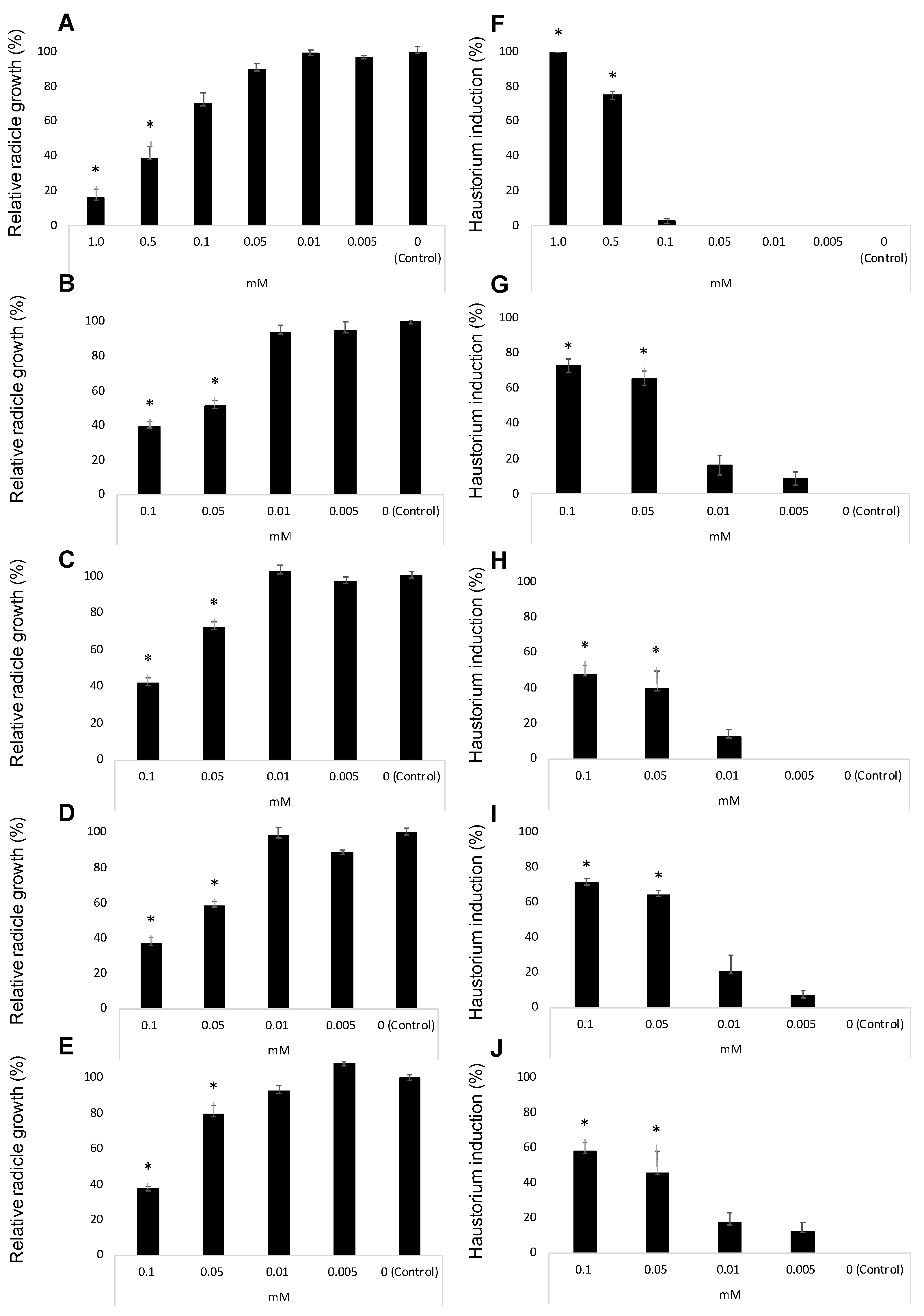
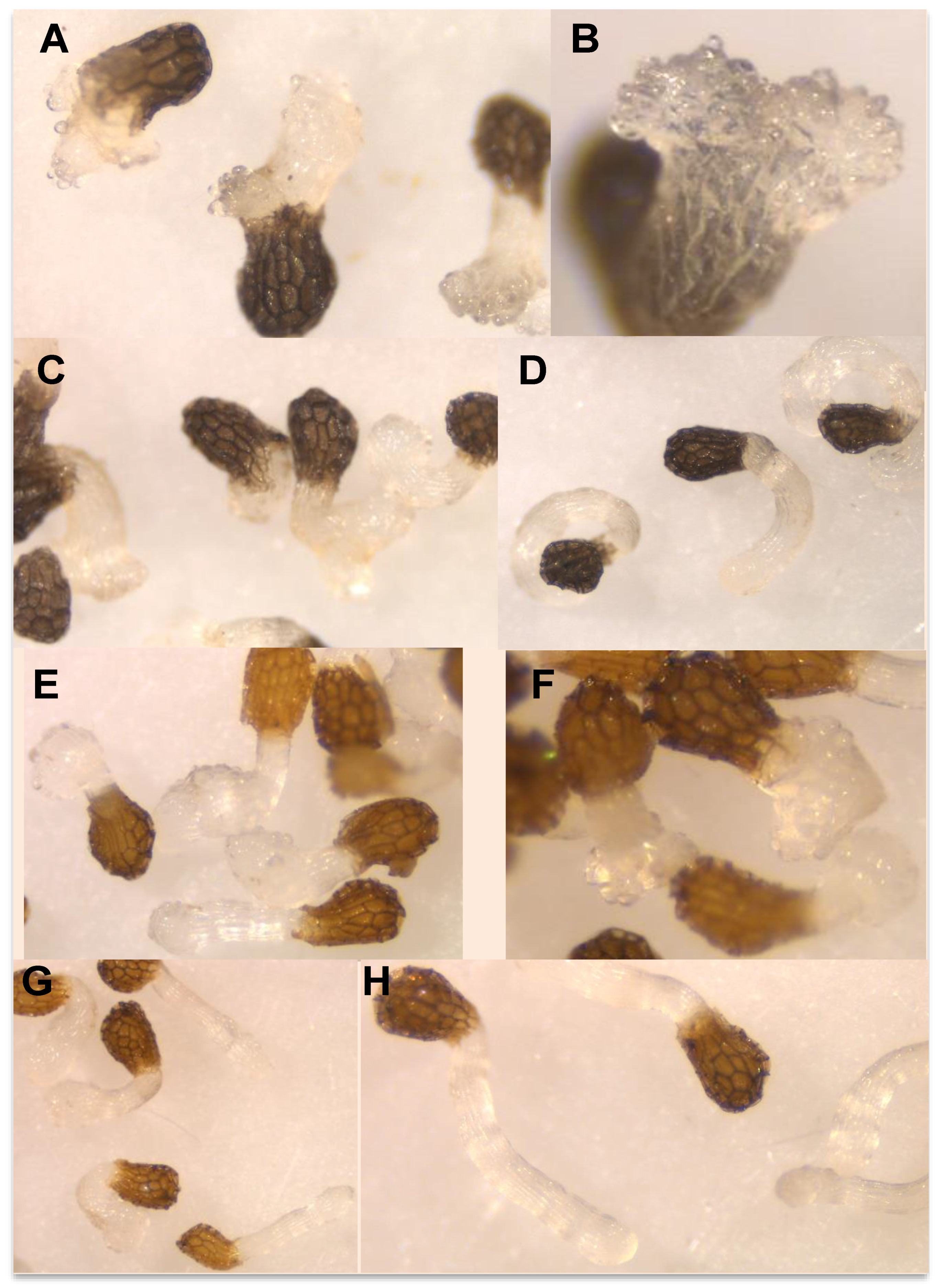
Figure 4A–E illustrate the inhibition of radicle growth and Figure 4F–J the induction of haustorium development observed during the evaluation of the activity of the three methyl derivatives of quercetin. Their activity was compared with the activity of quercetin and 2,6-dimethoxy-p-benzoquinone (DMBQ) the strongest haustorium-inducing factor active in radicles of other parasitic plants such as Striga spp. and Triphysaria spp. DMBQ is active inducing Triphysaria haustorium between 1 and 30 μM concentrations. At concentrations of 100 μM or higher DMBQ is toxic to Triphysaria roots [52]. In Striga species, the active range spans from 0.05 to 10 μM being toxic at 50 μM or higher [49,61]. Unlike Striga spp. and Triphysaria spp., broomrape species has been reported to do not respond to DMBQ with haustorium initiation [62,63,64,65] but the activity has been tested only at 10 μM [65] and the activity at higher concentrations has not been reported.
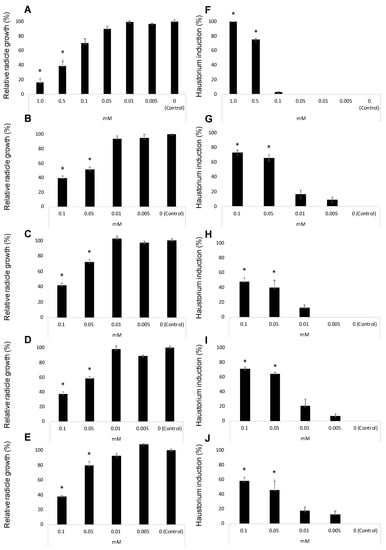
Figure 4.
Allelopathic effects of DMBQ (A,F), quercetin (B,G), 7,4′-O,O′-dimethylquercetin (6) (C,H), 3,7-O,O′-dimethylquercetin (7) (D,I), and 3,7,3′,4′-O,O′,O″,O‴-tetramethylquercetin (8) (E,J) in the radicle growth (A–E) and haustorium induction (F–J) of Phelipanche ramosa. * Indicates differences at the 0.05 level compared with the control. Error bars represent the standard error of the mean.
In our work, DMBQ induced the cessation of P. ramosa radicles growth and a swelling of P. ramosa tip with a formation of a papillae layer (Figure 5A–C). Figure 4A shows the radicle growth inhibition and Figure 4F the proportion of radicles that developed haustorium when P. ramosa was treated with DMBQ at a range of 1 mM to 5 μM showing that the active range for haustorium induction is found at concentrations of 0.5 mM or higher. Our results indicate that in P. ramosa radicles treated with DMBQ at 1 mM the radicle growth was only 16% of that of the control (Figure 4A, Figure 5A,B,H) with haustorium visible in 100% of the radicles (Figure 4F, Figure 5A,B) while in P. ramosa radicles treated with DMBQ at 0.5 mM the radicle growth was 39% of that of the control (Figure 4A, Figure 5H) with haustorium visible in 75% of the radicles (Figure 4F, Figure 5C). The haustorium-inducing effect of DMBQ on P. ramosa radicles disappeared at 100 μM and at lower concentrations (Figure 4F and Figure 5D). DMBQ did not induce visible signs of toxicity in P. ramosa as has been observed in other parasitic weeds which have been described as turning brown and die at concentrations higher than 50 μM [52,61]. Unlike more hydrophobic quinones, DMBQ is sufficiently soluble in water to make fresh working stocks at 1 mM directly in water, without the solvent DMSO usually used in labs to make stock solutions for haustorial induction assays of Phelipanche, Striga, and Triphysaria [49,65,66]. The effect of quercetin was evaluated at concentration range of 100 μM and 5 μM (Figure 4B,G). No visible signs of toxicity were observed in P. ramosa radicles. Quercetin induced haustorium in P. ramosa radicles at concentrations of 50 μM or higher. At 50 μM the average of radicle growth was only 51% of that of the control and 66% of the radicles carried haustorium. Among the three methyl derivatives evaluated, 3,7-O,O′-dimethylquercetin (7) showed the highest activity in the radicle growth inhibition and haustorium induction tests (Figure 4D,I). The growth inhibition at 50 μM by 7,4′-O,O′-dimethylquercetin (6) and 50 μM 3,7,3′,4′-O,O′,O″,O‴-tetramethylquercetin (8) was slightly reduced in comparison with quercetin and compound 7 as shown in Figure 4C,E. Furthermore, derivatives 6 and 8 induced lower haustorium development in comparison to quercetin and 3,7-O,O′-dimethylquercetin (7) (Figure 4H,J). Perception of haustorium-inducing factors promotes a cessation of parasite root growth with a rapid swelling developing an adhesive structure that attaches the parasite to the host surface from which the invasive organ subsequently develops [3]. In parasitic weeds such as Triphysaria and Striga several haustorium-inducing factors have been identified including phenolics, flavonoids, and p-benzoquinones with different concentrations and times of exposure required for optimal haustorium induction [67]. The length of the root that develops haustorium may depend on the strength and the concentration of the haustorium-inducing factor and the timing of detection. These processes have not been well characterized for broomrape species and until recently, it was believed to not being initiated by haustorium-inducing chemical agents [51,62,63,64,65]. Some authors observed the activity of quercetin in root growth inhibition without activity in cytodifferentiation [33]. Others, observed haustorium differentiation activity in quercetin [50]. In this work, we have characterized the effect of quercetin and its derivatives independently in P. ramosa radicle length and rate of haustorium induction since we cannot rule out the effect of quercetin acting specifically on root growth in addition to its haustorium-inducing effect.
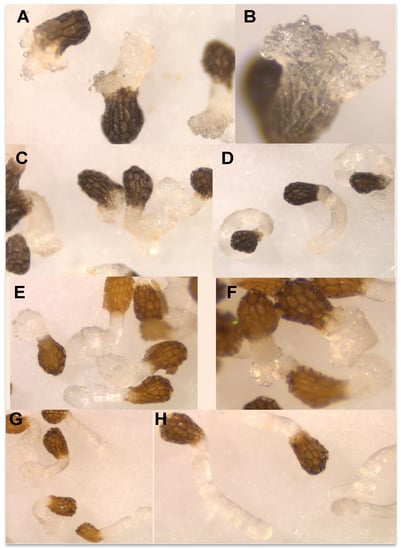
Figure 5.
Effects in P. ramosa radicle of (A) DMBQ 1 mM; (B) DMBQ 1 mM detail; (C) DMBQ 0.5 mM; (D) DMBQ 0.1 mM; (E) 7,4′-O,O′-dimethylquercetin (6) 0.1 mM; (F) 3,7-O,O′-dimethylquercetin (7) 0.1 mM; (G) 3,7,3′,4′-O,O′,O″,O‴-tetramethylquercetin (8) 0.1 mM; (H) control.
3. Materials and Methods
3.1. General Experimental Procedures
Optical rotations were measured in MeOH on a P-1010 digital polarimeter (Jasco, Tokyo, Japan), 1H and 13C NMR spectra were recorded at 500 and 400 and 125 and 1000 MHz in on Varian (Palo Alto, CA, USA) and Bruker (Karlsruhe, Germany). The same solvent was used as internal standard. The multiplicities were determined by DEPT spectrum [60] COSY, HSQC, HMBC and NOESY spectra were recorded using Bruker microprograms. ESI MS spectra were recorded on a 6120 Quadrupole LC/MS instruments (Agilent Technologies, Milan, Italy), respectively. Analytical and preparative TLC were performed on silica gel (Kieselgel 60, F254, 0.25 and 0.5 mm respectively) and on reversed phase (Kieselgel 60 RP-18, F254, 0.20 mm) plates (Merck, Darmstadt, Germany). The spots were visualized by exposure to UV radiation (253 nm), or by spraying first with 10% H2SO4 in MeOH and then with 5% phosphomolybdic acid in EtOH, followed by heating at 110 °C for 10 min. Column chromatography was performed using silica gel (Merck, Kieselgel 60, 0.063–0.200 mm). Quercetin and caffeic acid were purchased from Sigma-Aldrich Milano, Italy)
3.2. Plant Material and Growth Conditions
Buckwheat (Fagopyrum esculentum) roots and root exudates were obtained from the buckwheat accession PI 658422 collected in Nepal and kindly provided by USDA. Buckwheat seeds were surface sterilized with 4% sodium hypochlorite containing 0.02% Tween 20, rinsed three times with sterile distilled water and placed on moistened filter paper inside Petri dishes to allow germination. Four days later, germinated buckwheat seeds were transferred to pots filled with sterile perlite in a growth chamber (23/20 °C, 16/8 h day/night). Plants received Hoagland’s nutrient solution modified at one-quarter strength twice per week. For collection of roots, buckwheat plants were removed from the perlite, roots were carefully washed in distilled water, quickly dried with filter paper, immediately frozen and maintained at −80 °C until lyophilization. For determination of buckwheat allelopathic activity on Orobanche cumana and Phelipanche ramosa radicle growth, root exudates were collected from buckwheat accession PI 658422 and two sunflower control cultivars NR5 and P96. Three plants of each cultivar were grown as described above, removed from the perlite, their roots carefully washed and individually placed in tubes immersing the roots in sterile distilled water. After 24 h, the solutions containing the buckwheat and sunflower root exudates were collected and the total crop root contained in each tube weighed. Root exudate solution was adjusted with sterile distilled water to achieve equivalent concentrations of 0.02 g of crop root fresh weight /mL of hydroponic media (root exudate solution) and tested for allelopathic potential as described in Section 3.4 below. Fresh bunches of Lavandula stoechas in the flowering stage were purchased from the vegetable market in Algiers and a specimen was deposited at Ecole Nationale Supérieure d’Agronomie, ENSA, Algeria. Whole aerial parts of Dittrichia viscosa plant were collected fresh in Italy and Algeria from naturally occurring populations. A voucher specimen was deposited at the herbarium of the Ecole National Supérieure Agronomique in Algiers. After harvesting, leaves were detached from the stems and dried in a ventilated oven at 50 °C for two days. Seeds of two broomrape species: O. cumana, population collected in sunflower in Spain and P. ramosa, population collected in oilseed rape in France were used to determine allelopathic potential of tested metabolites.
3.3. Extraction Purification and Identification of Buckwheat Metabolites
Dried roots of buckwheat (6.2 g) were minced in a Blender mill and macerates overnight at dark in H2O-MeOH (1:1, 150 mL). The mixture was centrifuged at 7000 r.p.m. and the alcoholic-aqueous phase extracted first with n-hexane (3 × 150 mL) and then with CH2Cl2 (3 × 150 mL). The CH2Cl2 organic extracts were combined, dried (Na2SO4) and concentrated under vacuum to yield on oily brown residue (41.5 mg). This latter was fractionated by TLC, eluted with CHCl3-EtOAc-MeOH 6:2:2, affording a pure homogeneous solid identified as below reported as quercetin (1, 15 mg). In another experiment, the same extraction procedure was applied to buckwheat dried roots (5.5 g) yielding the CH2Cl2 extract as an oil. The latter was purified by TLC but eluted with the different solvent system CHCl3-EtOAc-MeOH 6:3:1, affording a homogeneous solid (4, 2.3 mg) identified as methyl ester of p-coumaric acid.
Quercetin (2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chromen-4-one) (1): 1H NMR, (CD3OD, 400 Mz) δ, 7.75 (1H, d, J = 2.1 Hz, H-2′), 7.63 (1H, dd, J = 8.5 and 2.1 Hz, H-6′), 6.90 (1H, d, J = 8.5 Hz H-5′), 6.41 (1H, d, J = 2.0 Hz, H-8), 6.20 (1H, d, J = 2.0 Hz H-6) these data are very similar to those previously recorded at 60 MHz in acetone-d6 [26]; ESI MS, m/z 303 [M + H]+.
p-Coumaric acid methyl ((E)-methyl 3-(4-hydroxyphenyl)acrylate) (4): 1H NMR (CDCl3, 500 MHz), δ, 7.67 (1H, d, J = 15.5 Hz, H-3), 7.46 (2H, d, J = 8.6 Hz, H-2′6′),) 6.88 (2H, d, J = 8.6 Hz, H-3′,5′), 6.33 (1H, d, J = 15.5 Hz, H-2), 3.84 (3H, s, OMe); these data are very similar to those previously recorded in CD3OD [27]; ESI MS, m/z 179 [M + H]+.
3.4. Apigenin and Methyl Ester of Caffeic from Lavandula stoechas
Dried L. stoechas plants (200 g) was minced in Blender mill and macerated overnight with MeOH-H2O (1:1, 1 L) and the alcoholic-aqueous extract than centrifuged and exhaustively extracted with CH2Cl2 as previously reported [25]. The organic extract was purified by combined column and TLC chromatography to afford apigenin as a yellow solid (2, 4.6 mg) and the methyl ester of caffeic acid (5, 5.2 mg).
Apigenin (4′,5,7-trihydroxyflavone) (2):1H NMR (CD3OD, 400 MHz), δ, 7.87 (2H, d, J = 8.9 Hz, H-2′,6′), 6.95 (2H, d, J = 8.9 Hz, H-3′,5′), 6.61 (1H, s, H-3), 6.46 (1H, d, J = 2.1 Hz, H-8), 6.22 (1H, d, J = 2.1 Hz, H-6); these data are very similar to those previously recorded in(CD3)2SO [43]; ESI MS, m/z 271 [M + H]+.
Methyl ester of caffeic acid ((E)-methyl 3-(3,4-dihydroxyphenyl)acrylate) (6):1H NMR (CD3OD, 500 MHz), δ, 7.56 (1H, d, J = 16.1 Hz, H-3), 7.05 (1H, br s, H-2′), 6.96 (1H, d, J = 8.2 Hz, H-5′), 6.79 (1H, d, J = 8.2 Hz, H-6′), 6.27 (1H, d, J = 16.1 Hz, H-2), 3.77 (3H, s, OMe) these data are very similar to those previously reported [42]; ESI MS, m/z 216 [M + Na]+, 195 [M + H]+, 163 [M − CH3OH]+.
3.5. 3-O-Acetylpadmatin from Dittrichia viscosa
Dried D. viscosa plant was minced in Blender mill and macerated overnight with MeOH-H2O (1:1, 1 L) and the alcoholic-aqueous extract than centrifuged and exhaustively extracted first with n-hexane and then with extracted with CH2Cl2 as previously reported [15]. The organic extract was purified by combined column and TLC chromatography to afford a homogeneous compound identified as below reported 3-O-acetylpadmatin (3, 20 mg).
3-O-Acetylpadmatin (Acetic acid 2-(3,4-dihydroxy-phenyl)-5-hydroxy-7-methoxy-4-oxo-chroman-3-yl ester) (3): [α]25D +40.0 (c 0.6) (lit. 26: [α]D +41.0 (c 0.84, MeOH)); 1H NMR (CDCl3, 400 MHz), δ, 11.46 (1H, br s, HO-5), 7.02 (1H, br s, H-2′), 6.87 (2H, br s, H-5′,6′), 6.10 (1H, br s, H-8), 6.04 (1H, br s, H-6), 5.83 (1H, d, J = 12.7 Hz, H-3), 5.22 (1H, d, J = 12.7 Hz, H-2), 3.81 3H, s, OMe), 2.05 (3H, s, MeCO) these data are very similar to those previously recorded at 200 MHz [26]; ESI MS m/z: 743 [2M + Na]+, 361 [M + Na]+.
3.6. Methylation of Quercetin
To quercetin (1, 30 mg) dissolved in MeOH (1 mL) was added an ethereal solution of diazomethane until yellow persisting color. After 2 h the reaction was stopped by evaporation under N2 stream. The residue was purified by TLC eluted with CHCl3-iso-PrOH (95:5) and three main derivatives were obtained. They are a tetramethyl- (8, 3.5 mg) and two dimethyl-quercetin derivatives (6 and 7, 6.6 and 3.6 mg, respectively).
7,4′-O,O′-Dimethylquercetin (6): 1H NMR (CD3OD, 500 MHz), δ, 7.71 (2H, br s, J = 2.1, Hz, H-2′,6′), 7.08 (1H, d, J = 8.0 Hz, H-5′), 6.62 (1H, brs, H-6), 6.33 (1H, br s = H-8), 3.96 (3H, s, 4′-OMe), 3.95 (3H, s, 7-OMe), 3.87 (3H, s OMe) these data are very similar to those previously recorded in CCl4 [58]; ESI MS m/z: 353 [M + Na]+.
3,7-O,O′-Dimethylquercetin (7): 1H NMR (CD3OD, 500 MHz), δ, 7.67 (1H, d, J = 2.1, Hz, H-2′), 7.58 (1H, dd, J = 8.5 and 2.1 Hz, H-6′), 6.92 (1H, d, J = 8.5 Hz, H-5′), 6.61 (1H, d, J = 2.2, H-6), 6.35 (1H, d, J = 2.2, H-8), 3.90 (3H, s, 7-OMe), 3.82 (3H, s, 3-OMe), 3.87 (3H, s OMe); these data are very similar to those previously recorded in CCl4 [59]; ESI MS m/z: 353 [M + Na]+.
3,7,3′,4′-O,O′,O′,O‴-Trimethylquercetin (8):1H NMR (CDCl3, 500 MHz), δ, 12.60 (1H, br s, HO-5), 7.74 (1H, dd, J = 8.6 and 2.7 Hz, H-6′), 7.70 (1H, d, J = 2.7, Hz, H-2′), 7.0 (1H, d, J = 8.6 Hz, H-5′), 6.46 (1H, d, J = 2.2, H-6), 6.36 (1H, d, J = 2.2, H-8), 3.97(6H, s, 2 x OMe), 3.88 (3H, s, MeO), 3.87 (3H, s OMe) these data are very similar to those previously recorded in CCl4 [59]; ESI MS m/z: 381 [M + Na]+.
3.7. Bioassay for Radicle Growth and Haustorium Induction
Allelopathic effects in radicle growth and haustorium induction by buckwheat and sunflower root exudates (0.02 g of root fresh weight /mL of root exudate solution), DMBQ (1 mM to 0.005 mM) and each of the following metabolites quercetin, apigenin, 3-O-acetylpadmatin (1–3), caffeic acid and p-coumaric acid methyl esters and the derivatives of quercetin 7,4′-O,O′- and 3,7-O,O′- dimethyl (6 and 7) and the 3,7,3′,4′-O,O′,O″,O‴-tetramethyl (8) (0.1 mM to 0.005 mM) was determined according to previous protocols [12,51,68,69]. Germination of broomrape seeds is achieved in the laboratory through a two-step process, a warm stratification called conditioning followed by an induction of germination by the synthetic strigolactone GR24 [70]. Broomrape seeds were surface sterilized by immersion in 0.5% (w/v) NaOCl and 0.02% (v/v) Tween 20, for 5 min, rinsed thoroughly with sterile distilled water, and dried in a laminar air flow cabinet. Approximately 100 seeds of each broomrape species were placed separately in 9 mm diameter glass fiber filter paper disks (GFFP) (Whatman International Ltd., Maidstone, UK) moistened with 50 μL of sterile distilled water and placed inside Petri dishes in incubators at 23 °C during 10 days to allow seed conditioning. GFFP disks containing conditioned broomrape seeds were transferred onto a sterile sheet of filter paper to remove the excess of water and transferred to new 10 cm sterile Petri dishes. Triplicate aliquots of 100 μL of each treatment described above, individually combined with the synthetic germination stimulant GR24 10−6 M were applied to GFFP discs. Treated seeds were incubated in the dark at 23 °C for 7 days and radicle growth and proportion of radicles that developed haustorium was determined for each GFFP disc using a stereoscopic microscope (Leica S9i, Leica Microsystems GmbH, Wetzlar, Germany).
3.8. Statistical Analysis
Percentage data were approximated to normal frequency distribution by means of angular transformation (180/π × arcsine (sqrt[%/100]) and subjected to analysis of variance (ANOVA) using SPSS software for Windows, version 21.0 (SPSS Inc., Chicago, IL, USA). The significant of mean differences between each treatment against the negative control was evaluated by the two-sided Dunnett test. Null hypothesis was rejected at the level of 0.05.
4. Conclusions
This manuscript reported for the first time the allelopathic potential of buckwheat root exudates and the effect of quercetin, isolated from buckwheat root, and its natural analogues apigenin and 3-O-acetylpadmatin isolated from L. stoechas and D. viscosa, on radicle growth of P. ramosa. SAR correlations were observed and discussed highlighting the importance for the activity of the quinone/hydroquinone oxo-reductive couple to impart activity. Besides reduction of radicle growth, haustorium induction was observed at the tip of P. ramosa radicles which swelled and a layer of papillae was formed. An additional haustorium assay was performed to study the haustorium inducing activity of quercetin in comparison with 2,6-dimethoxy-p-benzoquinone and a three partial methyl ether derivatives semisynthetized by quercetin. Results indicated that P. ramosa haustorium was induced by 2,6-dimethoxy-p-benzoquinone at concentrations of 1–0.5 mM and quercetin and its derivatives at concentration range 0.1–0.05 mM. In particular, the presence of two ortho-free hydroxy groups of C ring, like catechol, could be an important feature to impart activity while the carbon skeleton of B ring and substituents of both A and B rings are not essential. However, other experiments are needed to further support that the oxo-reductive mechanism is involved in reduction of radicle growth and haustorium induction activities.
Supplementary Materials
The Supplementary Materials are available online at https://www.mdpi.com/2223-7747/10/3/543/s1.
Author Contributions
M.F.-A., M.M., A.C., S.V. and A.E. designed the experimental work, M.F.-A., M.M., A.C., A.E. implemented the experiments, collected and analyzed the data, M.F.-A., M.M., A.C., A.E. wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
Financial support is acknowledged to M.F.-A. from the Spanish Ministry of Science and Innovation (RYC-2015-18961 and AGL2017-87693-R) and from CSIC-ALGOSUR research contract.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
Evidente is associated to Istituto di Chimica Biomolecolare del CNR, Pozzuoli, Italy.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Parker, C.; Riches, C.R. Parasitic Weeds of the World: Biology and Control; CAB International: Wallingford, UK, 1993. [Google Scholar]
- Parker, C. Observations on the current status of Orobanche and Striga problems worldwide. Pest Manag. Sci. 2009, 65, 453–459. [Google Scholar] [CrossRef]
- Fernández-Aparicio, M.; Delavault, P.; Timko, M. Management of infection by parasitic weeds: A review. Plants 2020, 9, 1184. [Google Scholar] [CrossRef]
- Barzman, M.; Barberi, P.; Birch, A.N.E.; Boonekamp, P.; Dachbrodt-Saaydeh, S.; Graf, B.; Hommel, B.; Jensen, J.E.; Kiss, J.; Kudsk, P.; et al. Eight principles of integrated pest management. Agron. Sustain. Dev. 2015, 35, 1199–1215. [Google Scholar] [CrossRef]
- Jeschke, P. Progress of modern agricultural chemistry and future prospects. Pest Manag. Sci. 2016, 72, 433–455. [Google Scholar] [CrossRef]
- Westwood, J.H.; Charudattan, R.; Duke, S.O.; Fennimore, S.A.; Marrone, P.; Slaughter, D.C.; Swanton, C.; Zollinger, R. Weed Management in 2050: Perspectives on the Future of Weed Science. Weed Sci. 2018, 66, 275–285. [Google Scholar] [CrossRef]
- Vurro, M.; Boari, A.; Evidente, A.; Andolfi, A.; Zermane, N. Natural metabolites for parasitic weed management. Pest Manag. Sci. 2009, 65, 566–571. [Google Scholar] [CrossRef]
- Evidente, A.; Fernandez-Aparicio, M.; Cimmino, A.; Rubiales, D.; Andolfi, A.; Motta, A. Peagol and peagoldione, two new strigolactone like metabolites isolated from pea root exudates. Tetrahedron Lett. 2009, 50, 6955–6958. [Google Scholar] [CrossRef]
- Evidente, A.; Cimmino, A.; Fernandez-Aparicio, M.; Andolfi, A.; Rubiales, D.; Motta, A. Polyphenols, including the new peapolyphenols A−C, from pea root exudates stimulate Orobanche foetida seed germination. J. Agric. Food Chem. 2010, 58, 2902–2907. [Google Scholar] [CrossRef] [PubMed]
- Evidente, A.; Andolfi, A.; Cimmino, A. Fungal phytotoxins for control of Cirsium arvense and Sonchus arvensis. Pest Technol. 2011, 5, 1–17. [Google Scholar]
- Evidente, A.; Cimmino, A.; Fernández-Aparicio, M.; Rubiales, D.; Andolfi, A.; Melck, D. Soyasapogenol B and trans-22-dehydrocampesterol from common vetch (Vicia sativa L.) root exudates stimulate broomrape seed germination. Pest Manag. Sci. 2011, 67, 1015–1022. [Google Scholar] [CrossRef] [PubMed]
- Cimmino, A.; Fernández-Aparicio, M.; Andolfi, A.; Basso, S.; Rubiales, D.; Evidente, A. Effect of fungal and plant metabolites on broomrapes (Orobanche and Phelipanche spp.) seed germination and radicle growth. J. Agric. Food Chem. 2014, 62, 10485–10492. [Google Scholar] [CrossRef] [PubMed]
- Cimmino, A.; Masi, M.; Evidente, M.; Superchi, S.; Evidente, A. Fungal phytotoxins with potential herbicidal activity: Chemical and biological characterization. Nat. Prod. Rep. 2015, 32, 1629–1653. [Google Scholar] [CrossRef] [PubMed]
- Cimmino, A.; Fernández-Aparicio, M.; Avolio, F.; Yoneyama, K.; Rubiales, D.; Evidente, A. Ryecyanatines A and B and ryecarbonitrilines A and B, substituted cyanatophenol, cyanatobenzo [1,3] dioxole, and benzo [1,3] dioxolecarbonitriles from rye (Secale cereale L.) root exudates: Novel metabolites with allelopathic activity on Orobanche seed germination and radicle growth. Phytochemistry 2015, 109, 57–65. [Google Scholar]
- Andolfi, A.; Zermane, N.; Cimmino, A.; Avolio, F.; Boari, A.; Vurro, M.; Evidente, A. Inuloxins A–D, phytotoxic bi-and tri-cyclic sesquiterpene lactones produced by Inula viscosa: Potential for broomrapes and field dodder management. Phytochemistry 2013, 86, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Cala, A.; Molinillo, J.M.G.; Fernandez-Aparicio, M.; Ayuso, J.; Alvarez, J.A.; Rubiales, D.; Macias, F.A. Complexation of sesquiterpene lactones with cyclodextrins: sSynthesis and effects on their activities on parasitic weeds. Org. Biomol. Chem. 2017, 15, 6500–6510. [Google Scholar] [CrossRef] [PubMed]
- Moeini, A.; Masi, M.; Zonno, M.C.; Boari, A.; Cimmino, A.; Tarallo, O.; Vurro, M.; Evidente, A. Encapsulation of inuloxin A, a plant germacrane sesquiterpene with potential herbicidal activity, in β-cyclodextrins. Org. Biomol. Chem. 2019, 17, 2508–2515. [Google Scholar] [CrossRef] [PubMed]
- Serino, N.; Boari, A.; Santagata, G.; Masi, M.; Malinconico, M.; Evidente, A.; Vurro, M. Biodegradable polymers to improve herbicidal effectiveness of Dittrichia viscosa plant organic extracts. Pest Man. Sci. 2020, 77, 646–658. [Google Scholar] [CrossRef] [PubMed]
- Ohsawa, R.; Nakatani, C. Inter-and intra-cultivar variations in the allelopathic effect of leaf aqueous extract of buckwheat (Fagopyrum esculentum Moench) on the growth of lettuce seedling. Fagopyrum 2005, 22, 21–24. [Google Scholar]
- Falquet, B.; Gfeller, A.; Pourcelot, M.; Tschuy, F.; Wirth, J. Weed suppression by common buckwheat: A review. Environ. Control Biol. 2015, 53, 1–6. [Google Scholar] [CrossRef]
- Kalinova, J.; Triska, J.; Vrchotova, N. Biological activity of phenolic compounds present in buckwheat plants. Allelopathy J. 2005, 16, 123–129. [Google Scholar]
- Kalinova, J.; Vrchotova, N. Level of catechin, myricetin, quercetin and isoquercitrin in buckwheat (Fagopyrum esculentum Moench), changes of their levels during vegetation and their effect on the growth of selected weeds. J. Agric. Food Chem. 2009, 57, 2719–2725. [Google Scholar] [CrossRef]
- Szwed, M.; Wiczkowski, W.; Szawara-Nowak, D.; Obendorf, R.L.; Horbowicz, M. Allelopathic influence of common buckwheat root residues on selected weed species. Acta Physiol. Plant. 2019, 41, 92. [Google Scholar] [CrossRef]
- Kalinova, J.; Vrchotova, N.; Triska, J. Exudation of allelopathic substances in buckwheat (Fagopyrum esculentum Moench). J. Agric. Food Chem. 2007, 55, 6453–6459. [Google Scholar] [CrossRef] [PubMed]
- Masi, M.; Pannacci, E.; Santoro, E.; Zermane, N.; Superchi, S.; Evidente, A. Stoechanones A and B, phytotoxic copaane sesquiterpenoids isolated from Lavandula stoechas with potential herbicidal activity against Amaranthus retroflexus. J. Nat. Prod. 2020, 83, 1658–1665. [Google Scholar] [CrossRef] [PubMed]
- Grande, M.; Piera, F.; Cuenca, A.; Torres, P.; Bellido, I.S. Flavonoids from Inula viscosa. Planta Med. 1985, 51, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, R.; Devadasu, C.; Srinivasa Babu, P. Isolation, characterization, and RP-HPLC estimation of p-coumaric acid from methanolic extract of durva grass (Cynodon dactylon Linn.)(Pers.). Int. J. Anal. Chem. 2015. [Google Scholar] [CrossRef]
- Dewick, P.M. Medicinal Natural Products—A Biosynthetic Approach; Wiley and Sons Ltd.: Chicester, UK, 2009. [Google Scholar]
- Osbourn, A.E.; Lanzotti, V. Plant-Derived Products; Springer: Drdrecht, Germany, 2009. [Google Scholar]
- Mottaghipisheh, J.; Iriti, M. Sephadex® LH-20, Isolation, and purification of flavonoids from plant species: A comprehensive review. Molecules 2020, 25, 4146. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.F.; Liu, S.S.; Song, Z.Q.; Xu, T.C.; Liu, C.S.; Hou, Y.G.; Wu, S.H. Naturally occurring flavonoids and isoflavonoids and their microbial transformation: A review. Molecules 2020, 25, 5112. [Google Scholar] [CrossRef]
- Turner, W.B.; Aldridge, D.C. Fungal Metabolites II; Academic Press: London, UK, 1983. [Google Scholar]
- Golisz, A.; Lata, B.; Gawronski, S.W.; Fujii, Y. Specific and total activities of allelochemicals identified in buckwheat. Weed Biol. Man. 2007, 7, 164–171. [Google Scholar] [CrossRef]
- Patil, B.S.; Pike, L.M.; Hamilton, B.K. Changes in quercetin concentration in onion (Allium cepa L.) owing to location, growth stage and soil type. New Phytol. 1995, 130, 349–355. [Google Scholar] [CrossRef]
- Hofmann, R.W.; Swinny, E.E.; Bloor, S.J.; Markham, K.R.; Ryan, K.G.; Campbell, B.D.; Jordan, B.R.; Fountain, D.W. Responses of nine Trifolium repens L. populations to ultraviolet-B radiation: Differential flavonol glycoside accumulation and biomass production. Ann. Bot. 2000, 86, 527–537. [Google Scholar] [CrossRef]
- Holasová, M.; Fiedlerová, V.; Smrčinová, H.; Orsák, M.; Lachman, J.; Vavreinová, S. Buckwheat—The source of antioxidant activity in functional foods. Food Res. Int. 2002, 35, 207–211. [Google Scholar] [CrossRef]
- Kirakosyan, A.; Kaufman, P.; Warber, S.; Zick, S.; Aaronson, K.; Bolling, S.; Chang, S.C. Applied environmental stresses to enhance the levels of polyphenolics in leaves of hawthorn plants. Physiol. Plant. 2004, 121, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Burczynski, F.; Campbell, C.; Pierce, G.; Austria, J.A.; Briggs, C.J. Rutin and flavonoid contents in three buckwheat species Fagopyrum esculentum, F. tataricum, and F. homotropicum and their protective effects against lipid peroxidation. Food Res. Int. 2007, 40, 356–364. [Google Scholar] [CrossRef]
- Brunori, A.; Sándor, G.; Xie, H.; Baviello, G.; Nehiba, B.; Rabnecz, G.; Végvári, G. Rutin content of the grain of 22 buckwheat (Fagopyrum esculentum Moench and Fagopyrum tataricum Gaertn.) varieties grown in Hungary. Eur. J. Plant Sci. Biotechnol. 2009, 3, 62–65. [Google Scholar]
- Suzuki, T.; Watanabe, M.; Iki, M.; Aoyagi, Y.; Kim, S.J.; Mukasa, Y.; Kokota, S.; Takigawa, S.; Hashimoto, N.; Noda, T.; et al. Time-course study and effects of drying method on concentrations of γ-aminobutyric acid, flavonoids, anthocyanin, and 2″-hydroxynicotianamine in leaves of buckwheat. J. Agric. Food Chem. 2009, 57, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Bystrická, J.; Vollmannová, A.; Kupecsek, A.; Musilová, J.; Poláková, Z.; Čičová, I.; Bojňanska, T. Bioactive compounds in different plant parts of various buckwheat (Fagopyrum esculentum Moench.) cultivars. Cereal Res. Commun. 2011, 39, 436–444. [Google Scholar] [CrossRef]
- Sugiura, M.; Naito, Y.; Yamaura, Y.; Fukaya, C.; Yokoyama, K. Inhibitory activities and inhibition specificities of caffeic acid derivatives and related compounds toward 5-lipoxygenase. Chem. Pharm. Bull. 1989, 37, 1039–1043. [Google Scholar] [CrossRef]
- Rabee, M.; Andersen, Ø.M.; Fossen, T.; Enerstvedt, K.H.; Abu Ali, H.; Rayyan, S. Acylated flavone O-glucuronides from the aerial parts of Nepeta curviflora. Molecules 2020, 25, 3782. [Google Scholar] [CrossRef] [PubMed]
- Breitmaier, E.; Voelter, W. Carbon-13 NMR Spectroscopy; VCH: Weinheim, Germany, 1987; pp. 183–280. [Google Scholar]
- Gripenberg, J. Flavones. In The Chemistry of Flavonoid Compounds; Geissman, T.A., Ed.; Pergamon Press, Inc.: Elmsford, NY, USA, 1962; pp. 406–440. [Google Scholar]
- Tsai, S.M.; Phillips, D.A. Flavonoids released naturally from alfalfa promote development of symbiotic Glomus spores in vitro. Appl. Environ. Microbiol. 1991, 57, 1485–1488. [Google Scholar] [CrossRef] [PubMed]
- Hungria, M.; Phillips, D.A. Effects of a seed color mutation on rhizobial nod-gene-inducing flavonoids and nodulation in common bean. Mol. Plant Microbe Interact. 1993, 6, 418–422. [Google Scholar] [CrossRef]
- Jia, Z.; Zou, B.; Wang, X.; Qiu, J.; Ma, H.; Gou, Z.; Song, S.; Dong, H. Quercetin induces H2O2 in Arabidopsis thaliana mediating resistance against Pseudomonas syringae. Biochem. Biophys. Res. Commun. 2010, 396, 522–527. [Google Scholar] [CrossRef]
- Wada, S.; Cui, S.; Yoshida, S. Reactive Oxygen Species (ROS) generation is indispensable for haustorium formation of the root parasitic plant Striga hermonthica. Front. Plant Sci. 2019, 10, 328. [Google Scholar] [CrossRef]
- Albrecht, H.; Yoder, J.I.; Phillips, D.A. Flavonoids promote haustoria formation in the root parasite Triphysaria versicolor. Plant Physiol. 1999, 119, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Aparicio, M.; Masi, M.; Maddau, L.; Cimmino, A.; Evidente, M.; Rubiales, D.; Evidente, A. Induction of haustorium development by sphaeropsidones in radicles of the parasitic weeds Striga and Orobanche. A structure-activity relationship study. J. Agric. Food Chem. 2016, 64, 5188–5196. [Google Scholar] [CrossRef]
- Jamison, D.S.; Yoder, J.I. Heritable variation in quinone-induced haustorium development in the parasitic plant Triphysaria. Plant Physiol. 2001, 125, 1870–1879. [Google Scholar] [CrossRef] [PubMed]
- Palmer, A.G.; Gao, R.; Maresh, J.; Erbil, W.K.; Lynn, D.G. Chemical biology of multi-host/pathogen interactions: Chemical perception and metabolic complementation. Annu. Rev. Phytopathol. 2004, 42, 439–464. [Google Scholar] [CrossRef]
- Andolfi, A.; Maddau, L.; Basso, S.; Linaldeddu, B.T.; Cimmino, A.; Scanu, B.; Deidda, A.; Tuzi, A.; Evidente, A. Diplopimarane, a 20-nor-ent-pimarane produced by the oak pathogen Diplodia quercivora. J. Nat. Prod. 2014, 77, 2352–2360. [Google Scholar] [CrossRef]
- Cala, A.; Masi, M.; Cimmino, A.; Molinillo, J.M.G.; Macias, F.A.; Evidente, A. (+)-epi-Epoformin, a phytotoxin fungal cyclohexenepoxide: Structure activity relationships. Molecules 2018, 23, 1529. [Google Scholar] [CrossRef] [PubMed]
- Andolfi, A.; Cimmino, A.; Villegas-Fernandez, A.M.; Tuzi, A.; Santini, A.; Melck, D.; Rubiales, D.; Evidente, A. Lentisone, a new phytotoxic anthraquinone produced by Ascochyta lentis, the causal agent of Ascochyta Blight in Lens culinaris. J. Agric. Food Chem. 2013, 61, 7301–7308. [Google Scholar] [CrossRef]
- Masi, M.; Nocera, P.; Zonno, M.C.; Tuzi, A.; Pescitelli, G.; Cimmino, A.; Boari, A.; Infantino, A.; Vurro, M.; Evidente, A. Lentiquinones A, B, and C, phytotoxic anthraquinone derivatives isolated from Ascochyta lentis, a pathogen of lentil. J. Nat. Prod. 2018, 81, 2700–2709. [Google Scholar] [CrossRef] [PubMed]
- Haraguchi, H.; Hashimoto, K.; Yagi, A. Antioxidative substances in leaves of Polygonum hydropiper. J. Agric. Food Chem. 1992, 40, 1349–1351. [Google Scholar] [CrossRef]
- Valesi, A.G.; Rodriguez, E.; Vander Velde, G.; Mabry, T.J. Methylated flavonols in Larrea cuneifolia. Phytochemistry 1972, 11, 2821–2826. [Google Scholar] [CrossRef]
- Berger, S.; Braun, S. 200 and More Basic NMR Experiments: A Practical Course, 1st ed.; Wiley-VCH: Weinheim, Germany, 2004. [Google Scholar]
- Keyes, W.J.; Palmer, A.G.; Erbil, W.K.; Taylor, J.V.; Apkarian, R.P.; Weeks, E.R.; Lynn, D.G. Semagenesis and the parasitic angiosperm Striga asiatica. Plant J. 2007, 51, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Joel, D.M.; Losner-Goshen, D. The attachment organ of the parasitic angiosperms Orobanche cumana and O. aegyptiaca and its development. Can. J. Bot. 1994, 72, 564–574. [Google Scholar] [CrossRef]
- Westwood, J.H.; Yoder, J.I.; Timko, M.P.; dePamphilis, C.W. The evolution of parasitism in plants. Trends Plant Sci. 2010, 15, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Cui, S.; Ichihashi, Y.; Shirasu, K. The haustorium, a specialized invasive organ in parasitic plants. Ann. Rev. Plant Biol. 2016, 67, 643–667. [Google Scholar] [CrossRef] [PubMed]
- Goyet, V.; Billard, E.; Pouvreau, J.B.; Lechat, M.M.; Pelletier, S.; Bahut, M.; Monteau, F.; Spichal, L.; Delavault, P.; Montiel, G.; et al. Haustorium initiation in the obligate parasitic plant Phelipanche ramosa involves a host-exudated cytokinin signal. J. Exp. Bot. 2017, 68, 5539–5552. [Google Scholar] [CrossRef]
- Wang, Y.; Steele, D.; Murdock, M.; Lai, S.; Yoder, J. Small-molecule screens reveal novel haustorium inhibitors in the root parasitic plant Triphysaria versicolor. Phytopathology 2019, 109, 1878–1887. [Google Scholar] [CrossRef]
- Bandaranayake, P.C.G.; Filappova, T.; Tomilov, A.; Tomilova, N.B.; Jamison-McClung, D.; Ngo, Q.; Inoue, K.; Yoder, J.I. A single-electron reducing quinone oxidoreductase is necessary to induce haustorium development in the root parasitic plant Triphysaria. Plant Cell 2010, 22, 1404–1419. [Google Scholar] [CrossRef]
- Fernández-Aparicio, M.; Moral, A.; Kharrat, M.; Rubiales, D. Resistance against broomrapes (Orobanche and Phelipanche spp.) in faba bean (Vicia faba) based in low induction of broomrape seed germination. Euphytica 2012, 186, 897–905. [Google Scholar] [CrossRef]
- Fernández-Aparicio, M.; Cimmino, A.; Evidente, A.; Rubiales, D. Inhibition of Orobanche crenata seed germination and radicle growth by allelochemicals identified in cereals. J. Agric. Food Chem. 2013, 61, 9797–9803. [Google Scholar] [CrossRef] [PubMed]
- Lechat, M.M.; Pouvreau, J.B.; Péron, T.; Gauthier, M.; Montiel, G.; Veronesi, C.; Todoroki, Y.; Le Bizec, B.; Monteau, F.; Macherel, D.; et al. PrCYP707A1, an ABA catabolic gene, is a key component of Phelipanche ramosa seed germination in response to the strigolactone analogue GR24. J. Exp. Bot. 2012, 63, 5311–5322. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).