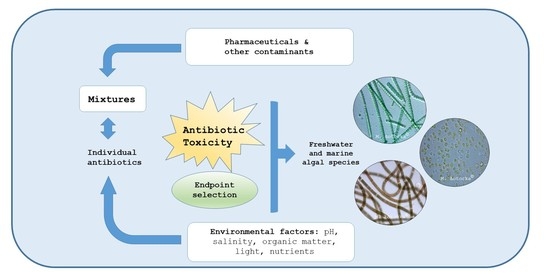
The Toxic Effects of Antibiotics on Freshwater and Marine Photosynthetic Microorganisms: State of the Art
Abstract
:1. Introduction
2. Summary of Available Toxicity Data on Individual Antibiotics
2.1. Biomass Endpoint Assessment
2.2. Biochemical Endpoints Assessment
3. The Mixture Effects
4. Environmental Factors Influencing the Toxicity of Antibiotics
5. Conclusions and Future Research Trends
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Boxall, A.B.A.; Fogg, L.A.; Blackwell, P.A.; Kay, P.; Pemberton, E.J.; Croxford, A. Veterinary medicines in the environment. Rev. Environ. Contam. Toxicol. 2004, 180, 1–91. [Google Scholar] [PubMed]
- Halling-Sørensen, B.N.N.S.; Nielsen, S.N.; Lanzky, P.F.; Ingerslev, F.; Lützhøft, H.H.; Jørgensen, S.E. Occurrence, fate, and effects of pharmaceutical substances in the environment—A review. Chemosphere 1998, 36, 357–393. [Google Scholar] [CrossRef]
- Siedlewicz, G.; Białk-Bielińska, A.; Borecka, M.; Winogradow, A.; Stepnowski, P.; Pazdro, K. Presence, concentrations and risk assessment of selected antibiotic residues in sediments and near-bottom waters collected from the Polish coastal zone in the southern Baltic Sea—Summary of 3 years of studies. Mar. Pollut. Bull. 2018, 129, 787–801. [Google Scholar] [CrossRef] [PubMed]
- Kümmerer, K. Pharmaceuticals in the Environment. Annu. Rev. Environ. Resour. 2010, 35, 57–75. [Google Scholar] [CrossRef] [Green Version]
- Pazdro, K.; Borecka, M.; Siedlewicz, G.; Biak-Bieliska, A.; Stepnowski, P. Analysis of the residues of pharmaceuticals in marine environment: State-of-the-art, analytical problems and challenges. Curr. Anal. Chem. 2016, 12, 202–226. [Google Scholar] [CrossRef]
- Branchet, P.; Arpin-Pont, L.; Piram, A.; Boissery, P.; Wong-Wah-Chung, P.; Doumenq, P. Pharmaceuticals in the marine environment: What are the present challenges in their monitoring? Sci. Total Environ. 2021, 766, 142644. [Google Scholar] [CrossRef] [PubMed]
- Aus der Beek, T.; Weber, F.A.; Bergmann, A.; Hickmann, S.; Ebert, I.; Hein, A.; Küster, A. Pharmaceuticals in the environment—Global occurrences and perspectives. Environ. Toxicol. Chem. 2016, 35, 823–835. [Google Scholar] [CrossRef]
- Szymańska, U.; Wiergowski, M.; Sołtyszewski, I.; Kuzemko, J.; Wiergowska, G.; Woźniak, M.K. Presence of antibiotics in the aquatic environment in Europe and their analytical monitoring: Recent trends and perspectives. Microchem. J. 2019, 147, 729–740. [Google Scholar] [CrossRef]
- Klein, E.Y.; Van Boeckel, T.P.; Martinez, E.M.; Pant, S.; Gandra, S.; Levin, S.A.; Goossens, H.; Laxminarayan, R. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc. Natl. Acad. Sci. USA 2018, 115, E3463–E3470. [Google Scholar] [CrossRef] [Green Version]
- Hughes, S.R.; Kay, P.; Brown, L.E. Global synthesis and critical evaluation of pharmaceutical data sets collected from river systems. Environ. Sci. Technol. 2012, 47, 661–677. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhang, W.; Li, J.; Yuan, M.; Zhang, J.; Xu, F.; Xu, H.; Zheng, X.; Wang, L. Ecotoxicological effects of sulfonamides and fluoroquinolones and their removal by a green alga (Chlorella vulgaris) and a cyanobacterium (Chrysosporum ovalisporum). Environ. Pollut. 2020, 263, 114554. [Google Scholar] [CrossRef]
- UNESCO; HELCOM. Pharmaceuticals in the Aquatic Environment of the Baltic Sea Region—A Status Report; (UNESCO Publishing No. No. 1), UNESCO Emerging Pollutants in Water Series; UNESCO: Paris, France, 2017. [Google Scholar]
- EU 2015/495. Commission Implementing Decision (EU) 2015/495 of 20 March 2015 Establishing a Watch List of Substances for Union-Wide Monitoring in the Field of Water Policy Pursuant to Directive 2008/105/EC of the European Parliament and of the Council. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32018D0840&rid=7 (accessed on 19 March 2021).
- Guo, J.; Boxall, A.; Selby, K. Do Pharmaceuticals Pose a Threat to Primary Producers? Crit. Rev. Environ. Sci. Technol. 2015, 45, 2565–2610. [Google Scholar] [CrossRef]
- Halling-Sørensen, B. Algal toxicity of antibacterial agents used in intensive farming. Chemosphere 2000, 40, 731–739. [Google Scholar] [CrossRef]
- Fu, L.; Huang, T.; Wang, S.; Wang, X.; Su, L.; Li, C.; Zhao, Y. Toxicity of 13 different antibiotics towards freshwater green algae Pseudokirchneriella subcapitata and their modes of action. Chemosphere 2017, 168, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Pleiter, M.; Gonzalo, S.; Rodea-Palomares, I.; Leganes, F.; Rosal, R.; Boltes, K.; Marco, E.; Fernandez-Pinas, F. Toxicity of five antibiotics and their mixtures towards photosynthetic aquatic organisms: Implications for environmental risk assessment. Water Res. 2013, 47, 2050–2064. [Google Scholar] [CrossRef]
- González-Pleiter, M.; Cirés, S.; Hurtado-Gallego, J.; Leganés, F.; Fernández-Piñas, F.; Velázquez, D. Chapter 20—Ecotoxicological Assessment of Antibiotics in Freshwater Using Cyanobacteria. In Cyanobacteria; Mishra, A.K., Tiwari, D.N., Rai, A.N., Eds.; Academic Press: Cambridge, UK, 2019; pp. 399–417. [Google Scholar]
- Liu, B.Y.; Nie, X.P.; Liu, W.Q.; Snoeijs, P.; Guan, C.; Tsui, M.T. Toxic effects of erythromycin, ciprofloxacin and sulfamethoxazole on photosynthetic apparatus in Selenastrum capricornutum. Ecotoxicol. Environ. Saf. 2011, 74, 1027–1035. [Google Scholar] [CrossRef]
- Test No. 201: Freshwater alga and cyanobacteria, growth inhibition test. In OECD Guidelines for the Testing of Chemicals in Section 2: Effects on Biotic Systems; OECD: Paris, France, 2011; Available online: https://search.oecd.org/env/test-no-201-alga-growth-inhibition-test-9789264069923-en.htm (accessed on 19 March 2021).
- European Commission. Technical Guidance Documents in Support of the Commission Directive 93/67/EEC on Risk Assessment for New Substances and the Commission Regulation (EC) No 1488/94 on Risk Assessment for Existing Substances; Off. of Pubication for the European Commission: Luxembourg, 1996; Available online: https://echa.europa.eu/documents/10162/16960216/tgdpart1_2ed_en.pdf (accessed on 19 March 2021).
- Ferrari, B.; Mons, R.; Vollat, B.; Fraysse, B.; Paxēaus, N.; Giudice, R.L. Environmental risk assessment of six human pharmaceuticals: Are the current environmental risk assessment procedures sufficient for the protection of the aquatic environment. Environ. Toxicol. Chem. 2004, 23, 1344–1354. [Google Scholar] [CrossRef] [Green Version]
- Nie, X.P.; Liu, B.Y.; Yu, H.; Liu, W.; Yang, Y. Toxic effects of erythromycin, ciprofloxacin, and sulfamethoxazole exposure to the antioxidant system in Pseudokirchneriella subcapitata. Environ. Pollut. 2013, 172, 23–32. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, S.; Zhang, J.; Li, X.; Gao, B. Stimulation effects of ciprofloxacin and sulphamethoxazole in Microcystis aeruginosa and isobaric tag for relative and absolute quantitation-based screening of antibiotic targets. Mol. Ecol. 2017, 26, 689–701. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chen, Q.; Hu, L.; Wang, M. Combined effects of binary antibiotic mixture on growth, microcystin production, and extracellular release of Microcystis aeruginosa: Application of response surface methodology. Environ. Sci. Pollut. Res. 2018, 25, 736–748. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chen, Q.; Zhang, J.; Dong, J.; Ao, Y.; Wang, M.; Wang, X. Long-term exposure to antibiotic mixtures favors microcystin synthesis and release in Microcystis aeruginosa with different morphologies. Chemosphere 2019, 235, 344–353. [Google Scholar] [CrossRef]
- Liu, L.; Wu, W.; Zhang, J.; Lv, P.; Xu, L.; Yan, Y. Progress of research on the toxicology of antibiotic pollution in aquatic organisms. Acta Ecol. Sin. 2018, 38, 36–41. [Google Scholar] [CrossRef]
- Teixeira, J.R.; Granek, E.F. Effects of environmentally-relevant antibiotic mixtures on marine microalgal growth. Sci. Total Environ. 2017, 580, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Selby, K.; Boxall, A.B. Effects of Antibiotics on the Growth and Physiology of Chlorophytes, Cyanobacteria, and a Diatom. Arch. Environ. Contam. Toxicol. 2016, 71, 589–602. [Google Scholar] [CrossRef] [Green Version]
- EU COM. 2019. Available online: http://ec.europa.eu/environment/water/water-dangersub/pdf/strategic_approach_pharmaceuticals_env.PDF (accessed on 19 March 2021).
- Grenni, P.; Ancona, V.; Caraccio, A.B. Ecological effects of antibiotics on natural ecosystems: A review. Microchem. J. 2018, 136, 25–39. [Google Scholar] [CrossRef]
- Falciatore, A.; Jaubert, M.; Bouly, J.P.; Bailleul, B.; Mock, T. Diatom Molecular Research Comes of Age: Model Species for Studying Phytoplankton Biology and Diversity. Plant Cell. 2020, 32, 547–572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- EMEA, 2006, European Medicines Agency. Committee For Medicinal Products For Human Use—Guideline on the Environmental Risk Assessment of Medicinal Products in Man, London, UK, 1 June 2006. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-environmental-risk-assessment-medicinal-products-human-use-first-version_en.pdf (accessed on 19 March 2021).
- Jiang, Y.; Liu, Y.; Zhang, J. Antibiotic contaminants reduced the treatment efficiency of UV-C on Microcystis aeruginosa through hormesis. Environ. Pollut. 2020, 261, 114193. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, S.; Zhang, J.; Gao, B. Growth, microcystin-production and proteomic responses of Microcystis aeruginosa under long-term exposure to amoxicillin. Water Res. 2016, 93, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Steinman, A.D.; Xue, Q.; Zhao, Y.; Xu, Y.; Xie, L. Effects of erythromycin and sulfamethoxazole on Microcystis aeruginosa: Cytotoxic endpoints, production and release of microcystin-LR. J. Hazard. Mater. 2020, 399, 123021. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Guo, P.; Peng, X.; Wen, K. Effect of erythromycin exposure on the growth, antioxidant system and photosynthesis of Microcystis flos-aquae. J. Hazard. Mater. 2015, 283, 778–786. [Google Scholar] [CrossRef]
- Qian, H.; Li, J.; Pan, X.; Sun, Z.; Ye, C.; Jin, G.; Fu, Z. Effects of streptomycin on growth of algae Chlorella vulgaris and Microcystis aeruginosa. Environ. Toxicol. 2012, 27, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Liu, Y.; Zhang, J. Mechanisms for the stimulatory effects of a five-component mixture of antibiotics in Microcystis aeruginosa at transcriptomic and proteomic levels. J. Hazard. Mater. 2021, 406, 124722. [Google Scholar] [CrossRef]
- Tomar, R.S.; Singh, B.; Jajoo, A. Effects of Organic Pollutants on Photosynthesis. In Photosynthesis, Productivity and Environmental Stress; Ahmad, P., Abass Ahanger, M., Nasser Alyemeni, M., Alam, P., Eds.; John Wiley & Sons Ltd: Chichester, UK, 2019; pp. 1–18. [Google Scholar] [CrossRef]
- Sigfridsson, K.G.; Bernát, G.; Mamedov, F.; Styring, S. Molecular interference of Cd2+ with photosystem II. Biochim. Biophys. Acta 2004, 1659, 19–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Machado, M.; Soares, E. Impact of erythromycin on a non-target organism: Cellular effects on the freshwater microalga Pseudokirchneriella subcapitata. Aquat. Toxicol. 2019, 208, 179–186. [Google Scholar] [CrossRef] [Green Version]
- Siedlewicz, G.; Żak, A.; Sharma, L.; Kosakowska, A.; Pazdro, K. Effects of oxytetracycline on growth and chlorophyll a fluorescence in green algae (Chlorella vulgaris), diatom (Phaeodactylum tricornutum) and cyanobacteria (Microcystis aeruginosa and Nodularia spumigena). Oceanologia 2020, 62, 214–225. [Google Scholar] [CrossRef]
- Chen, S.; Wang, L.; Feng, W. Sulfonamides-induced oxidative stress in freshwater microalga Chlorella vulgaris: Evaluation of growth, photosynthesis, antioxidants, ultrastructure, and nucleic acids. Sci. Rep. 2020, 10, 8243. [Google Scholar] [CrossRef]
- Majewska, M.; Harshkova, D.; Guściora, M.; Aksmann, A. Phytotoxic activity of diclofenac: Evaluation using a model green alga Chlamydomonas reinhardtii with atrazine as a reference substance. Chemosphere 2018, 209, 989–997. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Pan, X.; Zhang, D. Influence of ofloxacin on photosystems I and II activities of Microcystis aeruginosa and the potential role of cyclic electron flow. J. Biosci. Bioeng. 2015, 119, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Deng, C.; Zhang, D.; Wang, J.; Mu, G.; Chen, Y. Toxic effects of amoxicillin on the photosystem II of Synechocystis sp. characterized by a variety of in vivo chlorophyll fluorescence tests. Aquat. Toxicol. 2008, 89, 207–213. [Google Scholar] [CrossRef]
- Kvíderová, J.; Henley, W.J. The effect of ampicillin plus streptomycin on growth and photosynthesis of two halotolerant chlorophyte algae. J. Appl. Phycol. 2005, 17, 301–307. [Google Scholar] [CrossRef]
- Liu, W.; Ming, Y.; Huang, Z.; Li, P. Impacts of florfenicol on marine diatom Skeletonema costatum through photosynthesis inhibition and oxidative damages. Plant Physiol. Biochem. 2012, 60, 165–170. [Google Scholar] [CrossRef]
- Guo, J.; Peng, J.; Lei, Y.; Kanerva, M.; Li, Q.; Song, J.; Guo, J.; Sun, H. Comparison of oxidative stress induced by clarithromycin in two freshwater microalgae Raphidocelis subcapitata and Chlorella vulgaris. Aquat. Toxicol. 2020, 219, 105376. [Google Scholar] [CrossRef]
- Latowski, D.; Kuczyńska, P.; Strzałka, K. Xanthophyll cycle—a mechanism protecting plants against oxidative stress. Redox Rep. 2011, 16, 78–90. [Google Scholar] [CrossRef]
- Allocati, N.; Federici, L.; Masulli, M.; Di Ilio, C. Glutathione transferases in bacteria. FEBS J. 2009, 276, 58–75. [Google Scholar] [CrossRef]
- Cameron, J.C.; Pakrasi, H.B. Glutathione facilitates antibiotic resistance and photosystem I stability during exposure to gentamicin in cyanobacteria. Appl. Environ. Microbiol. 2011, 77, 3547–3550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, X.; Zhu, Y.; Wang, Y.; Zhao, Q.; Huang, H. Effects of three antibiotics on growth and antioxidant response of Chlorella pyrenoidosa and Anabaena cylindrica. Ecotoxicol. Environ. Saf. 2021, 211, 111954. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, Y.; Guo, P. Effect of florfenicol and thiamphenicol exposure on the photosynthesis and antioxidant system of Microcystis flos-aquae. Aquat. Toxicol. 2017, 186, 67–76. [Google Scholar] [CrossRef]
- Seoane, M.; Rioboo, C.; Herrero, C.; Cid, Á. Toxicity induced by three antibiotics commonly used in aquaculture on the marine microalga Tetraselmis suecica (Kylin) Butch. Mar. Environ. Res. 2014, 101, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aderemi, A.O.; Novais, S.C.; Lemos, M.F.L.; Alves, L.M.; Hunter, C.; Pahl, O. Oxidative stress responses and cellular energy allocation changes in microalgae following exposure to widely used human antibiotics. Aquat. Toxicol. 2018, 203, 130–139. [Google Scholar] [CrossRef] [Green Version]
- Rico, A.; Zhao, W.; Gillissen, F.; Lürling, M.; Van den Brink, P.J. Effects of temperature, genetic variation and species competition on the sensitivity of algae populations to the antibiotic enrofloxacin. Ecotoxicol. Environ. Saf. 2018, 148, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Backhaus, T.; Altenburger, R.; Boedeker, W.; Faust, M.; Scholze, M.; Grimme, L.H. Predictability of the toxicity of a multiple mixture of dissimilarly acting chemicals to Vibrio fischeri. Environ. Toxicol. Chem. 2000, 19, 2348–2356. [Google Scholar] [CrossRef]
- Backhaus, T. Environmental Risk Assessment of Pharmaceutical Mixtures: Demands, Gaps and Possible Bridges. AAPS J. 2016, 18, 804–813. [Google Scholar] [CrossRef]
- Magdaleno, A.; Saenz, M.E.; Juárez, A.B.; Moretton, J. Effects of six antibiotics and their binary mixtures on growth of Pseudokirchneriella subcapitata. Ecotoxicol. Environ. Saf. 2015, 113, 72–78. [Google Scholar] [CrossRef]
- Carusso, S.; Juárez, A.B.; Moretton, J.; Magdaleno, A. Effects of three veterinary antibiotics and their binary mixtures on two green alga species. Chemosphere 2018, 194, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Hagenbuch, I.M.; Pinckney, J.L. Toxic effect of the combined antibiotics ciprofloxacin, lincomycin, and tylosin on two species of marine diatoms. Water Res. 2012, 46, 5028–5036. [Google Scholar] [CrossRef] [PubMed]
- Białk-Bielińska, A.; Caban, M.; Pieczyńska, A.; Stepnowski, P.; Stolte, S. Mixture toxicity of six sulfonamides and their two transformation products to green algae Scenedesmus vacuolatus and duckweed Lemna minor. Chemosphere 2017, 173. [Google Scholar] [CrossRef]
- Yang, L.H.; Ying, G.G.; Su, H.C.; Stauber, J.L.; Adams, M.S.; Binet, M.T. Growth-inhibiting effects of 12 antibacterial agents and their mixtures on the freshwater microalga Pseudokirchneriella subcapitata. Environ. Toxicol. Chem. 2008, 27, 1201–1208. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Zhang, J.; Gao, B.; Feng, S. Combined effects of two antibiotic contaminants on Microcystis aeruginosa. J. Hazard. Mater. 2014, 279, 148–155. [Google Scholar] [CrossRef]
- Xu, S.; Liu, Y.; Zhang, J.; Gao, B. Proteomic mechanisms for the combined stimulatory effects of glyphosate and antibiotic contaminants on Microcystis aeruginosa. Chemosphere 2021, 267, 129244. [Google Scholar] [CrossRef]
- Christensen, A.M.; Ingerslev, F.; Baun, A. Ecotoxicity of mixtures of antibiotics used in aquacultures. Environ. Toxicol. Chem. 2006, 25, 2208–2215. [Google Scholar] [CrossRef]
- Zhang, Y.; Cai, X.; Lang, X.; Qiao, X.; Li, X.; Chen, J. Insights into aquatic toxicities of the antibiotics oxytetracycline and ciprofloxacin in the presence of metal: Complexation versus mixture. Environ. Pollut. 2012, 166, 48–56. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, Y.; Zhang, J. Antibiotics induced alterations in cell density, photosynthesis, microcystin synthesis and proteomic expression of Microcystis aeruginosa during CuSO4 treatment. Aquat. Toxicol. 2020, 222, 105473. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, H.; Tamura, I.; Abe, R.; Takanobu, H.; Nakamura, A.; Suzuki, T.; Hirose, A.; Nishimura, T.; Tatarazako, N. Chronic toxicity of an environmentally relevant mixture of pharmaceuticals to three aquatic organisms (alga, daphnia, and fish). Environ. Toxicol. Chem. 2016, 35, 996–1006. [Google Scholar] [CrossRef]
- Xiong, J.Q.; Govindwar, S.; Kurade, M.B.; Paeng, K.J.; Roh, H.S.; Khan, M.A.; Jeon, B.H. Toxicity of sulfamethazine and sulfamethoxazole and their removal by a green microalga, Scenedesmus obliquus. Chemosphere 2019, 218, 551–558. [Google Scholar] [CrossRef]
- Geiger, E.; Hornek-Gausterer, R.; Saçan, M.T. Single and mixture toxicity of pharmaceuticals and chlorophenols to freshwater algae Chlorella vulgaris. Ecotoxicol. Environ. Saf. 2016, 129, 189–198. [Google Scholar] [CrossRef]
- Vannini, C.; Domingo, G.; Marsoni, M.; De Mattia, F.; Labra, M.; Castiglioni, S.; Bracale, M. Effects of a complex mixture of therapeutic drugs on unicellular algae Pseudokirchneriella subcapitata. Aquat. Toxicol. 2011, 101, 459–465. [Google Scholar] [CrossRef] [Green Version]
- Claessens, M.; Vanhaecke, L.; Wille, K.; Janssen, C.R. Emerging contaminants in Belgian marine waters: Single toxicant and mixture risks of pharmaceuticals. Mar. Pollut. Bull. 2013, 71, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Shi, L.J.; Yuan, T. Growth inhibitive effect of typical antibiotics and their mixtures on Selenastrum capricornutum. J. Environ. Health 2013, 30, 475–478. [Google Scholar]
- Iswarya, V.; Sharma, V.; Chandrasekaran, N.; Mukherjee, A. Impact of tetracycline on the toxic effects of titanium dioxide (TiO2) nanoparticles towards the freshwater algal species, Scenedesmus obliquus. Aquat. Toxicol. 2017, 193, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Prata, J.C.; Lavorante, B.R.B.O.; Montenegro, M.D.C.; Guilhermino, L. Influence of microplastics on the toxicity of the pharmaceuticals procainamide and doxycycline on the marine microalgae Tetraselmis chuii. Aquat. Toxicol. 2018, 197, 143–152. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, J.; Tao, M.T.; Xu, C.M.; Chen, M. Quantitative characterization of toxicity interaction within antibiotic-heavy metal mixtures on Chlorella pyrenoidosa by a novel area-concentration ratio method. Sci. Total Environ. 2021, 762, 144180. [Google Scholar] [CrossRef]
- Lu, L.; Wu, Y.; Ding, H.; Zhang, W. The combined and second exposure effect of copper (II) and chlortetracycline on fresh water algae, Chlorella pyrenoidosa and Microcystis aeruginosa. Environ. Toxicol. Pharmacol. 2015, 40, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Sendra, M.; Moreno-Garrido, I.; Blasco, J.; Araújo, C. Effect of erythromycin and modulating effect of CeO2 NPs on the toxicity exerted by the antibiotic on the microalgae Chlamydomonas reinhardtii and Phaeodactylum tricornutum. Environ. Pollut. 2018, 242, 357–366. [Google Scholar] [CrossRef]
- Xin, X.; Huang, G.; Zhang, B. Review of aquatic toxicity of pharmaceuticals and personal care products to algae. J. Hazard. Mater. 2020, 124619. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, F.; Chen, X.; Zhang, J.; Gao, B. Cellular responses and biodegradation of amoxicillin in Microcystis aeruginosa at different nitrogen levels. Ecotoxicol. Environ. Saf. 2015, 111, 138–145. [Google Scholar] [CrossRef]
- Guo, R.X.; Chen, J.Q. Phytoplankton toxicity of the antibiotic chlortetracycline and its UV light degradation products. Chemosphere 2012, 87, 1254–1259. [Google Scholar] [CrossRef]
- Borecka, M.; Białk-Bielińska, A.; Haliński, Ł.P.; Pazdro, K.; Stepnowski, P.; Stolte, S. The influence of salinity on the toxicity of selected sulfonamides and trimethoprim towards the green algae Chlorella vulgaris. J. Hazard. Mater. 2016, 308, 179–186. [Google Scholar] [CrossRef] [PubMed]


| Compounds | Microorganism | Species | Exposure Time (Days) | Endpoint | Interaction | Effective Concentration [mg/L] | Reference |
|---|---|---|---|---|---|---|---|
| Binary mixtures of AMX, ERY, LVX, NOR, TCN | Green algae | Raphidocelis subcapitata | 3 | Growth rate | Synergism | 0.01–1500 | [17] |
| Cyanobacteria | Anabaena CPB4337 | ||||||
| CLA and 9 APIs | Green algae | Raphidocelis subcapitata | 3 | Growth rate | Buffering effects (synergistic and antagonistic effects) | 6.25–100 | [71] |
| Binary mixtures of AMP, AMX, CEP, CIP, GEN, VAN | Green algae | Raphidocelis subcapitata | 3 | Growth rate | Synergism | 1–50 | [61] |
| SMX and TMP | Green algae | Isochrysis galbana, Chaetoceros Neogracile, Nannochloropsis oculata | 20 | Growth rate | No interactive effect | 0.0000075 (SMX) 0.0000085 (TMP) | [28] |
| SMZ and SMX | Green algae | Scenedesmus obliquus | 4 (12) | Growth rate, chlorophyll, and carotenoid content, carbohydrate, fatty acid methyl ester (FAMEs) | - | >0.0011 (NOEC) 0.15–0.52 0.53 | [72] |
| Binary mixtures of TMP, SMX, SMZ, CTC, TCN, CIP, NOR, TYL, ROX, CLA | Green algae | Raphidocelis subcapitata | 3 | Growth rate | Additive, synergistic and antagonistic effects | 0.000001–0.01 | [65] |
| Binary mixtures of SPM, AMP | Cyanobacteria | Microcystis aeruginosa | 7 | Growth rate, microcystin synthesis, chlorophyll a content | Synergism and antagonism (mixture ratio-dependent) | >0.001 | [25] |
| OTC, OXO, ERY, FLO, FLU | Green algae | Raphidocelis subcapitata | 2 | Chlorophyll fluorescence kinetics | Synergistic and antagonistic effects | 0.13–42 | [68] |
| SPM, AMP | Cyanobacteria | Microcystis aeruginosa | 28 | Microcystin synthesis, SOD, CAD activity, MDA content, gene expression | Synergism | 0.0003 | [26] |
| Binary mixtures of TYL, LCM, CIP | Diatoms | Cylindrotheca closterium, Navicula ramosissima | 5 | Growth rate | Synergistic and additive effects | 1 | [63] |
| AMX, SPM | Cyanobacteria | Microcystis aeruginosa | 7 | Growth rate, chlorophyll a content, gene expression, microcystin synthesis | Antagonism and synergism at different mix ratios | 0.008 | [66] |
| CTC, OTC, ENR | Green algae | Raphidocelis Subcapitata, Ankistrodesmus fusiformis | 4 | Growth rate | Additive, synergistic and antagonistic effects | 0.1–10 | [62] |
| AMX, CIP, SPM, SMX, TCN | Cyanobacteria | Microcystis aeruginosa | 14 | Growth rate, chlorophyll fluorescence kinetics (Fv/Fm), proteomic responses, gene expression, ROS activity | - | 0.00005–0.0005 | [39] |
| Binary mixtures of CIP and 3 APIs | Green algae | Chlorella vulgaris | 4 | Growth rate | Synergism | <100 | [73] |
| A mixture of CIP, LCM, OFX, SMX, and 9 APIs | Green algae | Raphidocelis subcapitata | 3 | Genotoxic and proteomic effects, chlorophyll and carotenoids content, growth rate | - | 0.000026–0.000249 | [74] |
| TMP and 7 APIs | Diatom | Phaeodactylum tricornutum | 3 | Growth rate | - | 2.4 (EC10) | [75] |
| CIP, SMX | Cyanobacteria | Microcystis aeruginosa | Growth rate, microcystin synthesis, proteomic responses | Synergism | 0.00002–0.0001 | [24] | |
| Binary mixtures of CEF, CPX, 7—ACA, TCN, CTC | Green algae | Raphidocelis subcapitata | 3 | Growth rate | Additive and antagonistic effects | 0.0001–1 | [76] |
| TCN and titanium dioxide nanoparticles (TiO2 NPs) | Green algae | Scenedesmus obliquus | 3 | Growth rate | Additive and antagonistic effects | 0.15–0.6 (TCN) 1.4–6 (TiO2 NPs) | [77] |
| DOX/ microplastics | Green algae | Tetraselmis chuii | 4 | Growth rate, chlorophyll a content | Synergism | EC50 = 221 EC50 = 142 In the mixture: EC50 = 111 EC50 = 72 | [78] |
| CIP, OTC, and 3 metals | Green algae | Scenedesmus obliquus | 4 | Growth rate | Synergistic effects | 0.16 (CIP) 0.23 (OTC) | [69] |
| AMX, SMX, CIP, TCN, and glyphosate | Cyanobacteria | Microcystis aeruginosa | 10 | Proteomic responses, chlorophyll fluorescence kinetics (Fv/Fm), microcystin synthesis | Synergism | 0.00004–0.0002 | [67] |
| AMX, CIP, SMX, TCN, and copper sulfate (CuSO4) | Cyanobacteria | Microcystis aeruginosa | 20 | Growth rate, chlorophyll fluorescence kinetics (Fv/Fm) chlorophyll a content and microcystin synthesis, gene expression | Alleviated toxicity of CuSO4 in the presence of antibiotics, increased growth rate, Fv/Fm value, chlorophyll a content and microcystin synthesis | 0.01–0.05 | [34] |
| KAN, PAR, TOB, and Cu | Green algae | Chlorella pyrenoidosa | Synergism (weak antagonism) | [79] | |||
| CTC and copper (II) | Green algae | Chlorella pyrenoidosa | 4 | Chlorophyll fluorescence kinetics (Fv/Fm), MDA, protein content, SOD activity, | Synergism | 6.89–37.7 | [80] |
| Cyanobacteria | Microcystis aeruginosa | ||||||
| ENR and cerium oxide nanoparticles (CeO2 NPs | Green algae | Chlamydomonas reinhardtii | 3 | Growth rate, chlorophyll fluorescence kinetics, ROS activity | ENR toxicity decrease | 0.01 and 0.1 (ERY) 0.1 (CeO2 NPs) | [81] |
| Diatom | Phaeodactylum tricornutum |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, L.; Siedlewicz, G.; Pazdro, K. The Toxic Effects of Antibiotics on Freshwater and Marine Photosynthetic Microorganisms: State of the Art. Plants 2021, 10, 591. https://doi.org/10.3390/plants10030591
Sharma L, Siedlewicz G, Pazdro K. The Toxic Effects of Antibiotics on Freshwater and Marine Photosynthetic Microorganisms: State of the Art. Plants. 2021; 10(3):591. https://doi.org/10.3390/plants10030591
Chicago/Turabian StyleSharma, Lilianna, Grzegorz Siedlewicz, and Ksenia Pazdro. 2021. "The Toxic Effects of Antibiotics on Freshwater and Marine Photosynthetic Microorganisms: State of the Art" Plants 10, no. 3: 591. https://doi.org/10.3390/plants10030591